Abstract
The physical cues presented to stem cells by the substrate on or in which they exist have been shown to play a crucial role in regulation of their behavior. Until recently, most research has focused on the effects of substrate elasticity on differentiation capability rather than maintenance of long-term proliferation and plasticity. The main goal of the present study is to study the interaction of amniotic fluid-derived stem (AFS) cells with growth substrata of different elasticity, which may extend their application potentials. Here, we investigate the effects of elastic modulus (E′), on AFS cell proliferation, morphology, cell surface marker expression, and autocrine stimulation of cell migration. AFS cells cultured on substrates of different E′ exhibited significant changes in proliferation and morphology. Immunohistochemistry revealed increased expression of cell surface markers associated with mesenchymal stem cells (MSCs) (CD44, CD90, CD105, and N-cadherin) in cells cultured on softer substrates. Additionally, AFS cells cultured on softer substrates induced autocrine stimulation of migration. Therefore, tailoring the elastic modulus of biomaterials to specific stiffness values is an effective method to control stem cell properties, which may modulate the effectiveness of their therapeutic applications.
Keywords: Elastic modulus, Amniotic fluid-derived stem cells, Biomaterial, Cell therapy, Cell mobility
1. Introduction
Advances in stem cell biology and identification of available sources that supply safe, and effective cells for therapy promise to yield regenerative medicine applications that will be useful in clinical settings. A critical aspect is to identify external factors that will maximize the therapeutic potential of these cells and the therapies in which they can be applied. The mechanical signals presented to stem cells by the surrounding environment are crucial in determining their phenotype and activity. Prevalent in the literature is the work performed by Engler et al. (2006) in which it was demonstrated that the elastic modulus (E′) of polyacrylamide gels coated with collagen determined lineage selection of MSCs. Further research that followed these discoveries suggested that matching the E′ of an in vitro matrix or substrate to that of the target tissue will improve and guide differentiation of the cells. Approximate E′ values for many tissues in the body are depicted in Fig. 1 (Engler et al., 2006; Vanderhooft et al., 2009). Additional targets for the substrate elasticity are the effects on stem cell expansion efficiency in vitro, while maintaining their stem differentiation potential (Dellatore et al., 2008) and the effect on migration, and expansion of a variety of cell types including macrophages (Nemir et al., 2010), neural stem progenitor cells (Leipzig and Shoichet, 2009), and muscle stem cells (Raab et al., 2010). In the case of the latter, after culture on substrates mimicking E′ of muscle tissue, they engrafted more efficiently into rat leg muscle in vivo (Gilbert et al., 2010). These examples are evidence that substrate E′ strongly influences both differentiation, expansion, and engraftment of stem cells, and can be fine tuned for cellular control.
Fig. 1.
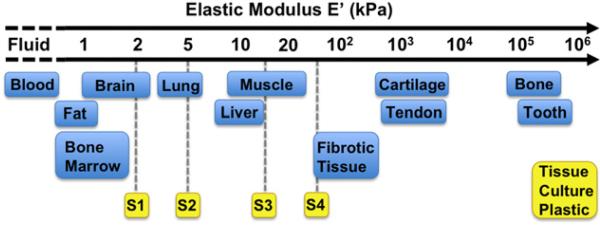
Plastic surfaces currently used for the majority of cell and tissue culture fail to replicate in vivo elastic moduli. S1, S2, S2, and S4 represent the physiologically-relevant substrates in this study.
Amniotic fluid-derived stem (AFS) cells are an attractive cell source for applications in regenerative medicine due to their high proliferation capacity, multipotency, immunomodulatory activity, and the lack of significant immunogenicity. Multipotent and expandable cells were first isolated from amniotic fluid by De Coppi et al. AFS cells expressed both embryonic stem cell and adult stem cell markers and could be expanded for over 250 passages (De Coppi et al., 2007; Delo et al., 2006; Kolambkar et al., 2007). These cells can be induced to differentiate into cells that represented each germ layer, such as adipogenic, osteogenic, myogenic, endothelial, neuronal, hepatic, and chondrogenic lineages. AFS cells possess several advantages over other commonly employed stem cells, such as embryonic stem cells (ESCs) and bone marrow-derived mesenchymal stem cells (MSCs). Unlike ESCs, AFS cells do not form teratomas, when injected into immune-deficient mice (Cananzi et al., 2009; De Coppi et al., 2007). Due to their location along the developmental timeline – they are “younger” than adult stem cells, in a developmental sense – AFS cells may have increased differentiation and expansion potential compared to MSCs (Valli et al., 2010). Additionally, isolation of AFS cells is a simpler process than that for isolation of both ESCs and MSCs. Large numbers of AFS cells can be isolated and expanded from as little as 2 mL of amniotic fluid.
Although AFS cells have many properties that support their clinical usefulness, little is known about the effects of growth substrata and the physical cues experienced by the cells. The use of elastic modulus as a tool to maintain or recover stemness in AFS and other types of stem cells is not well explored. It will be necessary to understand how to implement the appropriate environmental cues, including mechanical properties, for given applications in order to optimize success. Herein, we investigate the effects of substrate elastic modulus, E′, on AFSC expansion, morphology, and potential for cell therapy, by using substrates of varying elastic moduli with normalized surface composition. By querying these basic stem cell-substrate interactions, we hope to gain insight into culture methods potentially useful for future applications with these and other stem cells.
2. Materials and methods
2.1. Substrate preparation
Surfaces with E′ of 2, 5, 15, and 50 kPa (Excellness Biotech, Lausanne, Switzerland), and standard tissue culture plastic (~100,000 kPa), were coated with collagen to normalize surface composition and isolate stiffness as the experimental independent variable. Relative E′ values of the above surfaces in comparison with common bodily tissues are shown in Fig. 1. Type I rat tail collagen (BD Biosciences, Bedford, MA) was first dissolved in cold 0.1 N acetic acid to make a 1 mg/mL solution. This solution was diluted in serum-free α-MEM media (Hyclone, Logan, UT) to yield a 5 μg/mL collagen working solution. Wells were covered with the working solution and incubated at 37 °C overnight for collagen adsorption to the surfaces. Resulting collagen surface concentrations are reported by the manufacturer to be approximately 1 μg/cm2. Prior to cell seeding, wells were rinsed once with Dulbecco's phosphate-buffered saline (DPBS) to remove any remaining traces of acetic acid.
To verify normalized surface coating, two separate colorimetric stains were used for quantification of relative collagen. Aniline blue and methyl blue stains for collagen were performed after the collagen coating process. Briefly, coated wells and non-coated controls were washed with DPBS for 5 min before 15 min incubations with aniline blue or methyl blue reagents. The surfaces were washed with DPBS three times for 5 min each, after which the absorbance stained collagen was quantified using a Spectramax M5 Tunable Microplate Reader (Molecular Devices, Sunnyvale, CA). Aniline blue-stained surfaces were quantified at 620 nm while methyl blue-stained surfaces were quantified at 602 nm. Uncoated surfaces were used as controls.
2.2. AFS cell culture
Multipotent subpopulations of c-kit+ progenitor cells were isolated from human amniotic fluid as previously described (De Coppi et al., 2007), and expanded in culture. AFS cells can proliferate rapidly in culture without feeder cells for many passages, while maintaining chromosomal stability.
A1 and H1 AFS cell clones were expanded separately in culture using 150 mm diameter dishes until 75% confluence with Chang Media (α-MEM with 18% Chang B [Irvine Scientific, Santa Ana, CA], 15% ES-FBS [HyClone], 2% Chang C [Irvine Scientific]). Cells were detached from the substrate with Accutase (Innovative Cell Technologies, San Diego, CA) and counted prior to centrifugation. For proliferative assays, 10,000 A1 or H1 cells per mL Chang media were seeded per well in 24-well plates (n=3 per time point). The plates were then transferred to an incubator (37 °C, 5% CO2) and proliferation was determined using MTS mitochondrial metabolic assays (Promega, Madison, WI) at day 1, day 4, and day 7 of culture. Aliquots (100 μL) were removed from MTS incubations and absorbance readings determined at 490 nm using the Microplate Reader (Molecular Devices). Absorbance levels are directly proportional to the number of live cells. Media was changed on day 4.
To better visualize cell morphology, actin cytoskeletons were stained using a fluorescent phalloidin dye. Samples were first fixed in 4% paraformaldehyde (PFA) for 15 min, rinsed in 1× PBS for 5 min, followed by 0.2% Triton-X in 1× PBS for 5 min, and 2 more 5 min washes in 1× PBS. Fluorescein phalloidin (Invitrogen, Carlsbad, CA) was prepared in methanol to yield a 6.6 M solution that was diluted with 1× PBS containing 1% BSA to reach a final 165 nM phalloidin working concentration. This working solution was added to each well to completely coat the surface of the wells. Samples were incubated at room temperature (20 °C) for 15 min, followed by three washes with 1× PBS. Cells were counterstained with DAPI (4′,6-diamidino-2-phenylindole, dilactate, 16.67 μg/mL, Invitrogen) for 5 min, and washed three times with 1× PBS prior to fluorescent imaging (594 nm) with a Zeiss Axiovert (Carl Zeiss MicroImaging, LLC, Thornwood, NY) on day 1, day 4, and day 7 for subsequent morphological analysis.
2.3. Morphological quantification
Morphological characteristics of AFS cells were observed and quantified on day 1, 4 and 7 of culture using representative images of each group (n=12 or more). Three features were studied: eccentricity (a measure of roundness or even spreading), relative cell size, and extent of cell branching. To measure eccentricity, e, all cells were assumed to be elliptical in shape. Images of the cells were scaled with a hemocytometer, after which the semi-major axis (a) and semi-minor axis (b) were measured. The equation b2 – a2(1–e2) was used to provide values for e. Average cell size was calculated using the previously measured semi-major and semi-minor axes a and b. Size was determined by the equation for the area of an ellipse, A=π×a×b. To quantify the extent of cell branching, a metric was developed in which branching factor was defined as a length-weighted sum of the number of branched filipodia. Filipodia were defined as extensions of the cell away from the main cell body. For example, a spindle-shaped cell might have two short filipodia, resulting in a low branching factor, while a neuron-shaped cell with several long axon- and dendrite-like filipodia would receive a high branching factor.
2.4. Stem cell marker immunohistochemistry
AFS cells were immunostained with antibodies against CD44, CD90, CD105, N-cadherin, and CD117 (c-kit). Incubations were carried out at room temperature unless otherwise stated. Samples were first fixed in 4% PFA for 15 min and then rinsed three times in 1× PBS for 5 min. Nonspecific blocking was performed with Dako Antibody Protein-Block, Serum-Free (Dako, Carpinteria, CA) for 30 min. Next, samples were incubated with primary antibodies for 2 h: CD44 (raised in rat, cat. ab78947, Abcam, Cambridge, MA), CD90 (raised in rat, cat. 53016, BD Biosciences), CD105 (raised in rabbit, cat. ab107595, Abcam), N-cadherin (raised in mouse, 610921, BD Biosciences), and CD117 (raised in mouse, cat. sc-13508, Santa Cruz Biotechnology, Santa Cruz, CA). Each primary antibody was prepared in Dako Antibody Diluent at a 1:100 dilution. After incubation, samples were washed with 1× PBS three times. Samples were then incubated for 2 h with anti-rat, anti-rabbit, or anti-mouse DyLight 594 secondary antibodies (Jackson Immuno, West Grove, PA) as appropriate in Dako Antibody Diluent (1:100 dilution). Cells were counterstained with DAPI for 5 min, and washed three times with 1× PBS prior to fluorescent imaging. Negative controls were performed in parallel with the primary antibody incubations and included incubation with blocking solution in place of the primary antibody. No immunoreactivity was observed in the negative control sections. Samples were imaged with fluorescence at 594 nm with a Zeiss Axiovert.
2.5. AFS cell-induced transwell migration assay
To investigate the effect of substrate elasticity on the ability of AFS cells to induce migration, Transwell migration assays were performed using AFS cells as both the inducing and migrating populations. H1 AFS cells were expanded as described above, before being plated into 2 kPa or regular tissue culture 24-well plates at a density of 100,000 cells per well. After 24 h of culture in the wells, other H1 cells were seeded separately into Transwell inserts (Corning, Corning, NY) that had been coated in Type I collagen, at a density of 50,000 cells per insert. The inserts were then placed in the previously seeded 2 kPa and plastic wells, as well as 2 kPa cell-free control wells with Chang media.
After 4 h, inserts were fixed with 4% paraformaldehyde for 15 min. Cells still attached to the interior-side of the inserts (non-migratory) were removed with cotton swab applicators. Cells attached to the outside of the insert membranes were stained by placing each insert in 0.05% crystal violet solution for 20 min. Inserts were then washed two times in distilled water to remove excess dye. The insert membranes were then cut out of the inserts and mounted on glass slides for photography and analysis of migrated cells.
2.6. Statistical analysis
The data are generally presented as the means of number of replicates±the standard deviation. Values were compared using Student's t-test (2-tailed) with two sample unequal variance, and p<0.05 or less was considered statistically significant.
3. Results
3.1. Proliferation of AFS cells on substrates
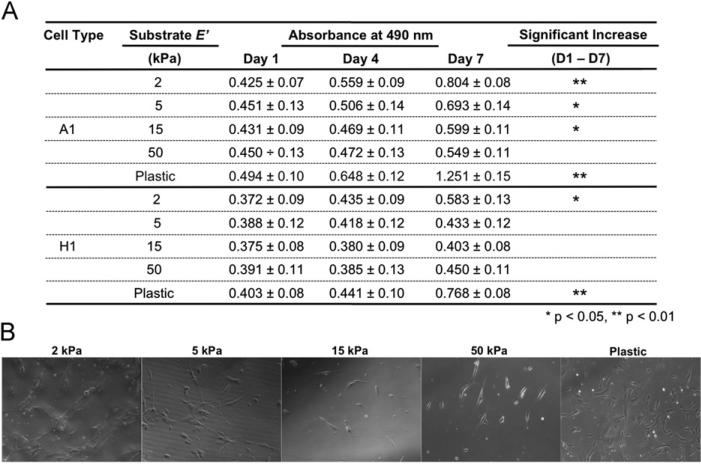
To study the effect of substrate elasticity on AFS cells, we used cell culture dishes with surface E′ of 2, 5, 15, and 50 kPa, and standard tissue culture plastic (plastic) (~100,000 kPa), coated with collagen to normalize surface composition and isolate stiffness as the experimental independent variable. Prior to testing the effect of different substrate elasticity, the amount of surface coating was verified by measuring absorbance of coated collagen stained with aniline blue and methyl blue. The different surfaces had absorbance values that were not significantly different from one another (data not shown), indicating similar collagen-coated surfaces. Culture of AFS cells on different surfaces resulted in different proliferation rates on substrates of different stiffness. MTS proliferative assays showed that each of the independent AFS cell lines, A1 and H1, displayed the highest proliferation on plastic (Fig. 2A), in which the absorbance increased significantly with time (p<0.01). Only on 2 kPa substrates a similar increase in absorbance was observed, but to a lesser degree and was less significant for H1 cells. Specifically, H1 cells showed significant increases between day 4 to day 7 and day 1 to day 7 (p<0.05), but not day 1 to day 4. In contrast, cells cultured on 5, 15, and 50 kPa substrates did not show a similar significant increase in proliferation. Microscopic images, taken during the time course of the experiment, showed that the number of cells in culture correlates well with the MTS data (Fig. 2B).
Fig. 2.
(A) Proliferative data of A1 and H1 AFS cells cultured on 2, 5, 15, and 50 kPa substrates and on tissue culture plastic. MTS assays were performed on days 1, 4, and 7 (n=3). Significance: *: p<0.05; **: p<0.01. (B) Representative phase contrast images of cells in culture at day 7.
3.2. Morphological analyses
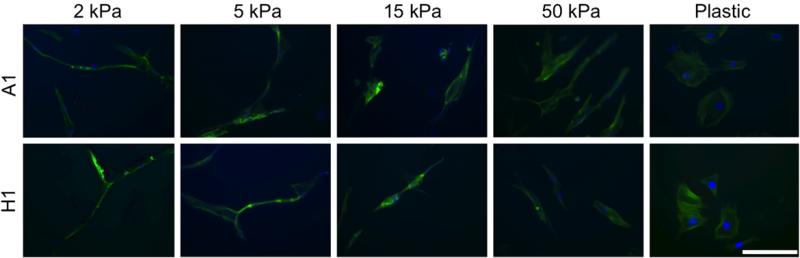
To study the effects of substrate elasticity on AFS cell morphology, images of representative cells (n=12 or more) were taken on days 1, 4, and 7 of culture (Fig. 3). Morphological trends were consistent between the A1 and H1 cell lines and across time-points. For this reason, and for simplicity, only images and numerical data from day 4 are presented. Control experiments with cells cultured on plastic showed spread out cells. In contrast, cell cultured on 2 and 5 kPa substrates displayed branching behavior and long extensions. On 15 and 50 kPa substrates, AFS cells appeared to maintain an extended spindle-shaped morphology.
Fig. 3.
Morphological observations of A1 and H1 AFS cells cultured on 2, 5, 15, and 50 kPa substrates and on tissue culture plastic. Images shown were taken on day 4 and are representative of the five groups and three time-points. Green—Phalloidin; Blue—DAPI; Scale bar: 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
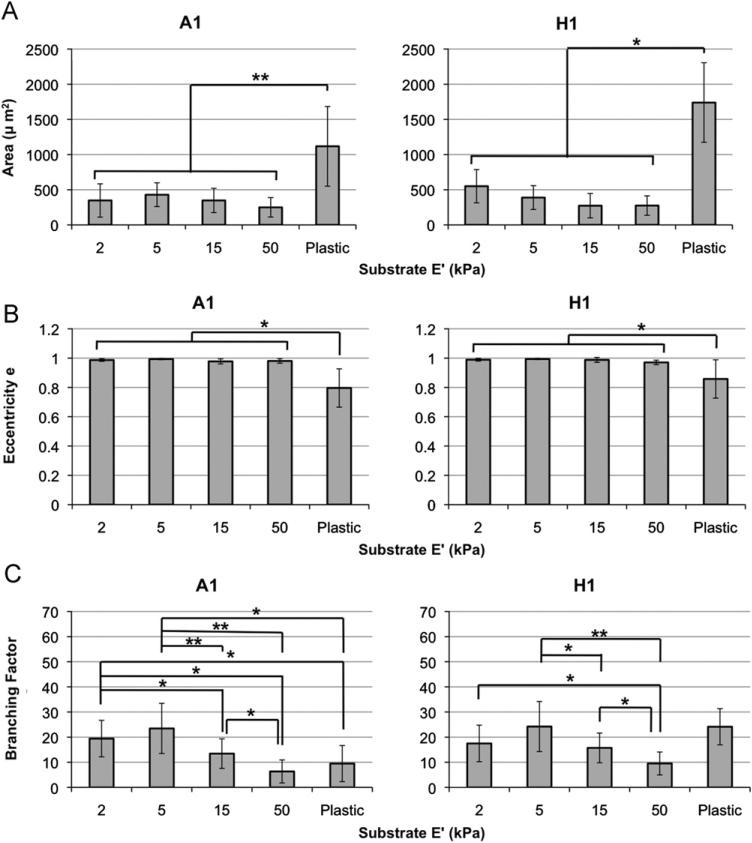
To further compare between the different substrates, the morphological characteristics of the cells, as described above, were numerically quantified. First, the average cell area was calculated by measuring the semi-major and semi-minor axes of the cells, which were assumed to be elliptical in nature. For both A1 and H1 cell lines, cells cultured on plastic were significantly larger than those cultured on any of the softer surfaces (p<0.01 and p<0.05, respectively, Fig. 4A). Cell area did not vary significantly between the 2, 5, 15, and 50 kPa groups.
Fig. 4.
Substrate elastic modulus influences A1 and H1 cell morphology. (A) The mean cell area of cells cultured on plastic substrates are significantly greater than those of cells cultured on 2, 5, 15, and 50 kPa substrates. (B) Eccentricity measurements revealed that cells cultured on plastic substrates are for the most part significantly rounder, or more evenly spread out. (C) Branching quantification revealed that, in general, cells cultured on 2 and 5 kPa substrates were significantly more branched than other groups. (Due the large amount of data in (C), all statistical comparisons shown should be assumed as *, unless otherwise noted.) (D) Schematic illustrating how cells were assumed to be elliptical in shape. Significance: *: p<0.05; **: p<0.01. Exceptions were 50 kPa versus plastic on day 7 (A1), and 2 kPa versus plastic on day 1 (H1).
Eccentricity, e, was used as a metric to determine the proclivity of the cells to spread evenly, become spindle-shaped, or spread linearly—characteristics that are common to cells of certain tissue types. A1 and H1 cells cultured on 2, 5, 15, and 50 kPa surfaces had e values almost equal to 1, indicating a more linear and not evenly rounded morphology (Fig. 4B). In contrast, cells cultured on plastic had e values of approximately 0.8, indicating a more evenly rounded shape. e values of cells cultured on plastic were significantly different from those cultured on the all softer surfaces.
Because the extent of cell branching was observed to vary between groups, a metric was developed in which branching factor was defined as a length-weighted sum of the number of cell extensions. A general trend emerged, in which the degree of branching peaked in cells cultured on 5 kPa substrates. Cells cultured on 15 and 50 kPa substrates displayed little branching behavior; they were more spindle-shaped or linear (Fig. 4C). For A1 line, the 5 kPa group had significantly greater branching than 15 and 50 kPa (p<0.01) and to a lesser degree than plastic (p<0.05). The 2 kPa group was insignificantly different than the 5 kPa group. For H1 line, similar trends were observed; however, the differences were less pronounced. The 5 kPa group had significantly greater branching than the 15 and 50 kPa (p<0.05 and 0.01, respectively) but not significantly different than plastic. The 2 kPa had significantly higher branching only compared to 50 kPa (p<0.05).
3.3. Cell surface marker expression
After demonstrating the significant differences in proliferative and morphological behavior due to the changes in E′ that were conserved between both AFS clones, we hypothesized that some underlying biological mechanism was being affected. Therefore, we queried the expression of a subset of classical MSC markers as well as CD117 or c-kit, a marker that was once expressed in similar cells and used for preliminary sorting by De Coppi et al. (2007).
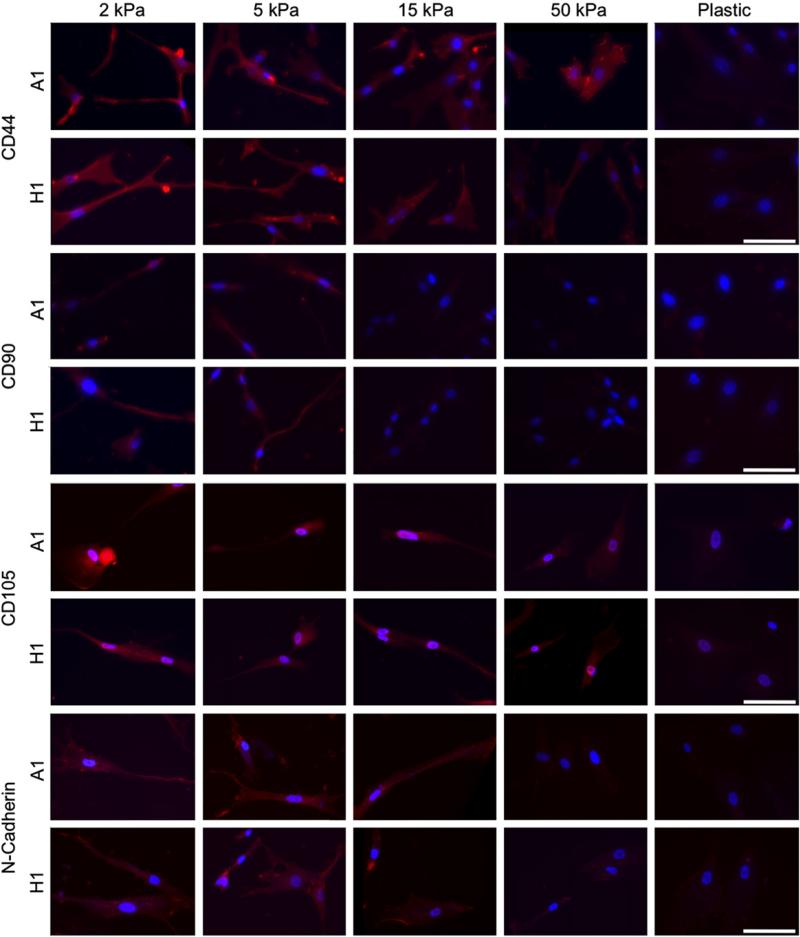
Substrate elasticity was shown to affect stem cell properties and behavior (Engler et al., 2006; Holst et al., 2010). We hypothesize that changes in stem cell properties may be associated with a change in cell surface protein (markers) expression. Accordingly, we examined the expression of typical MSCs markers such as CD44, CD90, CD105 and N-cadherin, under different substrate elasticity, by IHC staining (Fig. 5). CD44 and CD105 staining was observed in cells cultured on all substrates but weak in cells cultured on plastic. The staining appeared notably strongest on cells cultured on 2 and 5 kPa substrates, followed by less intense staining on the 15 and 50 kPa substrates, and the least intense staining on the plastic substrates. Likewise, strong CD90 staining was observed on cells cultured 2 and 5 kPa substrates, significantly less intense staining on the 15 and 50 kPa substrates and weak staining in cells cultured on plastic. Staining for N-cadherin was most intense in cells cultured on 2, 5, and 15 kPa substrates, with noticeably weaker stains on 50 kPa and plastic substrates. In general, the staining was localized to regions at the ends of cellular extensions or blebs protruding from the cell body. This suggests that at lower E′ levels, the cells had a better ability to interact with the substrate, and perhaps be more mobile.
Fig. 5.
Immunofluorescence shows increases in CD44, CD90, CD105, and N-cadherin expression in both A1 and H1 clones as substrate E′ decreases. Red—CD44, CD90, CD105, or N-cadherin as indicated; Blue—DAPI; Scale bar: 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. AFS cell-induced cell migration
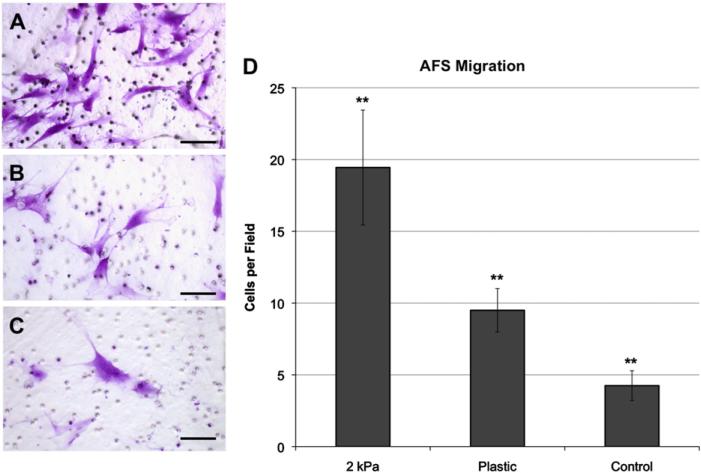
To test if culture on softer substrate induces AFS cell migration, we first cultured H1 AFS cells on soft 2 kPa substrate and plastic for 24 h. Subsequently, other H1 cells were seeded onto Transwell inserts placed above the previously seeded cells. As control, H1 AFS cells in Transwell inserts were placed into non-seeded substrates. Four hours later, the number of cells migrating from the upper side of the transwell to the lower side was measured (Fig. 6A–C). Significantly, more AFS cells migrated towards cells that were on soft 2 kPa substrates, compared to those on plastic, or in wells with control media (Fig. 6D, p<0.01). This suggests that the AFS cells cultured on softer substrates secreted more autocrine migratory cytokines.
Fig. 6.
AFS cells cultured on soft surfaces increase migration rates of other AFS cells. Crystal violet-stained AFS cells that migrated through Transwell inserts towards AFS cells cultured on (A) 2 kPa substrates, (B) plastic, or (C) Chang's media only. Average number of observed migrated (D) AFS cells per field of view. Scale bar: 50 μm. Significance: ** p<0.01.
4. Discussion
Some current practices in medical care do not necessarily treat the cause of a disease or condition, rather only the symptoms. In contrast, regenerative medicine-based treatments aim to not only treat symptoms, but to treat the cause by fixing or replacing the diseased or damaged tissue or organ (Atala, 2007). We previously describe the isolation of AFS cells and showed that they can proliferate in culture without feeder cells for many passages and showed no signs of transformation in culture or teratoma formation in vivo (De Coppi et al., 2007). AFS cells can be induced in vitro to give rise to cells of multiple lineages, as judged by marker expression and functional analyses. The purpose of this study was to investigate the influence of substrate mechanical properties on the properties of a special type of stem cells derived from amniotic fluid (AFS cells), in order to better design their culture conditions for therapeutic applications. The main finding of the current study were that AFS cells respond in changing their properties according to the E′ of the culture surface. We observed decreased proliferation rates as well as distinct morphologies dependent on the substrate E′ that were mostly conserved between two independent AFS clones. These qualitative observations were supported by quantitative data derived from measurements of the cells. We further showed that softer substrates (lower E′) supported stronger staining of typical MSCs markers. Lastly, AFS cultured on softer substrates induced a stronger autocrine migratory phenotype compared with AFS cells cultured on regular tissue culture plastic dishes. Collectively, the results indicate that AFS are capable of functional interaction with substrates of different elasticity and potentially can respond to changes the elasticity of the matrix surrounding them.
AFS cells are a promising cell source for cell therapy applications for several reasons. Amniotic fluid, as well as most other extraembryonic tissues, are often discarded at birth, making sourcing easy in comparison to obtaining human embryos or undergoing surgical operations for bone marrow. Furthermore, since AFS cells are isolated from discarded material, they do not suffer from the same concerns as ESCs; namely, the ethical issues associated with extracting cells from fetuses, and the susceptibility of teratoma formation upon ESC implantation in vivo (Cananzi et al., 2009; De Coppi et al., 2007; Marcus and Woodbury, 2008).
AFS cells share certain beneficial similarities with ESCs and MSCs (Pozzobon et al., 2010). Unlike most adult cells, AFS cells have been induced to form 3-D embryoid bodies similar to those derived from ESCs, which are used in developmental biology research to model embryogenesis (Valli et al., 2010). MSCs are implemented in cell therapy and tissue engineering applications for their ability to secrete a wide spectrum of bioactive trophic factors (Caplan, 2007). AFS cells appear to behave similarly, as demonstrated in a study in which regeneration of the sciatic nerve in rats was aided by neurotrophic factors secreted by embedded AFS cells (Pan et al., 2007). Likewise, in ongoing research we are currently investigating the role of AFS cells in wound healing, and observing beneficial effects from AFS-secreted factors. Also, like MSCs, AFS cells seem to have the ability to home to injury sites and tumors (Ghionzoli et al., 2010; Kidd et al., 2009). After migrating to these sites, the cells can deliver trophic factors, such as anti-inflammatory cytokines, immunomodulatory signals, and angiogenic factors. However, little work has been performed in order to understand how the mechanical microenvironment of stem cells affects the therapeutic capabilities of the cells.
The effect of substrate elasticity on stem cell lineage selection was previously shown with bone marrow-derived MSCs by Engler et al. (2006) and have been noted by others. For example, muscle cells cultured in vitro only developed sarcomeric striations of normal skeletal muscle if the substrate stiffness matched that of natural muscle. Furthermore, myogenesis of MSCs could be induced on such substrates without the use of any soluble factors (Chaudhuri et al., 2010). Similarly, varying stiffness was shown to control embryonic mesenchymal progenitor cell differentiation. On 30.6 MPa poly(ether sulfone)–poly(epsilon-caprolacone) (PES–PCL) substrates cells underwent osteogenesis, while 7.1 MPa PCL-only substrates induced chondrogenesis (Nam et al., 2011). However, analogous work with AFS cells has not been performed, and little or no morphological quantification has been documented. We showed that two independent lines of AFS cells displayed heightened branching and extended cellular extensions or blebs on 2 kPa and 5 kPa substrates, spindle-shaped morphology on 15 and 50 kPa substrates, and a large spread out morphology on plastic. After observing these morphological phenomena, similar to those observed in MSCs by Engler et al. (2006), we chose to investigate retention of characteristics of stem cells and therapeutic potential rather than E′-induced differentiation, as the latter has been thoroughly explored in a number of stem cell types.
Morphological differences in cells do not only occur in response to external environmental cues experienced by the cells, but also as a result of internal biological processes within the cells. For example, MSCs were primed for differentiation towards tissue types with similar E′. The MSCs were driven towards neural, muscular, and osteogenic lineages through culture on 0.1–1 kPa, 8–17 kPa, and 25–40 kPa polyacrylamide gels, respectively. On the other hand, hematopoetic stem cell expansion rates were altered depending on matrix elasticity (Holst et al., 2010). In both cases, protein expression and morphological changes were altered by the same changes in E′ (Engler et al., 2006; Holst et al., 2010). We tested the effects of E′ on the expression of cell surface markers associated with MSCs in order to develop culture conditions that will preserve their expression during in vitro culture, which is relevant to their therapeutic potentials. The results indicating that cell surface marker expression of CD44, CD90, CD105, and N-cadherin was increased in cells on softer substrates suggest that the decrease in E′ might mediate a recovery of some stem cell properties.
It is possible that loss of marker expression in cells cultured on plastic could be due to partial differentiation. Since one goal of stem cell expansion in culture is to maintain characteristics associated with stem cells, potential unwanted differentiation argues strongly for consideration of alternative surface substrates for culture, such as those explored in this study.
It was notable that the observed increases in staining intensity were often localized to regions in the extensions and blebs branching from the bodies of the cells. It is possible that decreased E′ in the microenvironment may induce cell mobilization in which CD44, CD90, CD105, and N-cadherin are actively expressed to aid in migration and engraftment of stem cells. CD90 (Thy-1) is a regulator of migration through both cell-cell and cell-matrix interactions (Rege and Hagood, 2006a, b) that is commonly used MSC marker (Augello et al., 2010). In hematopoietic stem cells (HSCs) filopodia are directly associated with mobile states, migration, and proliferation (Fong et al., 2012). Furthermore, during HSC homing and engraftment, CD44 expression is often expressed in the tips of filopodia (Avigdor et al., 2004). CD105 has been shown to promote migration in various forms of cancer (Benetti et al., 2008). One study showed that umbilical cord-derived MSCs expressing high levels of N-cadherin showed an increased migration and engraftment into a rat myocardial infarct model compared with MSCs expressing low levels of N-cadherin (Lee et al., 2012). These results suggest that there may be a correlation between N-cadherin expression and therapeutic efficacy of stem cells. Our data that the expression of N-cadherin (and other markers associated with MSCs) is increased in AFS cells cultured on softer substrates suggest that modulation of matrix elasticity could improve their therapeutic potential. As we move forward with future experiments, we are interested in further exploring gene expression profiles of AFS cells in low E′ environments using additional techniques such as FACS and qRT PCR, which can provide more quantitative data. Unfortunately, we found that it was difficult to harvest high quality RNA yields from the particular substrates implemented in our study, which is a problem we will have to address in future work.
To assess the effects of E′ on inducing mobility through an autocrine mechanism, we applied functional tests in which we assayed for AFS-induced cell migration in vitro as an effect of cytokines generated from cells cultured on the substrates of varying E′. Our data showing that AFS cells cultured on softer substrates induced migration of AFS cells likely through autocrine pathways suggest that AFS migratory autocrine stimulation may be increased in environments with soft stiffness characteristics like those found in vivo. Other types of MSCs have been well-documented to become active upon injury where they migrate and become cytokine factories (Caplan, 2007).
It should also be noted that we observed a general decrease in proliferation rates as the E′ of the substrate decreased, although it was not a linear trend. Both cell lines, showed a trend of inverse correlation between E′ and cell proliferation. However, the cells proliferated at the highest rate on plastic. This finding may be due to factors other than just the substrate E′ values, such as chemical composition, surface topography, cell–cell-contacts, etc. While expansion of cells to clinically applicable numbers is still one of the primary goals in stem cell culture, evidence exists where stem cells experience senescence when maintained in an inappropriate environment for too long. This raises the problem regarding balancing expansion with quiescence, or the retention of the characteristics that make stem cells useful. It may be that the lower E′ induces slower proliferation rates during quiescence and might be more conducive to prevent AFS senescence, and thus providing better cells for cellular therapies or generation of functional engineered tissues and organs. Specifically in the case of stem cells, they must be expanded to high cell numbers, but multipotency, mobility, and trophic delivery efficiency must be preserved. Since natural tissue microenvironments are viscoelastic and share no similarities with plastic surfaces (Keung et al., 2010), it is possible that classical tissue culture may actually limit stem cell and primary cell functionality. This is evident by the occurrence of cell senescence over time in culture. The A1 and H1 AFS cell clones used in this study had been cultured on plastic for many passages. The results obtained here show that it may be possible to “rescue” expression of markers associated with MSCs, simply by a transition to substrates (and potentially 3-D ECMs) of decreased elastic modulus E′. Additional work with other substrates, additional stem cell types, and a wider range of stem cell-related markers will be necessary to truly determine if this is the case. However, should it be so, this approach has the potential to be a powerful tool for increasing stem cell expansion efficiencies while most importantly, maintaining the cells in their true state of stemness.
Beyond recovery of MSC markers and potential for induction of cytokine secretion, this work may give us a glimpse into the changes that are induced within cells implanted or injected into in vivo environments. As presented in Fig. 1, the majority of the tissues in the body are soft. Cells delivered through cell therapy applications therefore almost always undergo a high E′ (expansion on plastic) to low E′ (in the tissue) transition. By mirroring this transition in our in vitro substrate platform, we may be modeling the mechanical aspects of this transition, providing a simple system for predicting mechanisms within various cell therapy administrations. To further investigate the theory that a branched migratory phenotype results in a more therapeutically effective cell population, we would like to study how changes in E′ affect the mobile state of the cells and apply mobile and less mobile cells in an in vivo engraftment model.
5. Conclusion
This study demonstrated that variations of substrate E′ directly influence expansion, morphology, MSC marker expression, and autocrine stimulation of cell migration. Tailoring the elastic modulus and other physical characteristics of biomaterials and culture microenvironments to specific physiological values might be an effective method for regulating the cell culture environment in order to improve efficacy of stem cells in cell therapy applications.
Acknowledgements
This research was supported by an NIH NIBIB Grant (R01EB008009).
REFERENCES
- Atala A. Engineering tissues, organs, and cells. Journal of Tissue Engineering and Regenerative Medicine. 2007;1:83–96. doi: 10.1002/term.18. [DOI] [PubMed] [Google Scholar]
- Augello A, Kurth TB, De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. European Cells and Materials. 2010;20:121–133. doi: 10.22203/ecm.v020a11. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Benetti A, Berenzi A, Gambarotti M, Garrafa E, Gelati M, Dessy E, Portolani N, Piardi T, Giulini SM, Caruso A, Invernici G, Parati EA, Nicosia R, Alessandri G. Transforming growth factor-beta1 and CD105 promote the migration of hepatocellular carcinoma-derived endothelium. Cancer Research. 2008;68:8626–8634. doi: 10.1158/0008-5472.CAN-08-1218. [DOI] [PubMed] [Google Scholar]
- Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reproductive BioMedicine Online. 2009;18(1):17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. Journal of Cellular Physiology. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Chaudhuri T, Rehfeldt F, Sweeney HL, Discher DE. Preparation of collagen-coated gels that maximize in vitro myogenesis of stem cells by matching the lateral elasticity of in vivo muscle. Methods in Molecular Biology. 2010;621:185–202. doi: 10.1007/978-1-60761-063-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Jr., Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Current Opinion in Biotechnology. 2008;19:534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delo DM, De Coppi P, Bartsch G, Jr., Atala A. Amniotic fluid and placental stem cells. Methods in Enzymology. 2006;419:426–438. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fong CY, Gauthaman K, Cheyyatraivendran S, Lin HD, Biswas A, Bongso A. Human umbilical cord Wharton's jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. Journal of Cellular Biochemistry. 2012;113:658–668. doi: 10.1002/jcb.23395. [DOI] [PubMed] [Google Scholar]
- Ghionzoli M, Cananzi M, Zani A, Rossi CA, Leon FF, Pierro A, Eaton S, De Coppi P. Amniotic fluid stem cell migration after intraperitoneal injection in pup rats: implication for therapy. Pediatric Surgical International. 2010;26:79–84. doi: 10.1007/s00383-009-2504-x. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS, Rasko JE. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nature Biotechnology. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- Keung AJ, Healy KE, Kumar S, Schaffer DV. Biophysics and dynamics of natural and engineered stem cell microenvironments. Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 2010;2:49–64. doi: 10.1002/wsbm.46. [DOI] [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. Journal of Molecular Histology. 2007;38:405–413. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Choi EK, Kang SK, Kim GH, Park JY, Kang HJ, Lee SW, Kim KH, Kwon JS, Lee KH, Ahn Y, Lee HJ, Cho HJ, Choi SJ, Oh WI, Park YB, Kim HS. N-cadherin determines individual variations in the therapeutic efficacy of human umbilical cord blood-derived mesenchymal stem cells in a rat model of myocardial infarction. Molecular Therapy. 2012;20:155–167. doi: 10.1038/mt.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. Journal of Cellular and Molecular. 2008;12:730–742. doi: 10.1111/j.1582-4934.2008.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J, Johnson J, Lannutti JJ, Agarwal S. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomaterialia. 2011;7:1516–1524. doi: 10.1016/j.actbio.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemir S, Hayenga HN, West JL. PEGDA hydrogels with patterned elasticity: novel tools for the study of cell response to substrate rigidity. Biotechnology and Bioengineering. 2010;105:636–644. doi: 10.1002/bit.22574. [DOI] [PubMed] [Google Scholar]
- Pan HC, Cheng FC, Chen CJ, Lai SZ, Lee CW, Yang DY, Chang MH, Ho SP. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. Journal of Clinical Neuroscience. 2007;14:1089–1098. doi: 10.1016/j.jocn.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Pozzobon M, Ghionzoli M, De Coppi P. ES, iPS, MSC, and AFS cells. Stem cells exploitation for Pediatric Surgery: current research and perspective. Pediatric Surgical International. 2010;26:3–10. doi: 10.1007/s00383-009-2478-8. [DOI] [PubMed] [Google Scholar]
- Raab M, Shin JW, Discher DE. Matrix elasticity in vitro controls muscle stem cell fate in vivo. Stem Cell Research & Therapy. 2010;1:38. doi: 10.1186/scrt38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1 as a regulator of cell–cell and cell–matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB Journal. 2006a;20:1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochimica et Biophysica Acta. 2006b;1763:991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli A, Rosner M, Fuchs C, Siegel N, Bishop CE, Dolznig H, Madel U, Feichtinger W, Atala A, Hengstschlager M. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene. 2010;29:966–977. doi: 10.1038/onc.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromolecular Bioscience. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]