Abstract
The majority of women gain more weight during pregnancy than what is recommended. Since gestational weight gain is related to short and long-term maternal health outcomes, it is important to identify women at greater risk of not adhering to guidelines. The objective of this study was to examine the relationship between body image and gestational weight gain. The Body Image Assessment for Obesity tool was used to measure ideal and current body sizes in 1,192 women participating in the Pregnancy, Infection and Nutrition Study. Descriptive and multivariable techniques were used to assess the effects of ideal body size and discrepancy score (current—ideal body sizes), which reflected the level of body dissatisfaction, on gestational weight gain. Women who preferred to be thinner had increased risk of excessive gain if they started the pregnancy at a BMI ≤26 kg/m2 but a decreased risk if they were overweight or obese. Comparing those who preferred thin body silhouettes to those who preferred average size silhouettes, low income women had increased risk of inadequate weight gain [RR = 1.76 (1.08, 2.88)] while those with lower education were at risk of excessive gain [RR = 1.11 (1.00, 1.22)]. Our results revealed that body image was associated with gestational weight gain but the relationship is complex. Identifying factors that affect whether certain women are at greater risk of gaining outside of guidelines may improve our ability to decrease pregnancy-related health problems.
Keywords: Body image, Body dissatisfaction, Gestational weight gain, Pregnancy
Introduction
More than half of all women in the United States are overweight or obese. In the last 25 years, we have seen a 16% increase in obesity prevalence among women of childbearing age [1, 2]. It has been hypothesized that one of the reasons for this increase is the greater amount of weight women have been allowed to gain during pregnancy [3]. From observational studies conducted in the US, roughly 30–40% of women gain weight within the recommended ranges, 20% gain less, and 40–50% gain more than what is recommended [4]. Women with excessive weight gain are less likely to lose this weight in the postpartum period and may, consequently, be at greater risk of beginning the next pregnancy at a higher weight status [5, 6] as well as have an increased risk of obesity later in life [7–9].
Gaining weight outside of recommended ranges can result in poorer birth outcomes due to increased maternal and fetal complications [10]. For example, excessive weight gain is associated with increased risk of caesarean section and macrosomia while inadequate weight gain can result in a low-for-gestational age infant and preterm birth [4]. In 1990, the Institute of Medicine (IOM) established recommendations for weight gain during pregnancy based on prepregnancy body mass index (BMI) [11]. The recommended weight gains according to prepregnancy BMI categories are as follows: 12.5–18 kg (28–40 lbs.) for women who start the pregnancy underweight (BMI <19.8 kg/m2), 11.5–16 kg (25–35 lbs.) for normal weight women (BMI of 19.8–26.0 kg/m2), 7–11.5 kg (15–25 lbs.) for overweight women (BMI of 26.0–29.0 kg/m2), and a weight gain of at least 6.8 kg (15 lbs.) for obese women (BMI >29.0 kg/m2) [11]. Given the importance of adequate weight gain during pregnancy, it is becoming increasingly important to identify women at risk of gaining weight outside of recommended ranges.
Pregnancy is the only time in a woman’s life when weight gain is encouraged and expected. The relationship between body image, which is a person’s perception of or attitude towards his or her own body [12, 13], and weight-related concerns in the nonpregnant state [12, 14, 15] suggests that body image may be related to weight gain during pregnancy, a time of immense physical and physiological changes. For example, a recent study found that women who were obese before pregnancy had greater weight and shape concerns before and during pregnancy than nonobese women [16]. In this study, we determined whether women who are more dissatisfied with their body shape and size before pregnancy are at higher risk of gaining outside of the recommended weight guidelines than women who are more comfortable with their body.
Perceptions and preferences related to body shape and size are thought to differ for Caucasians and African Americans. Previous research suggests that African American women are much more likely to prefer a larger body size compared to Caucasian women and that there are cultural norms within the African American community that support higher satisfaction with weight and appearance [13, 17–19]. Given these potential racial differences in body image, we examined whether race influences the effect of body image on gestational weight gain.
The relationship between body image preference and weight gain during pregnancy has not been explored sufficiently. Data from the Pregnancy, Infection and Nutrition study (PIN) was used to examine the following questions: (1) how does ideal body size preference relate to gaining weight according to IOM guidelines and (2) are women with greater body dissatisfaction more likely to gain outside of IOM-recommended ranges and is this relationship different for African American and Caucasian women? By identifying factors that affect whether certain women are at greater risk of gaining excessively or inadequately during pregnancy, we can potentially improve our ability to decrease pregnancy-related health problems for both mother and child.
Methods
Sample and Procedures
The PIN study is a longitudinal prospective cohort study identifying etiologic factors for preterm delivery (http://www.cpc.unc.edu/projects/pin). All medical charts of new prenatal patients at University of North Carolina hospitals between January 2001 and June 2005 were reviewed to identify potential participants. Eligible women included those who were older than 16, spoke English, were less than or equal to 20 weeks’ gestation on their second pre-natal visit, were planning to continue care or deliver at the study site, had access to a phone for telephone interviews, and were having singleton pregnancies. 2006 women, 63% of all eligible women, consented to participate in the PIN study. Of these 2006, we had weight gain and body image information for 1,290 women (64%); body image was assessed more than a year after recruitment began due to funding of a new grant related to gestational weight gain. Thirty-six women refused to complete the body image assessment, leaving 1,254 (63%); no differences in age, race, parity, education and income were found between those who completed the assessment and those who refused (all P-values ≥ .10). A subset of women experienced more than one pregnancy in this cohort. For these women, the first pregnancy (n = 43) was excluded as the second pregnancy was more likely to occur after funding of the new grant, when body image information was first collected. Other pregnancies excluded from the analysis were for women who experienced spontaneous abortion (n = 2), stillbirth (n = 7), or whose infants did not survive after birth (n = 10), leaving 1,192 (59%) for the analysis.
Women were interviewed during pregnancy at 15–20 weeks (clinic visit 1), 17–22 weeks (telephone interview 1), 24–29 weeks (clinic visit 2), 27–30 weeks (telephone interview 2), and in-hospital following delivery. Participants completed self-administered questionnaires about health behaviors, diet, physical activity, and psychosocial and psychological factors. Medical charts were abstracted following delivery. Women were compensated for participation with cash incentives. All study protocols were approved by the University of North Carolina, School of Medicine Institutional Review Board.
Measures
Potential Confounders
Participants reported their weight, race, age, parity, education, marital status, income, number of adults and kids in the household, smoking status in the first two trimesters, dieting history [20], and attitudes towards weight gain during pregnancy [21]. Pregravid BMI (kg/m2) was calculated from self-reported prepregnancy weight and height measured at screening (15–20 weeks’ gestation). Prepregnancy weights were checked for biological plausibility by comparing it to the weight recorded at the first prenatal visit. Large discrepancies were independently evaluated for reasonableness in light of gestational age at first prenatal visit. Unreasonable weights (3.8% of the sample) were replaced by imputed weights using a formula based on expected weight gain for a given gestational age [11]. Information on family income and number of household members was used to create the poverty level variable which represents percent of the 2001 poverty index according to the US Bureau of the Census [22]. Infant’s gestational age and information on pregnancy complications were abstracted from delivery logs and medical charts, respectively.
We created a directed acyclic graph, based on a review of the literature [23] that depicted the relationships between factors related to both gestational weight gain and body image. The diagram suggested that physical activity and diet during pregnancy were on the causal pathway from body image to gestational weight gain. Since the purpose of this study was to examine the independent effect of body image on gestational weight gain, physical activity and diet were not considered in further analyses.
Gestational Weight Gain
Total gestational weight gain is the difference between self-reported prepregnancy weight and weight measured at the last prenatal visit. This dependent variable was kept continuous during multivariable modeling.
Adequacy of Weight Gain
Adequacy of weight gain is the ratio of observed to expected gestational weight gain up to a woman’s last prenatal visit. This dependent variable was used to determine the level of adherence to IOM pregnancy weight gain guidelines [24, 25]. The 1990 IOM guidelines were used since they were in existence during the time of this cohort study. Expected gestational weight gain was calculated using the following formula: expected first-trimester total weight gain + [(gestational age at time of last weight measurement − 13 weeks) * expected rate of gain in second and third trimesters]. For underweight, normal weight, overweight, and obese women, expected total weight gains in the first trimester were 3.2, 2.2, 1.0, and 0.5 kg and expected rates were 0.5, 0.4, 0.3, and 0.23 kg/week, respectively [11].
Adequacy of weight gain was then categorized into inadequate, adequate and excessive weight gain using cut points corresponding to the IOM recommendations for weight gain; this approach has been used in previous studies [26, 27]. For example, the IOM recommends a gain of between 7.0 and 11.5 kg for overweight women (BMI >26.0–29.0 kg/m2) which corresponds to an adequacy ratio of 0.8–1.2 if the pregnancy is carried to term (40 weeks) [11]. Thus, overweight women who have an adequacy ratio of >1.2 were defined as having gained above IOM recommendations while those <0.8 gained inadequately. Since obese women are asked to gain at least 6.8 kg, we used this as the lower limit for the adequacy ratio and then adopted the 1.2 upper range similar to that of overweight women.
Body Image Assessment for Obesity
Body image was assessed during screening (clinic visit 1 at 15–20 weeks gestation) using the Body Image Assessment for Obesity (BIA-O) [28]. We used the BIA-O tool to assess current body size (CBS) and ideal body size (IBS). Participants were given 18 cards in random order with black and white line-drawn silhouettes depicting body sizes ranging from very thin (silhouette 1) to very obese (silhouette 18). Women were first asked to indicate their CBS, or which body size most resembled their pregravid body size. The cards were reshuffled and participants were asked to identify their IBS, the body size they would most like to resemble (when they are not pregnant). This tool has been found to have reasonable test–retest reliability (correlation coefficient = .77 for CBS and .93 for IBS) and is a valid measure of discrepancy score (correlation coefficient = .45–.48) [28].
Discrepancy scores (CBS-IBS) were calculated by subtracting IBS from CBS. Large absolute values of the score indicated higher levels of body dissatisfaction, with positive values indicating a preference for a body size smaller than the present level; negative values indicated a preference for a heavier size. For analysis, we categorized this variable into three categories that are as follows: those without discrepancy between their current and ideal sizes (represented by scores −1, 0, 1), those who prefer a heavier body size (scores ≤−2), and those who prefer a lighter body size (scores ≥2).
Williamson and colleagues [28] suggest that body image scores must be interpreted conditional on gender, race, and BMI and used a methodology based on norm scores. They developed these scores in a population of 1,209 adults of which 645 were non-pregnant women between the ages of 18–96 while our population consisted only of pregnant women within the ages of 16 and 47. Since our population was more homogenous, we did not develop normalization scores. However, we did condition on race and pregravid BMI in initial models and considered interactions between the exposures (discrepancy score/ideal body size), BMI and race.
IBS was categorized into three levels: light (silhouettes 1–4), average (5–8), and heavy (≥9) body size preference. Categories were created based on the authors’ perceptions of light, average and heavy silhouettes due to the absence of silhouette-specific BMI values. We assessed the influence of discrepancy score, light body size preference, and heavy body size preference on the outcome variables.
Analytic Approach
Descriptive statistics were calculated using Pearson’s chi-squared test and t tests. All potential confounders were tested first for effect measure modification using the Wald test for homogeneity (P <.10 criterion); they were then assessed as confounders using a 10% change in estimate criterion [29].
Separate multivariable models were created for the following dependent variables: excessive weight gain (dichotomous; adequate weight gain is referent) and inadequate weight gain (dichotomous; adequate weight gain is referent). Independent variables included discrepancy score (categorical), heavy body size preference (dichotomous; average body size is referent), and light body size preference (dichotomous; average body size is referent). Excessive and inadequate weight gain variables were modeled as dichotomous variables in separate models to facilitate the calculation of risk.
Poisson and exact logistic regression were used for multivariable analysis. To estimate relative risk ratios (RR), we attempted to use binomial regression but encountered problems of model non-convergence. Consequently, we used Poisson regression with a robust variance estimator since it yields incidence rate ratios that closely approximate RRs [30]. Exact logistic regression was used in models where the data were sparse. STATA (version 9.2; StataCorp LP, College Station, TX) was used to conduct Poisson regression models and LogXact was used to conduct exact logistic regression models (version 8; Cytel Inc., Cambridge, MA).
Results
The mean pregravid BMI for the study population was 25.77 ± 6.88 kg/m2. A significant difference in mean BMI (t test P <.0001) was found between Caucasians (24.49 ± 5.88 kg/m2) and African Americans (30.64 ± 8.15 kg/m2). The proportion of women entering pregnancy obese was higher among African Americans (50%) compared to Caucasians (17%). Mean total weight gained in the population was 15.21 ± 6.10 kg. Caucasian women gained more overall weight (15.76 ± 5.62 kg) than African American women (13.29 ± 7.44 kg) (t test P <.0001).
The majority of women (64%) gained excessively during pregnancy, as reflected in the mean adequacy of weight gain (observed/expected weight gain) of 1.54 ± 0.83. This value indicates that women gained, on average, 54% more weight than expected. African American women had a significantly higher mean adequacy of weight gain (1.71 ± .07) compared to Caucasian women (1.49 ± .03) (t test P <.0004). Among African American women, 67% gained excessively, 12% gained adequately, and 20% gained inadequately; among Caucasians, 63% gained excessively, 24% adequately, and 13% inadequately. Mean adequacy of weight gain increased with each category of pregravid BMI: 0.98 ± 0.28 for underweight, 1.37 ± 0.43 for average weight, 1.87 ± 0.74 for overweight and 2.07 ± 1.28 for obese women.
Approximately half of the women preferred a light body size (silhouettes 1–4) with only 3% preferring a heavy body size (silhouettes ≥ 9). Most women who preferred a light body size were between the ages of 25–34, Caucasian, of normal BMI before pregnancy, married, highly educated, and of high income (see Table 1). Though the majority of Caucasian women (60%) preferred a light body size, most African American women chose an average body size (63%) as their ideal. Women who preferred a heavier body size (only 3% of population) tended to be less educated (<16 years) and obese prior to pregnancy. The distribution of maternal characteristics differed significantly across levels of ideal body size preference with the exception of parity and prepregnancy dieting history (Table 1). Most women gained excessively during pregnancy, regardless of their ideal body shape preference.
Table 1.
Distribution of maternal characteristics by ideal body size preference in the pregnancy, infection, and nutrition study
| Maternal characteristics | Meane (±SDf) | % of the Study Population | % Light body size preference | % Average body size preference | % Heavy body size preference | P >chi2g |
|---|---|---|---|---|---|---|
| Age | 29.1 ± 5.7 | |||||
| ≤18 | 3.4 | 2.5 | 4.7 | 0.6 | .032 | |
| 19–24 | 18.7 | 15.5 | 21.7 | 30.8 | ||
| 25–29 | 28.2 | 29.0 | 27.3 | 25.6 | ||
| 30–34 | 33.5 | 36.3 | 30.6 | 25.6 | ||
| ≥35 | 16.2 | 16.6 | 15.7 | 15.4 | ||
| BMI | 25.8 ± 6.9 | |||||
| Underweight | 14.2 | 20.1 | 8.0 | .001 | ||
| Normal | 50.3 | 59.0 | 43.0 | 5.1 | ||
| Overweight | 11.5 | 10.8 | 12.8 | 5.1 | ||
| Obese | 24.0 | 10.1 | 36.2 | 89.7 | ||
| Racea | ||||||
| Caucasian | 72.5 | 82.0 | 62.9 | 46.2 | .001 | |
| African American | 19.3 | 10.8 | 28.2 | 41.0 | ||
| Education (years completed) | 15.5 ± 2.9 | |||||
| <12 | 6.9 | 4.9 | 8.1 | 23.1 | .001 | |
| 12 to <16 | 33.6 | 25.6 | 42.3 | 51.3 | ||
| 16+ | 59.4 | 69.5 | 49.6 | 25.6 | ||
| Marital status | ||||||
| Married | 75.1 | 81.8 | 69.3 | 43.6 | .001 | |
| Not marriedb | 24.8 | 18.2 | 30.7 | 56.4 | ||
| Percent of 2001 poverty line | 410.8 ± 226 | |||||
| <185% | 19.0 | 16.9 | 23.9 | 38.9 | .001 | |
| 185–350% | 18.8 | 16.6 | 25.0 | 22.2 | ||
| ≥350% | 54.7 | 66.6 | 51.2 | 38.9 | ||
| Parityc | ||||||
| Nulliparous | 46.8 | 47.7 | 45.8 | 43.6 | .750 | |
| 1 or more | 53.2 | 52.3 | 54.2 | 56.4 | ||
| Smokingd | ||||||
| No | 79.5 | 91.7 | 87.9 | 75.8 | .004 | |
| Yes | 9.2 | 8.3 | 12.1 | 24.2 | ||
| Dieted prepregnancy | ||||||
| No | 46.4 | 46.7 | 51.2 | 43.2 | .270 | |
| Yes | 49.2 | 53.3 | 48.9 | 56.8 | ||
| Pre-eclampsia | ||||||
| No | 95.1 | 97.7 | 93.2 | 76.9 | ||
| Yes | 5.0 | 2.4 | 6.8 | 23.1 | .001 |
97 women self-reported as “Other”
Includes single, divorced, separated, or widowed
Includes live births and still births
Maternal smoking in months 1–6
Mean and standard deviation are from originally continuous variables (age, prepregnancy BMI, education, and poverty were later categorized)
SD = Standard Deviation
Pearson chi-squared test
Discrepancy scores ranged from −6 to 10 with a mean of 1.28 ± 2.21; about 47% of women had a score reflecting no discrepancy (−1, 0, 1), 45% preferred a body size thinner than their CBS (scores ≥2), and 8% had a score indicating they preferred a body size heavier than their CBS (scores ≤−2). Mean scores for African Americans (1.66 ± 2.50) were significantly higher than Caucasians (1.20 ± 2.12) (t test P <.005). When stratified by BMI, mean discrepancy score between Caucasian and African American women was significantly different only among women who started the pregnancy in the normal BMI category (t test P <.0001); Caucasian women had a mean discrepancy score of 1.0 while African American women had a mean of 0.02.
Multivariable Results
For each model we first examined potential effect modification and confounding by all covariates. In particular, we examined interactions between body image, race and BMI, as recommended by Williamson and colleagues [28]; interactions were kept in the model if P <.10. Where necessary, results stratified by these variables are presented. Attitudes towards weight gain and dieting history were not found to be modifiers or confounders in any model.
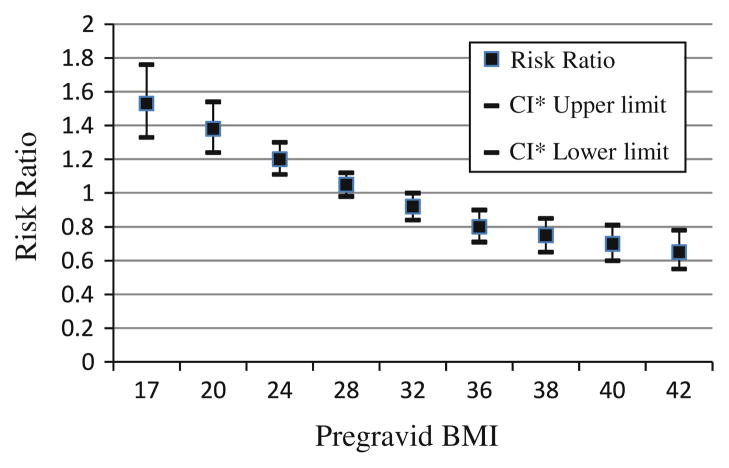
Using Poisson regression, we found that pregravid BMI modified the relationship between discrepancy score and adequacy of weight gain (Table 2). As shown in Fig. 1, risk of excessive weight gain decreased as pregravid BMI increased for women who preferred to be thinner (discrepancy score ≥2) compared to women with no discrepancy between their current and ideal body shapes; women who preferred to be heavier (discrepancy score ≥−2), however, had increasing risk of gaining excessively with increasing BMI (Table 2). There was no significant effect of body image discrepancy on inadequate weight gain.
Table 2.
Estimated effect of discrepancy scores ≥2 and ≤−2 on Adequacy of Weight Gain for Pregravid BMI Values
| BMI (kg/m2) | Inadequate weight gaina
n = 378
|
Excessive weight gainb
n = 1,015
|
||
|---|---|---|---|---|
| Discrepancy score ≥2 RR (95% CI) | Discrepancy score ≤−2 RR (95% CI) | Discrepancy score ≥2 RR (95% CI) | Discrepancy score ≤−2 RR (95% CI) | |
| 17 | 1.29 (0.83, 2.01) | 1.04 (0.51, 2.11) | 1.53 (1.33, 1.76) | 0.92 (0.66, 1.27) |
| 20 | 1.22 (0.84, 1.78) | 1.12 (0.68, 1.82) | 1.38 (1.24, 1.54) | 0.95 (0.77, 1.19) |
| 24 | 1.13 (0.83, 1.54) | 1.23 (0.72, 2.11) | 1.20 (1.11, 1.30) | 1.01 (0.82, 1.23) |
| 28 | 1.04 (0.80, 1.36) | 1.36 (0.55, 3.32) | 1.05 (0.98, 1.12) | 1.06 (0.76, 1.47) |
| 32 | 0.96 (0.75, 1.24) | 1.49 (0.39, 5.66) | 0.92 (0.84, 1.00) | 1.12 (0.67, 1.85) |
| 36 | 0.89 (0.67, 1.18) | 1.65 (0.27, 9.87) | 0.80 (0.71, 0.90) | 1.18 (0.59, 2.35) |
| 38 | 0.86 (0.63, 1.17) | 1.73 (0.23, 13.08) | 0.75 (0.65, 0.85) | 1.21 (0.55, 2.65) |
| 40 | 0.82 (0.58, 1.16) | 1.81 (0.19, 17.35) | 0.70 (0.60, 0.81) | 1.24 (0.51, 3.00) |
| 42 | 0.79 (0.54, 1.16) | 1.90 (0.16, 23.05) | 0.65 (0.55, 0.78) | 1.27 (0.48, 3.39) |
Poisson regression model adjusted for smoking in the first two trimesters and modified by pregravid BMI. The model is as follows: log (Y|X = x) = −2.3479 − 0.3767 (discrepancy score ≤ −2) + 0.5920 (discrepancy score ≥ 2) + 0.0546 (pregravid BMI) + 0.0243 (discrepancy score ≤ −2 * pregravid BMI) − 0.0196 (discrepancy score ≥ 2 * pregravid BMI) − 0.0508 (smoking in the first two trimesters).
Poisson regression model modified by pregravid BMI. The model is as follows: log (Y|X = x) = −1.3668 − 0.31067 (discrepancy score ≤ −2) + 1.0042 (discrepancy score ≥ 2) + 0.0414 (pregravid BMI) + 0.0132( discrepancy score ≤ −2 * pregravid BMI) − 0.0341 (discrepancy score ≥ 2 * pregravid BMI).
Fig. 1.
Influence (risk ratio) of discrepancy score ≥2 on excessive weight gain by pregravid BMI. CI confidence interval
Poverty level was identified as a modifier of the effect of light ideal body size (silhouettes 1–4) on inadequate weight gain (Table 3). Women greater than 185% of the poverty level had decreased risk of gaining inadequately if they preferred a light versus average body size [for middle income, RR = 0.64 (95% CI: 0.37, 1.10); for high income, RR = 0.83 (95% CI: 0.59, 1.18), respectively]. Women less than 185% of the poverty level, however, had 1.76 (95% CI: 1.08, 2.88) times the risk of gaining inadequately if they preferred a lighter body size (versus an average size). Pregravid BMI was held constant.
Table 3.
Crude and adjusted regression for the effect of ideal body size on inadequate and excessive weight gain
| Inadequate weight gain
|
Excessive weight gain
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | Robust SE | P | RR | 95% CI | Robust SE | P | ||
| Light vs. average IBS preference | |||||||||
| Crude n = 417 | 0.76 | 0.60, 0.95 | 0.09 | 0.02 | Crude n = 985 | 0.88 | 0.82, 0.94 | 0.03 | <0.01 |
| Adjusteda n = 387 | Adjustedc n = 984 | ||||||||
| <185% | 1.76 | 1.08, 2.88 | 0.44 | 0.02 | <16 years education | 1.11 | 1.00, 1.22 | 0.06 | 0.05 |
| 185 to <350% | 0.64 | 0.37, 1.10 | 0.18 | 0.10 | ≥16 years education | 0.92 | 0.83, 1.01 | 0.05 | 0.09 |
| ≥350% | 0.83 | 0.59, 1.18 | 0.15 | 0.31 | |||||
| Inadequate weight gain
|
Excessive weight gain
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | SE | P | OR | 95% CI | SE | P | ||
| Heavy vs. average IBS preference | |||||||||
| Crudeb n = 177 | 3.33 | 0.79, 19.77 | NA | 0.12 | Cruded n = 422 | 1.79 | 0.52, 9.58 | NA | 0.51 |
Poisson regression model adjusted for pregravid BMI; modified by poverty level
Exact logistic regression model; no modifiers or confounders
Poisson regression model adjusted for pregravid BMI; modified by maternal education
Exact logistic regression model; no confounders or modifiers
Education modified the relationship between light body size preference (silhouettes 1–4) and excessive weight gain. We adjusted for pregravid BMI (Table 3). We found that among women who were not college graduates (<16 years of education), those who preferred a thin body shape (silhouettes 1–4) were at higher risk of gaining excessively during pregnancy, [RR = 1.11 (95% CI: 1.00, 1.23)] when compared to those who preferred an average size (silhouettes 4–8).
Discussion
In this study, we determined that body image is associated with pregnancy weight gain and identified subgroups of women more likely to gain outside of established recommendations. We found that women with body dissatisfaction and women who had a thin ideal body size (silhouette ≤4) and were of lower education or lower income were at increased risk of gaining outside of recommended ranges. With an increasing number of women gaining outside of pregnancy weight gain guidelines, it is imperative that we can better identify at-risk women.
More than race, we found the relationship between body image and pregnancy weight gain to be influenced by pregravid BMI which often modified or confounded the relationship in most of our regression models. Notably, we found that as pregravid BMI increased, women who wanted to be thinner (discrepancy score ≥2) had decreasing risk of gaining above recommendations. There was also a clear distinction in risk between BMI categories. Women in the average and underweight BMI categories were at greater risk of excessive weight gain during pregnancy while women in the overweight and obese categories were at decreased risk of gaining excessively. We suspect a possible explanation may be that women in the normal and underweight categories may experience a relaxation of prepregnancy body image ideals and become more comfortable with weight gain as their pregnancy progresses. In contrast, obese women who prefer to be thinner may be more vigilant about gaining weight in the pregnancy period because they are starting the pregnancy at a higher weight and do not want to gain more weight. However, we can only hypothesize as to the possible reasons for the difference in risk of excessive weight gain between BMI categories. Current evidence points to the stability of body image across pregnancy—that as weight increases, so does ideal body size [31, 32] but as far as we know body image across pregnancy has not been examined in the context of BMI categories. Thus, we can only hypothesize as to the possible reasons for the difference in risk of excessive weight gain between BMI categories. We also examined attitudes towards pregnancy weight gain in our models but the majority of our population had positive weight gain attitudes. As a result, it was not found to influence the relationship between body image and gestational weight gain [33].
Women of low income or low education who preferred a thin body size (silhouettes 1–4) were at higher risk of not adhering to IOM recommendations. For women who preferred a thinner than average body size, lower education level increased risk of gaining excessively. In contrast, being low income increased risk of inadequate weight gain. Though we would expect that low income and low education would be highly correlated, our results may be explained by the lack of complete overlap of women in these categories. Slightly less than half (46%) of the 412 less educated women, those with less than 16 years of education, were classified as poor (less than 185% of the poverty level). However, the majority (84%) of the 227 low income women were less educated. We hypothesize that this combination among the lowest income women, of being both poor and less educated, may be identifying those who are income-constrained and, consequently, likely to suffer from food insecurity [34]. This inability to afford enough food during pregnancy could potentially influence the ability of the pregnant women to gain adequately. In contrast, a large proportion of women with lower education were not poor and their increased risk of excessive weight gain may be due to unhealthy lifestyle issues such as poor food choices and lack of physical activity. It is also interesting to note that heavy body size preference was not found to have a significant effect on risk of inadequate or excessive weight gain though this may be because few women chose a heavy body size as their ideal.
We expected race to be a modifier for all models based on knowledge from previous studies that race differentially affects body image preference and weight gain [17, 18, 35, 36]. Race more often confounded rather than modified the relationship between body image and gestational weight gain; however, we did find that the effect of preferring a heavy (versus average) body size on total weight gain differed significantly by race. We also found that African American women in our cohort had significantly higher body image discrepancy scores than Caucasians, indicating higher dissatisfaction with their body which is contrary to previous research [13, 17–19]. However, in our population, obese women had the greatest discrepancy between their current and ideal sizes. Since half of the African American study population was obese, this may explain the higher discrepancy score.
The results of our study must be considered within the context of its limitations. Women in their first trimester were asked to choose body sizes representing “current” and “ideal” prepregnancy body silhouettes. Being pregnant at the time they were asked may have affected their silhouette choices. However, the average gestational age at entry into the study was 15 ± 2.9 weeks. At that point in time, the women were not likely to have gained much above their non-pregnant state and, thus, pregnancy weight gain is not likely to have influenced this assessment.
Another limitation is that our models exploring the influence of heavy body size preference included several strata that were limited in number of exposed subjects; the number of women who preferred to be heavy was quite small (3%), and our ability to identify effect measure modifiers and confounders may be limited.
Body image categories of light, average and heavy were chosen by the authors based on our own preconceptions of which silhouettes should be in these categories. The Williamson silhouettes have not been assigned BMI ranges as those of Stunkard’s [37]; however, we believed the BIA-O represented a better tool for use in our population due to the increased number of silhouettes representing greater body fat and its applicability in the current overweight US population.
A strength of the study includes its prospective cohort design which allowed for the assessment of IBS and CBS prior to our outcome of interest. However, this is an observational study and we can not infer causality. We also minimized the possibility of bias relating to weight status by measuring prenatal height and checking self-reported weights for biological implausibility. Since gestational weight gain, the difference between self-reported prepregnancy weight and weight measured at the last prenatal visit was used to calculate adequacy of weight gain, a main outcome, it is possible that if a woman underreported her pregravid weight, this could place her as having gained beyond her IOM weight gain guidelines. Since the weight gain ranges are wide, however, it is unlikely that many women would be miscategorized and we should be able to see the same trend in our data
We recognize that our findings are only generalizable to women who have access to and receive prenatal care early in pregnancy. Despite this, we hope that future studies will be conducted among women from diverse backgrounds to replicate our findings and to further explore the influence of body image on pregnancy weight gain. Future studies of a qualitative nature are needed to help explain some of the results we found; for instance, why low income women are at greater risk of inadequate weight gain while those of lower education are at increased risk of excessive weight gain if they preferred a light body size (silhouette ≤4). Furthermore, qualitative studies among obese women are needed to explore whether the association between preferring to be thinner (discrepancy score ≥2) and decreased risk of gaining excessively is due to greater vigilance of weight gain as a result of starting out at a higher weight or some other reason. When taken in context with dietary and physical activity behaviors, exploration of the cognitive rationale that women of varying body image preferences formulate regarding weight gain during pregnancy can aid us in understanding how to intervene and help women achieve weight gain goals within the targeted range.
Acknowledgments
This study received support from the National Institute of Child Health and Human Development, National Institutes of Health (HD37584, HD39373), the National Institute of Diabetes and Digestive and Kidney Diseases (DK61981, DK56350, RR00046), and the Carolina Population Center.
Footnotes
Conflict of interest statements No conflicts of interest exist.
Contributor Information
Ushma J. Mehta, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA
Anna Maria Siega-Riz, Email: am_siegariz@unc.edu, Departments of Epidemiology and Nutrition, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA. Carolina Population Center, University of North Carolina, 123W. Franklin St., Chapel Hill, NC 27514, USA.
Amy H. Herring, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA. Carolina Population Center, University of North Carolina, 123W. Franklin St., Chapel Hill, NC 27514, USA
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. The Journal of the American Medical Association. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. The Journal of the American Medical Association. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Siega-Riz AM, Evenson KR, Dole N. Pregnancy-related weight gain–a link to obesity? Nutrition Reviews. 2004;62:S105–S111. doi: 10.1111/j.1753-4887.2004.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan M, Siega-Riz AM, Moos M, Deierlein A, Mumford S, Knaack J, et al. Evidence report/technology assessment. Agency for Healthcare Research and Quality; 2008. Outcomes of maternal weight gain. Report No.: 168. [PMC free article] [PubMed] [Google Scholar]
- 5.Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. American Journal of Clinical Nutrition. 2008;87(6):1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. International Journal of Obesity & Related Metabolic Disorders. 2000;24(12):1660. doi: 10.1038/sj.ijo.0801456. [DOI] [PubMed] [Google Scholar]
- 7.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstetrics and Gynecology. 2005;106(6):1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 8.Linne Y, Dye L, Barkeling B, Rossner S. Long-term weight development in women: A 15-year follow-up of the effects of pregnancy. Obesity Research. 2004;12(7):1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 9.Linne Y, Dye L, Barkeling B, Rossner S. Weight development over time in parous women–the SPAWN study–15 years follow-up. International Journal of Obesity and Related Metabolic Disorders. 2003;27(12):1516–1522. doi: 10.1038/sj.ijo.0802441. [DOI] [PubMed] [Google Scholar]
- 10.National Research Council and Institute of Medicine. Workshop report. Washington, DC: The National Academies Press; 2007. Influence of pregnancy weight on maternal and child health. [Google Scholar]
- 11.Institute of Medicine. Nutrition during pregnancy; Part I, weight gain. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 12.Grogan S. Body image and health: Contemporary perspectives. Journal of Health Psychology. 2006;11(4):523–530. doi: 10.1177/1359105306065013. [DOI] [PubMed] [Google Scholar]
- 13.Rucker CE, Cash TF. Body images, body-size perceptions, and eating behaviors among African-American and white college women. The International Journal of Eating Disorders. 1992;12(3):291–299. [Google Scholar]
- 14.Garner DM. Body image and anorexia nervosa. In: Cash TF, Pruzinsky T, editors. Body image: A handbook of theory, research, & clinical practice. New York: Guilford Press; 2002. p. 295. [Google Scholar]
- 15.Schwartz MB, Brownell KD. Obesity and body image. In: Cash TF, Pruzinsky T, editors. Body image: A handbook of theory, research, & clinical practice. New York: Guilford Press; 2002. p. 200. [Google Scholar]
- 16.Micali N, Treasure J, Simonoff E. Eating disorders symptoms in pregnancy: A longitudinal study of women with recent and past eating disorders and obesity. Journal of Psychosomatic Research. 2007;63(3):297–303. doi: 10.1016/j.jpsychores.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Lovejoy M. Disturbances in the social body: Differences in body image and eating problems among African American and white women. Gender & Society. 2001;15(2):239–261. [Google Scholar]
- 18.Perez M, Joiner TE., Jr Body image dissatisfaction and disordered eating in black and white women. The International Journal of Eating Disorders. 2003;33(3):342–350. doi: 10.1002/eat.10148. [DOI] [PubMed] [Google Scholar]
- 19.Smith DE, Thompson JK, Raczynski JM, Hilner JE. Body image among men and women in a biracial cohort: The CARDIA study. The International Journal of Eating Disorders. 1999;25(1):71–82. doi: 10.1002/(sici)1098-108x(199901)25:1<71::aid-eat9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, editor. Obesity. Philadelphia: Saunders; 1980. pp. 208–225. [Google Scholar]
- 21.Palmer JL, Jennings GE, Massey L. Development of an assessment form: Attitude toward weight gain during pregnancy. Journal of the American Dietetic Association. 1985;85(8):946–949. [PubMed] [Google Scholar]
- 22.Proctor BD, Dalaker J. US Bureau of the Census, Current population reports. Washington, DC: US Government Printing Office; 2001. Poverty in the United States: 2001. Report No.: Series P60–219. [Google Scholar]
- 23.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 24.Bodnar LM, Siega-Riz AM, Cogswell ME. High prepregnancy BMI increases the risk of postpartum anemia. Obesity Research. 2004;12(6):941–948. doi: 10.1038/oby.2004.115. [DOI] [PubMed] [Google Scholar]
- 25.Siega-Riz AM, Adair LS, Hobel CJ. Institute of medicine maternal weight gain recommendations and pregnancy outcome in a predominantly Hispanic population. Obstetrics and Gynecology. 1994;84(4):565–573. [PubMed] [Google Scholar]
- 26.Vahratian A, Siega-Riz AM, Savitz DA, Zhang J. Maternal pre-pregnancy overweight and obesity and the risk of cesarean delivery in nulliparous women. Annals of Epidemiology. 2005;15(7):467–474. doi: 10.1016/j.annepidem.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Mumford SL, Siega-Riz AM, Herring A, Evenson KR. Dietary restraint and gestational weight gain. Journal of the American Dietetic Association. 2008;108(10):1646–1653. doi: 10.1016/j.jada.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson DA, Womble LG, Zucker NL, Reas DL, White MA, Blouin DC, et al. Body image assessment for obesity (BIA-O): Development of a new procedure. International Journal of Obesity and Related Metabolic Disorders. 2000;24(10):1326–1332. doi: 10.1038/sj.ijo.0801363. [DOI] [PubMed] [Google Scholar]
- 29.Rothman KJ, Greenland S. Modern epidemiology. 2. Philadelphia, PA: Lippincott–Raven; 1998. [Google Scholar]
- 30.Zou G. A modified Poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 31.Skouteris H, Carr R, Wertheim EH, Paxton SJ, Duncombe D. A prospective study of factors that lead to body dissatisfaction during pregnancy. Body Image. 2005;2(4):347–361. doi: 10.1016/j.bodyim.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Duncombe D, Wertheim EH, Skouteris H, Paxton SJ, Kelly L. How well do women adapt to changes in their body size and shape across the course of pregnancy? Journal of Health Psychology. 2008;13(4):503–515. doi: 10.1177/1359105308088521. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari RM, Siega-Riz AM, Melvin CL. Influence of provider advice and maternal attitudes on weight change and physical activity during pregnancy and postpartum [dissertation] Chapel Hill, NC: University of North Carolina at Chapel Hill; 2008. [Google Scholar]
- 34.Laraia BA, Siega-Riz AM, Dole N, London E. Pregravid weight is associated with prior dietary restraint and psychosocial factors during pregnancy. Obesity (Silver Spring) 2009;17(3):550–558. doi: 10.1038/oby.2008.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neff LJ, Sargent RG, McKeown RE, Jackson KL, Valois RF. Black-white differences in body size perceptions and weight management practices among adolescent females. Journal of Adolescent Health. 1997;20(6):459–465. doi: 10.1016/S1054-139X(96)00273-X. [DOI] [PubMed] [Google Scholar]
- 36.Mvo Z, Dick J, Steyn K. Perceptions of overweight African women about acceptable body size of women and children. Curationis. 1999;22(2):27–31. doi: 10.4102/curationis.v22i2.719. [DOI] [PubMed] [Google Scholar]
- 37.Must A, Phillips SM, Stunkard AJ, Naumova EN. Expert opinion on body mass index percentiles for figure drawings at menarche. International Journal of Obesity and Related Metabolic Disorders. 2002;26(6):876–879. doi: 10.1038/sj.ijo.0801986. [DOI] [PubMed] [Google Scholar]