Abstract
In 1968, a child’s cranium was recovered from the banks of a northern Canadian river, and held in trust until the ‘cold case’ was re-opened in 2005. The cranium underwent re-analysis at the Centre for Forensic Research, Simon Fraser University, using recently developed anthropological, ‘bomb-pulse’ radiocarbon analysis and forensic DNA techniques. Craniometrics, skeletal ossification and dental formation indicated an age-at-death of 4.4 ±1 years. Radiocarbon analysis of enamel from two teeth indicated a year of birth between 1958–1962. Forensic DNA analysis indicated the child was male, and the obtained mitochondrial profile matched a living maternal relative of the presumed missing child. These multi-disciplinary analyses resulted in a legal identification 41 years after the discovery of the remains, highlighting the enormous potential of combining radiocarbon analysis with anthropological and mtDNA analyses in producing confident personal identifications for forensic cold cases dating to within the last 60 years.
Keywords: forensic science, forensic anthropology, mitochondrial DNA, ancient DNA, short tandem repeat, cold case, bomb-pulse, dental enamel, accelerator mass spectrometry, inter-disciplinary
In 1968, a hunter found a partial cranium on the banks of a river in Northern Canada. The cranium had initially been linked to a four-year-old boy who had been presumed drowned in the river in 1965. An anthropological assessment conducted in 1969 precluded a match with the missing child due to an error concerning age-at-death. The partial cranium had an erupted upper right second deciduous molar, which was mistakenly identified as a permanent first molar, resulting in a gross overestimation of age-at-death (i.e. a child of 7–9 years). Based on this error, the anthropologist concluded that “we are inclined to the belief that the cranium which we have examined is not part of the skeletal system of the missing child” (anonymized letter April 2, 1969 to RCMP).
For more than 35 years, the remains were held in trust by a local museum until new techniques would be available to identify the child and repatriate the remains. In 2005, the remains were submitted to the Ancient DNA Laboratory, Department of Archaeology, Simon Fraser University for multi-disciplinary scientific analysis in a renewed attempt to identify the child. Our analyses were deliberately conducted without background knowledge of the case as provided above. Our aim was to see if any mtDNA existed in the hard tissues, and if so, to advise the authorities of the feasibility of seeking surviving family members for further testing. The anthropological assessment of the hard tissues was also repeated, and associated botanical materials were analyzed for evidence as to geographic origin and seasonality. Given the elapsed time since death, we further sought to determine if application of the new technique of bomb-pulse dating might help determine when the child had been born and provide further indirect evidence of identity.
Methods and Materials
Anthropological Analysis
The remains included an incomplete, dry skeletonized cranium with one remaining erupted tooth (5-5, FDI nomenclature) and several unerupted, forming permanent teeth from the upper right maxilla. There was postmortem rodent gnawing on many edges of the cranium. The primary means of analysis was visual inspection. Craniometrics were recorded using standard equipment, and photographs were taken of the cranium and adherent plant matter using a Nikon D100 camera and a Keyence VHX-100 Digital Microscope. Methods for age-at-death estimation included dental formation, skeletal ossification, and circumference of the cranium.. Radiographs were taken of the developing dentition using a H.G. Fischer model FP200 portable X-ray unit at SFU to estimate age at death. We used standards of tooth formation for both native (1) and non-native children as both biological populations are represented among missing persons in northern Canada (2). Plant matter inside the neurocranium was observed and removed for identification in an effort to determine the season and location of deposition. This material consisted of a leaf and petioles (Fig. 1).
Fig. 1.

Plant matter from inside cranium: consistent with being Poplar leaf, bud scales and petioles (scale mm)
Radiocarbon Analysis
Recently, radiocarbon analysis of tooth enamel has proved to be a remarkably accurate indicator of year-of-birth for forensic cases dating to within the last 60 years (3–6). Above-ground nuclear weapons testing between 1955 and 1963 doubled the natural amount of radiocarbon (14C) in the atmosphere, which has since declined after the test-ban treaty in 1963 (7–11). Since living organisms naturally incorporate atmospheric carbon atoms into their tissues through the food chain, the bomb-pulse dating techniques can measure the amount of 14C in tooth enamel to indicate what year the tooth was formed, providing that the individual was born within the last 65 years (3–6). Typically, enamel from two teeth formed during different years are analyzed in order to distinguish whether the individual was born before or after the peak of the bomb tests, i.e. to distinguish whether the 14C measurement relates to the rising or falling part of the bomb-pulse curve.
Two teeth from the cranium (5-5, deciduous upper second molar; and 1–5, permanent successional second upper premolar) were prepared at the Department of Cell and Molecular Biology, Stockholm, Sweden and radiocarbon dated at the Center for Accelerator Mass Spectrometry, Lawrence Livermore National Laboratory, Livermore, California. The methods for enamel preparation and accelerator mass spectrometry (AMS) sample measurement and analysis are described in Buchholz et al. (3) and Spalding et al. (4) with slight adjustments to the scientific protocol to address the problem of incomplete mineralization of the upper right second premolar crown in this young child. Enamel purification by sonication in 10N NaOH was reduced from 3–5 days to 15 minutes. All other procedures were as described previously. The acid pretreatment to remove the outer surface of the enamel exposed to the base used for enamel purification was also adjusted due to the softer enamel in the deciduous tooth and incomplete mineralization of the permanent tooth. Pretreatment used 0.25N HCl rather than 1.0 N HCl for both teeth and exposure was reduced to 20 minutes for 1–5. All other AMS procedures were as described in (3).
DNA Analyses
The hard tissue samples were analyzed in the Ancient DNA laboratory in the Department of Archaeology at Simon Fraser University using a modified silica spin column method for the retrieval of degraded DNA (12). The Ancient DNA laboratory is specifically designed for and dedicated to ancient DNA analysis, and follows strict contamination control protocols including the use of protective clothing, and the separation of the pre-and post-PCR work. DNA was extracted from two unerupted teeth (CC1: permanent upper first premolar (1–4), and CC2: permanent first upper molar (1–6)) as well as a 2.41g portion of the skull (CC7: right occipital squama). The tooth and bone samples underwent a rigorous chemical decontamination process recommended for forensic and ancient DNA samples (13–15). The samples were immersed in 100% commercial bleach (6% sodium hypochlorite) for 7 min to remove surface contamination. The samples were then immersed in 1N HCl for 1 min and 1N NaOH for 1 min, rinsed in UltraPure™ DNase/RNase-free distilled water (Invitrogen, Carlsbad, CA) followed by UV irradiation for 60 min. The tooth and occipital bone samples were crushed into powder using a cryogenic mill. The resulting powder from each tooth, and the 2.41g of occipital bone were incubated overnight at 50°C in 3–5 ml of lysis buffer (0.5M EDTA, 0.25% SDS, and 2.0 mg/mL proteinase K) in three separate15 mL tubes. After centrifugation, the supernatant was transferred to an Amicon centrifugal filter device, Ultra-4 (Millipore, Billerica, MA). The extract was concentrated to less than 100 μL and then purified using QIAquick columns (Qiagen, Valencia, CA), from which approximately 100 μL of DNA solution were collected for each sample.
The analysis initially targeted mitochondrial DNA (mtDNA), which is present in higher copy numbers than nuclear DNA, making it easier to recover from degraded remains. PCR amplifications were conducted in a Mastercycler Personal (Eppendorf, Hamburg, Germany) in a 30 μL reaction volume containing 50 mM KCl, 10 mM Tris-HCl, 2.5 mM MgCl2, 0.2 mM dNTP, 1.0 mg/mL BSA, 0.3 μM each primer, 3.0 μL DNA sample and 2.5 U AmpliTaq Gold™ (Applied Biosystems). All PCR amplifications were run for 40 cycles at 94°C for 30 seconds (denaturing), 52°C for 30 seconds (annealing), and 72°C extension for 40 seconds, with an initial 12 minute denaturing period at 95°C. The samples were amplified using multiple overlapping primer sets (Integrated DNA Technologies, Coralville, LA) designed to amplify fragments of the hypervariable regions (HVI and HVII) of the human mitochondrial DNA genome (16). All amplifications (and resulting sequences) from both tooth extracts were repeated at least once. Extraction blanks and PCR negative controls were carried out for each PCR experiment. Five μL of PCR product were visualized via electrophoresis on a 2% agarose gel using SYBR Green™ staining. The remainder of the PCR products were purified using MinElute™ purification kits (Qiagen, Valencia, CA) and sent to Macrogen Inc, Seoul, Korea (www.macrogen.com) for sequencing. The obtained electropherograms were edited, aligned and compiled using ChromasPro software (www.technelysium.com.au). The consensus mtDNA sequence obtained from multiple overlapping PCR amplifications was compared to mtDNA profiles from SFU laboratory personnel, the SWGDAM database, and later to a putative family member. To determine the sex of the individual, the DNA extracts from CC1 and CC2 were also amplified using primers designed to target short fragments of the amelogenin gene on the X and Y chromosomes (17, 18).
mtDNA Analysis of Putative Relatives
Later in our investigations, a maternal DNA reference from a putative sister was obtained from a buccal swab. The DNA from the living relatives was analyzed only after a complete and replicated mtDNA profile was obtained for the cranium in order to avoid the possibility of contaminating the ‘cold case’ remains with the modern reference DNA sample (19). The FTA card underwent two separate DNA extractions (FC1, FC2), in the Modern DNA laboratory at the Centre for Forensic Research at Simon Fraser University. The FTA card was extracted using the Qiagen QIAamp DNA Investigator Kit following the manufacturer’s instructions. The HVI and HVII regions of the mtDNA control region were targeted using two primer sets (Integrated DNA Technologies, Coralville, LA) (mt1-1 5′-TAACTCCACCATTAGCACCC-3′, mt2-2 5′-TGATTTGAGGGAGGATGGTG-3′ and mt3-1 5′-CACCCTATTAACCACTCACG-3′, mt4-2 5′-GCTGGTGTTAGGGTTCTTTG-3′) as described in (20). PCR was run for 40 cycles at 94°C for 1min, 52°C for 1 min, and 72°C extension 1 min, with an initial 12 minute denaturing period at 95°C.
The preliminary mtDNA match observed between the cranium and the maternal reference, and determination of male sex of the cranium, led to the next step of seeking a paternal DNA reference from the putative father. It was expected that nuclear STR profiling of two samples would provide sufficient discriminatory power to evaluate presumed paternal relationship. The paternal reference (buccal swab) was shipped to Yang’s Lab for DNA extraction (FC5) in the Modern DNA Laboratory at the Centre for Forensic Research at Simon Fraser University, using the methods listed for the maternal reference above.
Aliquots of the DNA extracts from the remains (teeth and occipital bone) and putative relatives (father and sister) were sent to the Forensic DNA Laboratory within the Centre for Forensic & Security Technology Studies (BC Institute of Technology, Burnaby, BC) for mini-STR profiling. All samples were quantified using Applied Biosystems Quantifiler™ (Foster City, CA), a human DNA quantification kit using an Applied Biosystems 7500 Real-Time PCR System. The manufacturer’s protocol was followed for all samples except CC1 and CC2, which had 1 μL of sample and 1 μL distilled water (instead of 2 μL of sample).
Approximately 35pg of DNA from the occipital bone (CC7) extract was amplified in duplicate using Applied Biosystems AmpFlSTR MiniFiler™ kit in a 12.5 μL reaction. Similarly, 200pg of DNA from each familial sample was amplified. PCR was performed in a Perkin Elmer 2400 thermal cycler. Fragment analysis was performed on an Applied Biosystems 310 Genetic Analyzer using GeneMapper ID (v3.2). Genetic analysis samples were prepared in deionized formamide (1.5μL MiniFiler™ PCR product in 25μL of formamide/GS-500 LIZ). All other conditions were as per the manufacturer’s suggested protocol.
Results and Discussion
Elapsed Time since Death Based on Taphonomic Indicators
The cranium was devoid of soft tissue and grease and had acquired discoloration from prolonged contact with the soil. Several areas of the cranium had been gnawed by rodents. According to Klippel and Synstelian (21), in Tennessee, only those human remains that have been exposed for more than two years demonstrate gnaw marks, providing a minimum estimate for elapsed time since death. In a separate forensic case involving a child’s cranium from a similar region of Canada, remnants of nasal cartilage were still preserved after eight years, though the bones were more severely exfoliated (22). Based on these (weak) indicators, a rough estimate of elapsed time since death was judged to be 5 to 10 years prior to discovery. As the cranium was recovered in 1968, death was estimated to have occurred between 1958 and 1963.
Results of Anthropological Analysis
The ancestry or sex of the child could not be determined using conventional anthropological techniques since the individual was still very immature at death. There were no discrete traits of the available skeleton or teeth that might suggest First Nations ancestry (e.g., no enamel extensions, the oval window is visible, lambdoidal suture lacks wormian bones) (23).
Personal identification of a child is greatly assisted by an accurate and precise age-at-death determination. In this case, with only a cranium and partial upper dentition preserved, our options for determining age-at-death included: a) dental formation; b) skeletal ossification; and c) head circumference, with the results of the analyses summarized on Table 1.
TABLE 1.
Summary of age at death indicators
| Analytical Technique | Age-at-Death (years) |
|---|---|
| Stage of permanent tooth formation | 3.19–5.45 |
| Stage of deciduous root formation | 2.79–4.52 |
| Basi-occipital size/fusion | 4.6–6 years |
| Head circumference | >3.0 |
| Average age-at-death | 3.53–5.32 |
Dental formation
A radiographic evaluation of four forming right maxillary permanent teeth using native Canadian dental formation standards (1) yielded an age-at-death estimate of 4.0 years (ranging from 3.19 to 5.45 years; both sexes considered). Later, the removal of tooth 5-5 from the dental crypt revealed its incomplete root apices, which were judged to be at stage A½. For children of European ancestry, stage A½ for the maxillary second deciduous molar is attained by age 3.48+/−0.69 years with root completion by 3.92+/−0.60 years (2). Directly observable root apex formation is typically considered a more precise estimate of dental formation than that obtainable through radiographs alone (24); the formation of this tooth alone indicated an age-at-death from about 3.5 to 3.9 years.
Skeletal ossification
The basi-occipital component of the occipital bone is only partially fused to the rest of the occipital bone, indicating an age less than 5–6 years (25). Its dimensions (breadth =32.1mm, length 19.41mm) indicate an age greater than 4 years, 7 months (26).
Head circumference
The cranial circumference was 495mm, which was increased to 520 mm to allow for the missing soft tissue (27). Jones (28) provides head circumference measurements for children up to the age of 3 years; according to these criteria, this cranium falls within the 90–95th percentile for children aged 36 months (28). Based on cranial circumference, the age of the child is greater than 3 years.
In sum, dental and skeletal age indictors centre upon an age-at-death of 4.4 years (Table 1). For discussion purposes, the age-at-death is assumed to be 4.4±1 years.
Results of Botanical Analysis
The interior of the cranium yielded several botanical remains, the most diagnostic of which included four bud scales closely resembling ‘balsam poplar’ or black cottonwood (Populus balsamifera) (Fig. 1). Based on number and preservation of the leaf buds found within the cranium, poplar trees were likely growing within the depositional environment (provided that the leaf buds were introduced into the cranium through natural means such as wind or water). However, due to the widespread distribution of the balsam poplar, the botanical analysis could not provide any specific geographic information for the remains. The plant matter suggests that the inside of the neurocranium became accessible for the deposition of plant material from nearby Poplar trees in the spring season.
Results of Radiocarbon Analysis
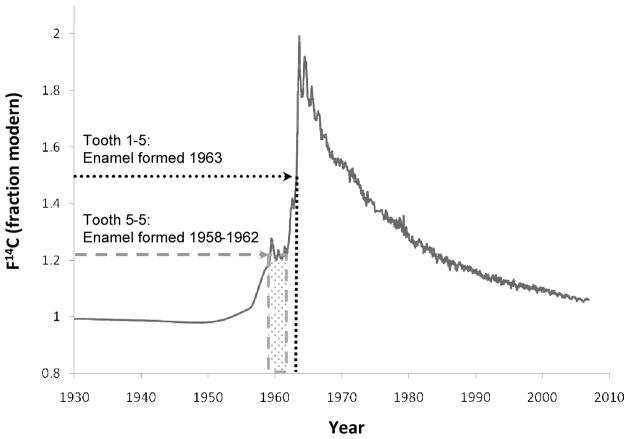
This case yielded radiocarbon values for two teeth (see Fig. 2). Tooth 5-5 (deciduous upper second molar) possessed a 14C/C concentration of 1.206±0.005 (Fraction modern) indicating that the average of the crown’s enamel formation span occurred between 1959 and 1961.7. Since tooth 5-5’s crown may form over a period from 11 weeks prenatal to a range from 6–11 months postnatal (22, 29–31), a reasonable estimate for the midpoint of crown enamel formation span is 1.5–6.5 months after birth. Conservatively, the 14C/C value for the crown’s enamel would correspond with a birth year between 1958 and 1962.
Figure 2.
Date of enamel formation based on the 14C/C concentration.. The solid line indicates the increase in atmospheric 14C/C caused by above-ground nuclear testing between 1955–63, and its subsequent decline (9–11). The vertical axis denotes 14C/C concentration using the F14C nomenclature (7) designed for reporting bomb-pulse data.
The enamel from tooth 1–5 (permanent upper second premolar) yielded a 14C/C concentration of 1.553±0.006 (Fraction modern) indicating an average of enamel formation in 1963. Tooth 1–5 was incomplete at the time of death, indicating that death occurred in or after 1963. Given the age-at-death estimate of 4.4±1 years (see above), the 14C values for tooth 1–5 are consistent with a year of birth between 1958 and 1960.
The 14C/C concentrations of tooth 5-5 and tooth 1–5 intersect on both the ascending and descending slopes of the bomb-pulse curve. However, the average dates of enamel formation on the descending portion of the curve would be 1984.1 to 1985.8 for tooth 5-5, and 1968.8 to 1970.7 for tooth 1–5. In that the deciduous tooth deposits its enamel before its successor, and considering the remains were recovered prior to 1984, it is clear that the later dates based on the descending portion of the bomb-pulse curve should be rejected.
In summary, the 14C dates in combination with the age-at-death estimate using anthropological techniques suggest that the child represented by the cranium was born between 1958 and 1962, and died between 1963 and 1968.
Results of mtDNA and Amelogenin Analysis
The initial analysis focused on the hypervariable regions (HVI and HVII) of the mtDNA control-region. More than 16 separate overlapping amplicons were sequenced to produce a consensus profile for the cranium, resulting in a total of 760bp of control region mtDNA. The obtained profile covered portions of both the HVI (16017–16411) and HVII (15–381) regions: A73-G, A263-G, 315.1-C, T16224-C, T16311-C (as compared with the revised Cambridge reference sequence (32)). Based on mutations T16224-C and T16311-C, the obtained mtDNA profile can be designated as haplogroup K, a haplogroup primarily found in European populations (33, 34). The mtDNA profile has no match among 4839 individuals in SWGDAM database (35) and the four closest sequences (with one base pair difference) are from Caucasian populations.
The authenticity of the obtained sequences was secured by multiple criteria, including: (i) the use of dedicated ancient DNA facilities equipped with UV filtered ventilation, positive airflow, and bench UV lights; (ii) a vigorous decontamination protocol of the bone samples before DNA extraction; (iii) the inclusions of blank extracts and PCR negative controls; (iv) DNA sequences obtained from different tooth/bone extracts, as well as sequences obtained from multiple amplification of the same extract that all yielded the same sequences; (v) the modern reference sequences were processed only after the analysis of the cranium had been completed; and (vi) the cranium’s mtDNA profile differed from that of DNA laboratory personnel and the forensic anthropologist by at least two base pairs.
In addition to mtDNA analysis, the DNA extracts were also amplified with primers designed to target short fragments of the amelogenin gene on the X and Y chromosomes. While the CC1 DNA extract failed to amplify, both the CC2 and CC7 extracts amplified the Y-chromosome amelogenin fragment, indicating that the cranium belonged to a male individual.
Results of Complete Biological Profile
These analyses, which were conducted initially without knowledge of the putative identity or date of disappearance, produced the following biological profile for the cranium: a male, most likely of European ancestry (based on the mtDNA haplogroup K profile), aged 4.4 ±1 year, born in the interval from 1958 to 1962 and who died between 1963–1968. His remains were sufficiently skeletonized that plant matter entered the skull in the Spring season.
Correspondence with the authorities indicated that there was a boy of European ancestry who had gone missing (and presumed drowned) in the area of the cranium’s discovery in early 1965, aged 4.63 years. Based on the correspondences between the cranium and the missing child, a maternal relative was contacted to provide a mtDNA reference for comparison.
Comparison of mtDNA with putative family references
The two DNA extracts from the maternal relative (FC1, FC2) both produced the same mtDNA profile as the cranium, amplifying 976bp of mtDNA from the HVI (15973–16569) and HVII (1–381) regions: A73-G, A263-G,315.1-C, T16224-C, T16311-C. The maternal reference also displayed a mutation at position T16519-C, a region that could not be amplified from the tooth or occipital DNA extracts. The profiles match at all other polymorphic areas.
In addition to the sibling, a paternal reference was also sought in order to add further evidence in support of the child’s identity through mini-STR analysis. The quantification of the familial reference samples yielded sufficient DNA for STR typing and gave full profiles suitable for comparison (data not shown). The two dental extracts (CC1, CC2) yielded no quantifiable nuclear DNA, however the occipital bone sample (CC7) yielded approximately 7 pg/uL of nuclear DNA. An attempt was made to amplify the limited nuclear DNA recovered in this sample in duplicate using AmpFlSTR MiniFiler™ kit. Mini-STR’s have been shown to be effective for amplifying DNA from degraded human remains (36). Previously, an internal validation study conducted at the British Columbia Institute of Technology (BCIT) determined that the optimal DNA input amount for the MiniFiler™ kit is 200 pg for a reaction volume of 12.5 μL (half of manufacture’s recommended volume). This validation study also tested a lower limit of DNA input down to 62 pg, which was successfully amplified using a variety of DNA sample types (data not shown). STR-typing was approached with extreme caution in this case given the low level (35pg) of input DNA. During the interpretation phase, it was apparent that the DNA profiles generated for CC7 contained extreme dropout due to stochastic effects (Table 2). Although limited conclusions can be drawn from the STR data, the presence of a Y allele indicated the remains are those of a male. Five loci yielded peak data although some peaks were sub-threshold. Of these, two loci (D18S51 and D2S1338) yielded a heterozygote peak pattern in one of two amplification replicates. Both heterozygote loci had an obligatory paternal allele present, consistent with a parent-child relationship although both the deceased and the putative father had alleles 17 and 20 at D2S1338 and therefore the obligatory allele cannot be determined without the mother’s sample. One locus (FGA) lacked an obligatory paternal allele although there was only a single, sub-threshold allele present in one of the replicates consistent with dropout. If one utilizes the four loci in agreement between the child and putative father (omitting FGA), a combined likelihood ratio of 14 is estimated (based on the Caucasian STR allele database) providing moderate support in favor of an identification.
TABLE 2.
Mini-STR Allele Information for the deceased. CC7 extract was amplified in duplicate (CC7-1 and CC7-2).
| Sample | D13S317 | D7S820 | Amel. | D2S1338 | D21S11 | D16S539 | D18S51 | CSF1PO | FGA |
|---|---|---|---|---|---|---|---|---|---|
| Cranium (CC7-1) | 11 | - | - | 17,20* | - | - | 14 | (11) | (18) |
| Cranium (CC7-2) | 11 | - | (X),Y | 17 | - | - | 13,14 | 10,(11) | - |
Paternal reference had alleles 17,20 at loci D2S1338
-indicates complete allelic dropout
( )indicates subthreshold allele (peak < 100 rfu)
Underlined allele indicates obligatory paternal allele present
Despite the minimal STR data in support of the identity of the missing child, the biological profile developed through anthropological, radiocarbon analysis, along with the mtDNA profile match with the maternal relative, provided the necessary evidence to secure a legal identification for the remains 41 years after the initial discovery. In October of 2009, the identity of the missing child was confirmed as that of the four-year old boy who had been presumed drowned in Northern Canada in 1965, and a death certificate was issued by authorities.
Combined mtDNA and Radiocarbon Approach
Identification of human remains that have elapsed time since death of more than 25 years poses several challenges. First, the DNA in these ‘cold case’ remains may be severely degraded, and therefore nuclear DNA may not be present in quantities sufficient for optimal STR analysis, as occurred in the case study presented here. Additionally, even when nuclear DNA is preserved, it may be difficult to locate or obtain a DNA reference sample from closely related individuals that would be suitable for comparison. Mitochondrial DNA profiles of the HVI and/or HVII regions are increasingly being used as a method of identification for forensic ‘cold cases’. Mitochondrial DNA is present in higher copy numbers per cell than nuclear DNA, thus increasing the chances of retrieving sufficient quantities of DNA for analysis. Additionally, the maternal inheritance pattern of mtDNA often allows for a greater likelihood of locating and obtaining a suitable reference sample from relatives.
However, mitochondrial profiles alone cannot usually provide definitive identification, especially if the mtDNA haplotype is a common one within a particular population. If mtDNA profiles are to be utilized for personal identification in forensic contexts, then it is crucial to know the frequencies of different mtDNA profiles within the populations (37). In mtDNA analyses of British (38), Croatian (39) and Nairobi (40) populations, the presence of common mtDNA haplotypes has ranged from 5–17%, obviously limiting the discriminatory power of certain haplotypes for personal identification. Additionally, less common issues such as such as heteroplasmy or point mutations may also complicate identifications (34).
While whole mitochondrial genome analysis using single-nucleotide polymorphisms may provide additional discriminatory power (41), this type of technique may be costly and time consuming. Furthermore, even whole mtDNA genomes may not separate maternal relatives in forensic context such as mass fatalities where siblings or maternal cousins are present within the same death assemblage.
The combination of mtDNA profiles and radiocarbon analysis, however, can provide a robust personal identification for those forensic cold cases dating to within the last half century. Since the first permanent molar crown takes less than four years to form starting at or shortly after birth (42), radiocarbon analysis of this tooth alone can provide an accurate and reasonably precise interval of birth for individuals born after 1955 (4). By dating the enamel of the third permanent molar (the last crown to form, in the interval between 9 and 12 years of age (42), the interval of birth may be extrapolated for individuals born from 1943 (4). With an average absolute error of 1.6 years, the radiocarbon method can provide a much more accurate and precise year of birth than does traditional anthropological analysis of the skeleton, especially for skeletally mature individuals (4, 43, 44). A precise interval of birth can provide strong corroborative evidence for a personal identification, as well as significantly narrowing down the list of missing persons where there is no presumptive ID. A combined mtDNA profile match and year-of-birth match can provide the evidence necessary for a ‘cold case’ legal identification, even if the mtDNA haplotype is a relatively common one.
In forensic cases where teeth are unavailable, the radiocarbon analysis of bone also can provide useful information concerning time of death. Unlike tooth enamel however, bone remodels throughout life, and therefore radiocarbon values of bone collagen will provide averages values for bone formation and remodeling, and may lag behind the actual date of death. Nevertheless, radiocarbon analysis of bone may be useful for determining whether the time of death occurred prior to 1950, or afterwards (45). Additionally, a comparison of radiocarbon value from slower remodeling cortical bone, and more rapidly remodeling trabecular bone may be useful for determining whether the bomb-carbon value fall before or after the peak of bomb-carbon in 1963 (46). Bone collagen radiocarbon analysis will be most useful in newborns and children whose tissues are forming rapidly and thus more accurately reflect the bomb-carbon values during deposition (46). However, radiocarbon analysis of tooth enamel can provide more precise information, and is preferable to bone when available (45, 46).
In mass graves or mass fatality contexts, a combined DNA and radiocarbon analysis approach provides the additional benefit of distinguishing between maternal relations. When DNA matches are based on matches with relatives (rather than known samples of the victim’s own DNA), distinguishing between childless siblings in commingled mass graves can be impossible, even when STR profiles can be generated (47). Though the siblings will have different STR profiles, they cannot be distinguished based on parental DNA references alone. If the siblings are of the same sex and closely related in age, anthropological age-at-death techniques may not be precise enough to discriminate between them, such as in the case of many adolescent brothers killed in the Srebrenica event in Bosnia and Herzegovina (47). Radiocarbon analysis of tooth enamel, in combination with STR and/or mtDNA analysis, has the potential to distinguish between siblings even one year apart in age, provided their birth year occurred after 1943.
While the particular anthropological, genetic and radiocarbon techniques discussed above have been applied separately in the past, this case study highlights the enormous potential of these combined techniques for ‘cold case’ identifications and other challenging forensic contexts dating to the last half century.
Acknowledgments
We thank Amy Mundorff for drawing our attention to Dr. Spalding’s research, Grace Zhang Hua and Marina Elliott for anthropological assistance, and Krista McGrath for lab assistance. Work was performed in part under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Footnotes
This research was supported in part by research grants including Social Science and Humanities Research Council of Canada’s RDI fund, SFU Discovery Park Fund and the Swedish Research Council.
References
- 1.Trodden BJ. Archaeological Survey of Canada Paper. Vol. 112. Ottawa: National Museums of Canada; 1982. A Radiographic Study of the Calcification and Eruption of the Permanent Teeth in Inuit and Indian Children. [Google Scholar]
- 2.Liversidge HM, Molleson T. Variation in crown and root formation and eruption of human deciduous teeth. Amer J Phys Anthrop. 2004;123(2):172–80. doi: 10.1002/ajpa.10318. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz BA, Spalding KL. Year of birth determination using radiocarbon dating of dental enamel. Surface and Interface Analysis. 2010;42(5):398–401. doi: 10.1002/sia.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spalding KL, Buchholz BA, Bergman L-E, Druid H, Frisén J. Age written in teeth by nuclear tests. Nature. 2005;437(7057):333–4. doi: 10.1038/437333a. [DOI] [PubMed] [Google Scholar]
- 5.Alkass K, Buchholz BA, Ohtani S, Yamamoto T, Druid H, Spalding KL. Age estimation in Forensic Sciences. Molecular & Cellular Proteomics. 2009;9(5):1022–30. doi: 10.1074/mcp.M900525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook GT, Dunbar E, Black SM, Xu S. A preliminary assessment of age at death determination using the nuclear weapons testing 14C activity of dentine and enamel. Radiocarbon. 2006;48(3):305–13. [Google Scholar]
- 7.Hua Q, Barbetti M. Review of tropospheric bomb 14C data for carbon cycle modeling and age calibration purposes. Radiocarbon. 2004;46(3):1273–98. [Google Scholar]
- 8.Reimer PJ, Brown TA, Reimer RW. Discussion: Reporting and calibration of post-bomb 14C data. Radiocarbon. 2004;46(3):1299–304. [Google Scholar]
- 9.Levin I, Hammer S, Kromer B, Meinhardt F. Radiocarbon observations in atmospheric CO2: Determining fossil fuel CO2 over Europe using Jungfraujoch observations as background. Science for the Total Environment. 2008;391:211–6. doi: 10.1016/j.scitotenv.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Levin I, Kromer B. The tropospheric 14CO2 level in mid-latitudes of the northern hemisphere (1959–2003) Radiocarbon. 2004;46(3):1261–72. [Google Scholar]
- 11.Levin I, Naegler T, Kromer B, Diehl M, Francey RJ, Gomez-Pelaez AJ, et al. Observations and modelling of the global distribution and long-term trend of atmospheric 14CO2. Tellus B. 2010;62(1):26–46. [Google Scholar]
- 12.Yang DY, Eng B, Waye JS, Dudar JC, Saunders SR. Improved DNA extraction from ancient bones using silica-based spin columns. Amer J Phys Anthrop. 1998;105:539–43. doi: 10.1002/(SICI)1096-8644(199804)105:4<539::AID-AJPA10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Kemp BM, Smith DG. Use of bleach to eliminate contaminating DNA from the surface of bones and teeth. Forensic Sci Int. 2005 Nov 10;154(1):53–61. doi: 10.1016/j.forsciint.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Watt K. MA Thesis. Burnaby, BC: Simon Fraser University; 2005. Decontamination techniques in ancient DNA analysis. [Google Scholar]
- 15.Malmström H, Svensson EM, Gilbert MTP, Willerslev E, Götherström A, Holmlund G. More on contamination: The use of asymmetric molecular behavior to identify authentic ancient Human DNA. Mol Biol Evol. 2007 Apr 1;24(4):998–1004. doi: 10.1093/molbev/msm015. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel MN, Huffine EF, Ryan JH, Holland MM, Parsons TJ. Improved mtDNA sequence analysis of forensic remains using a “mini-primer set” amplification strategy. J Forensic Sci. 2001 Mar;46(2):247–53. [PubMed] [Google Scholar]
- 17.Dudar JC, Waye JS, Saunders SR. Determination of a kinship system using ancient DNA, mortuary practice, and historic records in an upper Canadian pioneer cemetery. Int J Osteoarchaeol. 2003;13(4):232–46. [Google Scholar]
- 18.Yang DY, Eng B, Waye JS, Dudar JC, Saunders SR. A new strategy for DNA sex determination from ancient human skeletons. Amer J Phys Anthrop. 1998;26(Suppl):236. [Google Scholar]
- 19.Wilson MR, Dizinno JA, Polanskey D, Replogle J, Budowle B. Validation of mitochondrial-DNA sequencing for forensic casework analysis. Int J Legal Med. 1995 Oct-Nov;108(2):68–74. doi: 10.1007/BF01369907. [DOI] [PubMed] [Google Scholar]
- 20.Katzenberg MA, Oetelaar G, Oetelaar J, Fitzgerald C, Yang D, Saunders SR. Identification of historical human skeletal remains: a case study using skeletal and dental age, history and DNA. Int J Osteoarchaeol. 2005;15(1):61–72. [Google Scholar]
- 21.Klippel WE, Synstelien JA. Rodents as taphonomic agents: Bone gnawing by brown rats and Gray squirrels. J Forensic Sci. 2007;52(4):765–73. doi: 10.1111/j.1556-4029.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 22.Skinner MF, Anderson GS. Individualization and enamel histology: A case report in forensic anthropology. J Forensic Sci. 1991;36(3):939–48. [PubMed] [Google Scholar]
- 23.Scott GR, Turner CG. The Anthropology of Modern Human Teeth. Cambridge: Cambdrige University Press; 1997. [Google Scholar]
- 24.Reid DJ, Schwartz GT, Dean C, Chandrasekera MSS. A histological reconstruction of dental development in the common chimpanzee, Pan troglodytes. J Hum Evol. 1998;35(4–5):427–48. doi: 10.1006/jhev.1998.0248. [DOI] [PubMed] [Google Scholar]
- 25.Stewart TD. Essentials of Forensic Anthropology. Springfield, Il: Charles C. Thomas, Publisher; 1979. [Google Scholar]
- 26.Scheur L, Black S. Developmental Juvenile Osteology. San Diego: Academic Press; 2000. [Google Scholar]
- 27.Işcan MY, Helmer RP, editors. Forensic Analysis of the Skull: Craniofacial Analysis, Reconstruction, and Identification. New York: Wiley-Liss; 1993. [Google Scholar]
- 28.Jones KL. Smith’s Recognizable Patterns of Human Malformation. 6. Philadelphia: Elsevier; 2006. [Google Scholar]
- 29.Kraus BS, Jordan RE. The Human Dentition Before Birth. Philadelphia: Lea and Febiger; 1965. [Google Scholar]
- 30.Lunt RC, Law DB. A review of the chronology of calcification of deciduous teeth. J Am Dent Assoc. 1974;89(3):599–606. doi: 10.14219/jada.archive.1974.0446. [DOI] [PubMed] [Google Scholar]
- 31.Mahoney P. Human deciduous mandibular molar incremental enamel development. Amer J Phys Anthrop. 2011;144(2):204–14. doi: 10.1002/ajpa.21386. [DOI] [PubMed] [Google Scholar]
- 32.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23(2):147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 33.van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 2008;1039(29):E386–E94. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 34.Butler JM. Forensic DNA typing, biology, technology, and genetics of STR markers. New York: Elsevier Academic Press; 2005. [Google Scholar]
- 35.Monson KL, Miller KWP, Wilson MR, DiZinno JA, Budowle B. The mtDNA population database: an integrated software and database resource for forensic comparison. [accessed 15 March 9];Forensic Sci Comm. 2002 4(2) http://www.fbi.gov/hq/lab/fsc/backissu/april2002/miller1.htm. [Google Scholar]
- 36.Mulero JJ, Chang CW, Calandro LM, Green RL, Li Y, Johnson CL, et al. Development and Validation of the AmpF3STR® Yfiler™ PCR Amplification Kit: A Male Specific, Single Amplification 17 Y-STR Multiplex System. J Forensic Sci. 2006;51(1):64–75. doi: 10.1111/j.1556-4029.2005.00016.x. [DOI] [PubMed] [Google Scholar]
- 37.Crespillo M, Luque JA, Paredes M, Fernández R, Ramírez E, Valverde JL. Mitochondrial DNA sequences for 118 individuals from northeastern Spain. Int J Legal Med. 2000;114(1):130–2. doi: 10.1007/s004140000158. [DOI] [PubMed] [Google Scholar]
- 38.Piercy R, Sullivan KM, Benson N, Gill P. The application of mitochondrial DNA typing to the study of white Caucasian genetic identification. Int J Legal Med. 1993;106(2):85–90. doi: 10.1007/BF01225046. [DOI] [PubMed] [Google Scholar]
- 39.Gabriel MN, Calloway CD, Reynolds RL, Andelinovic S, Primorac D. Population Variation of Human Mitochondrial DNA Hypervariable Regions I and II in 105 Croatian Individuals Demonstrated by Immobilized Sequence-specific Oligonucleotide Probe Analysis. Croat Med J. 2001;42(3):328–35. [PubMed] [Google Scholar]
- 40.Brandstätter A, Peterson CT, Irwin JA, Mpoke S, Koech DK, Parson W, et al. Mitochondrial DNA control region sequences from Nairobi (Kenya): inferring phylogenetic parameters for the establishment of a forensic database. Int J Legal Med. 2004;118(5):306. doi: 10.1007/s00414-004-0466-z. [DOI] [PubMed] [Google Scholar]
- 41.Parsons T, Coble MD. Increasing the Forensic Discrimination of Mitochondrial DNA Testing through Analysis of the Entire Mitochondrial DNA Genome. Croat Med J. 2001;42(3):304–9. [PubMed] [Google Scholar]
- 42.Skinner MF, Goodman A. Anthropological uses of developmental defects of enamel. In: Saunders SR, Katzenberg A, editors. Skeletal Biology of Past Peoples: Research Methods. New York: Wiley-Liss; 1992. pp. 153–74. [Google Scholar]
- 43.Krogman WM, Işcan MY. The Human Skeleton in Forensic Medicine. 2. Springfield, IL: C. C. Thomas; 1986. [Google Scholar]
- 44.Alkass K, Buchholz BA, Druid H, Spalding KL. Analysis of 14C and 13C in teeth provides precise birth dating and clues to geographical origin. Forensic Sci Int. doi: 10.1016/j.forsciint.2010.12.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ubelaker DH, Buchholz BA. Complexities in the use of bomb-curve radiocarbon to determine time since death of human skeletal remains. [Accessed March 28, 11];Forensic Sci Comm. 2006 8(2) http://www.fbi.gov/about-us/lab/forensic-science-communications/fsc/jan2006/research/_01_research01.htm. [Google Scholar]
- 46.Ubelaker DH, Buchholz BA, Stewart JEB. Analysis of artificial radiocarbon in different skeletal and dental tissue types to evaluate date of death. J Forensic Sci. 2006;51(3):484–8. doi: 10.1111/j.1556-4029.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 47.Yazedjian L, Kesetovic R. The Application of Traditional Anthropological Methods in a DNA-Led Identification Process. In: Adams BJ, Byrd JE, editors. Recovery, Analysis, and Identification of Commingled Human Remains. New York: Springer-Verlag; 2008. pp. 271–85. [Google Scholar]