Abstract
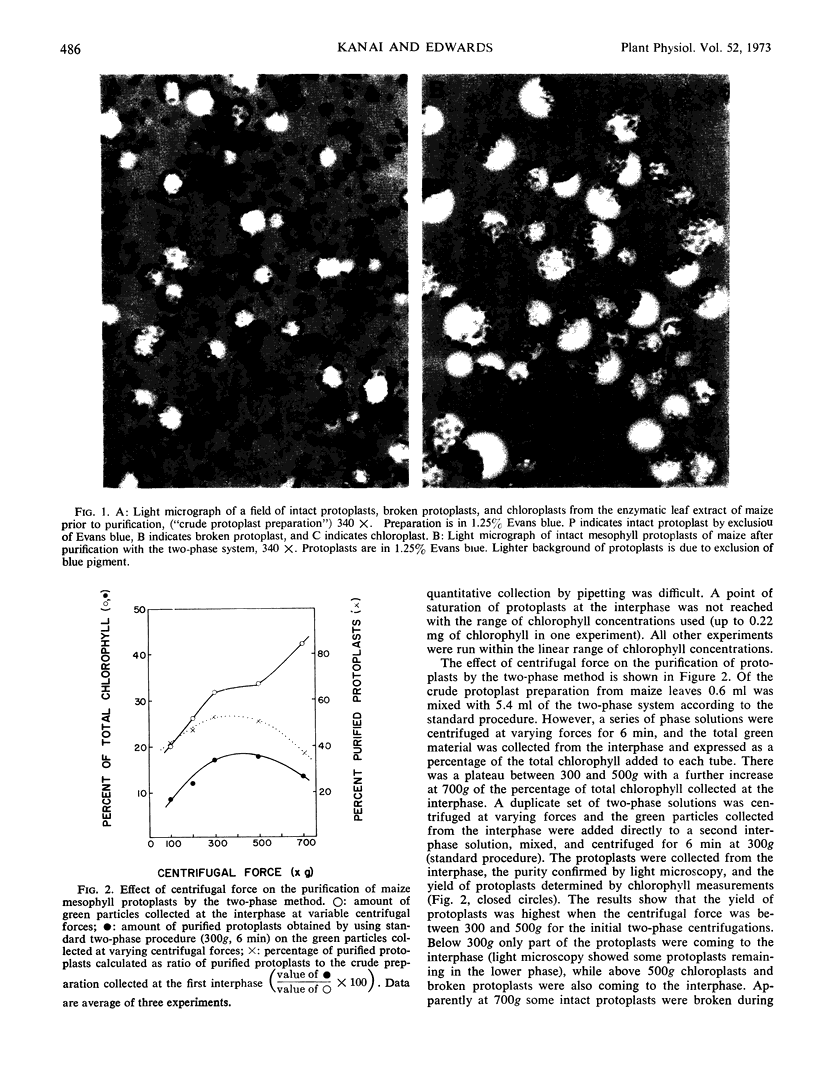
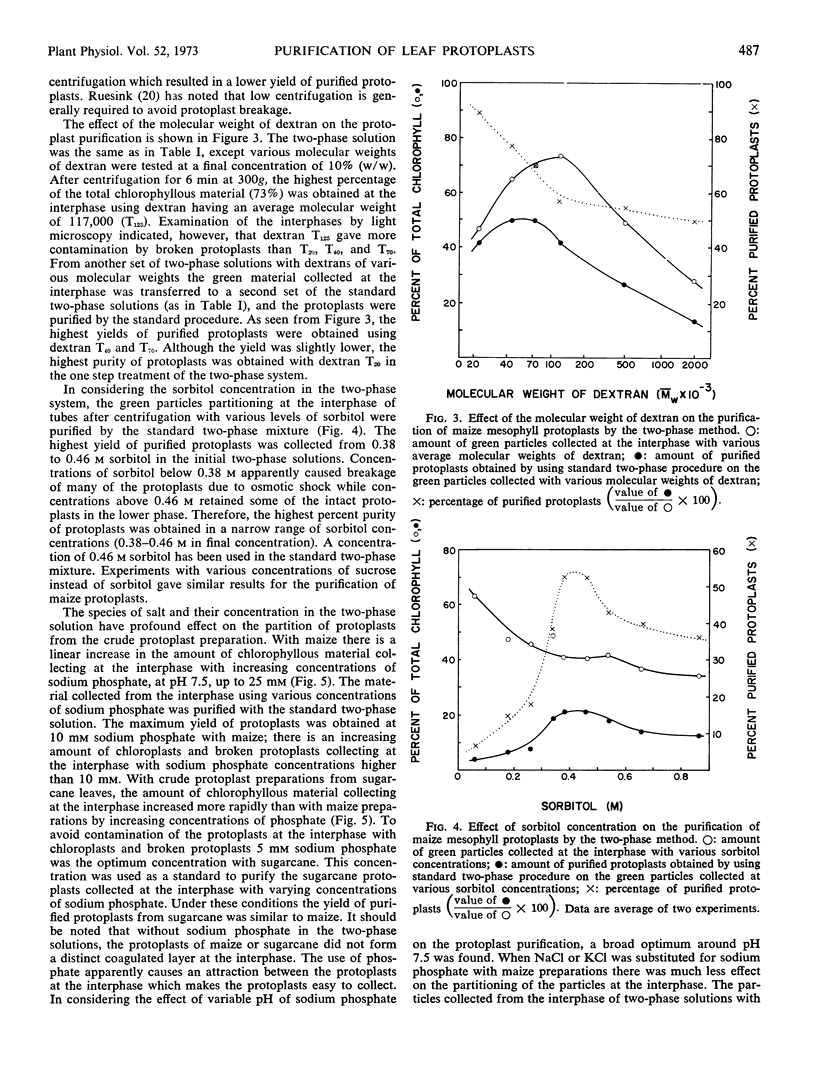
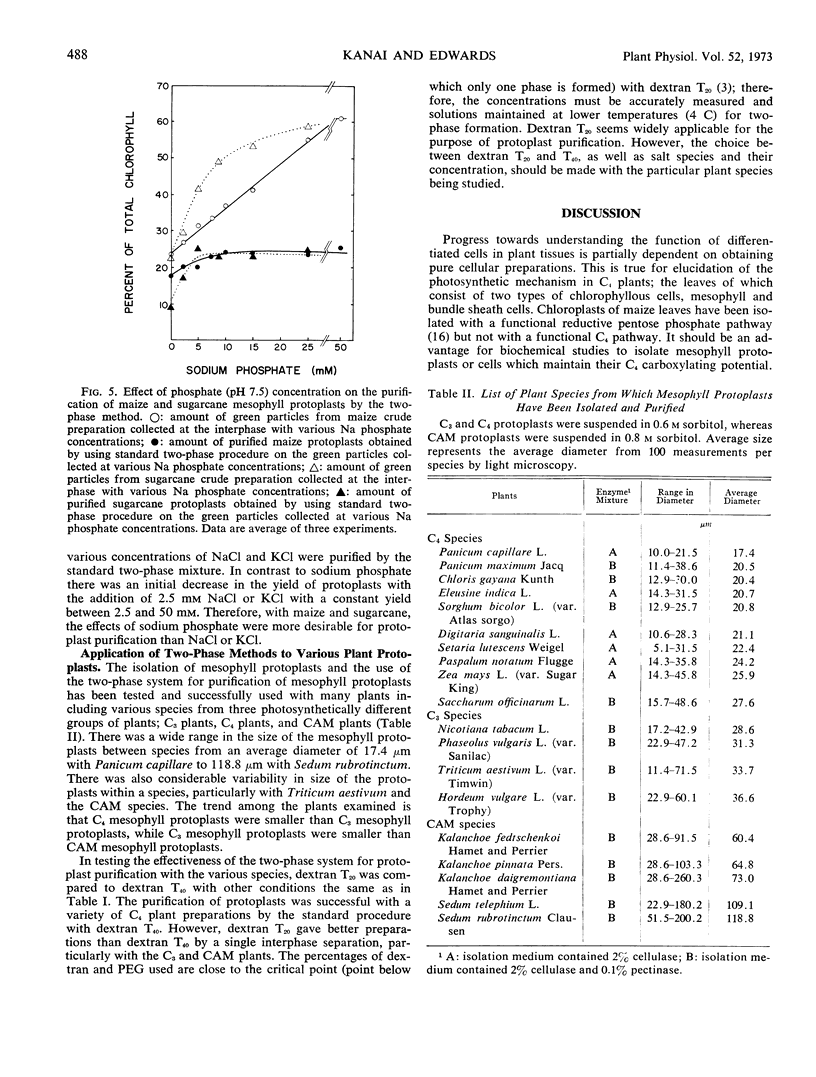
Enzymatic digestion of leaf segments with 2% cellulase, in combination with a pectinase in some species, yields intact protoplasts mixed with epidermal tissue, vascular tissue, broken protoplasts, and chloroplasts. Epidermal and vascular tissue are removed with sieves of various porosity. Intact protoplasts in the filtrate are separated from other components by an aqueous two-phase system which consists of dextran-polyethylene glycol, with sorbitol and sodium phosphate. Intact protoplasts partition at the interphase, while chloroplasts and broken protoplasts partition in the lower phase when the separation is facilitated by low speed centrifugation. The optimum conditions for purification of maize mesophyll protoplasts with high yields are centrifugation of the two-phase system at 300g for 6 minutes at 2 C with a mixture including 0.46 m sorbitol, 10 mm sodium phosphate, 5.5% polyethylene glycol 6000, and 10% dextran of average molecular weight of 20,000 to 40,000. The collection of protoplasts at the inter-phase was proportional to the amount of chlorophyll added over a wide range of concentrations regardless of the initial contamination of the preparation by other cellular debris. The two-phase system is applicable for protoplast purification from a wide variety of species, including C3, C4, and Crassulacean acid metabolism plants, regardless of protoplast size.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTSSON P. A., BAIRD G. D. Counter-current distribution of cells. Exp Cell Res. 1962 Nov;28:296–322. doi: 10.1016/0014-4827(62)90285-9. [DOI] [PubMed] [Google Scholar]
- ALBERTSSON P. A. Particle fractionation in liquid two-phase systems; the composition of some phase systems and the behaviour of some model particles in them; application to the isolation of cell walls from microorganisms. Biochim Biophys Acta. 1958 Feb;27(2):378–395. doi: 10.1016/0006-3002(58)90345-7. [DOI] [PubMed] [Google Scholar]
- Carlson P. S. The use of protoplasts for genetic research. Proc Natl Acad Sci U S A. 1973 Feb;70(2):598–602. doi: 10.1073/pnas.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Campbell W. H., Dittrich P., Black C. C. Distribution of carboxylation and decarboxylation enzymes in isolated mesophyll cells and bundle sheath strands of C 4 plants. Biochem Biophys Res Commun. 1973 Mar 17;51(2):461–467. doi: 10.1016/0006-291x(73)91279-5. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Black C. C. Isolation of Mesophyll Cells and Bundle Sheath Cells from Digitaria sanguinalis (L.) Scop. Leaves and a Scanning Microscopy Study of the Internal Leaf Cell Morphology. Plant Physiol. 1971 Jan;47(1):149–156. doi: 10.1104/pp.47.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY D. W., COCKING E. C. THE LARGE-SCALE ISOLATION OF PROTOPLASTS FROM IMMATURE TOMATO FRUIT. J Cell Biol. 1965 Jan;24:143–146. doi: 10.1083/jcb.24.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Francki R. I., Zaitlin M. Metabolism of separated leaf cells: I. Preparation of photosynthetically active cells from tobacco. Plant Physiol. 1971 Jul;48(1):9–13. doi: 10.1104/pp.48.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal D., Hew C. S., Latzko E., Gibbs M. Photosynthetic carbon metabolism of isolated corn chloroplasts. Plant Physiol. 1972 Apr;49(4):607–614. doi: 10.1104/pp.49.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]




