Abstract
Liver and kidney damage associated with polytrauma, endotoxic shock/sepsis, and organ transplantation, are among the leading causes of the multiple organ failure. Development of novel sensitive biomarkers that detect early stages of liver and kidney injury is vital for the effective diagnostics and treatment of these life-threatening conditions. Previously, we identified several hepatic proteins, including Argininosuccinate Synthase (ASS) and sulfotransferases which were degraded in the liver and rapidly released into circulation during Ischemia/Reperfusion (I/R) injury. Here we compared sensitivity and specificity of the newly developed sandwich ELISA assays for ASS and the sulfotransferase isoform SULT2A1 with the standard clinical liver and kidney tests Alanine Aminotransferase (ALT) and Aspartate Transaminase (AST) in various pre-clinical models of acute injury. Our data suggest that ASS and SULT2A1 have superior characteristics for liver and kidney health assessment in endotoxemia, Ischemia/Reperfusion (I/R), chemical and drug-induced liver injury and may be of high potential value for clinical applications.
Keywords: Biomarkers, Liver, ASS, SULT2A1, Sepsis, Toxicity, Shock
Introduction
Liver and kidney damage and failure due to various forms of intoxication and abdominal injury are significant sources of overall morbidity and mortality in the US and worldwide. Persistent hepatic injury occurs during viral hepatitis, fatty liver disease (steatohepatitis), drug or alcohol and autoimmune induced hepatitis. Clinical conditions are numerous where related hepatic and renal injuries are critical components of multi-organ failure caused by complex trauma including blast injury, septic shock, and graft failure after liver transplantation often leading to death of the patient. Specific biochemical markers have become mandatory in diagnosing dysfunction for a number of organs, for example myocardial infarction or hepatitis. The increase of ALT and AST in blood has been used in clinical practice for a long time for diagnostics of viral hepatitis of all types and alcoholic/toxic hepatitis, and monitoring of treatment. Several other enzymes such as isocitrate dehydrogenase [1] and anti-oxidative enzyme Glutathione-S-Transferase (GST) [2] were also shown as potential markers for viral hepatitis injury. γ-Glutamyl Transferase (γ-GT) has been used for diagnostics of hepatitis accompanied by cholestasis and hepatobiliary injury and viral hepatitis B and C [3,4]. Elevated GST levels in blood were detected in posttraumatic hepatic injury in primates, acute hepatotoxicity, and intestinal ischemia in rats [5-7]. However, diagnostic tests of toxic hepatitis or alcoholic liver disease reflect advanced stages of liver diseases with profound levels of hepatocellular death, and do not allow to distinguish between parenchymal and hepatic endothelial injury and/or to determine magnitude and reversibility of damage. Thus, a clinical need is apparent for specific, non-invasive pathogenically relevant biomarkers which would diagnose the magnitude and phase of liver injury for better diagnostics and control of treatment. We set out to develop biomarkers for liver focusing on molecules specific to the liver, which could be substrates for proteolytic cleavage. By using liver proteomic degradomics approach we identified several biomarker candidates, which were then tested in experimental liver I/R injury in rats [8]. The most promising molecules have been Argininosuccinate Synthase (ASS) and Estrogen Sulfotransferase (EST-1). We have found that hepatic ASS is subjected to proteolytic cleavage in the liver via pro-apoptotic caspase-3, which is activated upon hepatic toxic, ischemic or viral insult, and can be released in blood [8]. Subsequently, we discovered that SULT2A1 isoform of sulfotransferase is a more sensitive in detecting liver injury. Also, SULT2A1 possess a slightly greater value regarding specificity and assay development. In the current study we compared sensitivity and specificity of the newly developed ELISA assays for ASS and the SULT2A1 with standard clinical liver and kidney function assays for Alanine Aminotransferase (ALT) and Aspartate Transaminase (AST) in various pre-clinical models of acute toxicity. Our data suggest that ASS and SULT2A1 have superior characteristics for liver and kidney health assessment in endotoxemia, Ischemia/Reperfusion (I/R), chemical and drug-induced liver and kidney injury and may be of high potential value for clinical applications.
Materials and Methods
Reagents
Lipopolysaccharide (LPS) from Escherichia coli O111:B4, D-Galactosamine (D-Gal) and Carbon tetrachloride (CCl4) were purchased from Sigma (St. Louis, MO, USA). The levels of endogenous ASS and SULT2A1 in serum were determined by SW ELISA assays (Banyan Biomarkers, Inc.) using rabbit polyclonal antibodies as capture and mouse monoclonal as detection antibodies. Color development was accomplished using anti-mouse HRP-conjugated antibodies followed by TMB substrate incubation. ASS and SULT2A1 levels were calculated from a calibration curve using human recombinant proteins prepared according [9] as standards.
Transaminase activities
The alanine aminotransferase (ALT, C.E.2.6.1.2) and aspartate aminotransferase (AST, C.E.2.6.1.1) activities in animal blood were measured using end-point colorimetric tests (BioVision, Milpitas, CA) in duplicate according to the manufacturer’s instructions. .
Animals
Adult male Sprague-Dawley rats (200-225 g) and Balb/c mice (19-22 g) (Harlan Laboratories Inc., Indianapolis, IN) were used in this study. The animals were housed under constant temperature (22°C) and humidity with 12 h light/dark cycle and had access to chow and water as much as desired throughout the study. All experiments were performed in adherence with the National Institutes of Health guidelines for the use of experimental animals and were approved by the Institutional Animal Care and Use Committee of the University of Florida.
Rodent endotoxemia models
LPS/D-galactosamine acute liver injury
Lipopolysaccharide from E. coli (LPS, 10 μg/kg) plus D-galactosamine (D-Gal, 500 mg/kg), or LPS alone (100 μg/kg) or saline were injected intraperitoneally (i.p.) in Sprague-Dawley rats as described previously [10,11]. Blood was collected from heart of anesthetized animals at terminal time points 1 h, 2 h, 3 h and 24 h after the treatment, using at least 3 different rats for each time point.
Mouse endotoxemia model
For biomarker release studies Balb/c mice were given either i.p. E. coli LPS (15 mg/kg) alone or LPS followed in 1 h by injection with rASS (5 mg/kg).
Rat chemical hepatotoxicity models
Carbon tetrachloride (CCl4, diluted to 0.25 - 0.5 ml/kg in 0.2 ml olive oil) was injected as a bolus intraperitoneally (i.p.) in Sprague-Dawley rats. Blood was collected from heart of anesthetized animals at terminal time points 1 h, 6 h and 24 h after the treatment, using at least 3 different rats for each time point.
Rat model of liver ischemia/reperfusion injury
Adult male Sprague-Dawley rats (220-250 g) were anesthetized with 4% Isoflurane for 4 min in a chamber until a surgical level of anesthesia was achieved. Animals were placed on the heating pad and delivery of anesthetic gas continued via a nose cone throughout the surgery. A midline approximately 3-cm-long laparotomy was made, and the liver was exposed. The portal triad was exposed and occluded for 30 min using an atraumatic vascular clamp. After 30 min of normothermic ischemia, recirculation of the blood through the ischemic liver was achieved by removing the clamp for additional 10, 30 min, 1 and 3 h. At the end of reperfusion, blood was collected from heart; the liver was briefly perfused with cold Phosphate-Buffered Saline (PBS) to remove residual blood and taken for analysis. For comparison unilateral kidney I/R was performed in a similar manner as for liver with a renal artery and a vein dissected and occluded for 30 min. After 30 min of normothermic ischemia, recirculation of the blood through the ischemic kidney was restored for 30 min.
Statistics
Statistical analyses were performed using GraphPad Prism 5 software. Data were evaluated by 2-tailed unpaired t-test. The criterion for statistical significance was set at p<0.05 or p<0.01.
Results
ASS and SULT2A1 are sensitive biomarkers of endotoxicity/liver injury
To assess quantitatively the levels of endogenous ASS and in rat serum after LPS treatment alone and in combination with the liver injury priming agent D-Galactosamine (D-Gal) we used the ELISA assay developed at Banyan Biomarkers [9]. As seen in figure 1A, ASS significantly accumulated in serum within one h after i.p. injection of LPS (100 μg/kg), attained a maximum increase of ~25-fold over baseline at 2 h, and decreased but remained significantly elevated at 3 and 24 h. In contrast to ASS, serum ALT levels after injection of 100 μg/kg LPS alone did not change significantly (Figure 1B), serum AST levels changed significantly only 3 h after LPS injection (Figure 1B) and no pathomorphological injury to the liver was observed at 24 h after injection (data not shown). When rats (n=8) were treated with lower doses of LPS (10 μg/kg) together with D-Gal to sensitize liver to LPS exposure, serum ASS levels raised significantly at 1 h and achieved nearly 1000-fold levels over control (saline-treated) in the animals which were alive but exhibited strong signs of terminal illness (2 out of 8) (Figure 1D). For this group corresponding ALT/AST levels were 29/12-fold over the control level (Figures 1E and 1F). In rats recovered from treatment (3 out of 8), serum ASS levels declined to nearly baseline after 24 h, but were elevated over control rats (Figure 1D). Serum ALT activity was found to increase significantly at 2 h and was substantially elevated at 24 h after LPS/D-Gal injection compared to control values (Figure 1E). Serum AST levels changed significantly compared to control only 3 h after LPS/D-Gal injection (Figure 1F). The data shown above are in concordance with more prominent ASS accumulation in blood observed after i.p. injection of much higher doses of bacterial LPS in mice (15 mg/kg) (Figure 2A). It is worth noting that i.p. administered rASS is cleared from circulation over an 18 h time period in the absence of liver injury (Figure 2B) so that the LPS-related elevation at 18 h (Figure 2A) exceeds the normal clearance kinetics. SULT2A1 ELISA developed at Banyan Biomarkers also has high sensitivity for LPS intoxication. Following 24 h after i.p. injection of LPS only (Figure 6B) SULT2A1 serum level was ~200-fold over control level.
Figure 1. ASS is a potential biomarker of endotoxicity/liver injury.
Endotoxin i.p. injections in rats elicit strong ASS release in blood. A-C: LPS only (100 μg/kg); D-F: LPS (10 μg/kg) and D-Gal (500 mg/kg). ***-p<0.001 **-p<0.01; *-p<0.05 vs. control samples.
Figure 2. Serum ASS accumulates following LPS administration in mice.

A: Mice serum ASS levels induced by i.p. LPS (15 mg/kg) injection. B: Serum ASS kinetics following i.p. injection with rASS (5 mg/kg). Mean+S.D. and T-test analysis are shown. **-p<0.01; *-p<0.05 vs. control samples.
Figure 6. SULT2A1 accumulates in serum after rat treatment with CCI4 or LPS.
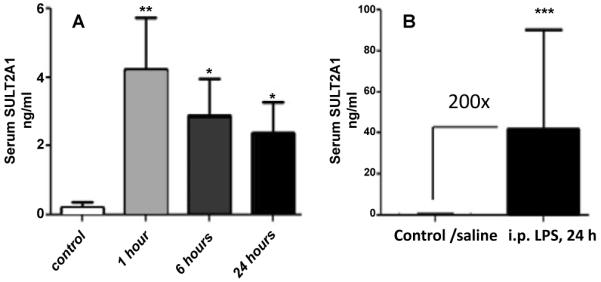
A: Time-course of serum levels of SULT2A1 after i.p. injection of 0.25 ml/kg of carbon tetrachloride in olive oil. B: SULT2A1 24 hours after i.p. injection of LPS (20 mg/kg). Control: solvent only. Mean+S.D. values are shown. **-p<0.01; *-p<0.05 vs. control samples.
ASS is a sensitive liver and kidney I/R injury biomarker
We assessed ASS in blood of rats subjected to liver Ischemia/Reperfusion (I/R) injury on different reperfusion time with a fixed 30 min of ischemia period. As figure 3 indicates, the magnitude of I/R injury depends largely on reperfusion time. Massive release and accumulation of ASS in blood was detected immediately after complete 30 min ischemia (0 reperfusion time), was reperfusion time-dependent and rapidly attained a steady state within 30 min, and persisted up until 180 min after initiation of reperfusion. Blood ASS levels correlated strongly with the severity of liver injury determined by histopathology of liver tissue (data not shown). As can be seen, ASS increase over sham treated animals was 23-fold immediately after ischemia as compared to ALT/AST which did not elevate at this time. Moreover, the magnitude of ASS increase has been 100-300-fold throughout 3 h reperfusion, whereas ALT/AST increase was 7 to 8-fold over sham-operated rats. The results obtained in unilateral kidney I/R studies show a sharp (~5-fold) increase of ASS concentration in rat blood after 30 min of normothermic ischemia followed by 30 min reperfusion (Figure 4A). No significant changes were observed in blood ALT (Figure 4B) and AST (Figure 4C) activities.
Figure 3. Liver injury markers correlate with I/R injury/reperfusion time.

A: ASS accumulation in blood; B and C: ALT and AST enzymatic assays in the same samples; N-naive (n=5); S-sham operated rats (n=4); 0 to 3 hours reperfusion (4-6 rats), 24 hours after partial reperfusion (n=4). ALT and AST were measured using end-point colorimetric tests in duplicate Mean+S.D and T-test analysis are shown.
Figure 4. ASS accumulates in serum as a response to kidney I/R injury.

Unilateral kidney Ischemia-Reperfusion. A: ASS ELISA; B: ALT; C: AST. For details please see Materials and Methods. Mean+S.D. and T-test analysis are shown. ***-p<0.001 vs. control samples.
ASS and SULT2A1 are potential biomarkers of chemical hepatotoxicity
ASS rapidly accumulated in plasma 1 h after CCl4 injection (~15-fold over control) and further increased at 24 h post-treatment (>50-fold over control) (Figure 5A). No significant increase in plasma ALT was detected at 1 h following injection; ALT was elevated at 24 h post-treatment 6-fold over control (Figure 5B). SULT2A1 level in serum also surged 1 h after CCl4 injection (~19-fold over control) and remained significantly elevated up to 24 h post-treatment (Figure 6A).
Figure 5. Serum ASS elevation reflecting chemical hepatotoxicity.

Plasma ASS accumulation after CCI4 i.p. injection (0.5 ml/kg in 0.2 ml veg. oil). A: ASS ELISA; B: ALT assay. For details please see Materials and Methods. Mean+S.D. and T-test analysis are shown. **-p<0.01; *-p<0.05 vs. control samples.
Discussion
In the previous studies we employed liver proteomic degradomics approach and identified several biomarker candidates, which were then preliminarily validated in experimental liver I/R injury and LPS/D-Gal injury in rats [8]. LPS/D-Gal treatment is an established model of targeted LPS-dependent liver injury accompanied by a massive hepatic apoptosis and release of amino transferases ALT and AST [12,13]. As seen on figure 1, rat liver injury induced by injection of bacterial LPS only (Figure 1A) or LPS and D-Gal combination (Figure 1D) was accompanied by a fast rise of serum ASS levels. At the same time-points changes of ALT (Figures 1B and 1E) and AST (Figures 1C and 1F) activities were far less pronounced if significant at all.
Our assessments of the ASS utility in the rat model of liver ischemia/reperfusion injury demonstrate high correlation of ASS levels in serum with duration of reperfusion and magnitude of liver injury (Figure 3A). Sensitivity on the early stages of liver injury and dynamic range of ASS ELISA far exceeds that of ALT/AST (Figures 3B and 3C). Strong ASS response to the kidney damage was found also in the rat model of kidney ischemia/reperfusion (Figure 4A). Again, here ASS ELISA was a better injury indicator than ALT/AST assays (Figures 4B and 4C). Exposure to high concentrations of toxic chemical compounds or drugs overused for medical or recreational purposes can cause liver and kidney damage [14-17]. Therefore important diagnostic need can be met with ELISA tests for ASS and SULT2A1 employed as illustrated for CCl4 (Figures 5A and 6A). Time/dose-dependent accumulation of ASS and SULT2A1 in blood strongly correlates with the toxicity levels achieved.
The use of ASS and SULT2A1 as components of biomarker panel for liver/kidney injury confers a number of advantages over existing ‘surrogate’ biomarkers: (i) ASS and SULT2A1 accumulated in blood earlier than ALT/AST (Figures 1,3,5 and 6), (ii) the diagnostic window was much larger with the fold increase ranged from 2 to 1000-fold depending on the injury magnitude (Figures 1 and 3-6), (iii) ASS and SULT2A1 declined faster than ALT/AST upon resolution of damage (Figure 1), and (iv) ASS/SULT2A1 play roles in pathogenesis of hepatic injury linking oxidative stress, liver function (ASS), and responses to toxic insults (SULT2A1). ASS is not found in erythrocytes or other blood cells. Thus, assessments of ASS and SULT2A1 in serum or plasma are not confounded by red blood cell hemolysis; unlike ALT or AST, both serum and plasma can be used for analysis. Ideally, whole blood can be employed in the future express diagnostics of ischemic liver damage, for example in doctor offices, or during emergency combat operations. Recently, the potential diagnostic value for liver type Arginase-I (Arg-I) and Carbamoyl Phosphate Synthetase-1 (CPS-1) was reported in rat liver ischemia/reperfusion [18-20]. While Arg-I and CPS-1 appear to be promising candidates as biomarkers for liver injury, the comprehensive studies of these enzymes have not been performed.
Our selection of liver biomarkers followed several criteria that a potential marker needs to fulfill for initiation of assay development: specificity of the marker to the organ of interest (tissue panel and protein expression level), comprehensive protein analysis for stability, antigenicity and isoform similarity. Ideally, biomarkers should employ biological substrates unique to the organ and, at the same time, provide information on injury mechanisms, a criterion that is used to distinguish biochemical markers from surrogate markers of injury since surrogate markers usually do not provide information on injury mechanisms. SULT2A1 and ASS fit criteria for biochemical markers: they are closely involved in major protective systems including liver and kidney. SULT2A1 represents detoxification function of liver [21]. In the liver, SULT2A1 plays an important role in bile acid homeostasis and protection against the toxic effects of bile acids. SULT2A1 is also found at minor levels in jejunum, ileum, cecum and kidney cytosol samples. Argininosuccinate Synthase (ASS) is a link between urea cycle and Nitric Oxide (NO) synthesis ([22]) which plays a major role in responses to ischemia, oxidative stress and toxins upon activation of inducible NO synthase in hepatocytes. In hepatic endothelial cells, constitutively expressed eNO synthase can utilize arginine supplied by consecutive actions of ASS and ASL and thereby provide the initial responses to injurious agents. As our prior experiments showed, if the endotoxin injection was followed by that of recombinant ASS, it significantly alleviated systemic septic symptoms and inflammation compared to untreated endotoxemia substantiating a hypothesis that systemic ASS release is an innate immune system response to endotoxic shock [9]. It should be noted that ASS is now recognized as a ubiquitous enzyme in mammalian tissues with the highest values (both mRNA and protein levels) found in the liver and kidneys [23]. Nevertheless, because liver is the largest rat organ [24], we consider it as the primary source of endogenous ASS in circulation even accounting for the possible input from non-hepatic tissues [25].
Conclusion
Our data suggest that ASS and SULT2A1 have superior characteristics over traditional biomarkers for liver and kidney health assessment in endotoxemia, I/R, chemical and drug-induced liver injury and may be of high potential value for clinical applications.
Acknowledgments
The authors thank Ms. Olena Glushakova and Ms. Sabrina Singleton for their expert technical assistance. This work was supported by the National Institutes of Health (NIH) grant [5R44DK074205] and NIH Recovery Act Administrative Summer Supplement [R44DK074205-03S1].
References
- 1.Chung YH, Jung SA, Song BC, Chang WY, Kim JA, et al. Plasma isocitrate dehydrogenase as a marker of centrilobular hepatic necrosis in patients with hyperthyroidism. J Clin Gastroenterol. 2001;33:118–122. doi: 10.1097/00004836-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Thorburn D, Bird GL, Spence E, MacSween RN, Mills PR. alpha-Glutathione S-transferase levels in chronic hepatitis C infection and the effect of alpha-interferon therapy. Clin Chim Acta. 1996;253:171–180. doi: 10.1016/0009-8981(96)06337-1. [DOI] [PubMed] [Google Scholar]
- 3.Colombatto P, Randone A, Civitico G, Monti Gorin J, Dolci L, et al. Hepatitis G virus RNA in the serum of patients with elevated gamma glutamyl transpeptidase and alkaline phosphatase: a specific liver disease? [corrected] J Viral Hepat. 1996;3:301–306. doi: 10.1111/j.1365-2893.1996.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 4.Reynaud M, Schellenberg F, Loisequx-Meunier MN, Schwan R, Maradeix B, et al. Objective diagnosis of alcohol abuse: compared values of carbohydrate-deficient transferrin (CDT), gamma-glutamyl transferase (GGT), and mean corpuscular volume (MCV) Alcohol Clin Exp Res. 2000;24:1414–1419. [PubMed] [Google Scholar]
- 5.Giffen PS, Pick CR, Price MA, Williams A, York MJ. Alpha-glutathione S-transferase in the assessment of hepatotoxicity--its diagnostic utility in comparison with other recognized markers in the Wistar Han rat. Toxicol Pathol. 2002;30:365–372. doi: 10.1080/01926230252929945. [DOI] [PubMed] [Google Scholar]
- 6.Khurana S, Corbally MT, Manning F, Armenise T, Kierce B, et al. Glutathione S-transferase: a potential new marker of intestinal ischemia. J Pediatr Surg. 2002;37:1543–1548. doi: 10.1053/jpsu.2002.36181. [DOI] [PubMed] [Google Scholar]
- 7.Redl H, Schlag G, Paul E, Davies J. Plasma glutathione S-transferase as an early marker of posttraumatic hepatic injury in non-human primates. Shock. 1995;3:395–397. [PubMed] [Google Scholar]
- 8.Svetlov SI, Xiang Y, Oli MW, Foley DP, Huang G, et al. Identification and preliminary validation of novel biomarkers of acute hepatic ischaemia/reperfusion injury using dual-platform proteomic/degradomic approaches. Biomarkers. 2006;11:355–369. doi: 10.1080/13547500600775110. [DOI] [PubMed] [Google Scholar]
- 9.Prima V, Wang A, Molina G, Wang KK, Svetlov SI. Inhibition of LPS toxicity by hepatic argininosuccinate synthase (ASS): novel roles for ASS in innate immune responses to bacterial infection. Int Immunopharmacol. 2011;11:1180–1188. doi: 10.1016/j.intimp.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Jones JJ, Fan J, Nathens AB, Kapus A, Shekhman M, et al. Redox manipulation using the thiol-oxidizing agent diethyl maleate prevents hepatocellular necrosis and apoptosis in a rodent endotoxemia model. Hepatology. 1999;30:714–724. doi: 10.1002/hep.510300324. [DOI] [PubMed] [Google Scholar]
- 11.Dokladny K, Kozak A, Wachulec M, Wallen ES, Menache MG, et al. Effect of heat stress on LPS-induced febrile response in D-galactosamine-sensitized rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R338–R344. doi: 10.1152/ajpregu.2001.280.2.R338. [DOI] [PubMed] [Google Scholar]
- 12.Zanobbio L, Palazzo M, Gariboldi S, Dusio GF, Cardani D, et al. Intestinal glucose uptake protects liver from lipopolysaccharide and D-galactosamine, acetaminophen, and alpha-amanitin in mice. Am J Pathol. 2009;175:1066–1076. doi: 10.2353/ajpath.2009.090071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JY, Yeon JD, Kim JY, Yoo ES, Yu YH, et al. Hepatoprotection by human epidermal growth factor (hEGF) against experimental hepatitis induced by D-galactosamine (D-galN) or D-GalN/lipopolysaccharide. Biol Pharm Bull. 2000;23:1243–1246. doi: 10.1248/bpb.23.1243. [DOI] [PubMed] [Google Scholar]
- 14.Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 15.Jones AL, Simpson KJ. Review article: mechanisms and management of hepatotoxicity in ecstasy (MDMA) and amphetamine intoxications. Aliment Pharmacol Ther. 1999;13:129–133. doi: 10.1046/j.1365-2036.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 16.Schreiner GE. Toxic nephropathy due to drugs, solvents and metals. Prog Biochem Pharmacol. 1972;7:248–284. [PubMed] [Google Scholar]
- 17.Rood AS, McGavran PD, Aanenson JW, Till JE. Stochastic estimates of exposure and cancer risk from carbon tetrachloride released to the air from the rocky flats plant. Risk Anal. 2001;21:675–695. doi: 10.1111/0272-4332.214143. [DOI] [PubMed] [Google Scholar]
- 18.Ikemoto M, Tsunekawa S, Toda Y, Totani M. Liver-type arginase is a highly sensitive marker for hepatocellular damage in rats. Clin Chem. 2001;47:946–948. [PubMed] [Google Scholar]
- 19.Langle F, Roth E, Steininger R, Winkler S, Mühlbacher F. Arginase release following liver reperfusion. Evidence of hemodynamic action of arginase infusions. Transplantation. 1995;59:1542–1549. [PubMed] [Google Scholar]
- 20.Ozaki M, Terada K, Kanazawa M, Fujiyama S, Tomita K, et al. Enzyme-linked immunosorbent assay of carbamoylphosphate synthetase I: plasma enzyme in rat experimental hepatitis and its clearance. Enzyme Protein. 1994;48:213–221. doi: 10.1159/000474991. [DOI] [PubMed] [Google Scholar]
- 21.Glatt H, Boeing H, Engelke CE, Ma L, Kuhlow A, et al. Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat Res. 2001;482:27–40. doi: 10.1016/s0027-5107(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 22.Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol. 2011;2:8–23. [PMC free article] [PubMed] [Google Scholar]
- 23.Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- 24.Schoeffner DJ, Warren DA, Muralidara S, Bruckner JV, Simmons JE. Organ weights and fat volume in rats as a function of strain and age. J Toxicol Environ Health A. 1999;56:449–462. doi: 10.1080/009841099157917. [DOI] [PubMed] [Google Scholar]
- 25.Nagasaki A, Gotoh T, Takeya M, Yu Y, Takiguchi M, et al. Coinduction of nitric oxide synthase, argininosuccinate synthetase, and argininosuccinate lyase in lipopolysaccharide-treated rats. RNA blot, immunoblot, and immunohistochemical analyses. J Biol Chem. 1996;271:2658–2662. doi: 10.1074/jbc.271.5.2658. [DOI] [PubMed] [Google Scholar]



