SUMMARY
Porphyromonas gingivalis is a periodontal pathogen that is also associated with preterm low-birthweight delivery. We investigated the transcriptional responses of human extravillous trophoblasts (HTR-8) to infection with P. gingivalis. Over 2000 genes were differentially regulated in HTR-8 cells by P. gingivalis. In ontology analyses of regulated genes, overpopulated biological pathways included mitogen-activated protein (MAP) kinase signaling and cytokine production. Immunoblots confirmed overexpression of the MAP kinase pathway components MEK3, p38 and Max. Furthermore, P. gingivalis infection induced phosphorylation and activation of MEK3 and p38. Increased production of interleukin (IL)-1β and IL-8 by HTR-8 cells was demonstrated phenotypically by enzyme-linked immunosorbent assay of HTR-8 cell lysates and culture supernatants. Hence, infection of trophoblasts by P. gingivalis can impact signal transduction pathways and modulate cytokine expression, outcomes that could disrupt the maintenance of pregnancy.
Keywords: cytokine, microarray, periodontal pathogens, preterm low birthweight
INTRODUCTION
A leading cause of infant mortality and morbidity is preterm delivery of low-birth-weight (PTLBW) infants, which accounts for about 12% of births in the USA (Hoyert et al., 2006). Bacterial infection is a major cause of PTLBW delivery (Goldenberg & Thompson, 2003; Schloesser et al., 2004; Bakardjiev et al., 2005), and epidemiological evidence has indicated an association between periodontal disease and PTLBW delivery (Xiong et al., 2006; Michalowicz & Durand, 2007). Porphyromonas gingivalis, a major periodontal pathogen, has been detected in the amniotic fluid in preterm labor (Leon et al., 2007), and in the placenta in pre-eclampsia (Barak et al., 2007). Furthermore, P. gingivalis infection results in pregnancy complications in rodent and rabbit animal models (Lin et al., 2003; Boggess et al., 2005; Belanger et al., 2008). There are several possible mechanisms by which periodontal bacteria or their products could contribute to PTLBW delivery. For example, periodontal organisms and their antigens could initiate an inflammatory response with systemic consequences that could trigger preterm labor (Offenbacher et al., 1998; Garcia et al., 2001). Alternatively, direct oral–hematogenous spread of bacteria to the gestational tissues may occur. Bacteremias are common in patients with periodontal disease (Kinane et al., 2005; Lockhart et al., 2008; Perez-Chaparro et al., 2008) and P. gingivalis is resistant to complement and neutrophil-mediated killing (Amano et al., 1992; Mydel et al., 2006; Slaney et al., 2006; Perez-Chaparro et al., 2008).
The placental–fetal barrier in the monohemochorial placenta of humans consists of a single layer of fetally derived trophoblasts. This trophoblast barrier is formed from a highly proliferative, migratory and invasive subpopulation of trophoblasts, known as extravillous trophoblasts, which invade the uterus and its vasculature. The resulting trophoblast layer is in direct contact with maternal blood and is essential for the adequate exchange of key molecules between the maternal and fetal circulation. Trophoblasts produce pro- and anti-inflammatory cytokines (Griesinger et al., 2001), which contribute to the immunological interplay between the fetal and maternal compartment and to the maintenance of pregnancy (Griesinger et al., 2001; Hirsch & Wang, 2005. Organisms that commonly cause fetoplacental infections are often intracellular, and include viruses such as cytomegalovirus (Stagno et al., 1986), protozoa such as Toxoplasma gondii (Jones et al., 2001) and bacteria such as Listeria monocytogenes (Mylonakis et al., 2002). Listeria, for example, invades trophoblasts both in vitro and in vivo and can induce apoptosis in placental cells (Disson et al., 2008). In addition, bacteria that have been identified in intrauterine infections are capable of modulating cytokine expression from trophoblasts, and imbalances in these inflammatory mediators can act as a trigger for the initiation of infection-induced preterm labor (Griesinger et al., 2001; Hirsch & Wang, 2005). P. gingivalis can invade trophoblasts and induce G1 arrest and apoptosis (Inaba et al., 2009). G1 arrest is associated with activation of extracellular signal-regulated kinase (ERK) 1/2 and signaling through cyclins and Rb (retinoblastoma protein). In this study we used microarrays to uncover the global transcriptional responses of trophoblasts to P. gingivalis. Signaling pathways leading to cytokine expression, predicted by differential gene expression, were verified at the protein level, and the ability of P. gingivalis to modulate cytokine production from trophoblasts was confirmed phenotypically.
METHODS
Bacterial and cell culture
P. gingivalis ATCC 33277 was grown anaerobically at 37°C in trypticase soy broth supplemented with yeast extract (1 mg ml−1), hemin (5 µg ml−1) and menadione (1 µg ml−1). The HTR-8/SVneo trophoblast cell line (henceforth called HTR-8 cells) was provided by Dr Charles Graham (Kingston, ON, Canada). Cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St Louis, MO) supplemented with 5% fetal bovine serum at 37°C in 5% CO2.
Transcriptional profiling
The P. gingivalis cells were reacted with HTR-8 cells at a multiplicity of infection (MOI) of 200 for 2 h at 37°C in 5% CO2. Cocultures were carried out in quadruplicate. The HTR-8 cells were lysed with Trizol (Invitrogen, Carlsbad, CA) before RNA extraction. RNA isolation, complementary DNA (cDNA) synthesis, labeled cRNA synthesis and chip hybridization were conducted as previously described (Handfield et al., 2005). Briefly, total RNA was extracted from Trizol-lysed cells, treated with DNase I, purified and quantified according to standard methods (Qiagen, Valencia, CA; and Affymetrix, Santa Clara, CA). The cDNA synthesis was performed according to the Affymetrix protocol (SuperScript double-stranded cDNA synthesis kit; Invitrogen) with 8 µg total cellular RNA used as a template to amplify messenger RNA species for detection. Double-stranded cDNA was purified, and used as a template for labeled cRNA synthesis. In vitro transcription was performed using a BioArray high-yield RNA transcript labeling kit (T7) (Enzo Life Science, Farmingdale, NY), to incorporate biotinylated nucleotides. The cRNA was subsequently fragmented and hybridized onto Genechip Human Genome (HG) U133-A Plus 2.0 oligonucleotide arrays (Affymetrix) with proper controls. Each sample was studied in parallel, and the samples were not pooled. The microarrays were hybridized for 16 h at 45°C, stained with phycoerythrin-conjugated streptavidin and washed according to the Affymetrix protocol (EukGE-WS2v4) using an Affymetrix fluidics station, and scanned with an Affymetrix GeneChip 3000 scanner. Expression data can be accessed using accession number GSE19810 at the NCBI GEO database.
Microarray data analysis was performed as previously described (Mans et al., 2006; Hasegawa et al., 2008). Briefly, expression filters were applied to remove Affymetrix controls and probe-sets whose signal was undetected across all samples. The signal intensity values of the resulting dataset were variance-normalized, mean-centered and ranked by their coefficients of variation. Normalization was performed to give equal weight to all probe-sets in the analysis, regardless of the order of magnitude of the raw signal intensity. To reduce the confounding effect of background signal variation on the analysis, only the half of the dataset demonstrating the most variation across samples was used to perform unsupervised hierarchical cluster analysis using Cluster software (Eisen et al., 1998). The resulting heat-map and cluster dendrograms were visualized with Treeview software to reveal the extent of characteristic host cell responses to each infection state, defined as identical treatments clustering together.
Following initial assessment of the host cell response to each condition, supervised analysis was performed to investigate differences in gene regulation among experimental conditions. For this analysis, the raw signal intensities were log2-trans-formed for all probe-sets that passed the initial expression filters, and were correlated using BRB Array Tools (Simon and Peng-Lam, National Cancer Institute, Rockville, MD). In each supervised analysis, biological replicates were grouped into classes according to their infection state during coculture experiments and probe sets significant at the P < 0.001 level between classes were identified. To test the ability of these significant probe sets to truly distinguish between the classes, leave-one-out-cross-validation (LOOCV) studies were preformed. In these LOOCV studies each array was left out in turn and a classifier was derived between the groups by selecting probe sets significant at P < 0.001. The significant probe sets were then used with several prediction models (compound covariate predictor, nearest neighbor predictor, and support vector machine predictor) to predict the class identity of the array that was left out and not included when the classification model was built. The significance (P < 0.001) of the LOOCV analysis was estimated using a Monte Carlo simulation with 2000 permutations of the dataset.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were populated using Pathway Express (Khatri et al., 2005), available at http://vortex.cs.wayne.edu/projects.htm.
Immunoblotting
Infected (MOI 200) or control HTR-8 cells were washed three times with phosphate-buffered saline and lysed in radioimmunoprecipitation assay buffer with proteinase inhibitors (Sigma). Proteins (25 µg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, electrophoretically transferred to nitrocellulose membranes (Millipore, Billerica, MA) and blocked with 5% skim-milk in Tris-buffered saline–0.1% Tween-20 (TBST). Membranes were incubated for 1 h with primary antibodies (Santa Cruz) diluted 1 : 200. After washing in TBST three times, membranes were reacted with the speciesappropriate peroxidase-coupled secondary antibody (1 : 1000). Visualization was performed with the enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ). Band intensities were scanned and quantified using the Kodak 1D Image Analysis Software v.3.6.1.
Detection of cytokines
Supernatants and cell lysates from infected (MOI 200) or control HTR-8 cells were collected and filter-sterilized. Interleukin (IL)-1β and IL-8 concentrations were investigated by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN), according to the manufacturer’s protocol. Experiments were conducted in triplicate.
RESULTS
Transcriptional profile of HTR-8 cells infected with P. gingivalis
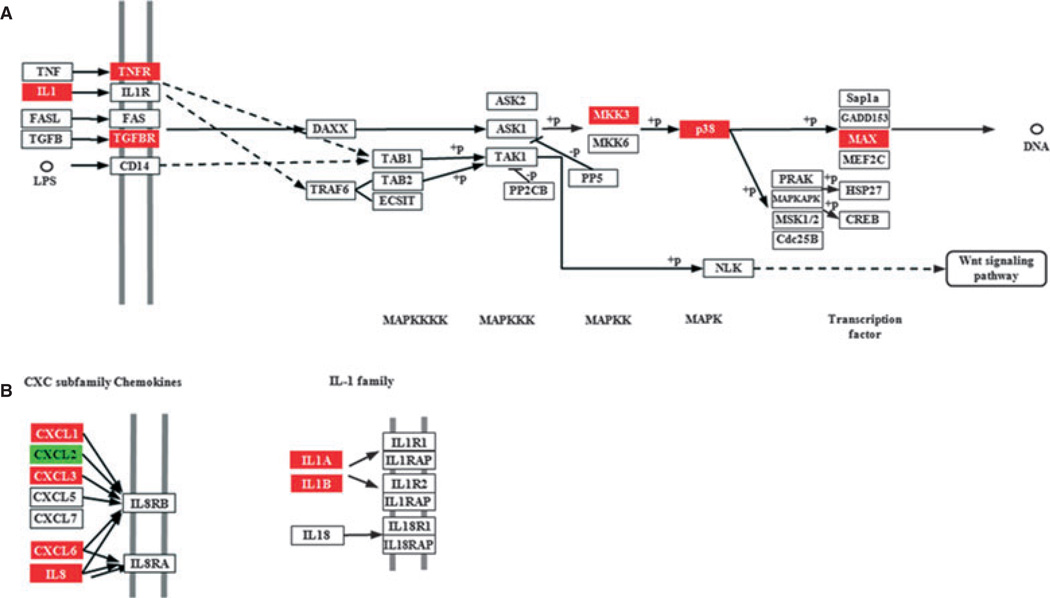
All samples of uninfected HTR-8 cells and P. gingivalis-infected cells were used to determine the overall similarity of the transcriptional responses. After elimination of probe sets whose signals were not greater than background levels on all arrays, signal intensity data for the 29,598 probe sets that passed the initial expression filters were included in an unsupervised cluster analysis and supervised class prediction analysis. The unsupervised hierarchical cluster analysis revealed an infection state-dependent host cell transcriptional profile, as biological replicates clustered together (data not shown). In a supervised analysis based on the infection state, at a significance level of P < 0.001, 2045 probe sets were differentially expressed. Assuming normality of the dataset, 2045 significant genes are 68-fold greater than the 30 probe sets that would be expected by chance at a significance threshold of P < 0.001, given that 29,598 probe sets passed the expression filter. To mine the array data for biologically relevant information, an ontology analysis of known metabolic pathways was performed using statistical algorithms in the Pathway Express software (Khatri et al., 2005). Pathways significantly (P < 0.05) overpopulated with differentially regulated genes (P < 0.001) included mitogen-activated protein kinase (MAPK) Signaling, Cell Cycle, and Apoptosis. Differential expression of genes involved in the cell cycle and in apoptosis is consistent with our previous work showing that P. gingivalis can induce G1 arrest and apoptosis in HTR-8 cells. Genes upregulated by P. gingivalis in the MAPK pathway included MEK3 (MKK3), p38 and Max (Fig. 1A). The MEK3-p38 pathway can regulate the expression of inflammatory cytokines (Patil & Kirk-wood, 2007; Schindler et al., 2007), and accordingly interrogation of the cytokine–cytokine receptor interaction pathway showed upregulation of proinflammatory cytokines including IL-1α and IL-1β, along with CXC chemokines IL-α CXCL1, CXCL3 and CXCL6 (Fig. 1B).
Figure 1.
Porphyromonas gingivalis infection impacts gene expression in HTR-8 cells. Pathways containing genes differentially regulated by P. gingivalis at P < 0.05, adapted from Pathway Express and using the Kyoto Encyclopedia of Genes and Genomes nomenclature (see text for details). Red indicates upregulation, green indicates downregulation and white indicates no change in expression. (A) p38 branch of the mitogen-activated protein (MAP) kinase pathway. (B) CXC and interleukin-1 (IL-1) family cytokines.
Correlation between messenger RNA, protein levels and activation status of MAPK signaling pathway
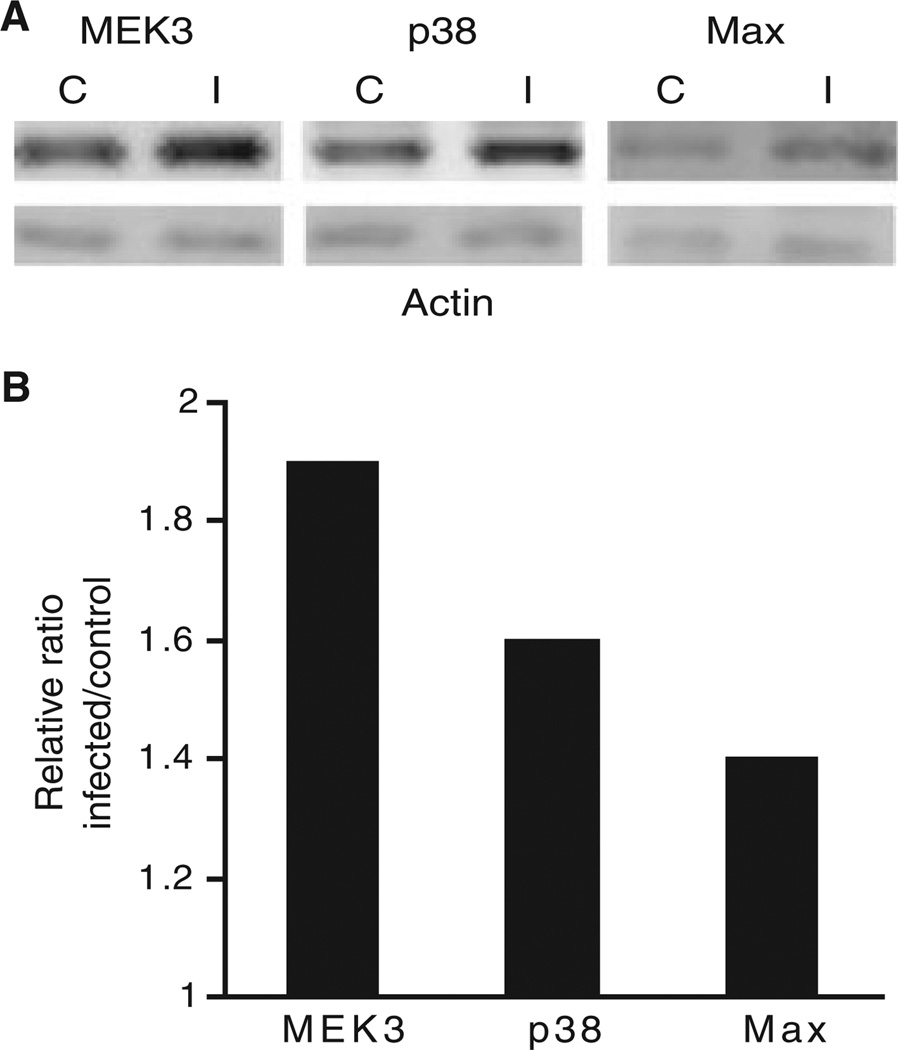
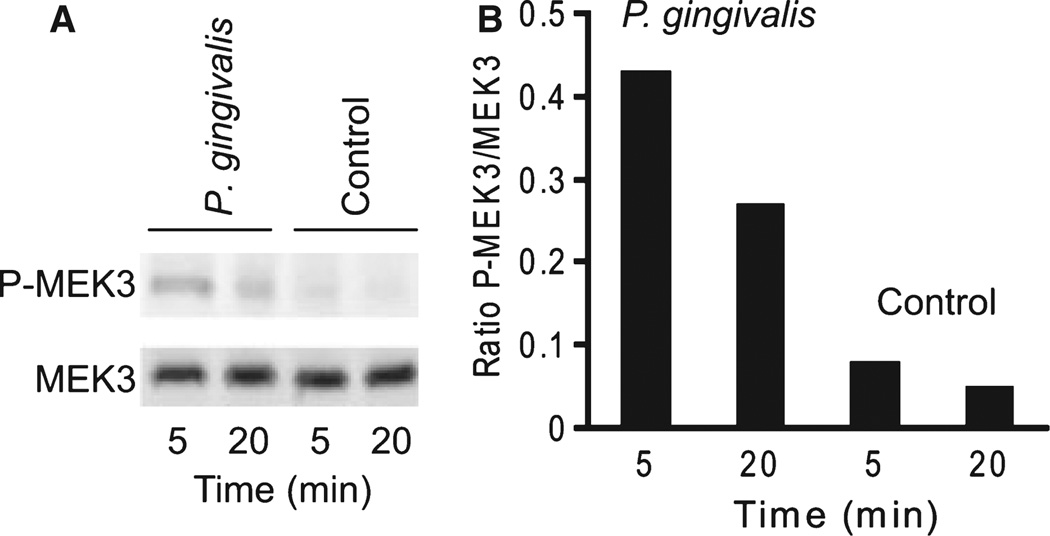
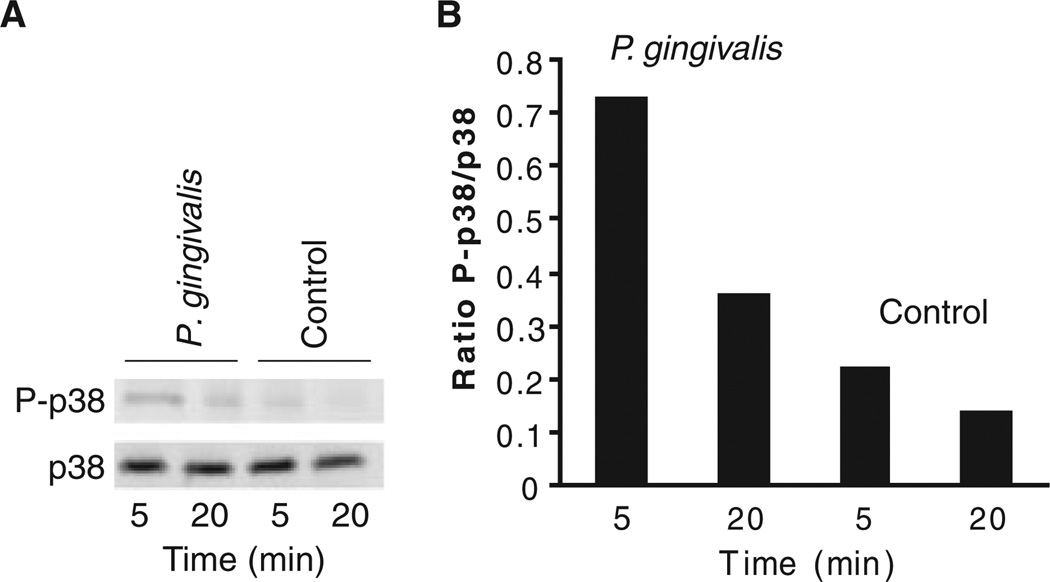
As messenger RNA levels do not necessarily reflect protein levels or activity, we examined protein levels of MEK3, p38 and Max in cells stimulated by P. gingivalis under the same conditions as the microarray experiment. Immunoblots (Fig. 2) showed increased expression of MEK3, p38 and Max, consistent with the transcription data. Furthermore, because the activity of the MAPK pathway depends on phosphorylation/ dephosphorylation of the components, we investigated the ability of P. gingivalis to induce activation of MEK3 and p38. As shown in Figs 3 and 4, P. gingivalis infection of HTR-8 cells resulted in increased phosphorylation of MEK3 and p38, indicating that P. gingivalis enhances both the level and activation status of components of the MAPK signaling pathway.
Figure 2.
HTR-8 genes upregulated by Porphyromonas gingivalis show a corresponding increase in protein levels. (A) Immunoblots of control uninfected (C) and P. gingivalis-infected (I) HTR-8 cells. The blots were probed with antibodies to MEK3, p38 and Max. Duplicate samples were probed with antibodies to β-actin (lower panel). (B) Densitometric analyses of the ratio of test protein band intensity to β-actin band intensity relative to the uninfected control. Data are representative of three independent experiments.
Figure 3.
MEK3 is phosphorylated in response to Porphyromonas gingivalis infection. (A) Immunoblots of control and infected HTR-8 cells probed with antibodies to MEK3 and phospho- (P) MEK3. (B) Densitometric analysis of the ratio of the P-MEK3 band to the MEK3 band. Data are representative of three independent experiments.
Figure 4.
p38 is phosphorylated in response to Porphyromonas gingivalis infection. (A) Immunoblots of control and infected HTR-8 cells probed with antibodies to p38 and phospho- (P) p38. (B) Densitometric analysis of the ratio of the P-p38 band to the p38 band. Data are representative of three independent experiments.
HTR-8 cytokine responses to P. gingivalis
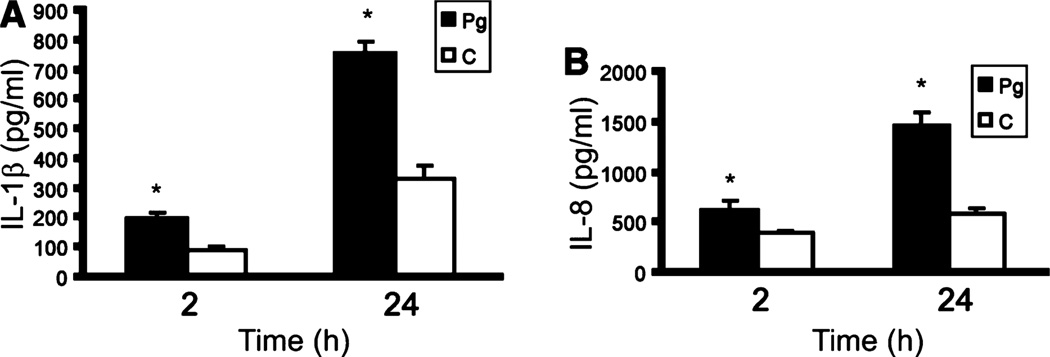
Activation of p38 can lead to stimulation of inflammatory cytokine secretion, and IL-1β and IL-8 were up-regulated at the transcriptional level in the array experiment. However, control of cytokine secretion is hierarchical and can occur at the transcriptional and posttranscriptional levels. Hence we assessed the phenotypic significance of the ontology analysis by directly assaying at the protein level the proinflammatory cytokines IL-1β and IL-8. Production of IL-1β and IL-8 by HTR-8 cells following stimulation with P. gingivalis was monitored over time (Fig. 5). Levels of both cytokines were elevated in response to P. gingivalis after 2 h of infection and continued to rise over a 24-h period, indicating that infection with P. gingivalis induces a proinflammatory phenotype in placental cells.
Figure 5.
Porphyromonas gingivalis induces interleukin (IL)-1β and IL-8 production in HTR-8 cells. Enzyme-linked immunosorbent assay of IL-1β in HTR-8 lysates (A) and IL-8 in HTR-8 cell supernatants (B) following coculture with P. gingivalis for 2 or 24 h. Control (C) cells were uninfected. Error bars indicate standard deviation (n = 3). *P < 0.05 (Student’s t-test) compared with control.
DISCUSSION
Trophoblasts are essential for the adequate exchange of key molecules between the maternal and fetal circulation, and form an important physical barrier that protects the fetus from microbial intrusion. Successful hematogenously derived fetal pathogens are capable of traversing the placenta, in some cases by invading and damaging trophoblasts (Disson et al., 2008). Accumulating evidence implicates P. gingivalis and other periodontal pathogens in fetal complications such as PTLBW delivery (Xiong et al., 2006; Michalowicz & Durand, 2007). The physiological status of the trophoblasts is important for normal pregnancy progression. Trophoblasts regulate transcriptional activity on a global scale according to environmental conditions (Fukushima et al., 2008), and following bacterial stimulation (Carvalho Neta et al., 2008). In the current study we found that stimulation of trophoblasts with P. gingivalis differentially regulated expression of over 2000 genes, indicating that P. gingivalis has a major affect on normal trophoblast function with the consequent potential for adverse placental function. The differentially regulated genes could be assembled into 18 significantly impacted KEGG pathways. These pathways include cell cycle and apoptosis, consistent with the ability of P. gingivalis to induce G1 arrest and apoptosis in trophoblasts (Inaba et al., 2009). Another pathway significantly impacted was MAPK, and upregulation of one branch of this pathway (MEK3, p38 and Max) was confirmed at the protein level. For many signaling pathways, such as MAPK, the level of constituent proteins is less important for information flow than is phosphorylation status. P. gingivalis induced phosphorylation, and therefore activation, of both MEK and p38, indicating that P. gingivalis infection of trophoblasts does stimulate signal transduction by the MAPK pathway.
The activation of the MEK3–p38 component of the MAPK pathway is predicted to lead to increased production of proinflammatory cytokines (Patil & Kirk-wood, 2007). This has relevance to PTLBW delivery, because adverse pregnancy outcomes can result from disruption of normal immune and inflammatory status (Goldenberg et al., 2000; Hirsch & Wang, 2005). Gestational tissues synthesize a variety of inflammatory mediators including cytokines and prostaglandins, which play important roles in normal pregnancy processes such as uterine contraction. Trophoblasts also express pattern recognition receptors and hence bacterial infection can evoke elevated levels of cytokines and prostaglandins, such as IL-1, IL-6, IL-8, IL-10, tumor necrosis factor-α and prostaglandin E2. Imbalances in these inflammatory mediators act as a trigger for the initiation of infection-induced preterm labor (Griesinger et al., 2001; Hirsch & Wang, 2005). Furthermore, proinflammatory cytokines can recruit and activate neutrophils, resulting in the release of matrix metalloproteinases that damage chorioamniotic membranes (Goldenberg et al., 2000). P. gingivalis infection induced higher levels of IL-1β and IL-8. Elevated production of both of these cytokines has been associated with PTLBW infants. Serum or vaginal IL-1β and IL-8 levels are also predictive of preterm delivery (Leitich, 2005; Torbe et al., 2007a,b; Bogavac & Brkic, 2009).
The interaction between P. gingivalis and trophoblasts differs from that between P. gingivalis and gingival epithelial cells in the oral cavity. The latter interaction results in antagonism of IL-8 production (Darveau et al., 1998) and suppression of apoptosis (Nakhjiri et al., 2001; Yilmaz et al., 2004; Mao et al., 2007) in an overall less disruptive interaction that may reflect a long-term evolutionary balanced relationship (Handfield et al., 2008). In contrast, trophoblasts and P. gingivalis have not had the opportunity to adapt to each other over evolutionary time and hence the organism may be a more acute pathogen in placental tissues. Recent evidence indicates that P. gingivalis is common in placental tissues both in pregnancies that proceed to term and in those that terminate prematurely (Katz et al., 2009). As a consequence, the presence of P. gingivalis alone may be insufficient to induce PTLBW delivery; nonetheless the ability of the organism to disrupt the transcriptome, signal transduction and cytokine production are characteristics that would make P. gingivalis a potent contributory factor in PTLBW delivery.
ACKNOWLEDGEMENTS
This study was supported by National Institutes of Health grant DE11111. Analyses were performed using BRB-ArrayTools developed by Dr. Richard Simon and the BRB-ArrayTools Development Team.
REFERENCES
- Amano A, Ishimoto T, Tamagawa H, Shizukuishi S. Role of superoxide dismutase in resistance of Porphyromonas gingivalis to killing by polymorphonuclear leukocytes. Infect Immun. 1992;60:712–714. doi: 10.1128/iai.60.2.712-714.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakardjiev AI, Stacy BA, Portnoy DA. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J Infect Dis. 2005;191:1889–1897. doi: 10.1086/430090. [DOI] [PubMed] [Google Scholar]
- Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with pre-eclampsia. J Periodontol. 2007;78:670–676. doi: 10.1902/jop.2007.060362. [DOI] [PubMed] [Google Scholar]
- Belanger M, Reyes L, von Deneen K, Reinhard MK, Progulske-Fox A, Brown MB. Colonization of maternal and fetal tissues by Porphyromonas gingivalis is strain-dependent in a rodent animal model. Am J Obstet Gynecol. 2008;199:86.e81–86.e87. doi: 10.1016/j.ajog.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Bogavac MA, Brkic S. Serum proinflammatory cytokine - interleukin-8 as possible infection site marker in preterm deliveries. J Perinat Med. 2009;37:707–708. doi: 10.1515/JPM.2009.115. [DOI] [PubMed] [Google Scholar]
- Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr., Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005;192:554–557. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Carvalho Neta AV, Stynen AP, Paixao TA, et al. Modulation of the bovine trophoblastic innate immune response by Brucella abortus. Infect Immun. 2008;76:1897–1907. doi: 10.1128/IAI.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP, Belton CM, Reife RA, Lamont RJ. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect Immun. 1998;66:1660–1665. doi: 10.1128/iai.66.4.1660-1665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disson O, Grayo S, Huillet E, et al. Conjugated action of two species-specific invasion proteins for feto-placental listeriosis. Nature. 2008;455:1114–1118. doi: 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Murata M, Hachisuga M, et al. Gene expression profiles by microarray analysis during matrigel-induced tube formation in a human extravillous trophoblast cell line: comparison with endothelial cells. Placenta. 2008;29:898–904. doi: 10.1016/j.placenta.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Garcia RI, Henshaw MM, Krall EA. Relationship between periodontal disease and systemic health. Periodontol 2000. 2001;25:21–36. doi: 10.1034/j.1600-0757.2001.22250103.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Saleh L, Bauer S, Husslein P, Knofler M. Production of pro- and anti-inflammatory cytokines of human placental trophoblasts in response to pathogenic bacteria. J Soc Gynecol Investig. 2001;8:334–340. [PubMed] [Google Scholar]
- Handfield M, Mans JJ, Zheng G, et al. Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell Microbiol. 2005;7:811–823. doi: 10.1111/j.1462-5822.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- Handfield M, Baker HV, Lamont RJ. Beyond good and evil in the oral cavity: insights into host–microbe relationships derived from transcriptional profiling of gingival cells. J Dent Res. 2008;87:203–223. doi: 10.1177/154405910808700302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Tribble GD, Baker HV, Mans JJ, Handfield M, Lamont RJ. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect Immun. 2008;76:2420–2427. doi: 10.1128/IAI.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168–183. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- Inaba H, Kuboniwa M, Bainbridge B, et al. Porphyromonas gingivalis invades human trophoblasts and inhibits proliferation by inducing G1 arrest and apoptosis. Cell Microbiol. 2009;11:1517–1532. doi: 10.1111/j.1462-5822.2009.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. Obstet Gynecol Surv. 2001;56:296–305. doi: 10.1097/00006254-200105000-00025. [DOI] [PubMed] [Google Scholar]
- Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. 2009;88:575–578. doi: 10.1177/0022034509338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Sellamuthu S, Malhotra P, Amin K, Done A, Draghici S. Recent additions and improvements to the Onto-Tools. Nucleic Acids Res. 2005;33:W762–W765. doi: 10.1093/nar/gki472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Riggio MP, Walker KF, MacKenzie D, Shearer B. Bacteraemia following periodontal procedures. J Clin Periodontol. 2005;32:708–713. doi: 10.1111/j.1600-051X.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- Leitich H. Secondary predictors of preterm labour. BJOG. 2005;112(suppl 1):48–50. doi: 10.1111/j.1471-0528.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- Leon R, Silva N, Ovalle A, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78:1249–1255. doi: 10.1902/jop.2007.060368. [DOI] [PubMed] [Google Scholar]
- Lin D, Smith MA, Elter J, et al. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 2003;71:5163–5168. doi: 10.1128/IAI.71.9.5163-5168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bactere-mia associated with toothbrushing and dental extraction. Circulation. 2008;117:3118–3125. doi: 10.1161/CIRCULATIONAHA.107.758524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans JJ, Baker HV, Oda D, Lamont RJ, Hand-field M. Distinctive characteristics of transcriptional profiles from two epithelial cell lines upon interaction with Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2006;21:261–267. doi: 10.1111/j.1399-302X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowicz BS, Durand R. Maternal periodontal disease and spontaneous preterm birth. Periodontol 2000. 2007;44:103–112. doi: 10.1111/j.1600-0757.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Mydel P, Takahashi Y, Yumoto H, et al. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2006;2:e76. doi: 10.1371/journal.ppat.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Paliou M, Hohmann EL, Calderwood SB, Wing EJ. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine. 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- Nakhjiri SF, Park Y, Yilmaz O, et al. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol Lett. 2001;200:145–149. doi: 10.1111/j.1574-6968.2001.tb10706.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Jared HL, O’Reilly PG, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998;3:233–250. doi: 10.1902/annals.1998.3.1.233. [DOI] [PubMed] [Google Scholar]
- Patil CS, Kirkwood KL. p38 MAPK signaling in oral-related diseases. J Dent Res. 2007;86:812–825. doi: 10.1177/154405910708600903. [DOI] [PubMed] [Google Scholar]
- Perez-Chaparro PJ, Gracieux P, Lafaurie GI, Donnio PY, Bonnaure-Mallet M. Genotypic characterization of Porphyromonas gingivalis isolated from subgingival plaque and blood sample in positive bacter-emia subjects with periodontitis. J Clin Periodontol. 2008;35:748–753. doi: 10.1111/j.1600-051X.2008.01296.x. [DOI] [PubMed] [Google Scholar]
- Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–811. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- Schloesser RL, Schaefer V, Groll AH. Fatal transplacental infection with non-typhoidal Salmonella. Scand J Infect Dis. 2004;36:773–774. doi: 10.1080/00365540410020802. [DOI] [PubMed] [Google Scholar]
- Slaney JM, Gallagher A, Aduse-Opoku J, Pell K, Curtis MA. Mechanisms of resistance of Porphyromonas gingivalis to killing by serum complement. Infect Immun. 2006;74:5352–5361. doi: 10.1128/IAI.00304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. J Am Med Assoc. 1986;256:1904–1908. [PubMed] [Google Scholar]
- Torbe A, Czajka R, Kordek A, Rzepka R, Kwiatkowski S, Rudnicki J. Maternal serum proinflammatory cytokines in preterm labor with intact membranes: neonatal outcome and histological associations. Eur Cytokine Netw. 2007a;18:102–107. doi: 10.1684/ecn.2007.0092. [DOI] [PubMed] [Google Scholar]
- Torbe A, Czajka R, Kordek A, Rzepka R, Kwiatkowski S, Rudnicki J. Value of vaginal fluid proinflammatory cytokines for the prediction of early-onset neonatal infection in preterm premature rupture of the membranes. J Interferon Cytokine Res. 2007b;27:393–398. doi: 10.1089/jir.2006.0127. [DOI] [PubMed] [Google Scholar]
- Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG. 2006;113:135–143. doi: 10.1111/j.1471-0528.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/ Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]