Abstract
Extracellular matrix (ECM)-based scaffolds, through their inherent bioactivity and molecular recognition signals, provide the ideal substrate for tissue engineering and regenerative applications. Collagen, the most abundant ECM protein, has proven itself to be a very versatile material with applications in many fields, including the leather and food industries, cosmetics, drug delivery, and tissue engineering. However, doubts persist about the optimal source of collagen for tissue engineering applications, given possible immunogenicity and disease transmission associated with animal sources and reduced bioactivity and availability of recombinant technologies. In this special edition, an attempt is made to elucidate the advantages of plant-derived human recombinant collagen and its applications in tissue engineering, particularly skin and wound healing. While results are promising, the widespread use of animal-derived collagen means that recombinant technologies may find applications in niche areas.
Without a suitable scaffold material, tissue engineering applications are unlikely to be therapeutically and clinically efficacious. Hence, the choice of a scaffold material and the form it takes is of vital importance. Synthetic materials have many desirable qualities, such as control over material properties, ease and consistency of synthesis, and options for functionalization. However, synthetic materials often suffer from in vivo toxicity and poor biodegradability. Extracellular matrix (ECM)-based scaffolds provide an ideal substrate for tissue engineering applications. Through their inherent bioactivity, molecular recognition, and biodegradability, they provide an ideal scaffold that cells recognize, infiltrate, and remodel, creating local microenvironments and gradients of chemotactic activity to drive neotissue formation. Collagen is the most abundant protein of the ECM in the human body, and thus, it stands out as an ideal candidate material for tissue engineering applications.
Collagen provides structural support and tensile strength to various tissues of the human body, including skin, ligaments, tendon, and bone. It consists of a right-handed triple helical structure with the repeating amino acid triplet sequence1: Glycine-X-Y, where X is often proline and Y is frequently hydroxyproline. The presence of the small amino acid Glycine at every third position on the polypeptide chain allows for close packing of the triple-helix along the collagen molecules central axis. Nonhelical, globular regions exist at either end of the collagen molecule, and prevent fibril formation before cleavage by appropriate proteinases. The properties that have contributed to its popularity as a scaffold material include its relatively low toxicity, natural degradation by matrix metalloproteinases, ease of crosslinking and functionalization as well as the homology of the amino acid sequences that exist between species.
The use of animal-extracted collagen in the fields of biomaterials, drug delivery, and tissue engineering is widespread.2–7 Researchers have mainly focused their efforts on the formulation of collagen into a wide variety of different forms, some of which have reached clinical translation. Collagen sponges have a wide range of applications varying from skin wound healing,8,9 to bone scaffolds10–15 to cardiac patches.16,17 Collagen hydrogels, due to their injectable nature and in situ self-assembly, have also been used for a variety of applications, including the myocardium,18,19 intervertebral disc,20,21 as a dermal substitute,22 for corneal applications23 and for regenerating cartilage.24,25 Aligned forms of collagen have been developed and utilized to act as a bridge for tendon,26–28 spinal cord,29 and peripheral nerve regeneration.30 Collagen films have primarily been used for wound healing,31 as they create a barrier between the wound and the external environment, thus reducing the possibility of infection. Collagen can be easily functionalized to add additional therapeutic benefit and bioactivity. For example, sponges and hydrogels can be functionalized with therapeutic genes11,15,32 or proteins,9,12 or used to deliver stem cells,16,19,33 while collagen microspheres not only have high capacity for loading, but also offer localized and sustained delivery of bioactive/therapeutic molecules, such as genes,34 protein,35 or stem cells.25,36
Despite the use of collagen in vitro, in preclinical models and in the clinic, there are a number of issues that continue to cause concern. One major obstacle to the clinical translation of these therapies is the source of the collagen used. As Figure 1 outlines, the main sources of collagen type I are porcine skin, bovine tendon, and human cadavers. Unfortunately, each of these has drawbacks associated with its use. The major drawback is possible disease transmission, allergic reactions,37 contamination with pathogens, as well as antigenicity and immunogenicity associated with the use of collagen telopeptides, although substantial evidence to prove this is lacking.38 In addition, batch-to-batch variability as well as cultural reasons add to the concern about animal-derived collagen. As a result, much effort has been made in the field of recombinant protein production39 to utilize expression systems that will allow the formation of recombinant human collagen in a consistent, efficient, and safe manner.
FIG. 1.
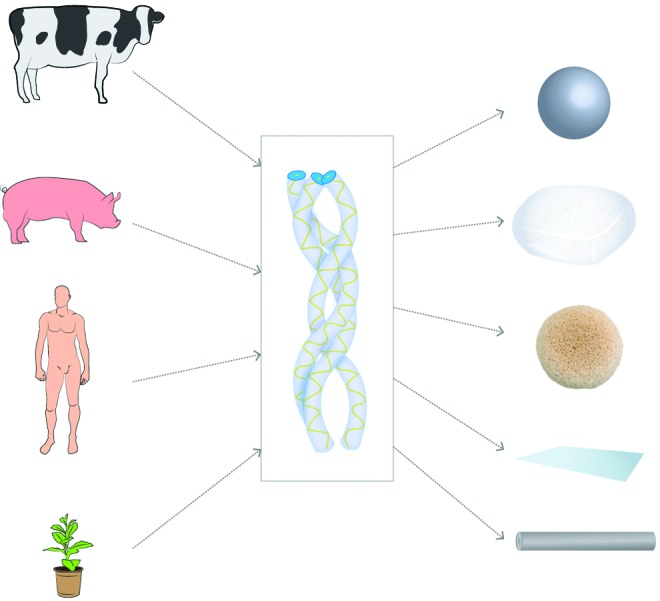
Collagen—sources and forms: the principal sources of collagen (bovine tendon, porcine skin, human cadavers, and human recombinant, from top to bottom) are displayed as well as some of the forms (microspheres, gels, sponges, films, and fibers, from top to bottom) into which collagen has been processed for tissue engineering applications. Color images available online at www.liebertpub.com/tea
The recombinant collagen has been developed in a number of different expression systems, including yeast, silkworm, mammalian cells, transgenic animals, and bacterial systems. Recent developments have seen the production of a post-translationally hydroxylated collagen in relatively large amounts using a transgenic tobacco plant.40 This collagen would theoretically bypass the concerns that exist with an animal-derived collagen. Recombinant collagens have been used in bone,14 ocular,41,42 and skin applications, as seen in this issue of Tissue Engineering. They have been used mostly as sponges, but also more recently as electrospun fibers and gels, and have been compared favorably with animal collagens with respect to processability and therapeutic efficacy.43 Despite these promising signs, recombinant technologies have suffered from a number of drawbacks, which include high cost, low yield, and the lack of cofactors or enzymes in the systems, which are critical to the stable formation of bioactive and biofunctional collagens. Because of these disadvantages, conveniently extracted animal collagen has remained the standard for use in both research and clinical.
While preliminary studies have shown the potential of recombinant collagens, whether produced in yeast or plants, it seems that until higher yields reduce its prohibitive price, researchers are unlikely to embrace its use over collagen from animal sources. However, possible uses for recombinant collagens could involve niche areas, such as the production of type II collagen, which is difficult to extract in large enough quantities from animals and has been associated with the induction of arthritis.44 Niche areas may be used as testing grounds to fully demonstrate the benefits of recombinant collagens over animal-derived collagen, and may help to bring the technology to the forefront of tissue engineering research and closer to clinical translation.
Acknowledgment
This authors wish to acknowledge financial support from Science Foundation Ireland under Grant No. 07/SRC/B1163.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gelse K. Poschl E. Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliver Rev. 2003;55:1531. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Stenzel K.H. Miyata T. Rubin A.L. Collagen as a biomaterial. Annu Rev Biophys Bio. 1974;3:231. doi: 10.1146/annurev.bb.03.060174.001311. [DOI] [PubMed] [Google Scholar]
- 3.Wallace D. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliver Rev. 2003;55:1631. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.H. Singla A. Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 5.Friess W. Collagen-biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 6.Glowacki J. Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 7.Zeugolis D.I. Raghunath M. Collagen–materials analysis and implant uses. In: Ducheyne P., editor; Healy K.E., editor; Hutmacher D.W., editor; Grainger D.W., editor; Kirkpatrick C.J., editor. Comprehensive Biomaterials. Amsterdam, The Netherlands: Elsevier Science; 2011. p. 261. [Google Scholar]
- 8.Garcia Y. Wilkins B. Collighan R.J. Griffin M. Pandit A. Towards development of a dermal rudiment for enhanced wound healing response. Biomaterials. 2008;29:857. doi: 10.1016/j.biomaterials.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 9.Pandit A. Ashar R. Feldman D. The effect of TGF-beta delivered through a collagen scaffold on wound healing. J Invest Surg. 1999;12:89. doi: 10.1080/089419399272647. [DOI] [PubMed] [Google Scholar]
- 10.Keeney M. Collin E. Pandit A. Multi-channelled collagen-calcium phosphate scaffolds: their physical properties and human cell response. Tissue Eng Part C Methods. 2009;15:265. doi: 10.1089/ten.tec.2008.0365. [DOI] [PubMed] [Google Scholar]
- 11.Keeney M. van den Beucken J.J.J.P. van der Kraan P.M. Jansen J.A. Pandit A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF(165) Biomaterials. 2010;31:2893. doi: 10.1016/j.biomaterials.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Visser R. Arrabal P.M. Becerra J. Rinas U. Cifuentes M. The effect of an rhBMP-2 absorbable collagen sponge-targeted system on bone formation in vivo. Biomaterials. 2009;30:2032. doi: 10.1016/j.biomaterials.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Cunniffe G.M. Dickson G.R. Partap S. Stanton K.T. O'Brien F.J. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. Mater Sci Mater M. 2010;21:2293. doi: 10.1007/s10856-009-3964-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y. Cui F.Z. Hu K. Zhu X.D. Fan D.D. Bone regeneration by using scaffold based on mineralized recombinant collagen. J Biomed Mater Res A. 2008;86:29. doi: 10.1002/jbm.b.30984. [DOI] [PubMed] [Google Scholar]
- 15.Tierney E.G. Duffy G.P. Hibbitts A.J. Cryan S.A. O'Brien F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J Control Release. 2012;158:304. doi: 10.1016/j.jconrel.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Holladay C. Duffy A. Chen X. Sefton M. O'Brien T. Pandit A. Recovery of cardiac function mediated by MSC and interleukin-10 plasmid functionalised scaffold. Biomaterials. 2012;33:1303. doi: 10.1016/j.biomaterials.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Xiang Z. Liao R. Kelly M.S. Spector M. Collagen-GAG scaffolds grafted onto myocardial infarcts in a rat model: a delivery vehicle for mesenchymal stem cells. Tissue Eng. 2006;12:2467. doi: 10.1089/ten.2006.12.2467. [DOI] [PubMed] [Google Scholar]
- 18.Thompson C.A. Nasseri B.A. Makower J. Houser S. McGarry M. Lamson T. Pomerantseya I. Chang J.Y. Gold H.K. Vacanti J.P. Oesterle S.N. Percutaneous transvenous cellular cardiomyoplasty. J Am Coll Cardiol. 2003;41:1964. doi: 10.1016/s0735-1097(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 19.Kouris N.A. Squirrell J.M. Jung J.P. Pehlke C.A. Hacker T. Eliceiri K.W. Ogle B.M. A nondenatured, noncrosslinked collagen matrix to deliver stem cells to the heart. Regen Med. 2011;6:569. doi: 10.2217/rme.11.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderon L. Collin E. Velasco D. Murphy M. O'Halloran D. Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cells Mater. 2010;20:134. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 21.Collin E. Grad S. Zeugolis D.I. Vinatier C.S. Clouet J.R. Guicheux J.J. Weiss P. Alini M. Pandit A. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32:2862. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Helary C. Bataille I. Abed A. Illoul C. Anglo A. Loudec L. Letourneur D. Meddahi-Pellé A. Giraud-Guille M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials. 2010;31:481. doi: 10.1016/j.biomaterials.2009.09.073. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y. Gan L. Carlsson D.J. Fagerholm P. Lagali N. Watsky M.A. Munger R. Hodge W.G. Priest D. Griffith M. A simple, cross-linked collagen tissue substitute for corneal implantation. Invest Ophthalmol Vis Sci. 2006;47:1869. doi: 10.1167/iovs.05-1339. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi T. Xu L. Kobayashi H. Taniguchi A. Kataoka K. Tanaka J. Encapsulation of chondrocytes in injectable alkali-treated collagen gels prepared using poly(ethylene glycol)-based 4-armed star polymer. Biomaterials. 2005;26:1247. doi: 10.1016/j.biomaterials.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Hui T.Y. Cheung K.M.C. Cheung W.L. Chan D. Chan B.P. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Kew S.J. Gwynne J.H. Enea D. Brookes R. Rushton N. Best S.M. Cameron R.E. Synthetic collagen fascicles for the regeneration of tendon tissue. Acta Biomater. 2012;8:3723. doi: 10.1016/j.actbio.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Awad H.A. Butler D.L. Harris M.T. Ibrahim R.E. Wu Y. Young R.G. Kadiyala S. Boivin G.P. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res. 2000;51:233. doi: 10.1002/(sici)1097-4636(200008)51:2<233::aid-jbm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Cheng X. Gurkan U.A. Dehen C.J. Tate M.P. Hillhouse H.W. Simpson G.J. Akkus O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29:3278. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Rub M.T. Billiar K.L. van Es M.H. Knight A. Rodriguez B.J. Zeugolis D.I. McMahon S. Windebank A.J. Pandit A. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein. Soft Matter. 2011;7:2770. [Google Scholar]
- 30.Daly W.T. Yao L. Abu-Rub M.T. O'Connell C. Zeugolis D.I. Windebank A.J. Pandit A. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials. 2012;33:6660. doi: 10.1016/j.biomaterials.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Maeda M. Kadota K. Kajihara M. Sano A. Fujioka K. Sustained release of human growth hormone (hGH) from collagen film and evaluation of effect on wound healing in db/db mice. J Control Release. 2001;77:261. doi: 10.1016/s0168-3659(01)00512-0. [DOI] [PubMed] [Google Scholar]
- 32.Holladay C. Keeney M. Greiser U. Murphy M. O'Brien T. Pandit A. A matrix reservoir for improved control of non-viral gene delivery. J Control Release. 2009;136:220. doi: 10.1016/j.jconrel.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Holladay C. Power K. Sefton M. O'Brien T. Gallagher W.M. Pandit A. Functionalized scaffold-mediated interleukin 10 gene delivery significantly improves survival rates of stem cells in vivo. Mol Ther. 2011;19:969. doi: 10.1038/mt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helary C. Browne S. Mathew A. Wang W. Pandit A. Transfection of macrophages by collagen hollow spheres loaded with polyplexes: a step towards modulating inflammation. Acta Biomater. 2012;8:4208. doi: 10.1016/j.actbio.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Chan B.P. Chan O.C.M. So K.F. Effects of photochemical crosslinking on the microstructure of collagen and a feasibility study on controlled protein release. Acta Biomater. 2008;4:1627. doi: 10.1016/j.actbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Chan B.P. Hui T.Y. Yeung C.W. Li J. Mo I. Chan G.C.F. Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28:4652. doi: 10.1016/j.biomaterials.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 37.Hori H. Hattori S. Inouye S. Kimura A. Irie S. Miyazawa H. Sakaguchi M. Analysis of the major epitope of the α2 chain of bovine type I collagen in children with bovine gelatin allergy. J Allergy Clin Immun. 2002;110:652. doi: 10.1067/mai.2002.127862. [DOI] [PubMed] [Google Scholar]
- 38.Lynn A.K. Yannas I.V. Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B. 2004;71:343. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 39.Ruggiero F. Koch M. Making recombinant extracellular matrix proteins. Methods. 2008;45:75. doi: 10.1016/j.ymeth.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Stein H. Wilensky M. Tsafrir Y. Rosenthal M. Amir R. Avraham T. Ofir K. Dgany O. Yayon A. Shoseyov O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules. 2009;10:2640. doi: 10.1021/bm900571b. [DOI] [PubMed] [Google Scholar]
- 41.Liu W. Merrett K. Griffith M. Fagerholm P. Dravida S. Heyne B. Scaiano J.C. Watsky M.A. Shinozaki N. Lagali N. Munger R. Li F. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials. 2008;29:1147. doi: 10.1016/j.biomaterials.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y. Griffith M. Watsky M.A. Forrester J.V. Kuffova L. Grant D. Merrett K. Carlsson D.J. Properties of porcine and recombinant human collagen matrices for optically clear tissue engineering applications. Biomacromolecules. 2006;7:1819. doi: 10.1021/bm060160o. [DOI] [PubMed] [Google Scholar]
- 43.Olsen D. Yang C. Bodo M. Chang R. Leigh S. Baez J. Carmichael D. Perala M. Hamalainen E.R. Jarvinen M. Polarek J. Recombinant collagen and gelatin for drug delivery. Adv Drug Deliver Rev. 2003;55:1547. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Morgan K. Clague R.B. Shaw M.J. Holt P.J. Native type II collagen-induced arthritis in the rat. I. Incidence and humoral response to collagen. Ann Rheum Dis. 1980;39:285. doi: 10.1136/ard.39.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]


