Abstract
Purpose.
We determined the mechanism by which all-trans retinoic acid (ATRA) inhibits experimental autoimmune uveitis (EAU) and determined the role of γδ T cells in this autoimmune disease.
Methods.
C57BL/6 (B6) mice were immunized with the uveitogenic, interphotoreceptor retinoid-binding protein1–20 peptide (IRBP1-20) in complete Freund's adjuvant (CFA), with or without a preceding ATRA treatment. Responses and pathogenic activity of Th1- and Th17-autoreactive T cells were compared, and the effects of ATRA on γδ T cells and CD25+ dendritic cell (DC) subset were determined. Interactions among uveitogenic T cells, DC subsets, and γδ T cells were investigated.
Results.
Administration of ATRA to B6 mice in which EAU was induced suppressed the response of Th17 autoreactive T cells, which was associated with decreased generation of the CD25+ DC subset and suppressed activation of γδ T cells. Adoptively transferred γδ T cells isolated from ATRA-treated mice showed a diminished ability to promote the activation of Th17 autoreactive T cells in vitro and in vivo compared to γδ T cells from untreated donors.
Conclusions.
ATRA inhibits the expansion of CD25+ DCs and γδ T-cell activation, thereby restraining the Th17 autoreactive T-cell response.
Keywords: autoimmunity, EAU, interleukin-17, Th17, retinoid acid, uveitis, γδ T cell
We previously reported that γδ T-cell activation is a crucial event leading to development of EAU, suggesting that therapies restraining γδ T-cell are important. Here, we show that the retinoic acid inhibits γδ-DC interaction and γδ T-cell activation, thereby restraining autoreactive T-cell response.
Introduction
Retinoic acids (RAs) are metabolites of vitamin A, but also can be produced endogenously by activated dendritic/macrophage cells.1–3 All-trans retinoic acid (ATRA), the major endogenously generated RA, is a ligand for all retinoic acid receptor (RAR) subtypes.4,5 Studies have shown that RAs are modulators of cell proliferation, differentiation, and morphogenesis. For example, RA promotes the differentiation of immature myeloid cells into mature cells,6,7 neutrophil maturation,8,9 and the generation of CD4+ T cells expressing the Treg specification factor (FoxP3), but decreases the frequency of cells expressing IL-17.10–15 RA also has been shown to have a suppressive effect on autoimmune inflammation, such as experimental autoimmune encephalomyelitis (EAE),16–18 experimental autoimmune uveitis (EAU),19,20 and arthritis,21–23 while improving the antitumor immune response and enhancing the effect of vaccination.6,24,25 The mechanisms by which RA suppresses the generation of autoimmune diseases, while enhancing tumor immunity, remain largely unclear.
We have reported previously that γδ T cells have a major role in regulating the Th17 autoreactive T-cell response in EAU.26–29 The proinflammatory effect of γδ T cells is augmented when γδ T cells become activated,28 suggesting that therapeutic approaches capable of restraining γδ T-cell activation might be exploited to manipulate Th17 responses. We also found that the generation of the CD25+ dendritic cell (DC) subset during immunization contributes to the increased activation of γδ T cells, leading to altered EAU susceptibility.30
To determine the mechanism by which RA affects autoimmune susceptibility, we examined the in vivo and in vitro effects of ATRA on autoreactive T cells, and on the regulatory interactions between uveitogenic T cells, CD25+ DCs, and γδ T cells. Here, we showed that administration of ATRA to mice after EAU induction significantly reduces the response of Th17 autoreactive T cells, which was associated consistently with decreased generation of CD25+ DCs and suppressed γδ T-cell activation. The consequences of these changes for the regulatory interactions between γδ T cells, CD25+ DCs, and Th17 autoreactive T cells in EAU are discussed.
Materials and Methods
Animals and Reagents
Female C57BL/6 (B6) and TCR-δ−/− mice (all 12–14 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME), and were housed and maintained in the animal facilities of the University of Southern California. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed. All animals were treated according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Recombinant murine cytokines (IL-4, IL-12, IL-23, and GM-CSF) were purchased from R&D Systems, Inc. (Minneapolis, MN). Phycoerythrin (PE)-conjugated antimouse IFN-γ, PE–antimouse CD11c (clone N418), PE–antimouse CD27 (clone 3A10), and fluorescein isothiocyanate (FITC)-conjugated antimouse IL-17 antibodies, antimouse CD25 (clone PC61) were purchased from Biolegend (San Diego, CA). Anti-γδTCR (clone GL3) and antimouse CD4 (clone GK1.5) antibodies were obtained from eBioscience (La Jolla, CA). ATRA and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). ATRA was dissolved in DMSO (vehicle), stored in aliquots at −20°C before use, and administered intraperitoneally (0.2 mg/mouse) on day −3 (3 days before immunization) and day 0 (immunization day). The RAR antagonists BMS 195614, which binds to the RAR-α subunit, and CD2665, which binds to the RAR-βγ subunits, were obtained from Tocris Bioscience (Ellisville, MO) and used at a final concentration of 1 μM.
γδ T-Cell Preparation
γδ T cells were purified from interphotoreceptor retinoid-binding protein1–20 peptide (IRBP1-20) immunized B6 mice as we reported previously.29,31 Nylon wool-enriched splenic T cells from immunized mice were incubated for 10 minutes at 4°C with FITC-conjugated antimouse γδTCR or αβTCR antibody, then for 15 minutes at 4°C with anti-FITC Microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The cells then were separated into bound and nonbound on an autoMACSTM separator column (Miltenyi Biotec GmbH). The purity of the isolated cell fraction was >95% to 99% as determined by flow cytometric analysis of PE-conjugated antibodies against αβ or γδ T cells.29,31 Resting cells were harvested from this isolate after culture in cytokine-free medium for 3 to 5 days, when they showed down-regulation of CD69 expression. Activated γδ T cells were prepared by incubating the resting γδ T cells with anti-γδTCR (GL3) and anti-CD28 antibodies (2 μg/mL) for 2 days.
Immunization Procedures and In Vitro Stimulation of In Vivo Primed T cells
Mice were immunized subcutaneously over 6 spots at the tail base and on the flank with 200 μL emulsion containing the uveitogenic peptide IRBP1-20 (150 μg/mouse; Sigma).29,32 The peptide was emulsified in complete Freund's adjuvant (CFA) (Sigma). All immunized mice also were injected intraperitoneally (IP) with a single dose of pertussis toxin (200 ng; Sigma). At day 13 after immunization, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column, then 1 × 107 cells in 2 mL RPMI 1640 medium (Cellgro, Manassas, VA) containing 10% fetal calf serum (FCS) in each well of a 6-well plate (Costar; Corning Life Sciences, Tewksbury, MA) were stimulated for 48 hours with 10 μg/mL IRBP1-20 in the presence of 1 × 107 irradiated syngeneic splenic antigen-presenting cells (APCs) in the presence of either IL-12 or IL-23 (10 ng/mL). Activated T-cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 hours in the same medium used for stimulation.
Assessment of the Proinflammatory Activity of γδ T cells
Enriched αβ responder T cells prepared from IRBP1-20-immunized TCR-δ−/− mice were used as responder T cells, and were stimulated in vitro for 5 days with immunizing antigen and APCs under Th1- or Th17 polarizing conditions (culture medium supplemented with 10 ng/mL IL-12 or IL-23, respectively). The responder T cells were cultured either alone or supplemented with 2% γδ T cells isolated from ATRA-treated or nontreated mice, then IL-17- and/or IFN-γ-producing αβ T cells were measured by cytoplasmic staining, followed by FACS analysis.
Determination of IL-17 by ELISA
Enriched T cells (3 × 104 cells/well) from the draining lymph nodes and spleens were prepared by nylon wool adherence and cultured at 37°C for 48 hours in 96-well microtiter plates with irradiated syngeneic spleen APCs (1 × 105) in the presence or absence of IRBP1-20, then a fraction of the culture supernatant was analyzed for IL-17 production using ELISA kits (R&D Systems, Inc.).
Induction of EAU by Cell Transfer
For induction of EAU by adoptive transfer, TCR-δ−/− mice received a single IP injection of γδ T cells (5 × 105) one day before immunization with IRBP1-20. Then, at 13 days after immunization, T cells were isolated from lymph node and spleen cells by passage through a nylon wool column and stimulated for 48 hours with 10 μg/mL IRBP1-20 in the presence of irradiated syngeneic APCs. Activated T-cell blasts were separated by Ficoll gradient centrifugation and transferred into B6 mice (2 × 106 activated cells per mouse). At 12 to 14 (average 13) days postimmunization is the best time point for harvesting draining lymph nodes and spleens to prepare in vivo primed autoreactive T cells. The decision is based on early kinetic studies, showing that the strongest antigen-specific proliferation and cytokine production are acquired 12 to 14 days after immunization.
Scoring of EAU
The mice were examined three times a week for clinical signs of EAU by indirect funduscopy. The pupils were dilated using 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions, and funduscopic grading of disease was performed using the scoring system described previously.33 For histopathologic evaluation, whole eyes were collected at the end of the experiment and immersed for 1 hour in 4% glutaraldehyde in phosphate buffer, pH 7.4, and transferred to 10% formaldehyde in phosphate buffer until processed. The fixed and dehydrated tissues were embedded in methacrylate, then 5 μm sections were cut through the pupillary-optic nerve plane, and stained with hematoxylin and eosin. Presence or absence of disease was evaluated blind by examining six sections cut at different levels for each eye. Disease was graded pathologically based on cellular infiltration and structural changes.34
Limiting Dilution Analysis (LDA)
TCR-δ−/− mice were administered with a single dose (5 × 105, IP) injection of γδ T cells purified from immunized mice, with or without ATRA treatment. At 14 days later, T cells were enriched from spleens and draining lymph nodes, and seeded in 24 replicates in two sets of 96-well flat-bottomed culture plates containing irradiated spleen APCs (1 × 105 per well) under Th1- or Th17-polarizing conditions, with one set of plates containing an optimal dose of immunizing peptide (10 μg/mL). Based on preliminary LDA estimates of IRBP-reactive cell frequencies, a fixed number of T cells (3 × 103–2 × 105) was seeded in each well, then 48 hours later, a fraction of the culture supernatant was analyzed for IFN-γ or IL-17 production, then the plates were pulsed with 0.5 μCi [3H]-thymidine/well for 6 hours, harvested, and assessed for isotope incorporation. Positive microcultures were defined as those in which lymphokine activity or incorporated thymidine exceeded the mean activity in control cultures (no responders) by more than three standard deviations.35–37 The frequency of responder T cells was obtained by the minimum estimates of precursor frequency calculated using a program developed to analyze the LDA data38 that uses the Poisson distribution to calculate the frequency of responder T cells with 99% confidence limits.
Generation of DCs
Bone-marrow derived DCs (BMDCs) were obtained as described by Zuo et al.26 Briefly, bone marrow was flushed from mouse femurs, and the cells (1 × 106/mL) were grown in filtered RPMI 1640 medium containing 10% FCS, 50 IU/mL penicillin, and 50 μg/mL streptomycin in the presence of 10 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA) and 10 ng/mL IL-4. Nonadherent cells were removed on day 3 and fresh medium added, then DCs were harvested on day 5.
Intracellular Staining and FACS Analysis
For intracellular staining, T cells (2 × 105 in 100 μL) were incubated for 4 hours to 50 ng/mL PMA, 1 μg/mL ionomycin, and 1 μg/mL brefeldin A (all from Sigma-Aldrich, St. Louis, MO), then were washed, fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience), stained intracellularly with antibodies against IFN-γ and IL-17, and analyzed on a FACScalibur flow cytometer (FACSCalibur; BD, Franklin Lakes, NJ).
Statistical Analysis
Experiments were repeated at least three times, usually more. Experimental groups typically were composed of four mice. The figures show data from a representative experiment using triplicates. Differences between the values for different groups were examined by the two-tailed t-test. Statistical analyses of clinical scores were performed using one-way ANOVA with Tukey post hoc analysis. A P value < 0.05 was considered significant.
Results
ATRA Inhibits the Activation of Il-17+ Autoreactive T cells
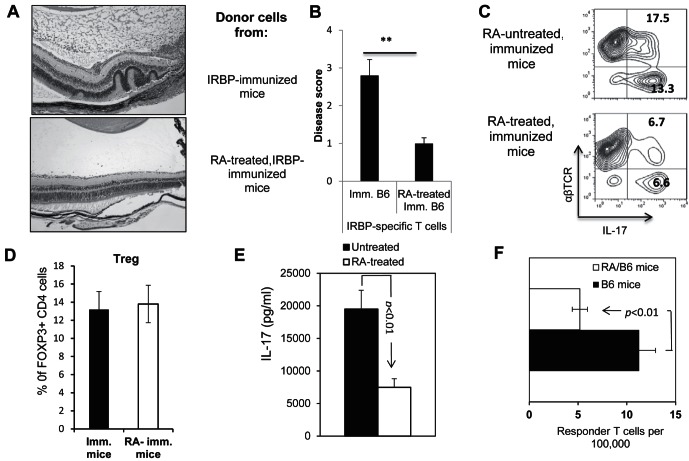
To determine whether RA affects the generation of uveitogenic T cells in EAU-prone B6 mice, particularly the newly characterized autoreactive T cells that express IL-17 (Th17), we randomly separated B6 mice into two groups, one of which received two IP injections of ATRA (200 μg/mouse) on day −3 (3 days before immunization) and day 0, while the other received DMSO (vehicle) only. Immediately after the second ATRA injection, the mice were immunized with a pathogenic dose (150 μg/mouse) of the IRBP1-20 peptide,29,31 and IRBP1-20-specific T cells were isolated 13 days after immunization by in vitro stimulation of enriched T cells with immunizing peptide and autologous irradiated adherent splenic APCs.29,31 The activated IRBP-specific T cells then were separated, characterized, and adoptively transferred to naïve B6 recipients (2 × 106 cells/mouse), and severity of disease induced by IRBP-specific T cells from ATRA-treated and untreated animals was compared by pathologic examination at 15 days after cell transfer. As shown, recipients of T cells from ATRA-treated donors had significantly milder disease than recipients of T cells from immunized donors not treated with RA (Figs. 1A, 1B). It is to note that the demonstrated disease was not induced maximally, because of the need of comparative study to reveal either enhancing or inhibitory effect. IRBP-specific T cells from ATRA-treated mice contained significantly reduced numbers of IL-17+ cells (Fig. 1C), but not appreciable altered numbers of regulatory T cells (Fig. 1D), suggesting that the decreased response was not attributed to increased number of regulatory T cells among the responder T cells. ELISA results (Fig. 1E) showed that responder T cells from ATRA-treated mice produced significantly less IL-17 than control mice, consistent with the cytoplasmic staining results.
Figure 1.
ATRA-treated B6 mice generate decreased numbers of Th17 autoreactive T cells after immunization. (A, B) Splenic T cells from IRBP1-20/CFA-immunized B6 mice with or without ATRA treatment (200 mM, IP on day −3 and day 0) were enriched and stimulated for 48 hours with an optimal dose of immunizing peptide (10 μg/mL) under Th17 polarizing conditions. Then, the activated T cells were separated by Ficoll gradient centrifugation on day 3 and transferred adoptively to syngeneic naïve B6 mice. (A) The pathology of a representative eye section from each group. (B) Summarized the disease score results from three independent studies, each with 5 mice per group. (C) Cytoplasmic staining of in vitro activated IRBP-specific T cells. Using the protocol described for (A, B), on day 5 after in vitro stimulation with the immunizing peptide, the activated T cells were separated by Ficoll gradient centrifugation, and stained intracellularly with PE-conjugated anti-αβTCR antibodies and FITC-conjugated anti-IL-17 antibodies, followed by FACS analysis. (D) Foxp3+ among responder T cells of RA-treated and nontreated, immunized mice. (E) ELISA assay. The culture supernatant from immunized splenic T cells from ATRA-treated or untreated mice was tested by ELISA for IL-17 after 48 hours of stimulation with the immunizing peptide IRBP1-20. (F) Responder T-cell numbers were evaluated by LDA as detailed in the Materials and Methods. The results shown are representative of those from >5 experiments. ** P < 0.01, statistically significant.
We previously established a system allowing the direct assessment of in vivo primed Th1 and Th17 autoreactive T cells by LDA.26 To determine whether ATRA suppressed the in vivo priming of IL-17+ autoreactive T cells, we measured the frequency of in vivo primed IL-17+ T cells and found that the frequency in immunized B6 mice was significantly lower in animals that received two doses of ATRA on day −3 and day 0. As shown in Figure 1F, immunized B6 mice generated approximately 12 IL-17+ T cells per 100,000 immunized responder T cells and this number was decreased by more than 50% (5 per 100,000 immunized responder T cells) in ATRA-treated mice.
ATRA Administration Inhibits γδ T-Cell Activation and Functional Differentiation
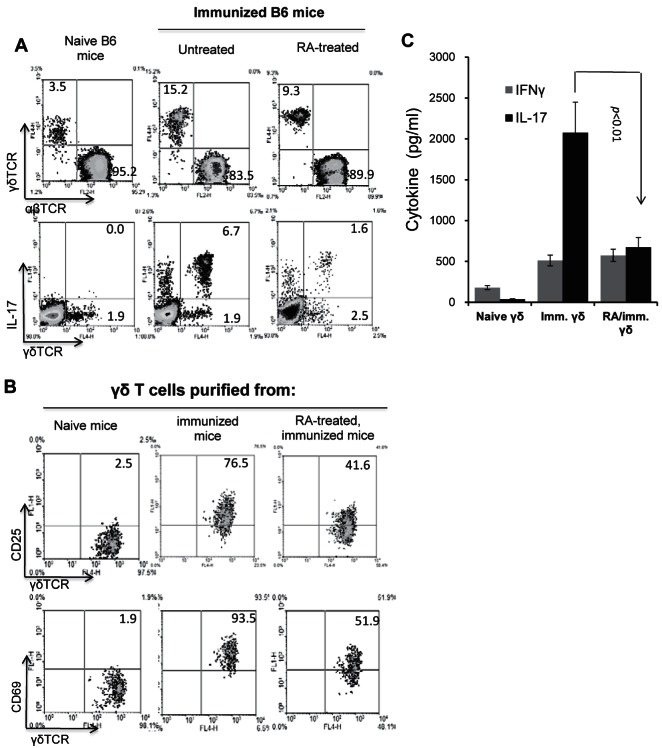
To determine the cellular mechanism involved in the suppressive effect of ATRA on the Th17 response, we compared the cellular components in the spleen and draining lymph nodes of ATRA-treated and untreated B6 mice. In this study, ATRA recipients received two doses of ATRA (200 μg/mouse) before immunization (day −3 and day 0). Then, 13 days after immunization, T cells isolated from the spleen, and draining lymph nodes were pooled and stimulated with the immunizing peptide under Th17 polarized conditions, and the cellular components in the gated CD3+ cells were analyzed immediately after separation (Fig. 2A). Although the percentage of γδ T cells in ATRA-treated immunized mice (9.3%) was higher than that in unimmunized naïve mice (3.5%), it was significantly lower than that in immunized mice not treated with ATRA (15.2%) (Fig. 2A). As shown in Figure 2B, immediately after sacrifice, only a low percentage of the γδ T cells in naïve mice expressed CD25 (2.5%) or CD69 (1.9%), whereas the majority of γδ T cells (76.5 or 93.5%) in immunized mice expressed these markers of activation. However, in ATRA-treated immunized mice, the frequencies of CD25+ (41.6%) or CD69+ (51.9%) γδ T cells were greatly reduced. Consistent with this, purified γδ T cells from ATRA-treated mice produced significantly decreased amounts of IL-17 compared to their non-ATRA–treated counterparts, with a minimal effect on IFN-γ production when tested immediately after separation in the absence of additional stimulation (Fig. 2C), showing that ATRA treatment inhibited not only γδ T-cell activation, but also their functional differentiation.
Figure 2. .
Altered γδ T-cell activation and expansion in ATRA-treated recipients. (A) Analysis of the αβTCR+, IL-17+γδTCR+, and IL-17−γδTCR+ cell populations in the CD3+ cells in naïve and IRBP1-20-immunized B6 mice, with or without prior ATRA administration. (B) γδ T cells in ATRA-treated mice express decreased levels of the T-cell activation molecules CD25 and CD69. (C) γδ T cells in ATRA-treated mice produce less IL-17. The results shown are representative of those from >5 experiments.
The Effect of γδ T cells on Activation of IRBP-Specific Th17 Cells Is Largely Abolished by ATRA
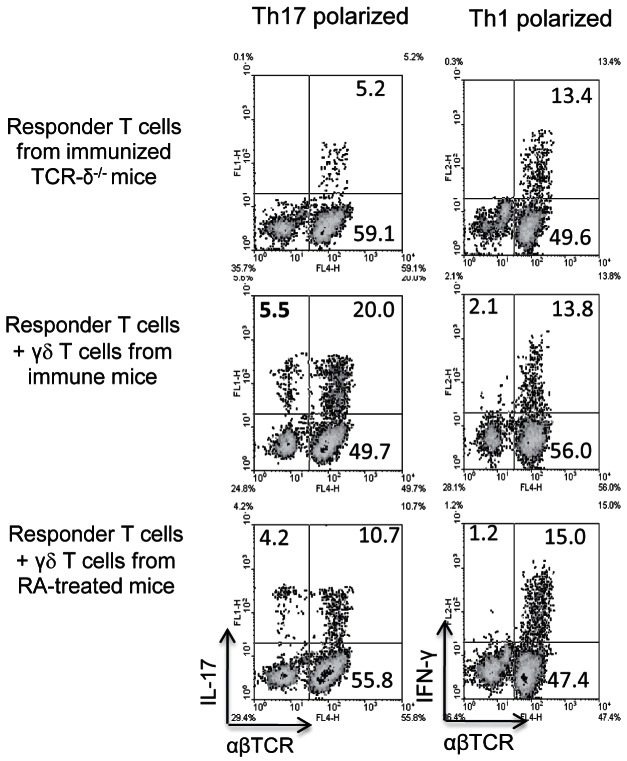
We previously reported that the proinflammatory activity of γδ T cells is not a stable feature, but fluctuates as the activation status of the γδ T cells changes.26,28,30 This suggests that factors affecting γδ T-cell activation also affect γδ T-cell–dependent autoimmune susceptibility. We have established an in vitro assay system for assessing the proinflammatory activity of γδ T cells, in which the in vitro generation of activated IRBP-specific Th17 cells from the in vivo primed T cells from immunized TCR-δ−/− mice is enhanced significantly when a small number of γδ T cells are added to the culture.27,28,30 Using this system, we examined whether γδ T cells isolated from ATRA-treated mice were more, or less, capable of promoting activation of IRBP-specific Th17 cells than those from untreated mice. As shown in Figure 3 (left panels), in cultures without addition of γδ T cells, only 5.2% of the αβ responder T cells expressed IL-17 and this number was increased substantially to 20% when the αβ responder T cells were supplemented with 2% of γδ T cells from immunized B6 mice. However, addition of the same number of γδ T cells from ATRA-treated immunized mice only increased the percentage of IL-17+ αβTCR+ T cells to 10.7%. In contrast, addition of γδ T cells did not significantly alter the percentage of IFNγ+αβTCR+ T cells (Fig. 3, right panels).
Figure 3. .
Pro-inflammatory activity of γδ T cells in immunized B6 mice is decreased after ATRA-treatment. Assessment of the proinflammatory effect of γδ T cells in vitro. Responder T cells were isolated from the spleen and draining lymph nodes of IRBP1-20/CFA-immunized TCR-δ−/− mice at 13 days after immunization. Then, T responder cells (1 × 106/well) were stimulated in 24-well plates for 48 hours with an optimal dose of immunizing peptide (10 μg/mL) under Th17 polarizing conditions. The control group contained responder αβ T cells alone, while the test groups aloes contained 2% (2 × 104/well) of γδ T cells isolated from ATRA-treated or untreated mice. After 5 days of in vitro stimulation, the activated T cells were separated and intracellularly stained with PE-conjugated anti–IFN-γ antibodies and FITC-conjugated anti–IL-17 antibodies, followed by FACS analysis. A representative study of more than 5 repeats is shown.
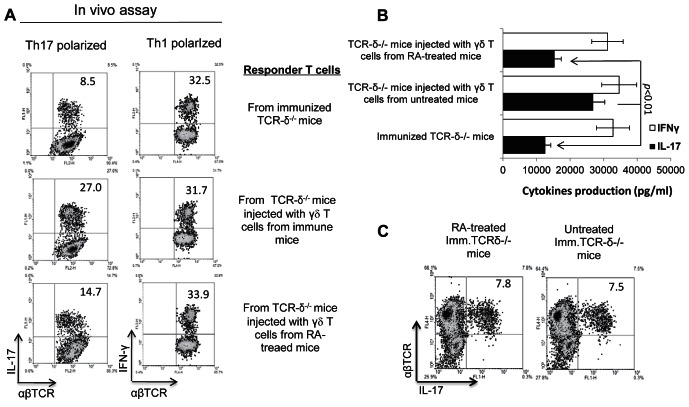
To determine whether the same occurred in vivo, we injected TCR-δ−/− mice IP with a small number of γδ T cells (2 × 105 cells/mouse) prepared from ATRA-treated and nontreated mice immunized with a pathogenic dose of IRBP1-20. Then T cells from the immunized TCR-δ−/− mice were stimulated in vitro with immunizing antigen and irradiated splenic APCs under Th17 or Th1 polarizing conditions, and the activated T cells separated on Ficoll and subjected to intracellular staining to assess the percentage of αβ T cells expressing IL-17 and IFN-γ. As shown in Figure 4A, TCR-δ−/− mice injected with γδ T cells from ATRA-treated immunized mice generated a significantly lower percentage (14.7%) of IL-17+ IRBP-specific αβ T cells than those that received γδ T cells from immunized, but not ATRA-treated, donors (27%). Consistent with this, ELISA results (Fig. 4B) showed that T cells from immunized TCR-δ−/− mice injected with ATRA-treated γδ T cells produced significantly lower amounts of IL-17 than mice that received non-ATRA–treated γδ T cells. The intracellular staining and ELISA results supported the conclusion that ATRA has less effect on IFN-γ+ autoreactive T cells (or Th1 cells) than IL-17+ T cells. We also compared the induced IRBP-specific, IL-17+αβTCR+ T cells between ATRA-treated and untreated, immunized TCR-δ−/− mice without administration of exogenous γδ T cells. The results showed that ATRA treatment did not significantly alter the induction of IL-17+αβTCR+ T cells in TCR-δ−/− mice (Fig. 4C), which is in sharp contrast to the response of wt-B6 mice (Fig. 1C), suggesting that γδ T cells have a crucial role in the regulation of ATRA on autoreactive T-cell responses.
Figure 4. .
In vivo pro-inflammatory activity of γδ T cells from ATRA-treated and untreated mice. (A) Splenic T cells isolated from IRBP-immunized mice with or without injection of γδ T cells from ATRA-treated or nontreated mice were stimulated with the immunizing peptide and APCs for 48 hours. Then, IL-17 and IFN-γ in the culture supernatant were measured by ELISA (B). (C) TCR-δ−/− mice, with or without precedent ATRA treatments, were immunized with the uveitogenic peptide IRBP1-20. The cytoplasmic staining procedure followed those described previously. The experiments were repeated more than 5 times.
ATRA Treatment Changes the Function of Splenic APCs
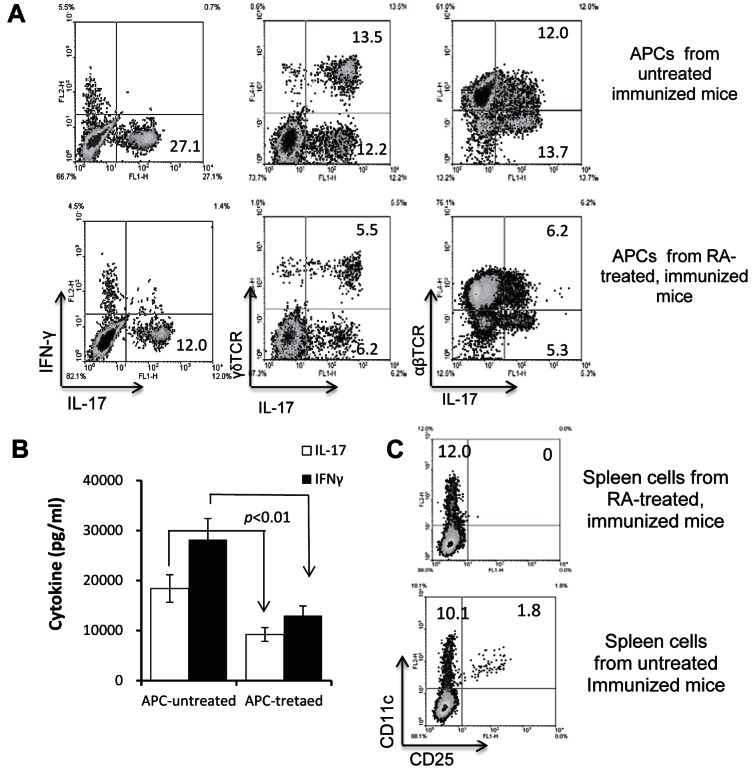
To distinguish the possibilities that ATRA directly affects γδ T-cell activity or it acts on DC/macrophages, leading to decreased activation of γδ T cells, splenic T cells from IRBP-immunized mice were stimulated with the immunizing antigen in the presence of splenic APCs from ATRA-treated or nontreated mice. Figure 5A shows that APCs from ATRA-treated mice had a significantly decreased ability to stimulate IL-17+ T-cell expansion (both γδ and αβ T cells) and to induce IL-17 production by the responder T cells (Fig. 5B). IFN-γ production also was decreased, albeit to a lesser extent (Fig. 5B). Because of our previous finding that Th17 responses are compromised if mice fail to generate adequate numbers of the CD25+ DC subset,30 we examined the percentage of these cells in the spleen of ATRA-treated or nontreated immunized mice. As shown in Figure 5C, in immunized mice not treated with ATRA, 10% of the splenic CD11c+ cells (or 1.8% of all spleen cells) expressed CD25 and CD11c, while, in ATRA-treated immunized mice, CD25+ DCs were undetectable.
Figure 5. .
Splenic APCs from ATRA-treated mice are functionally less effective in promoting activation of IL-17+ autoreactive T cells. (A) Responder T cells (1 × 106/well) from immunized B6 mice were stimulated in vitro for 48 hours in 24-well plates with IRBP1-20 in the presence of splenic APCs from ATRA-treated or nontreated mice for 5 days, then activated T cells were stained for expression of IL-17 and IFN-γ, αβTCR, or γδTCR. (B) ELISA assay. The culture supernatant from splenic T cells from immunized mice that were left untreated or treated with ATRA were tested for IL-17 production after 48 hours of stimulation with the immunizing peptide IRBP1-20 in the presence of splenic APCs from either ATRA-treated or untreated mice. (C) Recipient mice injected with ATRA generate adequate numbers of the CD25+ DC subset. Splenic cells from ATRA-treated and nontreated immunized mice were stained for expression of CD11c and CD25.
ATRA Alters BMDC Functional Differentiation
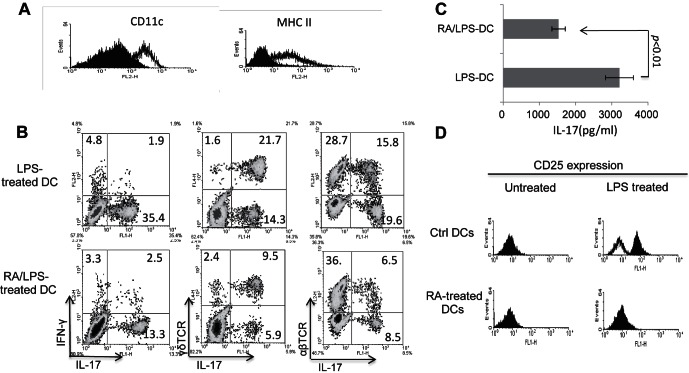
Having found that ATRA suppresses the development of CD25+ DCs, we wanted to determine whether this effect explained the immune suppression by ATRA and, therefore, examined whether the functional differentiation of BMDCs was affected by ATRA treatment in vitro. BMDCs were grown at 1 × 106 cells/mL in culture medium containing GM-CSF and IL-4 with or without ATRA for 5 days. Then, the cells were harvested and subjected to antibody staining, followed by FACS analysis. Figure 6A shows that 30 mM ATRA added on day 0 of culture significantly inhibited the differentiation of DCs, and that the growing cells expressed decreased levels of CD11c and MHC class II antigens. Moreover, functional tests showed that ATRA-treated DCs were poor APCs in the LPS-induced in vitro activation of Th17 autoreactive T cells, as they activated far fewer IL-17+ αβ T cells in the pool of in vivo primed responder T cells, as well as fewer IL-17+ γδ T cells (Fig. 6B) and responder T cells produced less IL-17 after exposure to ATRA-treated BMDCs than after exposure to untreated BMDCs (Fig. 6C). A further study showed that a significant percentage of the BMDCs cultured in medium containing GM-CSF and IL-4 expressed higher levels of CD25 when the culture medium was supplemented with LPS (1 μg/mL), and that addition of ATRA to the cultures completely blocked the LPS-driven induction of CD25+ cells (Fig. 6D).
Figure 6. .
ATRA inhibits the in vitro differentiation of BMDCs. (A) BMDCs (1 × 106/mL) were cultured in RPMI medium containing 10 ng/mL GM-CSF and IL-4 with or without addition of 30 mM ATRA to the culture on the first day of in vitro culture and day 3 of culture. Then, on day 5, the cells were harvested and stained for CD11c and MHC class II molecules, followed by FACS analysis. The filled curve is the result for ATRA-treated mice and the nonfilled curve for the non-ATRA–treated mice. (B) ATRA-treated DCs are less potent in stimulating γδ and IL-17+αβTCR+ T-cell activation. The responder T cells were enriched splenic T cells from IRBP-immunized mice. For in vitro stimulation, BMDCs were used as APCs. Top: LPS-treated BMDCs. Lower: ATRA- and LPS-treated BMDCs. After 5 days of in vitro stimulation with the immunizing antigen, the activated T cells were separated and stained intracellularly for IL-17 and IFN-γ, and surface stained for γδTCR or αβTCR. (C) Antigen-presenting activity of DCs cultured in the presence or absence of ATRA. The experimental set up was as in Figure 5B. Responder T cells obtained from IRBP1-20/CFA-immunized B6 mice were stimulated for 2 days with an optimal dose of immunizing peptide (10 μg/mL) under Th17 polarizing conditions, then IL-17 in the culture supernatants was measured by ELISA. (D) ATRA-treated DCs do not express CD25. BMDCs from untreated (upper) or ATRA-treated (lower) B6 mice were cultured in GM-CSF/IL-4 containing (10 ng/mL) culture medium for 5 days, then were stained with antimouse CD25 antibodies, followed by FACS analysis. The filled curve is anti-CD25–stained BMDCs and the nonfilled curve for same BMDCs stained with control antibodies.
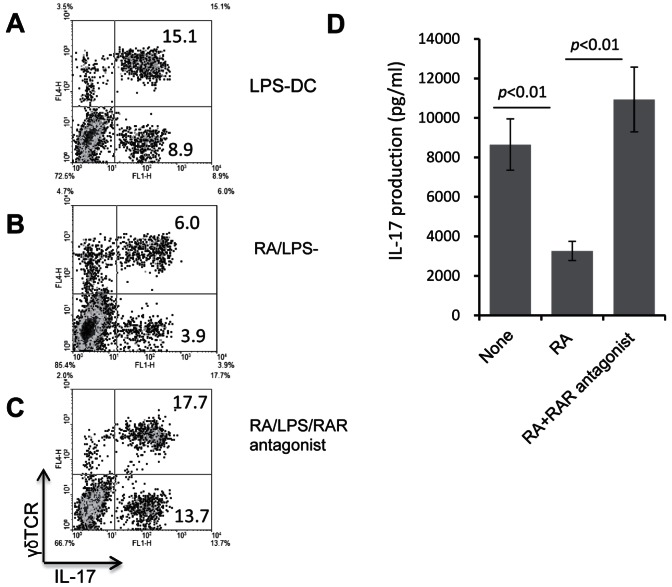
To confirm the effect of ATRA on BMDC functional differentiation and its relationship with the decreased activation of γδ T cells, we cultured bone marrow cells in medium containing GM-CSF, IL-4, and LPS, with or without supplementation with ATRA alone, or with ATRA plus two RA antagonists for 5 days, when the harvested DCs were used as APCs for in vitro stimulation of splenic T cells. Figure 7 shows that ATRA treatment again significantly diminished the ability of BDMCs to induce IL-17+ γδ T cells (top and middle panels), and that this effect was prevented by addition of the RA antagonists (middle and center panels). Furthermore, ELISA tests showed that ATRA treatment of BMDCs abolishes their support for IL-17 production by responder T cells and that this effect also was prevented by addition of the RA antagonists (Fig. 7D).
Figure 7. .
RA antagonists prevent the effect of ATRA on the differentiation of BMDCs. BMDCs were treated with LPS (A), LPS plus ATRA (B), or LPS plus ATRA plus two RA antagonists (BMS 195614 binding to RARα and CD2665 binding to RARβ/γ), both at a final concentration of 1 μM (C). The washed DCs were cultured with CD3-enriched splenic T cells from immunized B6 mice, then the activated T cells were separated and stained with the indicated antibodies. (D) The 48-hour culture supernatants from the experiments in Figure 5A were assayed for IL-17 using ELISA kits.
Discussion
Our previous studies showed that an induced Th17 autoreactive T-cell response in EAU was preceded by increased activation of γδ T cells,29,31 and increased generation of the CD25+ DC subset.30 Moreover, autoreactive Th17 responses are compromised in mice with dysfunctional γδ T cells26–29 and in mice incapable of generating CD25+ DCs during the immunization process.30 Based on these studies, we hypothesize that prevention of γδ T-cell activation or inhibition of CD25+ DC might allow us to restrain the intensity of the Th17 autoreactive T-cell response in EAU.28,29,31 γδ T cells can be activated via multiple pathways in the absence of TCR engagement, including ligation of NKG2D receptor,39 exposure to cytokines40 or TLR ligands,41 or binding to surface molecules expressed by myeloid cells.42 An effective way that can prevent γδ T-cell activation has not be investigated adequately to our knowledge. Using a well-established autoimmune disease model of EAU and an assay system established in our laboratory, we examined the mechanism by which RA treatment inhibits the autoimmune development. Our results supported the previous findings that RA-treated mice showed a significantly depressed autoreactive T-cell response, particularly with regard to the subset of autoreactive T cells that express IL-17. We also showed that ATRA treatment can, in vitro and in vivo, effectively inhibit the expansion of CD25+ DCs and γδ T-cell activation, thereby restraining the Th17 autoreactive T-cell response. The results of this study further support our previous report that CD25+ DCs possess strongest γδ T-cell stimulatory activity,30 and that control of γδ T-cell activation deserves to be a valuable way of controlling the Th17 autoreactive T cells in autoimmune disease. We also showed that decreased pathogenic T-cell response, rather than enhanced regulatory T-cell activity, can be attributed directly to the inhibitory effect of RA. Our results that the ATRA treatment, especially in vivo administration, has little effect on Foxp3+ cells, appears to differ from those of Keino et al.19 However, these two studies differed in a number of approaches, even though it is premature to predict the exact cause for the possible differences. For example, in the study of Keino et al., the examined Foxp3+ cells were from naïve mice, the stimulant was anti-CD3+CD28, and the response was in the presence of TGF-β. Our study targeted T cells of immunized mice after in vitro activation with the immunizing antigen and in the absence of TGF-β. All of these listed differences may have caused the difference in outcome.
RA, the active metabolite of vitamin A, has multiple effects on cell differentiation and survival by binding to two receptors, RARs and retinoid X receptors (RXRs), each of which has multiple isoforms.5,43–45 It also supports embryonic development, central nervous system function, and the immune response.46,47 With regard to immune responses, RA induces the differentiation of myeloid-derived suppressor cells to mature macrophages,3,6,48 and the differentiation and maturation of granulocytes.8,9 Treatment with RA leads to downregulation of TLR2 and the coreceptor CD14 on human monocytes.49 Recent studies showed that RA is a key regulator of Th17 responses, and that it balances the intensity of regulatory and effector T-cell responses.11,13 RA treatment inhibits the activation of T-helper type 17 (Th17) cells,10,11 but promotes the generation of Foxp3+ regulatory T cells.10,13,14,50 Studies on the effect of RA on autoimmunity have shown repeatedly an antiinflammatory effect in a number of autoimmune diseases, including EAE13,16–18 and EAU.19,20 The beneficial effect of RA in these studies was thought to be due to its inhibition of pathogenic Th17 responses.10,13,17,50,51 However, several lines of evidence demonstrate that the mechanism by which RA acts on immune responses is more complex than previously thought. RA does not always inhibit an immune response, but can also cause enhancement. For example, RA treatment during antitumor vaccination dramatically enhances the antitumor immune response by converting immature myeloid cells into more mature and less suppressive myeloid cells.6,7,24 Studies also have shown that, when RA suppresses the Th1 response, the accompanying Th2 response to the immunizing antigen is enhanced.18,52 Thus, despite its inhibitory effect on the development of autoimmune disease and on the activation of specific T-cell subsets, RA is not simply an immunosuppressant, but is capable of balancing pro- and antiinflammatory immune responses.11,43 Any effective use of RA for therapy will require a far better understanding of the mechanism by which in vivo and in vitro treatment with RA regulates immune responses.
The relationship among the depressed generation of CD25+ DCs, the diminished activation of γδ T cells, and the suppressed activation of IL-17+ autoreactive T cells in RA-treated mice will require further studies. Our present observations suggested that ATRA blocks the development of the CD25+ DC subset, which is essential for activation of Th17 autoreactive T cells, as we reported previously.30 It also seems likely that ATRA directly blocks the activation of γδ T cells, a key event leading to activation of Th17 autoreactive T cells.28,30 Further possibilities include that ATRA interrupts the reciprocal interactions between γδ T cells and DCs, which are necessary for eliciting a strong Th17 response. In depth studies on the mechanism by which RA affects autoimmune diseases may allow us to gain insights that will be essential for future therapeutic approaches. In our study, we showed that ATRA treatment can be used to manipulate γδ T-cell activation and, thereby, regulate the Th17 responses. The identification of the factors that modulate the regulatory activity of γδ T cells should improve our understanding of the mechanism by which γδ T cells regulate the autoimmune response. A growing body of evidence suggests that IL-17–expressing autoreactive T cells have a crucial role in the pathogenesis of autoimmune diseases.53–57 An improved understanding of the cellular and molecular mechanisms regulating the function of Th17 cells, the mechanism by which Th17 autoreactive T cells mediate autoimmune disease, and how regulatory T cells can be used to manipulate this particular T-cell response should greatly facilitate efforts at treating autoimmune diseases.
Acknowledgments
Supported in part by National Institutes of Health Grants EY018827, EY017373, EY00403, and EY003040.
Disclosure: D. Liang, None; A. Zuo, None; H. Shao, None; W.K. Born, None; R.L. O'Brien, None; H.J. Kaplan, None; D. Sun, None
References
- 1. Yokota A, Takeuchi H, Maeda N, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009; 21: 361–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011; 117: 6532–6541 [DOI] [PubMed] [Google Scholar]
- 3. Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol. 2007; 179: 3504–3514 [DOI] [PubMed] [Google Scholar]
- 4. Simeone A, Acampora D, Arcioni L, Andrews PW, Boncinelli E, Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990; 346: 763–766 [DOI] [PubMed] [Google Scholar]
- 5. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996; 10: 940–954 [PubMed] [Google Scholar]
- 6. Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003; 63: 4441–4449 [PubMed] [Google Scholar]
- 7. Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006; 66: 9299–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins SJ, Robertson KA, Mueller L. Retinoic acid-induced granulocytic differentiation of HL-60 myeloid leukemia cells is mediated directly through the retinoic acid receptor (RAR-a). Mol Cell Biol. 1990; 10: 2154–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawson ND, Berliner N. Neutrophil maturation and the role of retinoic acid. Exp Hematol. 1999; 27: 1355–1367 [DOI] [PubMed] [Google Scholar]
- 10. Elias KM, Laurence A, Davidson TS, et al. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008; 111: 1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T-cell differentiation mediated by retinoic acid. Science. 2007; 317: 256–260 [DOI] [PubMed] [Google Scholar]
- 12. Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-a favours regulatory T-cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007; 37: 2396–2399 [DOI] [PubMed] [Google Scholar]
- 13. Klemann C, Raveney BJE, Klemann AK, et al. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol. 2009; 174: 2234–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coombes JL, Siddiqui KRR, Rancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-b and retinoic acid-dependent mechanism. J Exp Med. 2007; 204: 1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007; 204: 1737–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Massacesi L, Castigli E, Vergelli M, et al. Immunosuppressive activity of 13-cis-retinoic acid and prevention of experimental autoimmune encephalomyelitis in rats. J Clin Invest. 1991; 88: 1331–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiao S, Jin H, Korn T, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-b-Driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008; 181: 2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Racke MK, Burnett D, Pak SH, et al. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995; 154: 450–458 [PubMed] [Google Scholar]
- 19. Keino H, Watanabe T, Sato Y, Okada AA. Oral administration of retinoic acid receptor-a/b-specific ligand Am80 suppresses experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011; 52: 1548–1556 [DOI] [PubMed] [Google Scholar]
- 20. Keino H, Watanabe T, Sato Y, Okada AA. Anti-inflammatory effect of retinoic acid on experimental autoimmune uveoretinitis. Br J Ophthalmol. 2010; 94: 802–807 [DOI] [PubMed] [Google Scholar]
- 21. Brinckerhoff CE, Coffey JW, Sullivan AC. Inflammation and collagenase production in rats with adjuvant arthritis reduced with 13-cis-retinoic acid. Science. 1983; 221: 756–758 [DOI] [PubMed] [Google Scholar]
- 22. Zhou X, Kong N, Wang J, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010; 185: 2675–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwok SK, Park MK, Cho ML, et al. retinoic acid attenuates rheumatoid inflammation in mice. J Immunol. 2012; 189: 1062–1071 [DOI] [PubMed] [Google Scholar]
- 24. Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001; 166: 5398–5406 [DOI] [PubMed] [Google Scholar]
- 25. Weiss FU, Marques IJ, Woltering JM, et al. retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009; 137: 2136–2145 [DOI] [PubMed] [Google Scholar]
- 26. Zuo A, Liang D, Shao H, Born WK, Kaplan HJ, Sun D. In vivo priming of IL-17+ uveitogenic T cells is enhanced by Toll ligand receptor (TLR)2 and TLR4 agonists via gd T-cell activation. Mol Immunol. 2012; 50: 125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nian H, Liang D, Zuo A, et al. Characterization of autoreactive and bystander IL-17+ T cells induced in immunized C57BL/6 mice. Invest Ophthalmol Vis Sci. 2012; 53: 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nian H, Shao H, O'Brien BA, Born WK, Kaplan HJ, Sun D. Activated gd cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest Ophthalmol Vis Sci. 2011; 52: 5920–5927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui Y, Shao H, Lan C, et al. Major role of gd T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009; 183: 560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang D, Zuo A, Shao H, et al. Role of CD25+ dendritic cells in the generation of Th17 autoreactive T cells in autoimmune experimental uveitis. J Immunol. 2012; 188: 5785–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nian H, Shao H, Zhang G, et al. Regulatory effect of T cells on IL-17+ uveitogenic T cells. Invest Ophthalmol Vis Sci. 2010; 51: 4661–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997; 109: 370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1-20-specific T cells. Exp Eye Res. 2006; 82: 323–331 [DOI] [PubMed] [Google Scholar]
- 34. Cerottini JC, MacDonald HR. Limiting dilution analysis of alloantigen-reactive T lymphocytes. V. Lyt phenotype of cytolytic T lymphocyte precursors reactive against normal and mutant H-2 antigens. J Immunol. 1981; 126: 490–496 [PubMed] [Google Scholar]
- 35. Kelso A, MacDonald HR, Smith KA, Cerottini JC, Brunner KT. Interleukin 2 enhancement of lymphokine secretion by T lymphocytes: analysis of established clones and primary limiting dilution microcultures. J Immunol. 1984; 132: 2932–2938 [PubMed] [Google Scholar]
- 36. Romero P, Dunbar PR, Valmori D, et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med. 1998; 188: 1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun D, Whitaker JN, Wilson DB. Regulatory T cells in experimental allergic encephalomyelitis. I. Frequency and specificity analysis in normal and immune rats of a T-cell subset that inhibits disease. Int Immunol. 1999; 11: 307–315 [DOI] [PubMed] [Google Scholar]
- 38. Strid J, Roberts SJ, Filler RB, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008; 9: 146–154 [DOI] [PubMed] [Google Scholar]
- 39. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009; 31: 331–341 [DOI] [PubMed] [Google Scholar]
- 40. Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells Selectively expand in response to pathogen products and environmental signals. Immunity. 2009; 31: 321–330 [DOI] [PubMed] [Google Scholar]
- 41. Aydintug MK, Roark CL, Chain JL, Born WK, O'Brien RL. Macrophages express multiple ligands for gammadelta TCRs. Mol Immunol. 2008; 45: 3253–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011; 35: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Du X, Tabeta K, Mann N, Crozat K, Mudd S, Beutler B. An essential role for RXRa in the development of Th2 responses. Eur J Immunol. 2005; 35: 3414–3423 [DOI] [PubMed] [Google Scholar]
- 44. Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007; 129: 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kane MA, Folias AE, Wang C, Napoli JL. Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J. 2010; 24: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duester G. Families of retinoid dehydrogenases regulating vitamin A function. Eur J Biochem. 2000; 267: 4315–4324 [DOI] [PubMed] [Google Scholar]
- 47. Pereira WF, Ribeiro-Gomes FL, Guillermo LVC, et al. Myeloid-derived suppressor cells help protective immunity to Leishmania major infection despite suppressed T-cell responses. J Leuk Biol. 2011; 90: 1191–1197 [DOI] [PubMed] [Google Scholar]
- 48. Liu PT, Krutzik SR, Kim J, Modlin RL. Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005; 174: 2467–2470 [DOI] [PubMed] [Google Scholar]
- 49. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T-cell responses. Nat Immunol. 2007; 8: 1086–1094 [DOI] [PubMed] [Google Scholar]
- 50. Santarlasci V, Maggi L, Capone M, et al. Rarity of human T helper 17 cells is due to retinoic acid orphan receptor-dependent mechanisms that limit their expansion. Immunity. 2012; 36: 201–214 [DOI] [PubMed] [Google Scholar]
- 51. Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003; 15: 1017–1025 [DOI] [PubMed] [Google Scholar]
- 52. Peng Y, Han G, Shao H, Wang Y, Kaplan HJ, Sun D. Characterization of IL-17+ interphotoreceptor retinoid-binding protein-specific T cells in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2007; 48: 4153–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Axtell RC, Xu L, Barnum SR, Raman C. CD5-ck2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006; 177: 8542–8549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially' polarize CD4+ TH-17 cells in relapsing EAE. Nat Immunol. 2007; 8: 172–180 [DOI] [PubMed] [Google Scholar]
- 55. Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003; 421: 744–748 [DOI] [PubMed] [Google Scholar]
- 56. Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004; 21: 467–476 [DOI] [PubMed] [Google Scholar]
- 57. Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T-cell population that induces autoimmune inflammation. J Exp Med. 2005; 201: 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]