Abstract
Chronic wounds, particularly diabetic ulcers, represent a main public health concern with significant costs. Ulcers often harbor an additional obstacle in the form of tunneled or undermined wounds, requiring treatments that can reach the entire wound tunnel, because bioengineered grafts are typically available only in a sheet form. While collagen is considered a suitable biodegradable scaffold material, it is usually extracted from animal and human cadaveric sources, and accompanied by potential allergic and infectious risks. The purpose of this study was to test the performance of a flowable gel made of human recombinant type I collagen (rhCollagen) produced in transgenic tobacco plants, indicated for the treatment of acute, chronic, and tunneled wounds. The performance of the rhCollagen flowable gel was tested in an acute full-thickness cutaneous wound-healing rat model and compared to saline treatment and two commercial flowable gel control products made of bovine collagen and cadaver human skin collagen. When compared to the three control groups, the rhCollagen-based gel accelerated wound closure and triggered a significant jumpstart to the healing process, accompanied by enhanced re-epithelialization. In a cutaneous full-thickness wound pig model, the rhCollagen-based flowable gel induced accelerated wound healing compared to a commercial product made of bovine tendon collagen. By day 21 post-treatment, 95% wound closure was observed with the rhCollagen product compared to 68% closure in wounds treated with the reference product. Moreover, rhCollagen treatment induced an early angiogenic response and induced a significantly lower inflammatory response than in the control group. In summary, rhCollagen flowable gel proved to be efficacious in animal wound models and is expected to be capable of reducing the healing time of human wounds.
Introduction
Modern life and increased life expectancy, accompanied by increased risk of disease, are associated with a heightened incidence of wounds requiring medical attention. Chronic wounds, such as venous and arterial ulcers, pressure ulcers, and diabetic ulcers, are often associated with advanced age, compromised blood circulation, patient immobility, and systemic illnesses.1,2 Each year, nearly 4 million individuals in the United States experience some form of chronic skin ulcer.3 These wounds are painful, unsightly, and can require limb amputation if left unattended. Chronic wounds pose a significant challenge to healthcare providers, who often adopt multidisciplinary approaches to reach effective results.4–6 Proper and timely intervention can impact time to healing, hospital length of stay and costs, pain levels, scar tissue, and overall patient quality of life.7–10
Wound repair and tissue regeneration are complex physiological processes, involving programmed matrix destruction for the purpose of renewed growth and reorganization.11–13 Collagens, the main structural proteins in vertebrates and many other multicellular organisms, provide unmatched structural integrity to the extracellular matrix (ECM).14–16 Collagen and collagen-derived metabolic components control many cellular functions relating to wound healing, including cell shape, differentiation, migration, and protein synthesis.17,18 Throughout the multistage wound-healing process, collagen and collagen-derived components provide indispensable support for cell aggregation and adhesion, clot formation, and adequate scar tissue generation. After clot formation, fibroblasts migrate into the wound region and begin to replace the blood clot with collagen. Fibroblasts biochemically alter the ECM by degrading fibrin and producing collagen. Biocompatible collagen-based wound dressings or tissue substitutes contribute to local haemostatic and chemotactic stimuli, while supplying a structural support scaffold upon which neotissue can be formed at enhanced rates.14,19 In addition, collagen dressings have other advantages over conventional dressings in terms of ease of application and being natural, nonimmunogenic, nonpyrogenic, hypoallergenic, and pain free. The study performed by Veves and Sheehan on 276 patients of diabetic foot ulcer divided equally into two groups, one group was treated with collagen and the second with other dressing materials, found that the healing was better in wounds of <6-month duration treated with collagen dressings.20
To date, animals and, to a lesser extent, cadavers are the primary collagen sources in the manufacturing of medical dressings. Such materials can provoke immune responses and involve risk of contamination with pathogens.21 In addition, collagen extracted from animals and humans undergoes irreversible cross-linking and harsh processing methods that compromise its biological and mechanical functions. A genetically engineered tobacco plant line expressing five human genes essential for the production of post-translationally modified human recombinant type I collagen (rhCollagen) was established.22 The extracted collagen raw material can be easily manipulated to form smooth and consistent fibrils, good for fabrication of collagen-based products.
Intimate contact between applied therapeutic matrices and deep wound beds of irregular geometries should expedite healing. Full coverage of such wounds with graft matrices that introduce the basic elements required for tissue regeneration is desirable. Therefore, in this study, we wanted to evaluate gel-type scaffolds that can address these types of wounds. The aim of the present study was to evaluate the ability of a flowable gel produced from rhCollagen (Vergenix™FG) to promote healing of deep wounds and to compare it to that of commercial flowable gel controls based on bovine or human cadaver collagen.
Materials and Methods
Flowable gel treatments
The following flowable gel formulations were used for the treatment of wounds in the animal wound-healing models: Test article: Human rhCollagen-based gel formulation, VergenixFG. The rhCollagen used as a raw material is a 2.5–3.5 mg/mL 10-mM HCl solution that was neutralized during the formulation. The product comes as a dry collagen sample, in the presence of excipients, which upon addition of saline turns into a gel. The product is sterilized by ETO (CollPlant, Ltd., Ness-Ziona, Israel), Commercial control 1: bovine tendon collagen-based flowable matrix with glycosaminoglycans and cross-linking, sterilized by irradiation (Control 1) and Commercial control 2: cadaveric human collagen-based flowable scaffold, cross-linked, sterilized by aseptic filling (Control 2). All products were prepared before use, according to the manufacturer's instructions.
Rat wound-healing animal model
Ethics approval
This study was approved by the Ethics Committee for Animal Use of the Hebrew University of Jerusalem Israel. Male Sprague-Dawley rats weighing 200–250 g were housed one per cage with free access to food and water, in a room with controlled humidity and temperature (22°C), on a 12-h light/dark cycle.
Operation procedure
Thirty-three rats were included in the study. Animals were anesthetized using isoflurane inhalation via a facemask. The dorsal aspect and flanks of the rat skin were clipped and scrubbed using betadine. One dorsal 1.4×1.4-cm full-thickness skin wound was created. Any ensuing bleeding was arrested before filling each wound either with Test or with Control 1 or 2. In addition, a saline control was used. Test or Control products were applied, so that the wound site was completely filled. Each treatment was given to nine animals. Control 2 was given to six animals. Subsequently, all wounds were bandaged with Tegaderm™ Film bandages (3M, St. Paul, MN). The entire trunk was then wrapped with a net. After treatment, the recovery of the animals was carefully monitored before returning them to their individual cages.
Clinical evaluation
All inflicted wound sites were inspected, evaluated, and photographed immediately postwounding and before the application of the Test and Control Products. In addition, wound sites were visually inspected and imaged by photography on days 1, 3, and 6 post-treatment.
Wound closure evaluation
Wound closure was assessed using computerized image analysis (ImageJ; NIH). Briefly, the extent of open wound area within the image was defined. Wound area was expressed in terms of percentage of wound area remaining with time, with the wound size measured immediately after injury taken as 100%.
Histological analysis of wound healing
Animals were sacrificed on days 1, 3, and 6 of the study; wound tissue together with surrounding marginal tissues was excised and processed for routine histology. Excised wound samples were fixated in 10% buffered formalin, embedded in paraffin, sectioned in 5-μm thickness, and stained using hematoxylin & eosin (H&E) for evaluation of inflammation and re-epithelialization. The severity of the inflammatory response was graded as minimal, mild, moderate, moderately severe, or severe. Each of these descriptions was assigned a numerical value: not present=0; minimal=1; mild=2; moderate=3; moderately severe=4; and severe=5. For re-epithelialization, the percent of closure evaluated in the H&E slides was reported. An accredited histopathologist was double blinded while performing all histopathology evaluations.
Statistical analysis
The data were tested with Student's t-test statistical analysis. All the results are presented with mean±standard deviation. Tests were considered statistically significant at p<0.05.
Porcine wound-healing animal model
Ethics approval
This study was approved by the Ethics Committee for Animal Use of Israel. Two domestic pigs, one weighing 37.3 kg and the other 38.9 kg at study initiation, were kept under environmentally controlled housing conditions throughout the entire study period, and maintained in accordance with approved standard operation procedures. The study was performed at Harlan Biotech (Ness-Ziona, Israel).
Operation procedure
After proper sedation, induction, and anesthesia, 12 full-thickness square wounds, measuring 2×2 cm, localized bilaterally along the animal's dorsal midline, were surgically created. Each wound site was then treated with the Test Product (7 wounds per pig, total 14 for both pigs) or Commercial Control 1 (5 wounds per pig, total 10 for both pigs), so that the wound site was completely filled. Subsequently, all wounds were bandaged with Tegaderm Film bandages (3M) and then covered with padded bandages to protect the wound sites. The entire trunk was then wrapped with an orthopedic stockinette to keep all the bandages in place. One animal was sacrificed on day 7 of the study, and the second animal was sacrificed on day 21. Wound tissues were collected from both animals for histopathological evaluation.
Clinical evaluation
All wound sites were visually inspected, evaluated, and photographed immediately postwounding, before application of the Test and Commercial products and, thereafter, twice weekly, until the respective time of sacrifice of each animal. All dermal reactions such as erythema and edema were also recorded and evaluated. The dermal reactions were evaluated using a common 5-point semiquantitative grading as shown: normal=0; slight=1; moderate=2; marked=3; and severe=4.
Wound closure evaluation
Wound closure was assessed using computerized image analysis (ImageJ; NIH). Wound area was expressed in terms of percentage of wound area remaining with time, with the wound size measured immediately after injury taken as 100%.
Histological analysis of wound healing
Wound samples were fixated in 10% buffered formalin, embedded in paraffin, and then sectioned. Sections of 5-μm thickness were stained using H&E for evaluation of foreign body response and re-epithelialization. All wounds were evaluated and used for analysis. Foreign body response evaluated the presence of giant cells in the wound, and scoring was performed in a 0–4 scale as follows: 0=no response; 1=minimal response; 2=mild response; 3=moderate response; and 4=marked response.
Additional sections were immunohistochemically evaluated for angiogenesis using von Willebrand factor (vWF) as a marker. Stained slides were evaluated for the presence and density of vWF-positive blood vessels within the wound bed. A 0–4 scale was used for scoring as follows: 0=no blood vessels; 1=minimal blood vessels; 2=mild blood vessels; 3=moderate blood vessels; and 4=marked blood vessels in the wound bed. An accredited histopathologist performed all histopathology evaluations.
Statistical analysis
The data were tested with Student's t-test statistical analysis. All results are presented with mean±standard deviation. Wounds from both pigs were combined for statistical analysis. Tests were considered to be statistically significant at p<0.05.
Results
Rat wound model
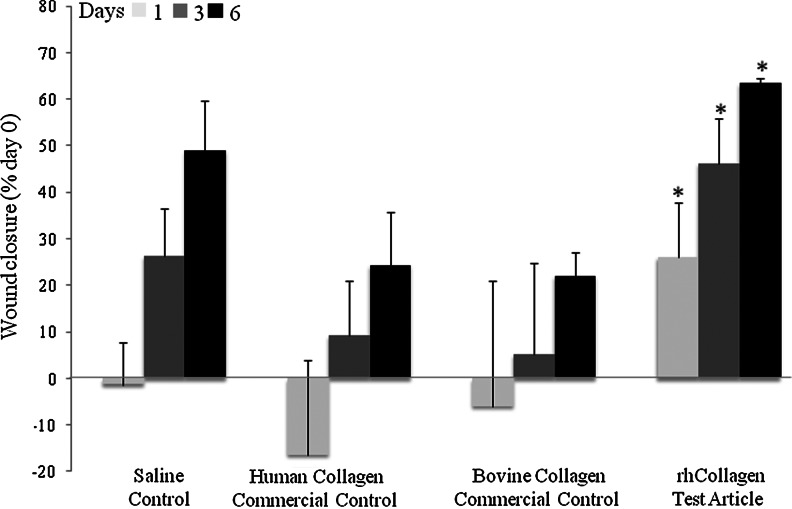
Large, full-thickness wounds created on the backs of healthy rats were treated and monitored for 6 days. Wounds treated with VergenixFG significantly accelerated wound closure when compared to standard treatment saline control and to both commercial controls (Fig. 1). Specifically, wound size significantly shrank within 24 h of treatment with VergenixFG. In contrast, a slight increase in wound size was noted in all other treatment groups, a common phenomenon among rodents, arising from a loose-skin phenotype.23 Furthermore, wound closure measured on both days 3 and 6 post-treatment occurred faster among VergenixFG-treated wounds, when compared to all other treatments; two-thirds of closure was achieved by day 6 in the VergenixFG-treated rats. Microscopical evaluation of treated wounds showed enhanced re-epithelialization in VergenixFG-treated wounds, when compared to both commercial control treatments (Fig. 2A, B), as manifested by keratinocyte proliferation and migration, the two early re-epithelialization events.24,25 Thus, rhCollagen jumpstarted the healing process by inducing an early and rapid reduction in wound size, after more advanced wound closure, when compared to control treatment.
FIG. 1.
Vergenix™FG significantly accelerates wound closure in a rat wound model. Full-thickness cutaneous wounds were created on rat backs (n=9 for saline (Saline control), control (Control 1), and VergenixFG, n=6 for Control 2) and treated with recombinant type I collagen (rhCollagen) flowable gel, saline control, bovine collagen flowable matrix commercial control (Control 1), or human collagen gel commercial control (Control 2). Wound closure was assessed on days 1, 3, and 6 post-treatment. Wound closure was measured as % closure from the time of wound infliction (*p<0.05).
FIG. 2.
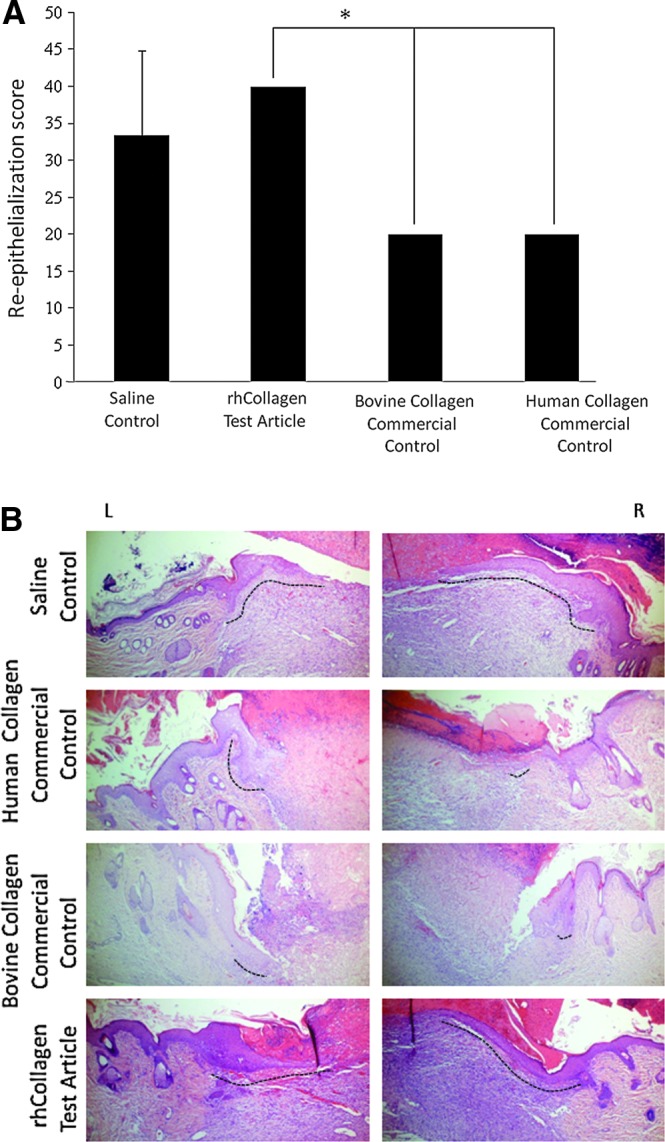
VergenixFG treatment induces re-epithelialization in rat wounds. Full-thickness cutaneous wounds were created on rats backs and treated with rhCollagen flowable gel, saline control, bovine collagen flowable matrix commercial control (Control 1), or human collagen gel commercial control (Control 2). (A) Microscopical wound re-epithelialization was assessed and scored on day 6, based on hematoxylin & eosin (H&E)-stained slides. (n=3) (*p<0.05). (B) Representative H&E sections showing right and left sides of wounds with marked re-epithelialization front (dashed line). Color images available online at www.liebertpub.com/tea
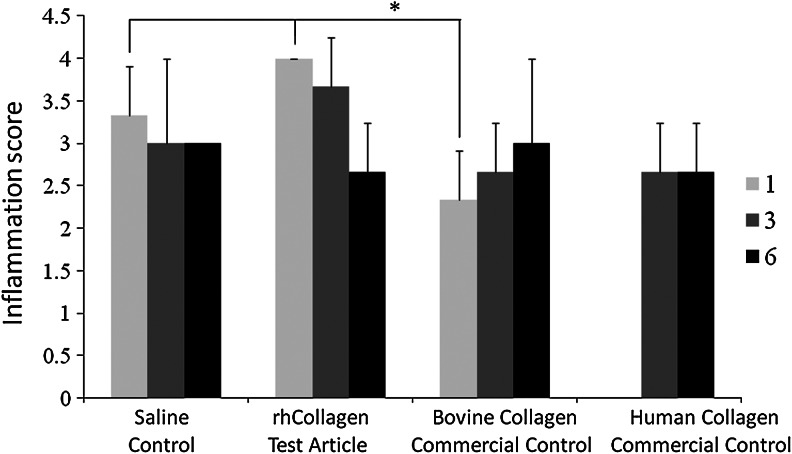
Inflammation of the wound area was scored, based on H&E slides. It was found that on day 1, the inflammatory response with rhCollagen treatment resembled the saline treatment, and was statistically enhanced compared to the bovine collagen control (Fig. 3). By day 6, this high-level inflammation decreased to match all other treatment levels. Early influx of inflammation and resolution is desired, as inflammation drives the repair process, followed by its resolution that is essential for healing, unlike most chronic wounds that are stalled at the inflammatory phase.
FIG. 3.
VergenixFG treatment results with high initial inflammation in rat wounds. Full-thickness cutaneous wounds were created on rats backs and treated with rhCollagen flowable gel, saline control, bovine collagen flowable matrix commercial control (Control 1), or human collagen gel commercial control (Control 2). Microscopical wound inflammation was assessed and scored on days 1, 3, and 6, based on H&E-stained slides. Human collagen Control (control) was not available for day-1 analysis (n=3) (*p<0.05).
The different populations were studied in the rat study, and no differences between the groups were observed (data not shown).
Pig wound model
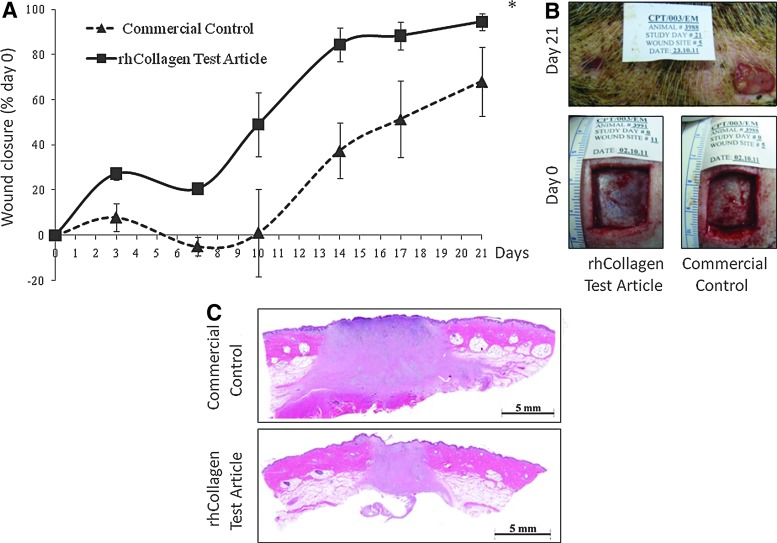
Wound closure was significantly faster after treatment with the VergenixFG, when compared to the bovine commercial control (p<0.01) (Fig. 4A). Moreover, a jumpstart in wound closure was documented on day 3 post-treatment in the VergenixFG-treated wounds. By day 21, rhCollagen-treated wounds reached a mean of 95% (±3.7%) closure, whereas control groups demonstrated a mean closure of 68% (±15.35%) (Fig 4A, B). VergenixFG-treated wounds reached full re-epithelialization at the wound site by the end of the study, while the epithelium had not fully closed the wound by day 21, in those treated with a commercial bovine-based gel (Fig. 4C).
FIG. 4.
VergenixFG significantly accelerates wound closure in a pig wound model. Full-thickness cutaneous wounds were inflicted on the backs of two domestic pigs and treated with rhCollagen flowable gel and commercially available bovine collagen flowable matrix. (A) Wound closure was monitored for 21 days (n=10 for Control 1, n=14 for Test until day 7, n=5 for Control 1l, and n=7 for Test until day 21) (*p<0.05). rhCollagen treatment jumpstarted healing on day 3. (B) Representative image of wound closure progression in rhCollagen-treated versus commercial control-treated wounds, as observed on day 21 post-treatment. (C) Representative images of H&E-stained samples collected from rhCollagen-treated and commercial control-treated wounds. Color images available online at www.liebertpub.com/tea
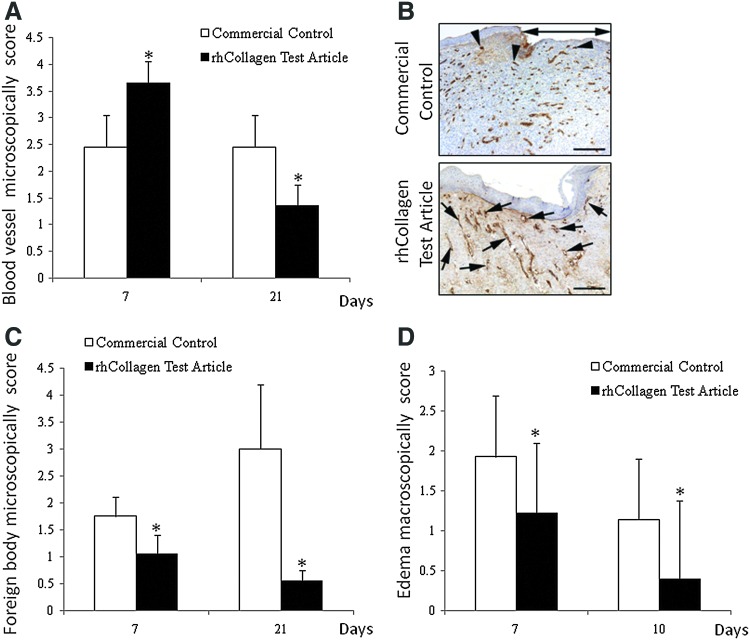
In addition to wound closure evaluation, any evident inflammatory dermal reaction, such as edema, in proximity to the wounding site margins was described. On days 0 and 3, no abnormalities were detected. A significant difference in the extent of edema was shown between the Control and Test articles on both days 7 and 10; lower levels of edema were observed after treatment with VergenixFG, compared to Control 1 treatment (Fig. 5D). After day 10, no differences in wound edema were evident (data not shown). During the H&E histopathological analysis, the residual scaffolds could be tracked, revealing that on day 7, little or no residual VergenixFG remained in the wound, while extensive amounts of residual bovine collagen-based control gel were evident in all treated wounds. The degree of foreign body inflammation was greater for the Control-treated wounds, when compared to those treated with the Test article; microscopic observation substantiated the macroscopic findings of enhanced local dermal edema (Fig 5C, D). On day 21, giant cell formation was lower in the VergenixFG-treated tissue samples when compared to the day-7 response of these samples, whereas in the control group, giant cell formation was higher.
FIG. 5.
VergenixFG induces early angiogenesis and reduces inflammatory response in a pig wound model. Full-thickness cutaneous wounds were created on the backs of two domestic pigs and then treated with either rhCollagen flowable gel or commercially available bovine collagen flowable matrix. Wounds were clinically evaluated for inflammatory response, and samples were harvested on days 7 and 21 for microscopical analysis of inflammatory and angiogenesis responses. Sections were stained with either H&E or anti-vWF antibody for determination of blood vessel formation, and then scored. (n=10 for Control, n=14 for Test until day 7, n=5 for Control, and n=7 for Test until day 21). (A) Blood vessel formation scores (*p<0.05). (B) Representative images of blood vessel formation immunohistochemistry. (C) Foreign body responses in VergenixFG-treated versus bovine collagen matrix-treated wounds. (D) Clinical wound assessment of inflammation of VergenixFG-treated versus bovine collagen matrix-treated wounds. Color images available online at www.liebertpub.com/tea
The density of blood vessels within the granulation tissue surrounding the wound was slightly higher on day 7 in wounds treated with VergenixFG when compared to those treated with Control 1. Furthermore, after an early increase in blood vessel formation, VergenixFG-treated wounds displayed a lower frequency of new blood vessels (vWF-positive cells) on day 21 (Fig 5A, B). These findings are indicative of accelerated wound healing, with faster progress through the natural phases of wound healing, inflammation, proliferation, and remodeling, when compared to wounds treated with a control gel.
Discussion
Chronic wounds, especially diabetic ulcers, present a serious public health concern due to their problematic healing process and a high rate of recurrence.26–28 Diabetic ulcers often harbor an additional obstacle in the form of tunneled or undermined wounds, calling for treatments that can fill the entire wound tunnel. Bioengineered scaffolds designed to promote wound healing are usually available in the sheet form and are ineffective in treating complex or irregularly shaped or hard to access wounds.29,30
A novel rhCollagen flowable gel product indicated for management of wound healing was evaluated and compared to two commercially available flowable gels made of bovine and cadaveric human collagen. The described rat study was preceded by multiple rat studies performed to select the best rhCollagen formulation (data not shown). The presented rat study purpose was to pre-evaluate the products in a small-animal study, before a large-animal study, and to help select the commercial controls to be used in the pig study.
Both animal models were tested in healthy animals and not in models that simulate chronic wounds. The decision was made because most of the chronic models do not manifest the full pathophysiology of chronic wounds such as diabetic wounds, including neuropathy, inflammatory process changes, and more. Secondly, as stated in the Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds—Developing Products for Treatment,31 there are no ideal animal models for chronic wounds or extensive burns; therefore, multiple animal models should be used to assess activity of wound treatment products, as was performed in this study. In the small-animal rat model, VergenixFG efficacy was compared to saline treatment, as well as to bovine and human collagen gel treatment. VergenixFG fostered advanced wound closure compared to all controls, including saline treatment; a notable jumpstart in wound closure was documented. Wound enlargement is often observed in rodent skin-healing processes, due to their loose-skin phenotype.23,32 Interestingly, while all wounds treated with control preparations showed some wound enlargement or static wound size on day 1, VergenixFG-treated wounds did not grow, but rather began to contract and heal. Wound enlargement was evident with the two collagen controls, which could be argued to be due to physical interference, but was evident to some extent also in the saline treatment group, where there is no physical interference. On the other hand, rhCollagen treatment induced early closure, supporting the beneficial action for wound healing that the rhCollagen treatment posses. The difference in wound closure/contraction in the initial healing is an outcome that can be translated into faster healing. Faster healing in humans can be further translated to better wound management, decreased complications such as wound infection, decrease in limb amputation, better compliance of patients, and eventually decreased health costs.
Histological analyses revealed that re-epithelialization of the wound bed via the hyperproliferative epidermal gulf were greater in rhCollagen-treated wounds when compared to the bovine and human cadaver collagen gel-treated wounds. Re-epithelialization of the wound is highly important, as it restores the skin barrier function.33 Furthermore, re-epithelialization is a proof that not only contraction occurs, but there is a true enhance in wound closure.
The rat study that was used as a preliminary study to select either Control 1 or Control 2 to the pig study showed that both controls exhibited similar wound closure and similar re-epithelialization and inflammation score. Control 1 was selected for the pig study, as it better resembled the rhCollagen formulation in terms of the collagen preparation. In Control 2, human collagen is derived from donated human skin. The epidermal and dermal cells are removed to result in a matrix of preserved human dermal tissue, including its native protein, collagen structure, blood vessel channels, and other biochemical components. On the other hand, the bovine collagen commercial control gel (Control 1) is made of collagen extracted in techniques that result in a clean collagen solution, similar to the clean rhCollagen extract that is later engineered to form the rhCollagen gel.
The close resemblance of porcine to human skin makes this model a widely acceptable one and the closest to humans for cutaneous wound-healing studies.34 VergenixFG treatment accelerated wound closure in the porcine model, when compared to a commercial bovine flowable matrix control. As in the rat model, healing was jumpstarted at early stages, with 27% wound closure already recorded during the first observation session, versus 7.9% closure in control-treated wounds. Moreover, full re-epithelialization was seen in the Test article-treated wounds, while only partial re-epithelialization was achieved after control treatment (Fig. 4A–C).
Angiogenesis is an important process that has to take place in wound healing.35 In the pig study, blood vessel count was higher on day 7 and lower on day 21 upon the rhCollagen treatment (Fig. 5A, B). Higher blood vessel score at day 7 indicates good vessel growth and effective healing process. Toward full repair of the wound, the scar has fewer blood vessels than in the middle of the healing process. This was demonstrated in this study by the rhCollagen treatment. It can be claimed as a transition between wound healing phases, corresponding to natural and accelerated healing.36,37
Inflammation is a highly effective component of the innate initial reaction of the body to injury and one that normally leads to tissue repair and restoration of function. Leukocytes, including neutrophils and macrophages, infiltrate the wounded area and assist in cleaning and removing damaged tissue debris and foreign particles. Once in the wound site, activated macrophages release several important growth factors and cytokines, initiating granulation tissue formation.37 If there is no resolution of inflammation, wound healing will not occur and will lead to chronic wounds, such as in diabetic wounds.38 Hence, inflammation is necessary for wound healing, but needs to be resolved for the repair to continue and move to the proliferative phase. In the foreign body response, giant cells composed of cluster of macrophages persist at the site of implantation, sometimes for years. Their presence indicates that there is a chronic inflammatory response that distinguishes it from wound healing, thus not a desired phenomenon.39 The respective inflammatory response observed in the treated pigs was documented. Control 1 treatment induced high-level skin edema around lesions on days 7 and 10 post-treatment compared to VergenixFG. In correlation with the macroscopical evaluation, histopathological analyses revealed more giant cell formation that increased with time, in the bovine-treated wounds. However, a very slight reaction that decreased with time was observed in the group of wounds treated with VergenixFG. Giant cell formation, manifested as a foreign body response, can delay wound repair.40–42 Both the rhCollagen and the bovine collagen of the control treatment are of different species than the pig; therefore, it raises a question why with rhCollagen, the inflammatory reaction was normal and to a lesser extent than with the control treatment. It may be argued that the rhCollagen is of pure nature verses collagen extracted from animals, as was demonstrated by Stein et al.22 In addition, other factors may affect the inflammatory reaction, including the degree of the cross-linking of the collagen scaffold and the type of crosslinker.43
To conclude, VergenixFG rhCollagen flowable gel, accelerated healing of cutaneous full-thickness animal wounds when compared to the rate of healing achieved by the tested commercial controls. Furthermore, VergenixFG jumpstarted the wound closure process, stimulated an early angiogenic response, and reduced the inflammatory response. Implementing this treatment in human wounds may be advantageous in deep and inaccessible wounds, possible shorting the time to healing.
Disclosure Statement
S.S., S.R., T.A., T.H.A., and F.G. are employed by CollPlant, Ltd. E.T. is a clinical adviser for CollPlant, Ltd. O.S. is the founder and Chief Scientific Officer of CollPlant, Ltd.
References
- 1.Blumberg S.N. Berger A. Hwang L. Pastar I. Warren S.M. Chen W. The role of stem cells in the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2011;96:1. doi: 10.1016/j.diabres.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 2.White-Chu E.F. Flock P. Struck B. Aronson L. Pressure ulcers in long-term care. Clin Geriatr Med. 2011;27:241. doi: 10.1016/j.cger.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Chow I. Lemos E.V. Einarson T.R. Management and prevention of diabetic foot ulcers and infections: a health economic review. Pharmacoeconomics. 2008;26:1019. doi: 10.2165/0019053-200826120-00005. [DOI] [PubMed] [Google Scholar]
- 4.Alexiadou K. Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3:4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attinger C.E. Brown B.J. Amputation and ambulation in diabetic patients: function is the goal. Diabetes Metab Res Rev. 2012;28(Suppl 1):93. doi: 10.1002/dmrr.2236. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh P. Attinger C. Abbas Z. Bal A. Rojas N. Xu Z.R. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab Res Rev. 2012;28(Suppl 1):107. doi: 10.1002/dmrr.2245. [DOI] [PubMed] [Google Scholar]
- 7.Gottrup F. Jorgensen B. Karlsmark T. News in wound healing and management. Curr Opin Support Palliat Care. 2009;3:300. doi: 10.1097/SPC.0b013e328331d40c. [DOI] [PubMed] [Google Scholar]
- 8.Helberg D. Mertens E. Halfens R.J. Dassen T. Treatment of pressure ulcers: results of a study comparing evidence and practice. Ostomy Wound Manage. 2006;52:60. [PubMed] [Google Scholar]
- 9.Reddy M. Gill S.S. Kalkar S.R. Wu W. Anderson P.J. Rochon P.A. Treatment of pressure ulcers: a systematic review. JAMA. 2008;300:2647. doi: 10.1001/jama.2008.778. [DOI] [PubMed] [Google Scholar]
- 10.Wasiak J. Cleland H. Campbell F. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2008:CD002106. doi: 10.1002/14651858.CD002106.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Kondo T. Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2012;203:93. doi: 10.1016/j.forsciint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Miyagi K. Sharma P.R. Price R.D. Please close this skin wound. Br J Hosp Med (Lond). 2011;72:M162. doi: 10.12968/hmed.2011.72.sup11.m162. [DOI] [PubMed] [Google Scholar]
- 13.Velnar T. Bailey T. Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 14.Purna S.K. Babu M. Collagen based dressings—a review. Burns. 2000;26:54. doi: 10.1016/s0305-4179(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmajr R. Olsen B.R. Structure, molecular biology, and pathology of collagen. Ann N Y Acad Sci. 1990;580:1. [PubMed] [Google Scholar]
- 16.Fleischmajer R. Collagen fibrillogenesis: a mechanism of structural biology. J Invest Dermatol. 1986;87:553. doi: 10.1111/1523-1747.ep12455728. [DOI] [PubMed] [Google Scholar]
- 17.Rocnik E.F. Chan B.M. Pickering J.G. Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest. 1998;101:1889. doi: 10.1172/JCI1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka S. Koyama H. Ichii T. Shioi A. Hosoi M. Raines E.W., et al. Fibrillar collagen regulation of plasminogen activator inhibitor-1 is involved in altered smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2002;22:1573. doi: 10.1161/01.atv.0000028002.60919.4d. [DOI] [PubMed] [Google Scholar]
- 19.Fleck C.A. Chakravarthy D. Understanding the mechanisms of collagen dressings. Adv Skin Wound Care. 2007;20:256. doi: 10.1097/01.ASW.0000269310.00145.e2. [DOI] [PubMed] [Google Scholar]
- 20.Veves A. Sheehan P. Pham H.T. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137:822. doi: 10.1001/archsurg.137.7.822. [DOI] [PubMed] [Google Scholar]
- 21.Hori H. Hattori S. Inouye S. Kimura A. Irie S. Miyazawa H., et al. Analysis of the major epitope of the alpha2 chain of bovine type I collagen in children with bovine gelatin allergy. J Allergy Clin Immunol. 2002;110:652. doi: 10.1067/mai.2002.127862. [DOI] [PubMed] [Google Scholar]
- 22.Stein H. Wilensky M. Tsafrir Y. Rosenthal M. Amir R. Avraham T., et al. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules. 2009;10:2640. doi: 10.1021/bm900571b. [DOI] [PubMed] [Google Scholar]
- 23.Davidson J.M. Animal models for wound repair. Arch Dermatol Res. 1998;290(Suppl):S1. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]
- 24.D'Souza S.J. Vespa A. Murkherjee S. Maher A. Pajak A. Dagnino L. E2F-1 is essential for normal epidermal wound repair. J Biol Chem. 2002;277:10626. doi: 10.1074/jbc.M111956200. [DOI] [PubMed] [Google Scholar]
- 25.Werner S. Krieg T. Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 26.Harding K. Queen D. Chronic wounds and their management and prevention is a significiant public health issue. Int Wound J. 2010;7:125. doi: 10.1111/j.1742-481X.2010.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y. Huang S. Fu X. Liu H. Ran X. Lu S., et al. Epidemiology of chronic cutaneous wounds in China. Wound Repair Regen. 2011;19:181. doi: 10.1111/j.1524-475X.2010.00666.x. [DOI] [PubMed] [Google Scholar]
- 28.Posnett J. Franks P.J. The burden of chronic wounds in the UK. Nurs Times. 2008;104:44. [PubMed] [Google Scholar]
- 29.Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine. 2012;41:384. doi: 10.1007/s12020-012-9619-x. [DOI] [PubMed] [Google Scholar]
- 30.Pinzur M.S. Slovenkai M.P. Trepman E. Shields N.N. Guidelines for diabetic foot care: recommendations endorsed by the Diabetes Committee of the American Orthopaedic Foot and Ankle Society. Foot Ankle Int. 2005;26:113. doi: 10.1177/107110070502600112. [DOI] [PubMed] [Google Scholar]
- 31.Guidance for Industry Chronic Cutaneous Ulcer and Burn Wounds—Developing Products for Treatment, U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Center for Devices and Radiological Health (CDRH) Wound Repair Regen. 2006;9:258. doi: 10.1046/j.1524-475x.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 32.Lindblad W.J. Animal models in wound healing research: do we need more? Wound Repair Regen. 2000;8:81. doi: 10.1046/j.1524-475x.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 33.Hanson D. Langemo D. Thompson P. Anderson J. Hunter S. Understanding wound fluid and the phases of healing. Adv Skin Wound Care. 2005;18:360. doi: 10.1097/00129334-200509000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Meyer W. Schwarz R. Neurand K. The skin of domestic mammals as a model for the human skin, with special reference to the domestic pig. Curr Probl Dermatol. 1978;7:39. doi: 10.1159/000401274. [DOI] [PubMed] [Google Scholar]
- 35.Tonnesen M.G. Feng X. Clark R.A. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 36.Shilo S. Roy S. Khanna S. Sen C.K. MicroRNA in cutaneous wound healing: a new paradigm. DNA Cell Biol. 2007;26:227. doi: 10.1089/dna.2006.0568. [DOI] [PubMed] [Google Scholar]
- 37.Appleton I. Wound healing: future directions. IDrugs. 2003;6:1067. [PubMed] [Google Scholar]
- 38.Li J. Chen J. Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol. 2007;25:9. doi: 10.1016/j.clindermatol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Khanna S. Biswas S. Shang Y. Collard E. Azad A. Kauh C. Bhasker V. Gordillo G.M. Sen C.K. Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyriakides T.R. Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90:986. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 41.Sonmez K. Bahar B. Karabulut R. Gulbahar O. Poyraz A. Turkyilmaz Z., et al. Effects of different suture materials on wound healing and infection in subcutaneous closure techniques. B-ENT. 2009;5:149. [PubMed] [Google Scholar]
- 42.Brodbeck W.G. Anderson J.M. Giant cell formation and function. Curr Opin Hematol. 2009;16:53. doi: 10.1097/MOH.0b013e32831ac52e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothamel D. Schwarz F. Sager M. Herten M. Sculean A. Becker J. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16:369. doi: 10.1111/j.1600-0501.2005.01108.x. [DOI] [PubMed] [Google Scholar]