Abstract
Tissue engineering scaffolds are commonly formed using proteins extracted from animal tissues, such as bovine hide. Risks associated with the use of these materials include hypersensitivity and pathogenic contamination. Human-derived proteins lower the risk of hypersensitivity, but possess the risk of disease transmission. Methods engineering recombinant human proteins using plant material provide an alternate source of these materials without the risk of disease transmission or concerns regarding variability. To investigate the utility of plant-derived human collagen (PDHC) in the development of engineered skin (ES), PDHC and bovine hide collagen were formed into tissue engineering scaffolds using electrospinning or freeze-drying. Both raw materials were easily formed into two common scaffold types, electrospun nonwoven scaffolds and lyophilized sponges, with similar architectures. The processing time, however, was significantly lower with PDHC. PDHC scaffolds supported primary human cell attachment and proliferation at an equivalent or higher level than the bovine material. Interleukin-1 beta production was significantly lower when activated THP-1 macrophages where exposed to PDHC electrospun scaffolds compared to bovine collagen. Both materials promoted proper maturation and differentiation of ES. These data suggest that PDHC may provide a novel source of raw material for tissue engineering with low risk of allergic response or disease transmission.
Introduction
Collagen, an important biological component of the extracellular matrix (ECM), provides structural support for many tissues. Collagen exhibits both flexibility and great tensile strength and is a major constituent of load-bearing tissues, such as ligaments, tendons, bone, and skin.1,2 Of the many varieties of collagen, type 1 is the most abundant,3 comprising over 90% of the fibrous protein found in the human body.4 In skin, collagen type I comprises 80%–85% of the dermal matrix and, as a result, is a common material choice for treatments to address the dermal volume depletion due to atrophy or migration,5–7 to prepare the wound bed for split-thickness autografting,8–11 or to serve as a dermal matrix analog for skin tissue engineering.12–17
For skin tissue engineering applications, the decellularized human, porcine, or bovine dermis is often used as a scaffolding material as it maintains the natural architecture of the dermis,18 promotes rete ridge formation,19 and contains basement membrane proteins to prevent epidermal blistering.18,20,21 Despite these benefits, the long processing time22,23 combined with the inability to tailor the structure of the scaffolds and the inherent variability between sheets, reduces their widespread use for engineered skin (ES) applications. More commonly, the collagen raw material, obtained from bovine24 and equine25 sources, is processed to form collagen scaffold materials, including electrospun mats,26,27 lyophilized sponges,28 and hydrogels.29 Collagen hydrogels have been successfully used to produce ES for the treatment of chronic wounds.30,31 Bovine type I collagen sponges and electrospun mats have been shown to promote in vitro full-thickness skin development15–17 and ES formed with bovine collagen sponges have been shown to reduce donor skin required to close burn wounds,32 inhibit wound contraction compared to a widely meshed autograft, grow with the patient, and facilitate vascularization.33
Although the ES fabricated using collagen obtained from bovine and human sources has proved useful for the treatment of acute and chronic wounds and as an in vitro diagnostic tool,34 there are risks associated with the use of these materials, including human allergic reactions and pathogenic contamination.35,36 With the growing use of bovine collagen implants, questions have been raised regarding their immunogenicity in humans. Approximately 3% of the population is allergic to bovine collagen with severe hypersensitivity reported in some cases.37,38 Although these bovine collagen preparations are of low immunogenicity, they are still foreign proteins. Their antigenicity is not low enough to escape an immunologic response in all patients. The major disadvantage of bovine collagen is a treatment-associated hypersensitivity reaction that consists of redness and swelling at the treatment site. These reactions occurred in 1.3%–3% of patients treated with Zyderm I or II (the Zyderm® collagen implant is a sterile device composed of a highly purified bovine dermal collagen that is dispersed in phosphate-buffered physiological saline containing 0.3% lidocaine). These allergic reactions are believed to be an immunological response to bovine collagen.39
Additionally, the Centers for Disease Control and Prevention and the FDA both concluded that a significant statistical association exists between bovine collagen used in dermal fillers and dermatomyositis.40 Autologous and cadaveric human collagens have a lower risk of hypersensitivity, but possess the risk of disease transmission. Methods engineering recombinant human proteins using bacteria41 or plant material36,42 provide an alternate source of these materials without the risk of disease transmission or concerns regarding variability. In prior studies, the plant-derived human collagen (PDHC) type I supported cell growth at a level equivalent to or superior than human tissue-derived collagen42 suggesting that plant-derived collagen is a promising raw material for the manufacturing of tissue engineering scaffolds with high levels of reproducibility and without the risk of disease transmission.
This study evaluates the use of PDHC type I as a platform to support in vitro skin cell growth and attachment and as a raw material for tissue engineering scaffold fabrication and ES development. We hypothesize that human-derived collagen will be comparable to bovine collagen in terms of scaffold fabrication and microstructure. It is anticipated that human collagen scaffolds will support cell adhesion and growth at a more rapid rate than bovine collagen and elicit a less robust immune response when human macrophages are exposed to the acellular scaffolding material. The ability of PDHC type I sponges to support keratinocyte, fibroblast, and endothelial cell proliferation was first compared with commercially available type I collagen matrices. Electrospun and lyophilized collagen scaffolds were then fabricated using the novel PDHC and a widely used bovine collagen source. Scaffold's physical and mechanical properties were evaluated in the acellular scaffolds. The cell viability and organization on each scaffold type were evaluated and the ability of the scaffolds to support skin maturation was assessed. Additionally, interleukin (IL)-1 beta production by activated human THP-1 macrophages was assessed after the macrophages were exposed to each scaffold type for 24 h.
Materials and Methods
PDHC formation
Human recombinant Type I collagen was prepared from transgenic tobacco plants.36 In brief, transgenic tobacco plants were successfully transformed with all five human genes optimized for expression in plants. These include two human genes encoding recombinant heterotrimeric collagen type I (rhCOL1) with the human prolyl-4-hydroxylase and lysyl hydroxylase 3 enzymes, responsible for key post-translational modifications of collagen. Plants coexpressing all five vacuole-targeted proteins generated intact procollagen yields of 2% of the extracted total soluble proteins. The procollagen was converted to allele-collagen by plant ficin and further fibrillated by several salting out steps and ultrafiltration to yield 3 mg/mL of pure rhCollagen in 10 mM HCl. Plant-extracted rhCOL1 formed thermally stable triple helical structures. Amino acid sequencing displayed 100% identity to the human collagen protein sequence. In addition, circular dichroism analysis of rhCOL1 yielded comparable spectra to that of human skin type I collagen (CalBiochem), confirming a similar structural conformation between rhCOL1 and human skin collagen.
Lyophilized collagen scaffolds
Lyophilized collagen scaffolds were prepared from PDHC (CollPlant) and comminuted bovine hide collagen (SEMED F; Kensey Nash). This specific bovine collagen source was utilized as it has been previously utilized for cultured skin substitutes (i.e., ES) both in laboratory experiments and for the treatment of full-thickness burns in human clinical trials.28,32,33 For PDHC sponges, collagen powder was first mixed with 10 mM HCl at a concentration of 3 mg/mL. The collagen solution was mixed with a fibrillogenesis buffer (200 mM Na2PO4, pH 11.2) at a ratio of 9:1 and incubated for 16 h at 27°C. Fibrils were collected by centrifugation (10 min, 3000g), the supernatant removed, and the pelleted fibrils lyophilized. A solution of the collagen fibrils in 0.5 M acetic acid (0.6 wt./vol.%) was created by mixing at 300 rpm for 30 min. The solution was then cast into a Teflon-lined 316L stainless steel casting frame and frozen at three different cooling rates: slow (placement in −20°C for 2 h), medium (placement in a −80°C freezer for 2 h), or rapid (placement in a −80°C ethanol bath for 2 h). Frozen castings were then lyophilized. For bovine collagen sponges, fibrous bovine collagen powder (SEMED F; 0.60 wt./vol.%) was first milled (IKA Works, Inc.) until all collagen particles passed easily through a 1-mm sieve. A presolution of collagen in 0.5 M acetic acid (0.6 wt./vol.%) was created and mixed at 300 rpm for 72 h at 4°C. The presolution was then homogenized by a high-speed overhead mixer (5200 rpm; IKA Works, Inc.) for 6 h at 4°C. The collagen solution was then degassed by centrifugation (1800 rpm, 10 min, 4°C), and then immediately cast under the same casting/freezing procedures as the PDHC. The lyophilized collagen scaffolds were physically crosslinked by vacuum dehydration at 140°C for 24 h, and then chemically crosslinked in a solution of 5 mM 1-ethyl-3-3-dimethylaminopropylcarbodiimide hydrochloride (EDC; Sigma) in 100% ethanol for 24 h to increase biostability via production of amide and ester crosslinks. This dual method crosslinking strategy has been previously shown to decrease the degradation rates and improve scaffold mechanics to a greater extent than each method alone.43
Electrospun collagen scaffolds
Electrospun collagen scaffolds were fabricated using 12, 16, and 20 wt./vol.% solution of acid-soluble bovine collagen (SEMED S) or nonfibrillated PDHC in hexafluoropropanol (HFP; Sigma). Bovine collagen was solubilized in HFP for 48 h before spinning and PDHC was solubilized for 20 min before spinning. Scaffolds were spun at rates between 4 and 8 mL/h a potential of 30 kV onto an 8.5-cm2 grounding plate that was positioned perpendicular to the tip of the needle. All electrospun collagen scaffolds were physically crosslinked by vacuum dehydration at 140°C for 24 h, and then chemically crosslinked in a solution of 25 mM EDC in 100% ethanol for 24 h.
Scanning electron microscopy
The morphology of the bovine and plant-derived human scaffolds was examined by scanning electron microscopy (Sirion; FEI). Punch biopsies from all scaffolds were collected from six distinct regions within a scaffold and mounted onto aluminum stubs with a carbon tape (Ted Pella), sputter coated with gold-palladium, and imaged in a secondary electron mode with a 5-kV accelerating voltage. A group of 24 images for each sample was collected and analyzed using ImageJ (NIH Freeware). For lyophilized collagen scaffolds, the pore diameter was calculated for 100 pores total for each type of collagen and each freezing rate. Fiber diameter measurements were collected from 100 fibers from each collagen type and solution concentration. The average pore size and fiber diameter±standard deviation were reported.
Mechanical testing
Mechanical properties of the acellular scaffolds were quantified using uniaxial tensile testing. Each acellular scaffold (n=6 per group) was crosslinked, hydrated using HEPES-buffered saline (HBS), and punched into dog-bone-shaped tensile specimens with a width of 4 mm and gauge length of 20 mm. The samples were secured in the grips of a tensile tester (TestResources 100R) and loaded until failure at a rate of 2 mm/s, to prevent scaffold dehydration. Ultimate tensile strength (UTS) and linear stiffness were calculated for all scaffolds and reported as average±standard deviation.
In vitro biocompatibility
To assess the in vitro biocompatibility of the plant-derived human collagen and its ability to support cell attachment and growth, commercial wound dressings made of different sources of collagen and from rhCollagen were cut into discs using a 6-mm punch biopsy tool under sterile conditions. Collagen disks samples included a wound dressing manufactured by CollPlant Ltd. (CP) containing 100% PDHC, fibrillated, crosslinked, and lyophilized and three commercial wound dressing controls made of bovine collagen (COM1, COM2, and COM3). Commercial product 1 (COM1) is made of 100% native, fibrillated, type I bovine collagen which has been crosslinked, and lyophilized, COM2 is composed of collagen (90%) and alginate (10%) and combines the structural support of collagen and the gel-forming properties of alginate is, COM3 consists of a is sterile, freeze-dried composite of 55% collagen and 45% oxidized regenerated cellulose, formed into a sheet ∼3 mm thick. The information presented on the commercial products is what is made public. Each disc was placed in a well of a 96-well plate (n=6 per group).
Primary human dermal fibroblasts (nHDF), primary human endothelial cells, and primary human epidermal keratinocytes (nHEK) (Promocell) were harvested, counted, and seeded at a density of 25×103–50×103 cells (25 μL), per well. Plates were incubated in a humidified incubator at 37°C, 5% CO2, for 2–4 h, to allow cell attachment to the discs, followed by the addition of 200 μL fresh cell media, specific to each cell type. Control samples included discs incubated with media in the absence of cells. Plates were incubated for an additional 72 h. After 3 days, the discs were gently removed with forceps, placed in clean wells, and supplemented with 100 μL of fresh media. Ten microliter WST-1 reagent (Roche) was added and cell proliferation measured as instructed. An average absorbance at 450 nm±standard deviation was reported. WST-1 is a cell proliferation assay based on a metabolic activity. As the same number of cells was seeded on top of the different scaffolds, quantification of viable cells after a few days indicates the differences between the proliferation of the cells on the different scaffolds.
Macrophage culture and IL-1 beta assay
To assess immunogenicity of these scaffolds, differentiated human THP-1 cells were seeded (1.0×106 cells/well) on bovine or human electrospun collagen matrices in six-well plates (n=6 per group) and differentiated using phorbol, 12-myristate, 13-acetate (20 ng/mL) for 48 h as described previously.44 The cells were cultured in RPMI1640 supplemented with 10% heat inactivated fetal bovine serum and 1% antibiotic–antimycotic under standard culture conditions (37°C, 5% CO2). After differentiation for 48 h, the bovine and plant-derived human scaffolds containing cells were moved to fresh six-well plates containing 1 mL media and incubated for another 24 h. Media were collected with dead cells removed via centrifugation. The supernatant was assayed for cytokine levels. Levels of IL-1 beta in culture media were measured using commercially available ELISA kits (R&D Systems) as per the manufacturer's recommendation.44
ES formation
ES was prepared from bovine and PDHC scaffolds (12% electrospun and medium freezing rate lyophilized). Collagen scaffolds were crosslinked as described above, disinfected in sterile 70% ethanol for 24 h, and then rinsed in HBS for 15 min×5 exchanges of solution and finally soaked in a medium for 1 h. HDF and HEK, isolated from surgical discard tissue with the Institutional Review Board Approval, were serially inoculated onto all scaffolds. HDF were inoculated onto electrospun or lyophilized scaffolds at a density of 5.0×105 cells/cm2 and incubated at 37°C and 5% CO2 in a custom-formulated skin growth medium45 for 3 days. Scaffolds were then inoculated with HEK at a density of 1.0×106 cells/cm2. One day following HEK inoculation (engineering skin day 1), the ES was placed onto a perforated stainless steel platform covered by a cotton pad to establish an air–liquid interface and incubated up to 21 days with media replaced daily.
Histology
Biopsies for histology from each sample were collected at days 7, 14, and 21, embedded in OCT™ (VWR) in a cross section and cryosectioned. Sections were stained with hematoxylin and eosin (H&E) and imaged with light microscopy. Bright field images were collected using Olympus DPController software (Olympus) with a total of six samples per condition per time point.
Cellular metabolism
On days 7, 14, and 21, 4-mm punch biopsies were collected from the ES fabricated with plant-derived human or bovine electrospun and lyophilized collagen scaffolds (two punches/graft, six grafts per time point). Each biopsy punch was placed into a separate well of a 24-well plate and a standard MTT assay was performed on the punch biopsies following a protocol previously described.46 The amount of the MTT-formazan product released was measured at 590 nm on a microplate reader (SpectraMax 190; Gemini) with values reported as mean optical density±standard deviation.
Surface electrical capacitance measurement
Surface electrical capacitance (SEC) directly measures skin surface hydration, which is related to barrier function.47 Low SEC readings corresponding to reduced levels of surface hydration and the formation of an epidermal barrier. SEC measurements were collected from ES fabricated with human or bovine electrospun and lyophilized collagen scaffolds using the NOVA dermal phase meter (DPM 9003; NOVA Technology). On culture days 7, 14, and 21, measurements were collected from three sites on each piece of ES (six grafts per scaffold type) and the SEC values are expressed in DPM units as mean±standard deviation.
Mechanical testing of ES
Mechanical properties of the ES were quantified following the same procedures used for the acellular scaffolds. ES was cultured for 14 days at the air–liquid interface before mechanical testing. Small dog bone punches (width 3 mm, gauge length 10 mm) were used to create tensile ES specimens, which were loaded in tension at a rate of 1 mm/s until failure. UTS and linear stiffness were calculated and reported as average±standard deviation.
Statistical analysis
For all quantitative assays, either repeated measures analysis of variance or Student's t-tests were performed. The data were presented as mean±standard error of the means with a p<0.05 considered statistically significant.
Results
Scaffold processing
As optimal processing protocols for fabricating scaffolds using the PDHC were being developed, it was observed that the plant-derived collagen was visually more soluble in acetic acid and HFP than collagen from bovine hide. For electrospinning solutions, plant-derived collagen was rapidly solubilized by HFP and formed a low viscosity, a clear solution suitable for spinning within 20 min (Table 1). In contrast, bovine collagen-HFP solutions were extremely thick and white in color for the first 2–4 h after mixing, and a decrease in viscosity throughout the mixing process until an optimal viscosity was reached after 48 h of spinning (Table 1). At this time, the electrospinning solution remained cloudy and white. The fibrillogenesis process for the plant-derived collagen resulted in a semi-solid white pellet of fibrils that were easily separated from the supernatant and lyophilized. The dry pellets were directly weighed and put into a solution with the acetic acid, no milling was required. After mixing for 20 min at 300 rpm, the solution was homogeneous and easily casted. Bovine collagen, in its as-received form, contained rough, thick fibers that require milling to ensure that all of the collagen particles were of the same size. If the collagen sponge-making process was performed without milling, large irregular masses of collagen within the sponge structure were present (data not shown). To ensure that the collagen-acetic acid slurry was as homogeneous as possible, several mixing steps were performed (Table 1). The freezing process for both types of collagen was identical.
Table 1.
Fabrication Steps and Duration for Plant-Derived Human and Bovine Collagen Scaffolds
| Scaffold type | Fabrication step (time) | Total time (h) | ||||
|---|---|---|---|---|---|---|
| Plant-derived human collagen sponge | Fibrillogenesis (16 h) | Fibril collection and lyophilization (24 h) | Collagen solution mixing (20 min) | Casting (2 h) | Lyophilization (24 h) | 66.3 |
| Bovine collagen sponge | Collagen milling (1 h) | Presolution mixing (72 h) | Homogenize and degass (7 h) | Casting (2 h) | Lyophilization (24 h) | 106 |
| Plant-derived human collagen electrospun scaffold | Solution mixing (20 min) | Electrospinning 40 min per 90×90×0.5 mm scaffold | 1 | |||
| Bovine collagen electrospun scaffold | Solution mixing (48 h) | Electrospinning 75 min per 90×90×0.5 mm scaffold | 49.25 | |||
Effect of source material on physical and mechanical properties of lyophilized collagen sponges
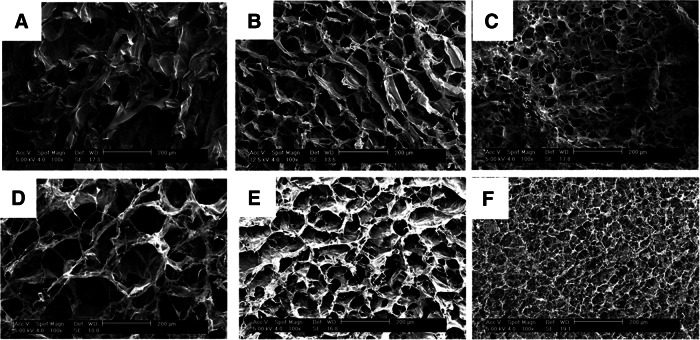
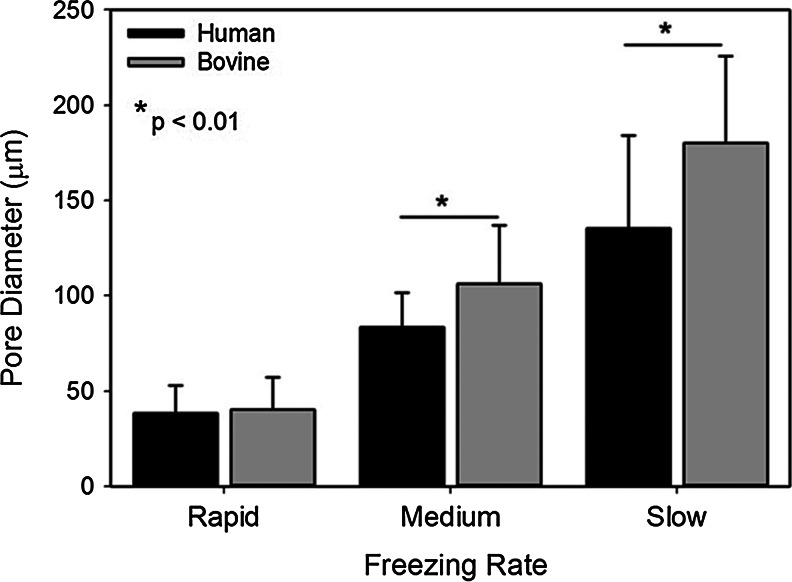
Collagen sponges fabricated using PDHC were characterized by an interconnected pore structure where pores were defined by thick struts of collagen (Fig. 1A–C). The pore size increased with a decreasing cooling rate with the rapid freezing rate producing pores ∼4-fold smaller than the slow freezing rate (Fig. 2). In bovine hide collagen sponges, a similar structure was observed with slightly thinner reticulations surrounding the pores. As the freezing rate increased, the bovine collagen sponge pore size also decreased with an average pore size 180.1±45.8 μm in a slow freezing rate sponge group (frozen at −20°C) and an average pore diameter of 40.4±17.0 μm in the rapid freezing rate sponge group (frozen in a −80°C ethanol bath) (Fig. 2). Plant-derived human and bovine collagen sponges were similar in structure; however, in the medium and slow sponge groups, the bovine collagen sponges possessed a larger average pore size than the equivalently processed human collagen sponges (Fig. 2).
FIG. 1.
Collagen sponges fabricated via lyophilization of a 0.6 wt./vol.% solution of plant-derived human (A–C) or bovine collagen (D–F) in 0.5 M acetic acid. Casting frames were frozen in −20°C air [slow; (A, D)], −80°C air [medium; (B, E), or −80°C ethanol [rapid; (C, F). Scale bar=200 μm.
FIG. 2.
Pore size in collagen sponges as a function of the freezing rate and collagen source. The average pore size in sponges fabricated using plant-derived human collagen are significantly smaller under the medium (−80°C air) and large (−80°C ethanol bath) freezing conditions.
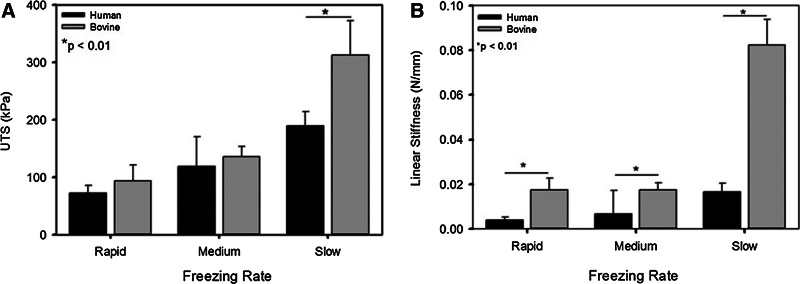
Tensile testing revealed the UTS to be significantly lower for the slow freezing rate PDHC sponges (189.1±25.5 kPa) than for the bovine collagen sponge (312.6±59.6 kPa) frozen using the slowest freezing rate. However, no statistical significance was observed between the rapid and medium freezing rate sponges of the two different collagen types (Fig. 3A). The linear stiffness of the PDHC sponges was statistically smaller than the bovine collagen sponges in all cases, with the most significant difference being between the human collagen slow frozen human collagen sponge (0.02±3.9E-03 N/mm) and the slow frozen bovine collagen sponge (0.08±0.01 N/mm) (Fig. 3B).
FIG. 3.
Mechanical properties of acellular collagen sponges fabricated using plant-derived human or bovine collagen. (A) Ultimate tensile strength (UTS) is significantly greater in large pore size bovine sponges with no statistical difference measured between small or medium bovine or human collagen sponges. (B) Bovine collagen sponges were observed to be stiffer in each freezing condition.
Effect of source material on physical and mechanical properties of electrospun collagen scaffolds
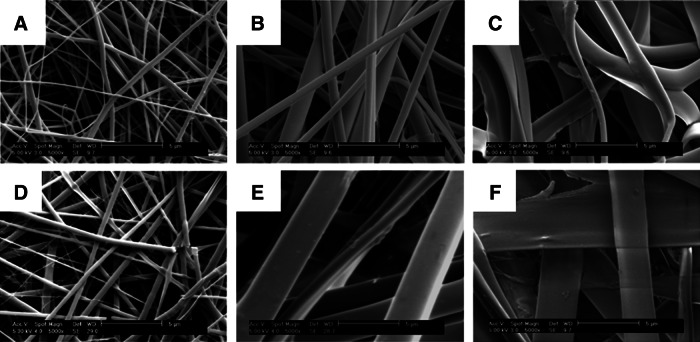
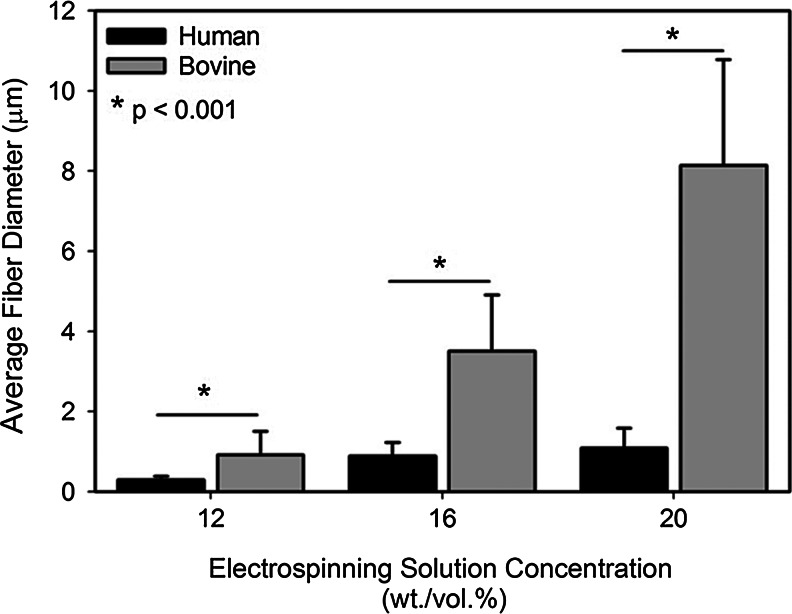
The fiber diameter in electrospun collagen scaffolds was controlled using the collagen-HFP solution concentration. Electrospun fibers formed using PDHC (Fig. 4A–C) were thinner, more uniform in diameter, and more round than bovine-derived collagen scaffolds (Fig. 4D–F). At high solution concentrations, bovine collagen fibers displayed a ribbon-like morphology, while the human collagen scaffold contained a collection of both rounded and ribbon fibers (Fig. 4C, F). With both collagen sources, the average collagen fiber diameter scaled positively with a collagen solution concentration; however, with bovine collagen solutions, the fiber diameter was significantly more affected by the solution concentration. The fiber diameter increased only 3.7-fold from a 12 to 20 wt./vol.% concentration in the plant-derived collagen fibers; whereas a ninefold increase was observed in the bovine collagen scaffolds (Fig. 5). For all solution concentrations, the fiber diameter of human collagen scaffolds was smaller than the bovine collagen scaffolds, with the scaffolds of a 20% collagen concentration having the largest disparity (Fig. 5). As scaffold morphology was dramatically different in solutions with concentrations higher than 12 wt./vol.%, the mechanical properties of only these scaffolds were evaluated.
FIG. 4.
Fibrous collagen scaffolds fabricated via electrospinning of plant-derived human (A–C) or bovine collagen (D–F). Electrospun scaffolds were spun using (A, D) 12, (B, E) 16, and (C, F) 20 wt./vol.% solutions of collagen in hexafluoroisopropanol. Fibrous human collagen scaffolds were characterized by thinner, more uniform rounded fibers. In contrast, bovine collagen fibers exhibited ribbon-like morphologies at concentrations greater than 12 wt./vol.%. Scale bar=5 μm.
FIG. 5.
The collagen fiber diameter as a function of an electrospinning solution concentration. An average fiber diameter scales positively with a solution concentration. The fiber diameter in bovine collagen scaffolds is significantly greater than plant-derived human collagen in all scaffolds.
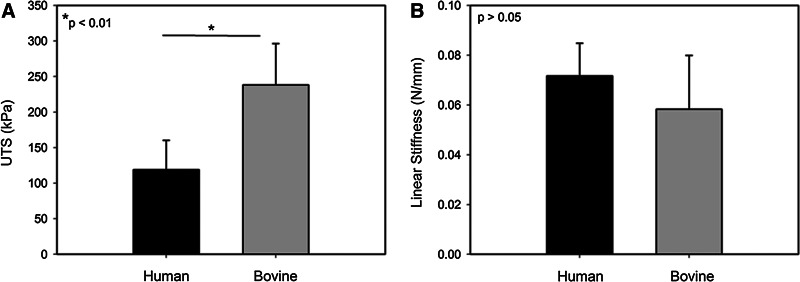
The electrospun collagen scaffolds strength was significantly reduced when formed with PDHC versus bovine collagen, 118.9±41.4 kPa and 238.2±58.2 kPa, respectively, (Fig. 6A). In contrast, no statistical difference in linear stiffness was found between human and bovine collagen scaffolds (Fig. 6B).
FIG. 6.
Mechanical properties of 12 wt./vol.% electrospun collagen scaffolds fabricated using bovine-derived or plant-derived human collagen. (A) UTS is significantly greater in bovine scaffolds; however, no statistical difference was found in linear stiffness (B).
Scaffold biocompatibility and immunogenicity
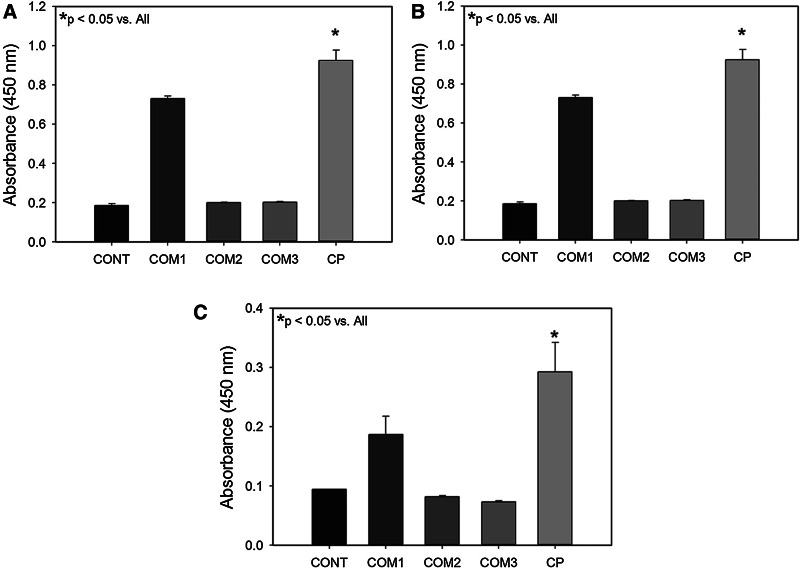
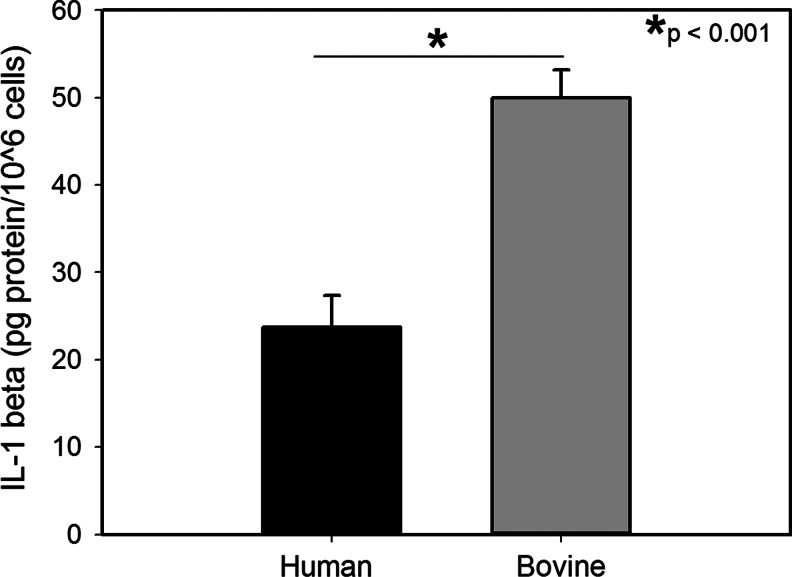
The ability of a scaffold to facilitate cell attachment and proliferation is critical for its use as an in vitro culture platform and as a surrogate ECM for tissue engineering. PDHC (CP) was found to support the endothelial, fibroblast, and keratinocyte cell attachment and proliferation (Fig. 7). Primary cell proliferation in all cell types tested was significantly better in scaffolds made of the PDHC compared to commercial scaffolds made of bovine collagen or bovine collagen with either alginate or oxidized cellulose (Fig. 7A–C). Activated THP-1 macrophages produced approximately twice the amount of IL-1 beta per million cells when exposed to electrospun bovine collagen scaffolds compared to PDHC scaffolds (p<0.001) (Fig. 8).
FIG. 7.
Cell proliferation on plant-derived human collagen wound dressings (CP) or commercially available wound dressings (COM1, COM2, and COM3). Control samples with no cells (CONT). Proliferation of (A) human dermal fibroblasts, (B) human dermal endothelial cells, and (C) human epidermal keratinocytes.
FIG. 8.
Interleukin (IL)-1 beta production (pg) per million activated THP-1 macrophages. Activated macrophages were incubated with bovine- or plant-derived human collagen electrospun scaffolds for 24 h before collection of the media and analysis via an enzyme-linked immunosorbent assay.
Influence of source material on ES development and viability
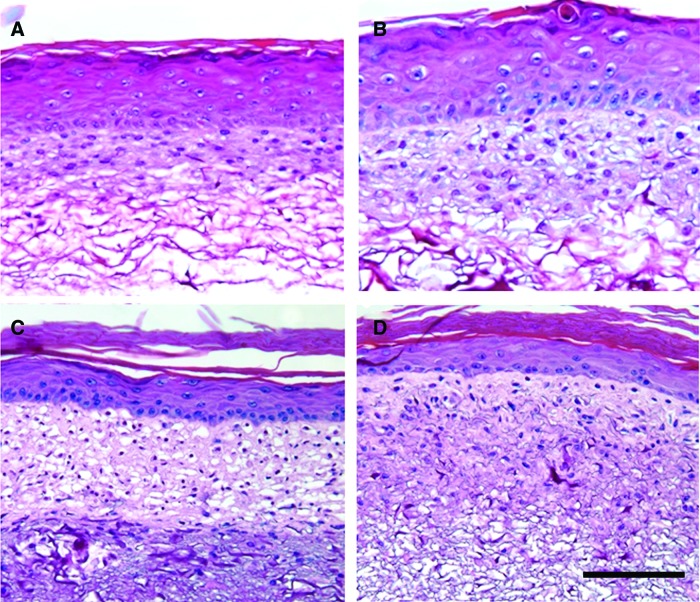
H&E-stained histological sections of ES fabricated using plant-derived human and bovine-derived collagen showed dense groups of fibroblasts as well as distinct, stratified epidermal layers in both groups. By day 14, the stratum corneum had formed as well as a continuous basal cell layer (Fig. 9). In electrospun human collagen scaffolds, the fibroblast infiltration into the scaffolds was more restricted than in the bovine collagen scaffolds with cells penetrating approximately half the distance into the scaffold (Fig. 9C, D). No marked differences in skin morphology were observed between ES fabricated with plant-derived human or bovine collagen (Fig. 9A, B).
FIG. 9.
Hematoxylin and eosin-stained histological sections of engineered skin at culture day 14 fabricated using plant-derived human (A, C) and bovine (B, D) collagen. (A, B) Sponge and (C, D) electrospun scaffold format. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
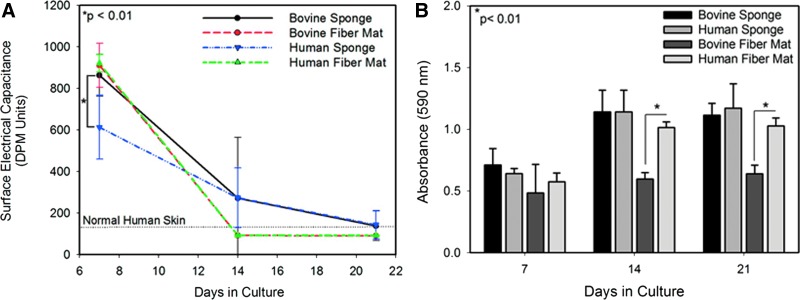
Epidermal differentiation, quantified using SEC, occurred in all groups of ES. At day 7, all ES was characterized by moist surfaces and high-capacitance readings. By day 14, epidermal differentiation continued resulting in the formation of keratinized layers at the surface of the grafts (Fig. 9) and reduced SEC readings (Fig. 10A). In both electrospun formats, the ES reached normal human values of capacitance. By day 21, all ES was at or below normal human values with no statistical difference between groups (Fig. 10A).
FIG. 10.
(A) Surface electrical capacitance of engineered skin made using bovine- and plant-derived human collagen. (B) Cell metabolism, measured by MTT, in engineered skin as a function of culture time and scaffold type. No statistical difference was observed between skin fabricated with human and bovine lyophilized collagen sponges at any time point. Cell metabolism was greater in engineered skin with an electrospun human scaffold compared to bovine electrospun scaffolds. Color images available online at www.liebertpub.com/tea
Cellular metabolism was assessed using an MTT assay in ES made from both collagen sponges and electrospun scaffolds. The assay revealed no statistical difference in cellular metabolism in ES fabricated using human or bovine sponges throughout the culture period (Fig. 10B). Cell metabolism in ES fabricated with electrospun collagen was sensitive to the collagen source. For both days 14 and 21, cellular metabolism was significantly enhanced on plant-derived human scaffolds compared to the bovine scaffolds (Fig. 10B).
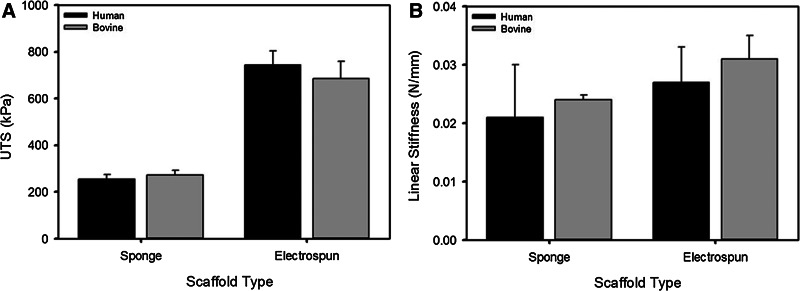
After 14 days in culture, the mechanical properties of the ES were evaluated. Despite significant differences in linear stiffness in the acellular collagen sponges, the linear stiffness of the ES fabricated with human and bovine sponges were not statistically different (Fig. 11B). UTS of the ES was equivalent between plant-derived human and bovine collagen scaffolds of the same type with electrospun groups displaying both a greater strength and stiffness compared to the sponge groups (Fig. 11).
FIG. 11.
(A) UTS and (B) linear stiffness of engineered skin at culture day 14 as a function of the scaffold type and source material.
Discussion
PDHC represents a novel source of collagen for biomedical applications that combines the benefits of the biocompatibility of human proteins without the risk of disease transmission. The structure of scaffolds formed with the plant-derived human and bovine collagen was similar in all cases with the general trend of thicker reticulations and larger fiber diameters for bovine materials (Figs. 1, 3, 4, and 7). In general, the PDHC is less viscous than bovine collagen due to the monomeric nature of recombinant human collagen (no beta and gamma structures). Additionally, the PDHC powder is ∼80% solid compared to >97% solid in the bovine collagen, thus equivalently fabricated human collagen solutions would have less total collagen concentration. The solution concentration and solution viscosity are both inversely related to the fiber diameter in electrospun solutions.46,47
For all sponge material, strength and stiffness scaled positively with pore size (Fig. 3). As large pore-sized materials contained both thicker collagen reticulations and a larger radius of curvature, it is expect that the mechanical properties would scale positively with the pore size. As the human collagen sponges were characterized by smaller pore sizes and thinner collagen reticulations, their mechanical properties would be anticipated to be lower. This connection between the pore size and mechanical properties is in agreement with prior studies of collagen and collagen-glycosaminoglycan sponges.28,48 In addition to the macrostructural control of the mechanical properties, less extensive fibrillogenesis in the human collagen sponges and higher hydrophilicity of the human collagen, which promotes the inclusion of water molecules in the scaffold is another possible contributor to reduced mechanics in these scaffolds. For electrospun collagen, no evidence of d-banding or other signs of an ultrastructure was found within the bovine or human fibers via transmission electron microscopy analysis (data not shown); thus, differences in the macrostructure are likely responsible for the variations observed in mechanical properties.
Primary human keratinocytes and fibroblasts were able to adhere to and proliferate on all the human and bovine scaffolds. All scaffolds promoted a stratified skin construct with an epidermal barrier beginning to form by day 14 (Figs. 9 and 0). No difference in cell metabolism was measured in skin fabricated with human or bovine collagen sponges; however, human electrospun scaffolds appeared to support enhanced cell viability compared to the bovine electrospun scaffold (Fig. 10). This could be the result of enhanced cell–cell communication in the human scaffold. A thick, predominantly cellular layer of fibroblasts is present on top of the fibroblast-infiltrated human collagen scaffold. It is possible that as fibroblasts were inoculated onto the human scaffold, the more compliant human scaffolds collapse slightly reducing the overall fibroblast infiltration and promoting a cell-dense fibroblast layer. This theory is support by the histological images of the electrospun PDHC skin, where there is an increase in the scaffold density observed directly under the fibroblast dense layer (Fig. 9C). Alternately, the human collagen scaffold might be more susceptible to degradation by MMPs and was remodeled more rapidly than the bovine collagen.
The thick layer of fibroblasts at the upper layers of the electrospun PDHC scaffold is also believed to be the cause of the decrease in SEC values observed with this scaffold at the 7-day time point (Fig. 10). A dense fibroblast layer has been previously shown to promote basal cell proliferation and barrier function with poor epidermal differentiation observed when the fibroblast layer is sparse.28 It is hypothesized that the dense fibroblast layer in the PDHC electrospun mat promoted more rapid initial epidermal differentiation. As all other scaffolds maintained fibroblast viability and epidermal differentiation, the SEC values were not statistically different at time points past 7 days (Fig. 10).
Mechanical properties of the ES in all cases were not statistically different (Fig. 11) despite the improved acellular scaffold mechanics observed in the bovine scaffolds (Figs. 3A, B, and 6A). The epidermis of ES has previously been shown to dominate the mechanical properties of the composite ES construct15; thus, the modest differences in scaffold mechanics are overshadowed by the vast increase in skin mechanics provided by a properly differentiated epidermal layer. In prior studies, it has been shown that the mechanical properties of ES are more significantly correlated with proper cell growth and epidermal differentiation and to a far lesser extent scaffold properties.15,28 As values for UTS of ES range (0.01 and 0.7 MPa49,50) are significantly less than native human skin (UTS=2.7–10 MPa51), alternate strategies are needed to increase tissue biomechanics. Currently, composite scaffolding and mechanical stimulation are being utilized to improve dermal strength through the use of higher strength synthetic polymers and via stimulation of ECM deposition, respectively.17,52
Conclusions
The current study showed that PDHC can easily be formed into two common scaffold types, electrospun nonwoven scaffolds and lyophilized sponges, for tissue engineering with similar architectures to standard bovine collagen scaffolds. The processing of this raw material into the scaffolds was significantly less labor intensive than bovine collagen likely due to the purity, increased water content, and monomeric nature of the human recombinant collagen. The in vitro data suggest that these new materials may provide biocompatible scaffolds for skin tissue engineering with less risk of an allergic response or disease transmission. Future studies are planned to investigate the function and immune response of engineered tissues grafted to full-thickness wounds.
Acknowledgments
This work was partially supported by the National Science Foundation under Grant No. EEC-0914790.
Disclosure Statement
The primary and corresponding author lists no conflict of interests. Authors S.S. and O.S., hold positions at Collplant Ltd., the manufacturer of the plant-derived collagen materials.
References
- 1.Di Lullo G.A. Sweeney S.M. Korkko J. Ala-Kokko L. San Antonio J.D. Mapping the ligand-binding sites and disease-associated mutation the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 2.Brincat M. Kabalan S. Studd J.W. Moniz C.F. de Trafford J. Montgomery J. A study of the decrease of skin collagen content, skin thickness, and bone mass in postmenopausal women. Obstet Gynecol. 1987;70:840. [PubMed] [Google Scholar]
- 3.van der Rest W.J. Dublet B. Champliaud M. Fibril associated collagens. Biomaterials. 1990;11:28. [PubMed] [Google Scholar]
- 4.Pachence J.M. Berg R.A. Silver F.H. Collagen: its place in the medical device industry. Med Device Diagn. 1987;9:49. [Google Scholar]
- 5.Cockerham K. Hsu V.J. Collagen-based dermal fillers: past, present, future. Facial Plast Surg. 2009;25:106. doi: 10.1055/s-0029-1220650. [DOI] [PubMed] [Google Scholar]
- 6.Jae-Ho S. Sangsoo P. Fillers for soft tissue augmentation: a materials perspective. J Tissue Eng Regen Med. 2011;8:A1. [Google Scholar]
- 7.Klein A.W. Skin filling: collagen and other injectables of the skin. Dermatol Clin. 2001;19:491. doi: 10.1016/s0733-8635(05)70290-4. [DOI] [PubMed] [Google Scholar]
- 8.de Carvalho V.F. Paggiaro A.O. Isaac C. Gringlas J. Ferreira M.C. Clinical trial comparing 3 different wound dressings for the management of partial-thickness skin graft donor sites. J Wound Ostomy Continence Nurs. 2011;38:643. doi: 10.1097/WON.0b013e3182349d2f. [DOI] [PubMed] [Google Scholar]
- 9.Horch R.E. Stark G.B. Comparison of the effect of a collagen dressing and a polyurethane dressing on the healing of split thickness skin graft (STSG) donor sites. Scan J Plast Reconstr Surg Hand Surg. 1998;32:407. doi: 10.1080/02844319850158499. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood J.E. Dearman B.L. Comparison of a sealed, polymer foam biodegradable temporizing matrix against integra(R) dermal regeneration template in a porcine wound model. J Burn Care Res. 2012;33:163. doi: 10.1097/BCR.0b013e318233fac1. [DOI] [PubMed] [Google Scholar]
- 11.Fitton A.R. Drew P. Dickson W.A. The use of a bilaminate artificial skin substitute (Integra (TM)) in acute resurfacing of burns: an early experience. Br J Plast Surg. 2001;54:208. doi: 10.1054/bjps.2000.3525. [DOI] [PubMed] [Google Scholar]
- 12.Kuroyanagi Y. Yamada N. Yamashita R. Uchinuma E. Tissue-engineered product: allogeneic cultured dermal substitute composed of spongy collagen with fibroblasts. Artif Organs. 2001;25:180. doi: 10.1046/j.1525-1594.2001.025003180.x. [DOI] [PubMed] [Google Scholar]
- 13.Rnjak-Kovacina J. Wise S.G. Li Z. Maitz P.K.M. Young C.J. Wang Y.W. Weiss A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials. 2011;32:6729. doi: 10.1016/j.biomaterials.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 14.MacNeil S. Biomaterials for tissue engineering of skin. Mater Today. 2008;11:26. [Google Scholar]
- 15.Ebersole G.C. Anderson P.M. Powell H.M. Epidermal differentiation governs engineered skin biomechanics. J Biomech. 2010;43:3183. doi: 10.1016/j.jbiomech.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Powell H.M. Boyce S.T. Wound closure with EDC cross-linked cultured skin substitutes grafted to athymic mice. Biomaterials. 2007;28:1084. doi: 10.1016/j.biomaterials.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Powell H.M. McFarland K.L. Butler D.L. Supp D.M. Boyce S.T. Uniaxial strain regulates morphogenesis, gene expression, and tissue strength in engineered skin. Tissue Eng Part A. 2010;16:1083. doi: 10.1089/ten.TEA.2009.0542. [DOI] [PubMed] [Google Scholar]
- 18.Krejci N.C. Cuono C.B. Langdon R.C. McGuire J. In vitro reconstitution of skin: fibroblasts facilitate keratinocyte growth and differentiation on acellular reticular dermis. J Invest Dermatol. 1991;97:843. doi: 10.1111/1523-1747.ep12491522. [DOI] [PubMed] [Google Scholar]
- 19.Medalie D.A. Eming S.A. Collins M.E. Tompkins R.G. Yarmush M.L. Morgan J.R. Differences in dermal analogs influence subsequent pigmentation, epidermal differentiation, basement membrane, and rete ridge formation of transplanted composite skin grafts. J Transplant. 1997;64:454. doi: 10.1097/00007890-199708150-00015. [DOI] [PubMed] [Google Scholar]
- 20.Grinnell F. Toda K. Lamke S.C. Reconstitution of human epidermis in vitro is accompanied by transient activation of basal keratinocyte spreading. Exp Cell Res. 1987;172:439. doi: 10.1016/0014-4827(87)90402-2. [DOI] [PubMed] [Google Scholar]
- 21.Guo M. Grinnell F. Basement membrane and human epidermal differentiation in vitro. J Invest Dermatol. 1989;93:372. [PubMed] [Google Scholar]
- 22.Medalie D.A. Eming S.A. Tompkins R.G. Yarmush M.L. Krueger G.G. Morgan J.R. Evaluation of human skin reconstituted from composite grafts of cultured keratinocytes and human acellular dermis transplanted to athymic mice. J Invest Dermatol. 1996;107:121. doi: 10.1111/1523-1747.ep12298363. [DOI] [PubMed] [Google Scholar]
- 23.Bannasch H. Stark G.B. Knam F. Horch R.E. Fohn M. Decellularized dermis in combination with cultivated keratinocytes in a short- and long-term animal experimental investigation. J Eur Acad Dermatol Venereol. 2008;22:41. doi: 10.1111/j.1468-3083.2007.02326.x. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira S. Yang L. Dijkstra P.J. Ferraz M.P. Monteiro F.J. Heparinized hydroxyapatite/collagen three-dimensional scaffolds for tissue engineering. J Mater Sci Mater Med. 2010;21:2385. doi: 10.1007/s10856-010-4097-2. [DOI] [PubMed] [Google Scholar]
- 25.Malicev E. Radosavljevic D. Velikonja N.K. Fibrin gel improved the spatial uniformity and phenotype of human chondrocytes seeded on collagen scaffolds. Biotechnol Bioeng. 2007;96:364. doi: 10.1002/bit.21038. [DOI] [PubMed] [Google Scholar]
- 26.Powell H.M. Supp D.M. Boyce S.T. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials. 2008;29:834. doi: 10.1016/j.biomaterials.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Martins A. Araujo J.V. Reis R.L. Neves N.M. Electrospun nanostructured scaffolds for tissue engineering applications. Nanomedicine. 2007;2:929. doi: 10.2217/17435889.2.6.929. [DOI] [PubMed] [Google Scholar]
- 28.Powell H.M. Boyce S.T. EDC crosslinking improves cultured skin substitute strength and stability. Biomaterials. 2006;27:5821. doi: 10.1016/j.biomaterials.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L. Sun J. Chen X. Wang G. Jiang B. Fan H. Zhang X. In vivo cartilage engineering with collagen hydrogel and allogenous chondrocytes after diffusion chamber implantation in immunocompetent host. Tissue Eng Part A. 2009;15:2145. doi: 10.1089/ten.tea.2008.0268. [DOI] [PubMed] [Google Scholar]
- 30.Falanga V. Apligraf treatment of venous ulcers and other chronic wounds. J Dermatol. 1998;25:812. doi: 10.1111/j.1346-8138.1998.tb02510.x. [DOI] [PubMed] [Google Scholar]
- 31.Streit M. Braathen L.R. Apligraf - a living human skin equivalent for the treatment of chronic wounds. Int J Artif Organs. 2000;23:831. [PubMed] [Google Scholar]
- 32.Boyce S.T. Kagan R.J. Yakuboff K.P. Meyer N.A. Rieman M.T. Greenhalgh D.G. Warden G.D. Cultured skin substitutes reduce donor site harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235:269. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyce S.T. Kagan R.J. Meyer N.A. Yakuboff K.P. Warden G.D. Cultured skin substitutes combined with Integra to replace native skin autograft and allograft for closure of full-thickness burns. J Burn Care Rehabil. 1999;20:453. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 34.Medina J. de Fraissinette A.D. Chibout S.D. Kolopp M. Kammermann R. Burtin P. Ebelin M.E. Cordier A. Use of human skin equivalent Apligraf for in vitro assessment of cumulative skin irritation potential of topical products. Toxicol Appl Pharmacol. 2000;164:38. doi: 10.1006/taap.1999.8875. [DOI] [PubMed] [Google Scholar]
- 35.Hori H. Hattori S. Inouye S. Kimura A. Irie S. Miyazawa H. Sakaguchi M. Analysis of the major epitope of the α2chain of bovine type I collagen in children with bovine gelatin allergy. J Allergy Clin Immunol. 2002;110:652. doi: 10.1067/mai.2002.127862. [DOI] [PubMed] [Google Scholar]
- 36.Stein H. Wilensky M. Tsafrir Y. Rosenthal M. Amir R. Avraham T. Ofir K. Dgany O. Yayon A. Shoseyov O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-I collagen in transgenic tobacco. Biomacromolecules. 2009;10:2640. doi: 10.1021/bm900571b. [DOI] [PubMed] [Google Scholar]
- 37.Siegle R.J. McCoy J.P., Jr. Schade W. Swanson N.A. Intradermal implantation of collagen. Humoral immune responses associated with clinical reactions. Arch Dermatol. 1984;120:183. [PubMed] [Google Scholar]
- 38.Castrow F.F., II Krull E.A. Injectable collagen implant–update. J Am Acad Dermatol. 1983;9:889. doi: 10.1016/s0190-9622(83)70204-5. [DOI] [PubMed] [Google Scholar]
- 39.Liu B. Xu Z. Ruirao Y. Wang J. Wang Z. Harrell R. The use of type I and type III injectable human collagen for dermal fill: 10 years of clinical experience in China. Semin Plast Surg. 2005;19:241. [Google Scholar]
- 40.Cukier J. Beauchamp R.A. Spindler J.S. Spindler S. Lorenzo C. Trentham D.E. Association between bovine collagen dermal implants and a dermatomyositis or a polymyositis-like syndrome. Ann Intern Med. 1993;118:920. doi: 10.7326/0003-4819-118-12-199306150-00002. [DOI] [PubMed] [Google Scholar]
- 41.Liu W. Merrett K. Griffith M. Fagerholm P. Dravida S. Heyne B. Scaiano J.C. Watsky M.A. Shinozaki N. Lagali N. Munger R. Li F. Recombinant human collagen for tissue engineered corneal substitutes. Biomaterials. 2008;29:1147. doi: 10.1016/j.biomaterials.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Shilo S. Dgany O. Rosental M. Amir R. Tal T. Yaari A. Avrahan T. Stein H. Ofir K. Kredy-Farhan L. Amitai H. Lapidot N. Shoseyov O. Novel recombinant human collagen for wound healing. Wound Repair Regen. 2009;17:A15. [Google Scholar]
- 43.Drexler J.W. Powell H.M. DHT crosslinking of electrospun collagen. Tissue Eng Part C. 2011;17:9. doi: 10.1089/ten.TEC.2009.0754. [DOI] [PubMed] [Google Scholar]
- 44.Ganesh K. Das A. Dickerson R. Khanna S. Parinandi N.L. Gordillo G.M. Sen C.K. Roy S. Prostaglandin e2 induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J Immunol. 2012;189:2563. doi: 10.4049/jimmunol.1102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swope V.B. Supp A.P. Schwemberger S. Babcock G. Boyce S. Increased expression of integrins and decreased apoptosis correlate with increased melanocyte retention in cultured skin substitutes. Pigment Cell Res. 2006;19:424. doi: 10.1111/j.1600-0749.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 46.Powell H.M. Boyce S.T. Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal–epidermal skin substitutes. J Biomed Mater Res A. 2008;84:1078. doi: 10.1002/jbm.a.31498. [DOI] [PubMed] [Google Scholar]
- 47.Sisson K. Zhang C. Farach-Carson M.C. Chase D.B. Rabolt J.F. Fiber diameters control osteoblastic cell migration and differentiation in electrospun gelatin. J Biomed Mater Res. 2010;94A:1312. doi: 10.1002/jbm.a.32756. [DOI] [PubMed] [Google Scholar]
- 48.Berthod F. Saintigny G. Chretien F. Hayek D. Collombel C. D'Amour O. Optimization of thickness, pore-size, and mechanical properties of a biomaterial designed for deep burn coverage. Clin Mater. 1994;15:259. doi: 10.1016/0267-6605(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 49.Powell H.M. Boyce S.T. Engineered human skin fabricated using electrospun collagen-PCL blends: morphogenesis and mechanical properties. Tissue Eng. 2009;15:2177. doi: 10.1089/ten.tea.2008.0473. [DOI] [PubMed] [Google Scholar]
- 50.LaFrance H. Yahia L. Germain L. Guillot M. Auger F.A. Study of the tensile properties of living skin equivalents. Biomed Mater Eng. 1995;5:195. [PubMed] [Google Scholar]
- 51.Silver F.H. Freeman J.W. DeVore D. Viscoelastic properties of human skin and processed dermis. Skin Res Tech. 2001;7:18. doi: 10.1034/j.1600-0846.2001.007001018.x. [DOI] [PubMed] [Google Scholar]
- 52.Drexler J.W. Powell H.M. Regulation of electrospun scaffold stiffness via coaxial core diameter. Acta Biomater. 2011;7:1133. doi: 10.1016/j.actbio.2010.10.025. [DOI] [PubMed] [Google Scholar]