Abstract
Background
The calcimimetic cinacalcet lowers parathyroid hormone (PTH), calcium (Ca) and phosphorus (P) in dialysis patients with secondary hyperparathyroidism (SHPT). We explored serum P changes in dialysis patients treated with cinacalcet, while controlling for vitamin D sterol and phosphate binder (PB) changes, based on data from the pan-European observational study ECHO.
Methods
Patients were categorized by serum P change (decreased/unchanged/increased) at 12 months after starting cinacalcet and subcategorized by vitamin D sterol and PB dose changes (decreased/unchanged/increased). The impact of PTH, Ca and P, and vitamin D sterol, PB and cinacalcet doses (absolute values and/or change) was evaluated. Predictors of P change were explored using univariate and multivariate general linear models (GLM) and logistic regression analysis.
Results
At Month 12, 661 (41%) of 1607 patients had decreased, 61 (4%) unchanged and 400 (25%) increased serum P, while 485 patients had missing data. In 45% of the patients with serum P reduction, vitamin D was either increased or unchanged and P binders decreased or unchanged. PTH was a key predictor of serum P reduction, with an estimated 3% decrease in P per 10% reduction in PTH. Changes in vitamin D sterol and PB doses were not generally significant factors in GLM and regression analyses.
Conclusions
The serum P reduction observed in a significant proportion of dialysis patients after adding cinacalcet to an existing therapeutic regimen for SHPT appears to result mainly from PTH reduction, rather than from changes in vitamin D sterol or PB doses. Financial support for the ECHO study was provided by Amgen.
Keywords: chronic kidney disease, cinacalcet, secondary hyperparathyroidism, serum phosphorus
Introduction
Chronic kidney disease (CKD) is accompanied by impaired metabolism of calcium (Ca), phosphorus (P) and vitamin D, leading to secondary hyperparathyroidism (SHPT). CKD-mineral and bone disorder (CKD-MBD) [1] is characterized by parathyroid gland hyperplasia, progressive elevation of serum parathyroid hormone (PTH) levels and impaired P and Ca homeostasis, together with abnormal bone metabolism and vascular and soft tissue calcification [2]. These abnormalities are associated with an increased risk of bone fractures, cardiovascular morbidity and mortality and parathyroidectomy [1–7].
The pathological contribution of hyperphosphataemia to vascular calcification and cardiovascular morbidity and mortality in dialysis patients is increasingly being recognized [8–12]. Indeed, elevated serum P is associated with a higher observed hazard ratio (HR) for death in epidemiological studies than are elevated Ca and PTH [12–14]. Recent guidelines from the Kidney Disease: Improving Global Outcomes (KDIGO) group have highlighted the importance of tight control of serum P, suggesting that levels should be maintained as close to normal as possible and should also guide the choice of pharmacological SHPT treatment [15].
Ca supplements, calcitriol or other active vitamin D analogues, and phosphate binders (PB), together with dietary P restriction, have been the cornerstone of management of SHPT in dialysis patients. However, it is difficult to control PTH and P simultaneously with these measures alone, and the new KDIGO guidelines present a further challenge. Dietary P sources include P-rich inorganic additives in processed foods and beverages, as well as natural protein-rich foods [16]. A source of P that may be overlooked is bone, especially in the setting of elevated PTH levels [17–19]. This bone-derived phosphorus is not removed by PB, which bind phosphate only within the gastrointestinal tract. Moreover, Ca-based binders and high doses of vitamin D sterols tend to promote hypercalcaemia and hyperphosphataemia, which can exacerbate cardiovascular and soft-tissue calcification [20] and necessitate treatment interruption, risking further progression of SHPT. Parathyroidectomy may ultimately need to be considered if medical measures are unsuccessful.
The calcimimetic cinacalcet (Mimpara/Sensipar®, Amgen Inc., Thousand Oaks, CA, USA) offers an alternative mechanism of PTH-lowering in SHPT. It enhances the sensitivity of the parathyroid calcium-sensing receptors to extracellular Ca [21] and substantially lowers serum PTH, P and Ca in dialysis patients, as shown in clinical studies in which the majority of patients had also received treatment with vitamin D sterols or PB [22, 23].
The present analysis evaluates the serum P-lowering potential of cinacalcet, while controlling for PB and vitamin D sterol changes, using data from the pan-European ECHO study (Evaluation of the Clinical Use of Mimpara in Haemodialysis and Peritoneal Dialysis Patients, an Observational Study). Results are published elsewhere [24].
Methods
ECHO methodology
ECHO was a multicentre, part-retrospective and part-prospective observational study in 1865 dialysis patients who were receiving cinacalcet (as part of an overall treatment regimen for SHPT) at the initiative of their physicians. The methodology has been described previously in detail [24]. Patients were enrolled between July 2005 and October 2006 at a total of 187 sites in 12 Western European countries. Patients provided informed consent where required by local regulations. No treatment algorithm was provided for study purposes, nor were any additional clinic visits or laboratory/diagnostic tests conducted. Medical history, comorbidities, concurrent medication and laboratory data were obtained from patients' records and recorded in case report forms.
Data were collected retrospectively 6 months before the start of cinacalcet, at initiation of cinacalcet (baseline) and retrospectively or prospectively (depending on the time of enrolment) for the next 12 months. Patients could remain in the study if cinacalcet was discontinued. Time windows were prespecified for analysis of serum PTH, P and Ca. These were ±6 weeks for PTH (−3 months at baseline) and ±2 weeks for P and Ca. PTH levels measured by a biointact assay were arbitrarily converted to iPTH values by multiplying them by 1.95 [25]. Utilization of cinacalcet, vitamin D sterols and PB was also analysed. Dosages of vitamin D sterols were converted to oral calcitriol equivalent doses, by dividing dosage by the following: eight for IV paricalcitol, four for oral paricalcitol, four for IV alfacalcidol and two for oral alfacalcidol or IV calcitriol [26].
Analysis of P-lowering effect
For the present analysis, ECHO patients were categorized according to whether their serum P was decreased, unchanged (within 0.1 mg/dL) or increased at 12 months, compared with baseline values. In order to examine the effect of vitamin D sterols and PB, these data were subcategorized according to whether the dose of each agent was decreased, unchanged or increased at 12 months compared with baseline. The algorithm used to categorize PB changes is shown in Table 1. Additionally, we evaluated PTH, Ca and P, vitamin D sterols and cinacalcet doses by serum P change category. Regression analysis was used to assess the relationship between the change in P and PTH from baseline to Month 12, in all patients and separately in the different PB categories (decrease/no change versus increase). The interaction between the PB dose change categories was also assessed.
Table 1.
Algorithm for the classification of PB dose change in patients with decreased serum phosphorus at Month 12 and unchanged, newly started or increased vitamin D sterols
| Criterion 1 | Criterion 2 | Criterion 3 | Classification |
|---|---|---|---|
| Same set of PB at baseline and Month 12 | No dose change for all PB | No change | |
| Increase or no dose change for all PB doses | At least one dose increase | Increase | |
| Decrease or no dose change for all PB doses | At least one dose decrease | Decrease | |
| Different set of PB at baseline and Month 12, or unknown types of PB at both time points | Number of PB with a dose increasea > number of PB with a dose decreaseb | Increase in overall dosec | Increase |
| Decrease in overall dose | No change | ||
| Overall dose not changed | Increase | ||
| Number of PB with a dose increase < number of PB with a dose decrease | Increase in overall dose | No change | |
| Decrease in overall dose | Decrease | ||
| Overall dose not changed | Decrease | ||
| Number of PB with a dose increase = number of PB with a dose decrease | Increase in overall dose | Increase | |
| Decrease in overall dose | Decrease | ||
| Overall dose not changed | No change |
aIncludes starting a new PB.
bIncludes stopping an existing PB.
cOverall dose is the sum of all PB doses, derived at baseline and Month 12.
Predictors of P change were explored using univariate and multivariate logistic regression analysis and general linear models (GLM). The covariates examined were as follows: treatment centre characteristics, baseline patient characteristics [age, gender, ethnic group, previous renal transplant (yes/no), parathyroidectomy (yes/no), dialysis modality, dialysis vintage and time on haemodialysis per week] as well as serum P, PTH, Ca and albumin at baseline and changes in PTH and Ca and cinacalcet, PB and vitamin D sterol doses between baseline and 12 months. Patients with missing covariate data were excluded from the models.
The GLM response variable was the percentage change in serum P from baseline to month 12 (log-transformed, due to skewedness of the data). The logistic regression analyses modelled the probability of a P decrease versus an increase or no change. Multivariate models were constructed via a stepwise iterative procedure, according to the results of univariate modelling. Sensitivity analyses were conducted to evaluate the impact of missing covariate data. The GLM and logistic regression analyses were repeated with continuous data split into quartiles and missing data included as a subgroup for each covariate.
A two-sided P-value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 8.2.
Results
Demographic and baseline characteristics
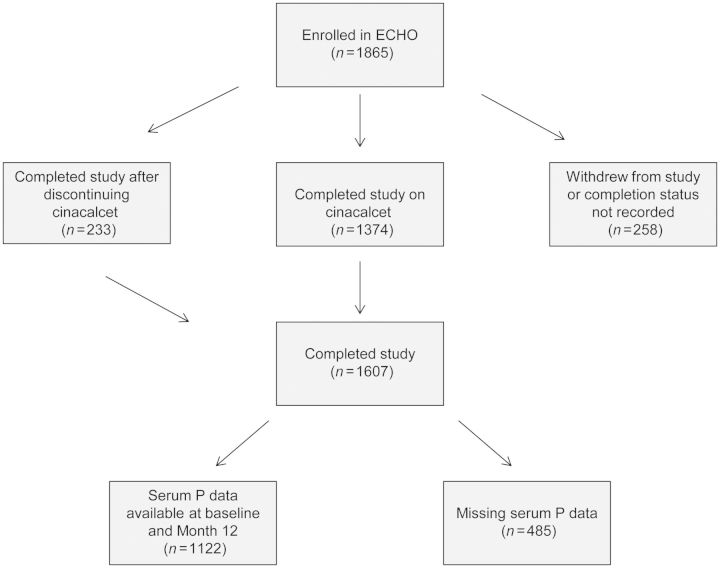
Baseline and demographic data for all 1865 patients enrolled in ECHO are shown in Supplementary data, Table S1. The mean (SD) age was 58.1 (15.0) years; median (Q1, Q3) PTH 721.0 (506.5, 1050.0) pg/mL and median P 5.9 (1.6) mg/dL. Four hundred and fifty-three patients (24%) discontinued cinacalcet: the main reasons were renal transplantation (5%), PTH oversuppression (4%), nausea and vomiting (3%), non-compliance (1%) and parathyroidectomy (1%). A total of 1607 patients (86%) completed 12 months of follow-up after starting cinacalcet (1374 who were still taking cinacalcet), 254 (14%) withdrew and four had no completion status recorded. Baseline and Month 12 serum P data were available for 1122 patients who completed the study (Figure 1).
Fig. 1.
Patient disposition.
PTH and phosphorus
Six hundred and sixty-one (35% overall; 41% of those who completed the study) patients were categorized as having decreased serum P at Month 12, 61 (3%; 4%) had unchanged and 400 (21%; 25%) had increased serum P. Month 12 serum P data were missing in a total of 743 patients (258 patients who did not complete the study or had no completion status recorded and 485 who completed the study but did not have serum P recorded within the prespecified window at Month 12). These patients did not differ in demographics and baseline characteristics from those with decreased, unchanged or increased serum P or the overall ECHO population (Supplementary data, Table S1).
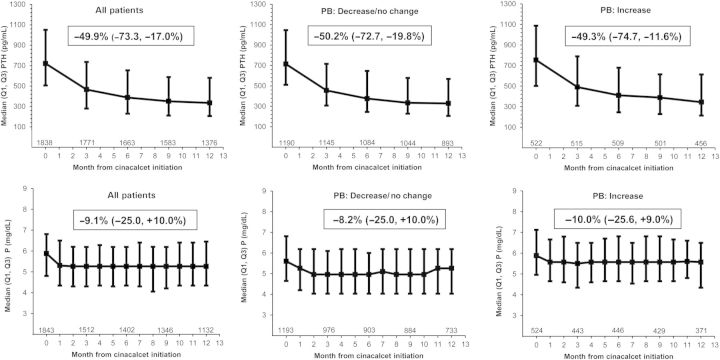
The evolution of median values for serum PTH and P over the course of the study (available data for all enrolled patients) is shown in Figure 2. The individual median P decreased by 9.1% (Q1, Q3 change −25.0, +10.0%) between baseline and Month 12, while PTH decreased by 50% (−73.3, −17.0%). (Patients with baseline and Month 12 data only.)
Fig. 2.
Evolution of serum PTH and phosphorus, overall and by PB change category. For each timepoint, values are shown for all patients with available data. The box within each graph shows the median (Q1, Q3) individual change from baseline (cinacalcet start) to Month 12 (patients with baseline and Month 12 values only, thus patient numbers differ slightly from the graphs).
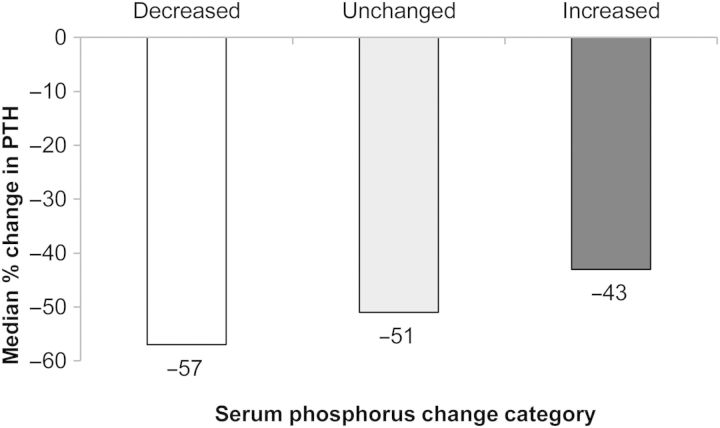
Although a decrease in median PTH was seen in all P change categories, patients with decreased serum P also showed the largest decrease in PTH (Figure 3). Regression analysis confirmed a statistically significant relationship between change in serum PTH and change in serum P, with an estimated overall 3% decrease in serum P for every 10% reduction in PTH.
Fig. 3.
Median percentage change in PTH from baseline to Month 12 by serum phosphorus change category.
Impact of vitamin D sterol, PB and cinacalcet dose changes
In all categories of PB dose change, a reduction in serum P was observed: the median change was −8.2% (−25.0, 10.0%), in patients with a decrease or no change in PB, and −10.0% (−25.6, 9.0%) in patients with a PB dose increase (Figure 2). For the former group, there was an estimated 2% decrease in serum P for every 10% reduction in PTH, while the latter showed an estimated 5% decrease in serum P for every 10% reduction in PTH (P = 0.027 for between-group comparison) (data not shown).
Table 2 shows vitamin D sterol and PB changes (combined) as categorical parameters in patients with a change in serum P. These data show that for 173 (26%) of 661 patients with decreased serum P, the decrease could potentially be attributed to concomitant changes in vitamin D sterols and PB (i.e. decreased vitamin D sterol dose and increased/unchanged PB, or unchanged vitamin D sterol and increased PB dose). However, for 295 (45%) of these 661 patients with P reduction (those with increased/unchanged vitamin D sterol and decreased/or unchanged PB), changes in vitamin D sterols and PB do not explain the serum P decrease. In 122 (31%) of the 400 patients with increased serum P, the increase could potentially be explained by changes in vitamin D sterols and PB (i.e. vitamin D sterol dose was increased and PB dose decreased/unchanged, or vitamin D sterols unchanged and PB dose decreased).
Table 2.
Vitamin D sterol and PB dose changes (categorical) in patients with a change in serum phosphorus (P) at 12 months.a
| Dose change category |
Serum P change category |
||
|---|---|---|---|
| Vitamin D sterolb | PB | Decrease (n = 661) n (%) | Increase (n = 400) n (%) |
| Decreased | Decreased | 69 (10.4) | 32 (8.0) |
| Unchanged | 37 (5.6) | 32 (8.0) | |
| Increased | 49 (7.4) | 33 (8.3) | |
| Unknown | 2 (0.3) | 1 (0.3) | |
| Unchanged | Decreased | 89 (13.5) | 54 (13.5) |
| Unchanged | 95 (14.4) | 59 (14.8) | |
| Increased | 87 (13.2) | 39 (9.8) | |
| Unknown | 9 (1.4) | 7 (1.8) | |
| Increased | Decreased | 61 (9.2) | 38 (9.5) |
| Unchanged | 50 (7.6) | 30 (7.5) | |
| Increased | 73 (11.0) | 44 (11.0) | |
| Unknown | 2 (0.3) | 3 (0.8) | |
| Unknown | Decreased | 11 (1.7) | 8 (2.0) |
| Unchanged | 9 (1.4) | 7 (1.8) | |
| Increased | 18 (2.7) | 9 (2.3) | |
| Unknown | 0 (0.0) | 4 (1.0) | |
aItalic text indicates combinations of changes that would tend to promote serum P reduction; bold indicates changes that would tend to promote P increase.
bVitamin D dose is measured in Oral Calcitriol Equivalents.
Analysis of vitamin D sterol and cinacalcet dose changes relative to serum P change categories did not show a consistent pattern and there was no consistent relationship between cinacalcet dose and median serum P levels (not shown). Of the 453 patients who discontinued cinacalcet, 74 had decreased, 44 increased and 6 unchanged serum P, while 329 had missing serum P data.
GLM and logistic regression analysis
GLM analysis identified eight significant predictors of serum P decrease: dialysis vintage, hours of haemodialysis per week at cinacalcet start, history of parathyroidectomy, baseline PTH, absolute and percentage change in PTH from baseline to Month 12, change in cinacalcet dose (categorical: decrease versus no change/increase) and absolute change in vitamin D dose. Logistic regression analysis showed three significant predictors: dialysis vintage, and absolute and percentage change in PTH from baseline to Month 12 (Table 3 and Supplementary Table S2).
Table 3.
Significant predictors of serum phosphorus (P) change by univariate general linear modelling (GLM; log-transformed) and logistic regression analysis
| Parametera | No. of patients | GLM |
Logistic regression |
||
|---|---|---|---|---|---|
| Estimated serum P change, % (95% CI) | P-valueb | Odds ratio (95% CI) | P-valuec | ||
| Baseline characteristics | |||||
| Dialysis vintage (months) | 1119 | −0.03 (−0.06, −0.01)d | 0.004 | 1.002 (1.000, 1.003)e | 0.047 |
| Hours of HD/wk at cinacalcet start | 960 | −0.87 (−1.49, −0.25)d | 0.006 | 1.029 (0.982, 1.079)e | 0.212 |
| History of parathyroidectomy: yes versus no | 1113 | −6.19 (−11.98, −0.02)f | 0.050 | 1.256 (0.797, 1.981)g | 0.323 |
| PTH | |||||
| At cinacalcet start (per 100 pg/mL) | 1114 | −0.42 (−0.75, −0.09)d | 0.012 | 1.016 (0.992, 1.040)e | 0.187 |
| Absolute change baseline to Month 12 (per 10 pg/mL) | 1004 | 1.02 (0.69, 1.35)d | <0.001 | 0.950 (0.925, 0.975)e | <0.001 |
| % change baseline to Month 12 (per 10%) | 1004 | 0.91 (0.60, 1.22)d | <0.001 | 0.938 (0.912, 0.964)e | <0.001 |
| Change in cinacalcet dose | |||||
| Decrease versus no change/increase | 1095 | −4.78 (−9.25, −0.09)f | 0.046 | 1.072 (0.764, 1.506)g | 0.687 |
| Change in vitamin d sterol dose | |||||
| Absolute change (per 10 µg/week) | 1053 | 14.27 (1.91, 28.13)d | 0.023 | 0.787 (0.354, 1.751)e | 0.558 |
aGLM models log (Month 12 P/baseline P) with the specified parameter and baseline P as covariates, while logistic regression models the probability of a P decrease versus no change/increase with the specified parameter and baseline P as covariates. Full results are shown in Supplementary Table S2.
Patients with unknown or missing covariate values have been removed from analysis.
bUnpaired t-test.
cLog-likelihood estimate from models excluding and including the specified parameter as a covariate.
dContinuous, GLM: LS mean estimated mean change in P per 10 unit change in the parameter.
eContinuous, LR: an odds ratio >1 or <1 indicates a higher probability of P decrease with each unit increase or decrease, respectively.
fCategorical, GLM: an LS mean reduction was observed in both categories. Therefore, a negative estimate indicates a greater reduction in the first, while a positive estimates a greater reduction in the second category.
gCategorical, LR: an odds ratio >1 indicates a higher probability of serum P decrease in the first category, while a value <1 indicates a higher probability of a P decrease in the second category.
Results of multivariate GLM and logistic regression analysis showed a similar pattern. Multivariate GLM found seven statistically significant predictors of serum P change: baseline dialysis vintage, serum albumin and P, absolute and percentage change in PTH from baseline to Month 12, and change in vitamin D sterol dose (absolute and categorical: increase versus no change/decrease). Multivariate logistic regression analysis revealed three significant predictors: baseline serum P and dialysis vintage and PTH percentage change from baseline to Month 12.
PB dose change categories (increase versus decrease, increase versus decrease/no change or decrease versus increase/no change) and study centre characteristics were not found to be significant factors in any analysis (univariate/multivariate GLM or logistic regression). Sensitivity analyses (categorizing the continuous covariates into quartiles and including missing covariate data) revealed that a significant reduction in serum P was seen in all but the lowest quartiles of PTH at baseline and change in PTH at Month 12, respectively. Patients with a higher baseline PTH and those with a greater reduction at Month 12 had significantly greater reductions in serum P than those with lower baseline PTH or a smaller PTH change, respectively. Longer dialysis vintage and low serum albumin (≤3.5 g/dL) were also associated with significant reductions in serum P (Table 4; GLM results only shown).
Table 4.
Sensitivity analysisa
| Parameter/category | n (%) | Estimated % change in P (95% CI)b | Differencec | P-value |
|---|---|---|---|---|
| Dialysis vintage (months) | ||||
| ≤19.9 | 265 (23.6%) | −1.92 (−5.37, 1.53) | Reference | |
| >19.9–47.4 | 274 (24.4%) | −2.77 (−6.17, 0.63) | −0.85 (−5.69, 3.99) | 0.731 |
| 47.4–98.3 | 285 (25.4%) | −6.94 (−10.27, −3.61) | −5.02 (−9.82, −0.22) | 0.041 |
| >98.3 | 295 (26.3%) | −8.26 (−11.54, −4.98) | −6.34 (−11.11, −1.58) | 0.009 |
| Missing | 3 (0.3%) | −22.53 (−54.96, 9.90) | −20.61 (−53.22, 12.00) | 0.216 |
| Serum albumin (g/dL) at cinacalcet start | ||||
| ≤3.5 | 273 (24.3%) | −8.66 (−12.08, −5.24) | Reference | |
| >3.5 to <3.8 | 227 (20.2%) | −3.19 (−6.93, 0.55) | 5.47 (0.41, 10.54) | 0.035 |
| ≥3.8–4.1 | 276 (24.6%) | −3.95 (−7.34, −0.57) | 4.71 (−0.11, 9.52) | 0.056 |
| >4.1 | 246 (21.9%) | −4.55 (−8.14, −0.95) | 4.11 (−0.86, 9.08) | 0.105 |
| Missing | 100 (8.9%) | −4.51 (−10.15, 1.12) | 4.15 (−2.45, 10.75) | 0.218 |
| PTH (pg/mL) at cinacalcet start | ||||
| ≤506.5 | 268 (23.9%) | −1.12 (−4.55, 2.31) | Reference | |
| >506.5 to <721.0 | 270 (24.1%) | −3.98 (−7.40, −0.56) | −2.86 (−7.71, 1.98) | 0.247 |
| ≥721.0–1050.0 | 285 (25.4%) | −7.16 (−10.48, −3.83) | −6.03 (−10.82, −1.25) | 0.014 |
| >1050.0 | 291 (25.9%) | −7.62 (−10.92, −4.33) | −6.50 (−11.26, −1.74) | 0.008 |
| Missing | 8 (0.7%) | −14.61 (−34.48, 5.27) | −13.49 (−33.65, 6.68) | 0.190 |
| PTH (pg/mL) change from baseline to Month 12 | ||||
| ≤−643.4 | 259 (23.1%) | −10.18 (−13.62, −6.74) | Reference | |
| >−643.4 to <−348.0 | 237 (21.1%) | −9.74 (−13.34, −6.14) | 0.44 (−4.54, 5.42) | 0.863 |
| ≥−348.0 to −95.9 | 263 (23.4%) | −4.08 (−7.50, −0.67) | 6.10 (1.25, 10.94) | 0.014 |
| >−95.9 | 245 (21.8%) | 4.39 (0.85, 7.93) | 14.57 (9.63, 19.51) | <0.001 |
| Missing | 118 (10.5%) | −6.84 (−11.95, −1.73) | 3.34 (−2.82, 9.50) | 0.288 |
| PTH % change from baseline to Month 12 | ||||
| ≤−73.3 | 254 (22.6%) | −11.04 (−14.52, −7.56) | Reference | |
| >−73.3 to <−49.9 | 253 (22.5%) | −8.33 (−11.82, −4.85) | 2.70 (−2.22, 7.63) | 0.283 |
| ≥−49.9 to −17.0 | 251 (22.4%) | −3.93 (−7.43, −0.43) | 7.10 (2.17, 12.04) | 0.005 |
| ≥−17.0 | 246 (21.9%) | 3.89 (0.34, 7.43) | 14.92 (9.96, 19.89) | <0.001 |
| Missing | 118 (10.5%) | −6.84 (−11.95, −1.72) | 4.20 (−1.99, 10.38) | 0.184 |
aEffects of covariates (categorical fields) on percentage change in serum phosphorus (P) from baseline to Month 12 (by GLM univariate analysis).
bModelling P % change from baseline with the specified parameter and baseline P as covariates.
cDifference versus reference category.
Discussion
In this post hoc analysis of data from the pan-European ECHO study, we explored serum P changes in dialysis patients treated with cinacalcet. Of 1607 dialysis patients who completed this observational study, 661 (41%) had decreased, 61 (4%) unchanged and 400 (25%) increased, serum P at Month 12, while 485 patients had missing data. Our main findings were that (i) the serum P reduction appeared to result mainly from PTH reduction, rather than from changes in vitamin D sterol or PB doses, and (ii) the magnitude of P reduction was related to that of PTH reduction.
A significant reduction in serum P was seen in all but the lowest quartiles of PTH at baseline and change in PTH at Month 12, respectively. Patients with a higher baseline PTH and those with a greater reduction at Month 12 had the greatest reductions in serum P. This subgroup probably represents those patients with the highest bone turnover and bone-derived P load. Although a decrease in PTH was consistently found to be an important factor in determining serum P reduction, absolute serum P and PTH values (log-transformed) at baseline were not correlated (data not shown). Due to our observational study design, it is possible that P changes preceded the PTH changes. However, this is unlikely, as we were able to correct for the impact of several important serum P-modulating interventions. Moreover, these P changes occurred after starting cinacalcet, which acts primarily on PTH, and not directly on serum P. Other recent data from very large patient populations show that PTH and P levels are directly correlated in dialysis patients [27, 28]. Moreover, analysis of PTH evolution over time also shows that changes in PTH are associated with concomitant changes in serum P levels [29]. Improved control of PTH levels may reduce bone turnover and bone-derived P. Indeed, recent studies have documented the efficacy of cinacalcet in reducing increased total and bone-specific alkaline phosphatase [30]. As bone biopsy data were not collected in ECHO, we were unable to histologically evaluate any changes in bone turnover that followed the initiation of cinacalcet. There was also no collection of data on circulating 25-hydroxyvitamin D or biomarkers of bone formation or resorption.
Patients with increased serum P at 12 months (n = 400) had the smallest median decrease in PTH (Figure 2). The serum P increase could not be accounted for by changes in PB and vitamin D sterol doses in a substantial proportion of these patients (n = 219): their median (Q1, Q3) PTH change was −39 (−63, −5)%, versus −46 (−74, −14)% (n = 110) in those in whom PB and vitamin D sterol changes might potentially explain the serum P increase. Increased serum P might indicate unresponsiveness to, or suboptimal uptitration of, cinacalcet [24] or perhaps a subgroup of patients with lower bone turnover in whom the skeleton was less able to buffer the phosphate excess. We repeated the univariate and multivariate logistic regression analysis, modelling an increase in serum P versus no change/decrease. PTH change again was the key determinant of P change, with absolute and percentage change in PTH being significant predictors (P < 0.001 in univariate analysis). Overall, only 221 patients (12%) had increased PTH at Month 12.
Dialysis vintage and lower baseline serum albumin (≤3.5 g/dL) were also predictors for serum P change. Further analysis showed that dialysis vintage was correlated with PTH at cinacalcet start (data not shown) and other reports indicate that the prevalence of parathyroidectomy (as a marker of severe SHPT) increases with dialysis vintage [31, 32]. As cinacalcet achieves the greatest PTH reduction in those with the most severe SHPT [24], one might expect to see the largest serum P reduction in these patients. As longer dialysis vintage may be associated with a greater risk of malnutrition [33], low serum albumin might identify a subgroup of malnourished patients with long-standing and severe SHPT, in whom the main source of serum P is bone resorption rather than dietary intake. It could be speculated that changes in dietary protein and P intake might have influenced serum P changes. Serum albumin is also influenced by other factors such as inflammation [34]. We did not study additional markers of nutritional status such as normalized protein catabolic rate or subjective global assessment, or other markers of inflammation.
Changes in vitamin D sterols and PB do not explain the serum P change in a substantial proportion of patients in our study and were not generally significant factors in the analyses, suggesting that such changes were less important than changes in PTH in our patients. Change in vitamin D sterol dose was significant in GLM analysis when evaluated as an absolute value, but not when analysed as a percentage, suggesting that the effect seen in the model was just an artefact of the difference in vitamin D sterol dose at baseline. Cinacalcet dose change was not a significant factor in determining serum P reduction, although there was only a very modest uptitration of cinacalcet dose in ECHO in the majority of patients [24]. A total of 453 patients discontinued cinacalcet: for patients who were still taking cinacalcet at Month 12, the mean daily dose was 57 mg (median 60 mg, cf 30 mg at baseline) at this time point. The corresponding vitamin D sterol dose was 1.1 (SE 0.04) µg/week, measured in oral calcitriol equivalents.
As patients were selected on the basis of poorly controlled SHPT, it is possible that our observations do not apply to the general dialysis population. Limitations of ECHO include its observational and partly retrospective design [24], with patients being treated and monitored according to local practices: treatment guidelines changed during the study and there were regional differences in types and dosages of vitamin D sterols and PB used [35]. Forty-five per cent of study sites did not provide information about the type of PTH assay used; of those that did, most sites (94%) employed second- or third-generation serum intact PTH assays [35]. Although different types of PTH assays can give different absolute values [36–38], trends in PTH reduction were similar for the different assay types [35]. Moreover, it is unlikely that an important number of participating centres changed the PTH assay used during the observation period. Other factors that might influence serum P that we did not evaluate include changes in dialysis schedule or dialysate calcium levels, type of PB used (calcium- versus non-calcium-based), compliance with treatment and dietary changes.
The large amount of missing data could potentially have introduced bias into the analyses, but sensitivity analysis showed that results were similar when patients with missing covariate data were included in the model. As analytical and within-subject variation in serum P might theoretically have influenced the results, an additional sensitivity analysis was conducted, using a 5% threshold to define serum P change. This had no effect on the results of the logistic regression analysis.
Despite its limitations, results of the present analysis suggest that the serum P reduction achieved in a significant proportion of dialysis patients after adding cinacalcet to an existing SHPT regimen results mainly from PTH reduction, rather than from changes in vitamin D sterol or PB doses.
Supplementary Data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Funding
Amgen was involved in the design and conduct of the ECHO study and the present analysis and provided funding for both.
Conflict of interest statement
The authors disclose the following: E.Z.—honoraria for lectures from Amgen and Genzyme; D.F.—speaker and/or consultancy fees from Amgen, Genzyme and Shire and research funding from Amgen; S.J.—scientific consultant for Amgen, Abbott, Genzyme and Swedish Orphan and honoraria from Amgen, Abbott, Genzyme and Swedish Orphan; F.M.—honoraria for scientific consulting and symposia from Amgen, Genzyme and Shire; P.U.—honoraria for scientific consulting and symposia and clinical research grants from Amgen, Shire and Roche; Ma.R.—scientific consultant for Amgen and honoraria from Genzyme, Abbott and Swedish Orphan; M.V.—research grants from Abbott and Genzyme, and advisory board participation for Abbott and Genzyme: B.D. and N.M. are employees of Amgen. The Amgen staff had the opportunity to review the present manuscript.
Supplementary Material
Acknowledgements
The ECHO study and present analysis were sponsored by Amgen (Europe) GmbH. We would also like to thank all the ECHO investigators (listed in supplementary material) and Julia Balfour, Dundee, Scotland, and Caterina Hatzifoti, Amgen (Europe) GmbH for assistance with the writing of the manuscript.
References
- 1.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. doi:10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Slatopolsky E, Brown A, Dusso A. Pathogenesis of secondary hyperparathyroidism. Kidney Int Suppl. 1999;73:S14–S19. doi: 10.1046/j.1523-1755.1999.07304.x. doi:10.1046/j.1523-1755.1999.07304. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. doi:10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. doi:10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 5.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 6.Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. doi:10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. doi:10.1111/j.1523-1755.2005.00185. [DOI] [PubMed] [Google Scholar]
- 8.Giachelli CM. The emerging role of phosphate in vascular calcification. J Kidney Int. 2009;75:890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruska KA, Saab G, Mathew S, et al. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Semin Dial. 2007;20:309–315. doi: 10.1111/j.1525-139X.2007.00300.x. doi:10.1111/j.1525-139X.2007.00300. [DOI] [PubMed] [Google Scholar]
- 10.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. doi:10.1161/01.RES.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 11.Kanbay M, Goldsmith D, Akcay A, et al. Phosphate—the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009;27:220–230. doi: 10.1159/000197562. doi:10.1159/000197562. [DOI] [PubMed] [Google Scholar]
- 12.Covic A, Kothawala P, Bernal M, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1506–1523. doi: 10.1093/ndt/gfn613. doi:10.1093/ndt/gfn613. [DOI] [PubMed] [Google Scholar]
- 13.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. doi:10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 14.Danese MD, Belozeroff V, Smirnakis K, et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1423–1429. doi: 10.2215/CJN.01060308. doi:10.2215/CJN.01060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO Clinical practice guidelines for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76(Suppl. 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 16.Uribarri J. Phosphorus additives in food and their effect in dialysis patients. Clin J Am Soc Nephrol. 2009;4:1290–1292. doi: 10.2215/CJN.03950609. doi:10.2215/CJN.03950609. [DOI] [PubMed] [Google Scholar]
- 17.Drake TG, Albright F, Castleman B. Parathyroid hyperplasia in rabbits produced by parenteral phosphate administration. J Clin Invest. 1937;16:203–206. doi: 10.1172/JCI100848. doi:10.1172/JCI100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raisz LG. Bone resorption in tissue culture. factors influencing the response to parathyroid hormone. J Clin Invest. 1965;44:103–116. doi: 10.1172/JCI105117. doi:10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruska KA, Mathew S, Lund R, et al. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. doi:10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moe SM, Drueke TB. Management of secondary hyperparathyroidism: the importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium–phosphorus product. Am J Nephrol. 2003;23:369–379. doi: 10.1159/000073945. doi:10.1159/000073945. [DOI] [PubMed] [Google Scholar]
- 21.Nemeth EF, Steffey ME, Hammerland LG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. doi:10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. doi:10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg JS, Culleton B, Wong G, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. doi:10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 24.Urena P, Jacobson SH, Zitt E, et al. Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice—the ECHO observational study. Nephrol Dial Transplant. 2009;24:2852–2859. doi: 10.1093/ndt/gfp144. doi:10.1093/ndt/gfp144. [DOI] [PubMed] [Google Scholar]
- 25.Martin KJ, Juppner H, Sherrard DJ, et al. First- and second-generation immunometric PTH assays during treatment of hyperparathyroidism with cinacalcet HCl. Kidney Int. 2005;68:1236–1243. doi: 10.1111/j.1523-1755.2005.00517.x. doi:10.1111/j.1523-1755.2005.00517. [DOI] [PubMed] [Google Scholar]
- 26.Messa P, Macario F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45. doi: 10.2215/CJN.03591006. doi:10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant. 2011;26:1938–1947. doi: 10.1093/ndt/gfq304. doi:10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 28.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. doi:10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilpatrick RD, Gill KS, Block GA. [SA-PO2403] Exploring the Relationship between Temporal Trends in PTH, P and Ca in HD Patients.. Abstract presented at the American Society of Nephrology Annual Meeting; 16–21 November 2010; Denver, CO. [Google Scholar]
- 30.Shigematsu T, Akizawa T, Uchida E, et al. Long-term cinacalcet HCl treatment improved bone metabolism in Japanese hemodialysis patients with secondary hyperparathyroidism. Am J Nephrol. 2009;29:230–236. doi: 10.1159/000156717. doi:10.1159/000156717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malberti F, Marcelli D, Conte F, et al. Parathyroidectomy in patients on renal replacement therapy: an epidemiologic study. J Am Soc Nephrol. 2001;12:1242–1248. doi: 10.1681/ASN.V1261242. [DOI] [PubMed] [Google Scholar]
- 32.Kestenbaum B, Andress DL, Schwartz SM, et al. Survival following parathyroidectomy among United States dialysis patients. Kidney Int. 2004;66:2010–2016. doi: 10.1111/j.1523-1755.2004.00972.x. doi:10.1111/j.1523-1755.2004.00972. [DOI] [PubMed] [Google Scholar]
- 33.Chertow GM, Johansen KL, Lew N, et al. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000;57:1176–1181. doi: 10.1046/j.1523-1755.2000.00945.x. doi:10.1046/j.1523-1755.2000.00945. [DOI] [PubMed] [Google Scholar]
- 34.Kaysen GA. Serum albumin concentration in dialysis patients: why does it remain resistant to therapy? Kidney Int Suppl. 2003:S92–S98. doi: 10.1046/j.1523-1755.64.s87.14.x. doi:10.1046/j.1523-1755.64.s87.14. [DOI] [PubMed] [Google Scholar]
- 35.Vervloet M, Bencova V, Malberti F, et al. ‘Real-World’ use of cinacalcet for managing SHPT in different European countries: analysis of data from the ECHO observational study. Clin Nephrol. 2010;74:198–208. doi: 10.5414/cnp74198. [DOI] [PubMed] [Google Scholar]
- 36.Joly D, Drueke TB, Alberti C, et al. Variation in serum and plasma PTH levels in second-generation assays in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2008;51:987–995. doi: 10.1053/j.ajkd.2008.01.017. doi:10.1053/j.ajkd.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Ates F, Koken T, Demir S, et al. Comparison of three different immunoassay methods for the evaluation of intact parathyroid hormone levels in hemodialysis patients. Scand J Clin Lab Invest. 2011;71:227–231. doi: 10.3109/00365513.2011.555563. doi:10.3109/00365513.2011.555563. [DOI] [PubMed] [Google Scholar]
- 38.Souberbielle J-CP, Roth H, Fouque DP. Parathyroid hormone measurement in CKD. Kidney Int. 2009;77:93–100. doi: 10.1038/ki.2009.374. doi:10.1038/ki.2009.374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.