Abstract
Light acts as environmental signal to control animal behavior at various levels. The Drosophila larval nervous system is used as a unique model to answer basic questions on how light information is processed and shared between rapid and circadian behaviors. Drosophila larvae display a stereotypical avoidance behavior when exposed to light. To investigate light dependent behaviors comparably simple light-dark preference tests can be applied. In vertebrates and arthropods the neural pathways involved in sensing and processing visual inputs partially overlap with those processing photic circadian information. The fascinating question of how the light sensing system and the circadian system interact to keep behavioral outputs coordinated remains largely unexplored. Drosophila is an impacting biological model to approach these questions, due to a small number of neurons in the brain and the availability of genetic tools for neuronal manipulation. The presented light-dark preference assay allows the investigation of a range of visual behaviors including circadian control of phototaxis.
Keywords: Neuroscience, Issue 74, Developmental Biology, Neurobiology, Behavior, Molecular Biology, Cellular Biology, Physiology, Anatomy, Light, preference test, Drosophila, larva, fruit fly, visual behavior, circadian rhythm, visual system, animal model, assay
Introduction
Here we describe a behavioral assay based on the larval preference for dark (or light). Larvae react with a strong and stereotypic photonegative response during foraging stages (L1 to early L3)1. The assay is aimed to assess the photophobic behavior of the larva and compares the light or dark preference of a group of larvae moving freely in a Petri dish coated with agar. This behavioral assay not only provides information about the sensitivity, integration and temporal plasticity of the visual system, it further provides hints on how light sensitivity and its process is controlled by the circadian system.
The Drosophila larval eye (also termed Bowlig Organ; BO), is the main organ for light perception. Each eye is composed of 12 photoreceptors (PR), eight PRs express the green-sensitive rhodopsin6 (rh6) and four PRs express the blue-sensitive rhodopsin5 (rh5)2,3. In addition to PRs, also class IV multidendritic neurons, which cover the larval body wall, have been identified to respond to noxious light intensities4,5. It is also known that pacemaker neurons situated in the central larval brain express the light sensitive protein Cryptochrome (Cry) that acts as clock intrinsic blue light sensor within the brain6,7. Intriguingly photophobicity of wild type animals shows a circadian component at different time points during the course of day and night when testing with this assay. Responses to light of foraging L3 larva showed stronger photophobicity at dawn and lower photophobicity at dusk when tested for light-dark preference7. Interestingly only Rh5-PRs are required for light avoidance, while Rh6-PRs are dispensable. Both, Rh5-PRs and Rh6-PRs are involved in resetting the molecular clock by light8. The Cry pathway must be coordinated with the other light-sensing pathways to orchestrate an appropriate behavioral output in the course of the day. Acetylcholine in PRs plays an essential role in light avoidance behavior as well as entrainment of the molecular clock. Blocking acetylcholine neurotransmission from PRs to circadian pacemaker neurons reduces the photophobic response in the light-darkness preference assay8. Employing the same assay, two symmetrical pairs of neurons have been recently identified to switch the light preference of the third larval instar of Drosophila9. These two pairs of neurons may be functioning during late larval stages, when animals leave the food to presumably find an appropriate pupariation site. However, the question of how the visual pathways interact and control larval visual behavior in a circadian manner remains largely unanswered. The light preference assay allows comparisons among circadian time points, fly lines and circadian state under different light qualities. The assay is easily prepared and inexpensive and has been useful previously in several labs to describe and study light derived behavior in the larva.
Protocol
1. Larval Rearing
Keep fly strains or genetic crosses in mass culture at 25 °C on corn meal medium under a 12-hr light-12-hr dark cycle in a fly incubator equipped with light and timer.
Dilute backer yeast in water to form a fluid paste (10 g of backer yeast diluted with 3-4 ml distilled H2O). Add a small drop to the corn meal food and cover the vials. Let dry for at least one hour to avoid adult flies sticking to the yeast paste. Putting a small drop of yeast diluted in water on the surface of the food can enhance oviposition. Alternatively baker yeast paste, 20% acetic acid (AcOH) (Sigma Aldrich, Switzerland A6283-100ML) can be used. For this, dip the tip of a Q-tip on the solution and spread on the corn meal food surface.
Put adult flies (at least four days old, but not older than ten days) in corn meal food vials. Put enough adults into each vial in order to obtain sufficient larvae to repeat the preference test several times and allow statistical comparison. For genetic crosses, we typically use a minimum of 20 females and 5-10 males per vial, but some lines require more females to obtain a sufficient larval offspring.
Allow adults to lay eggs for 12 hr and transfer them to new vials. Adults can be transferred in the morning and the evening. We usually transfer adults for seven to ten days and if required take new adults.
Keep the egg collections taken every 12 hr in the incubator and let grow the larvae at 25 °C, 12-hr light-12-hr dark cycle and 60% humidity. To test for circadian components of visual behavior move larvae to constant darkness (DD) 48 hr after egg collection (corresponding to two-light-dark cycles). For this, use a separate incubator without light but keep all other conditions such as temperature and humidity identical. Be sure to transfer the vials to the DD incubator just before the switch to the dark phase. Before moving the vials to another incubator put vials in a cardboard box or wrap the vials with aluminum foil to prevent light exposure during the transfer (packing in cardboard box or wrapping has to be done during the "lights-on phase" prior to the shift between incubators). Prevent the animals from any light exposure after this point and until experiments start.
After 4 days (84 to 108 hr from egg laying) collect early L3 (feeding larvae) at the time points to be tested (see 4. Light preference test, point. 4.4.).
2. Test Setup
Perform experiments in a dark room with constant temperature and humidity conditions.
To keep two quadrants of the Petri dish in darkness, cover the lid with black tape and aluminum foil. In order to do so, mark the center of the lid circumference. Also mark four points in the border of the lid with 90 ° of separation among them. To make it easier, draw a 20 cm square divided in four quadrants of 10 cm length on a sheet of paper or with help of a computer. Cut 10 cm squares of aluminum foil and black tape (Figure 1A). Cover two opposite quadrants by gluing a square of aluminum foil, as first layer and black tape covering it, be careful to also cover the border of the lid. In the past two slightly distinct forms of this assays were used. We here use the "quarter-plate" assay, in which the Petri dish is divided in four equal quarters10. In the alternative "half-plate" assay the Petri dish is divided half (half exposed to light, half in darkness)1,7,8. Until today no significant differences between the two assays has been published.
Glue a quadrant sheet such as the one used for marking the lids right under the light source on the table. Mark the circumference of a Petri dish centered on the quadrants intersection. Use the marks as reference to place the test plate always in the same position under the light source.
Install a lamp, either a simple white light bulb (Phillips, Softtone 5W) or a LED lamp (LED lamp, 80012 White, Osram) above the Petri dish with the help of an iron support stand (Fisher Scientific, S47808). Adjust the height in a way that the whole test plate is homogenously illuminated. We strongly recommend the use of LED lamps since they emit more specific wavelengths than conventional light bulbs. Moreover LEDs emit less heat.
Adjust light intensity with the help of a photometer (Environment Meter PCE EM882). To achieve the required light intensity of 350-760 lux, move the lamp up or down as required. Plugging the lamp to an adjustable power supply gives more flexibility to change intensities without moving the lamp (Figure 1B).
3. Plates Preparation
Make 200 ml of 2.5 % agar (Sigma-Aldrich, Switzerland A5093-500G) with double distilled water (Millipore).
Place Petri dishes on a table (90-mm diameter; Greiner Bio-One GmbH, 4550 Kremsmeinster, Austria) in rows to allow pouring hot agarose.
Boil the agarose in a microwave until the solution is completely transparent and fluid. Make sure that liquid solution does not contain bubbles. CAUTION: Transport carefully to the table since the solution can be very hot!
Pour hot agarose into the Petri dishes until the whole surface is homogenously covered with a thin layer of the solution, about two or three millimeters are enough. Be quick to prevent agarose solidifying before coating all plates. Let the plates cool down. Store plates at room temperature and use them only on the same day of preparation.
4. Light Preference Test
Work under red light conditions in the experiment room to prevent light influence before the test since Drosophila is not able to sense red wavelengths of light. Use a red light bulb (Phillips, PFE712E*8) mounted in a lamp to illuminate the place to work.
Maintain temperature through all experiments at 25 °C. Control room temperature by means of a heater or cooling device if required.
Take some food from the vials reared for two-day cycles under constant darkness (from section 1). Since larvae are usually digging on the surface of the food, take the uppermost layer (about 5 mm deep) with help of a spatula (Fisher Scientific, 14-373-25A). Spread the food on the outer side of a Petri dish lid, add some water and mix gently with the spatula.
Collect feeding L3 larvae from the food. As light preference will be tested, choosing only early/feeding L3 larvae is crucial since the negative phototaxis is assured. Late/wandering L3 larvae or big larva crawling on the food presumably already switched their photobehavior. Feeding L3 larvae (negative phototactic) can be recognized because their anterior spiracles are open and protruded to the outside in a finger-like form. The posterior spiracles have three openings each, and four groups of large branched hairs. The salivary glands extend to the second abdominal segment11. Staging larva under a microscope is convenient whereas no white light should be used. A red light lamp is necessary to illuminate the larvae if staging under stereoscopic microscope before experiments is required.
Wash larvae briefly in tap water and collect early-feeding third-instar larvae (still in red light). Before transferring the larvae to the test plate, take the larvae with a wet paintbrush and carefully absorb excess water with a paper towel or filter paper. Do not dry larvae too excessively since it could harm the animal and influence its behavior.
Using the wet paintbrush carefully transfer the larvae to plates. Place a group of approximately 30 larvae in the center of the plate. Cover the plate with the lid already prepared with the quadrants and set the plate under the light source ready for experiment (see section 2).
Turn on the white light lamp and start the timer. Let the larva move freely on the plate for 5 min, then quickly remove the lid and count the number of larvae in the dark and in the light quadrants. Marking the position of each larva with a marker can ease the counting. Alternatively take a picture of the plate and count a posteriori. Larvae showing unclear light preference, like larvae crawling on the walls or burrowing into agar should be considered as neutral preference and just be included in the total number of larvae when the light preference index is calculated.
After counting, discard the plate with larvae and replace with a new test plate for the next experiment. Collect used plates in an autoclave plastic bag for later handling and disposal.
Once you have performed a sufficient number of experiments a new genotype can be tested. 10-15 trials per genotype are sufficient for analysis.
5. Data Analysis
For convenience transfer the data to a datasheet as Excel (Microsoft) or Origin (Origin Lab) on a computer for further statistical analysis. Arrange in one column the number of animals in darkness, in a second column the number of animals in light quadrants and in the third the total of animals in the plate (including "neutral" larvae).
Calculate the preference index (PREF) for the darkness for each experiment using the following formula: PREF(darkness) = (number of larvae in dark - number of larvae in light)/total number of larvae
Compare statistically a set of data with an appropriate analysis for groups. Here, we use a Wilcoxon test to compare statistically two groups. An ANOVA test with a Tukey's multiple-comparison post hoc test can be done, if normal distribution assumption is fulfilled in a manifold group comparison.
Make graphs that show clearly comparisons among lines and time points along the day cycle. We use Origin Software (Origin Lab) to test statistical significance and generate appropriate graphs, but any other statistical software can be helpful.
Representative Results
Following the protocol described above, we tested light-dark preference in early third larval stadium of wild type Canton-S flies at two different circadian times CT0 and CT12. Adults were reared 12-hr light-12-hr dark and left to lay eggs for 12 hr. Larvae grow the first two days under the same light-dark regime. Since we wanted to test circadian modulation under constant conditions (free running of the circadian clock), larvae were then transferred to constant darkness for the next three days until test was performed (Figure 3A).
Here, we used 350 lux since it has been shown that this is the optimal light intensity to detect differences of light response of Drosophila larva along the circadian cycle in comparison to other intensities (70 and 600 lux)7. Differences in light sensitivity, and therefore in light response is observed when comparing CT0 with CT12. In Drosophila, CT0 coincides with dawn, the half cycle between CT0-CT12 is considered the subjective day and from CT12 to CT24 the subjective night12. Light photosensitivity is higher briefly after the transition from darkness to light, when almost 77% of the larvae prefer the darkness compared with almost 69% of dark preference at the early subjective night (Figure 3B). This is also reflected by the dark preference index (PREF(darkness)) calculated with the formula presented above for each experiment. Averaging all repetitions we obtained a preference index of 0.52 for CT0 and of 0.36 for CT12. Using the Wilcoxon test (Origin) a statistical significant difference between two time points is shown (p=0.0229).
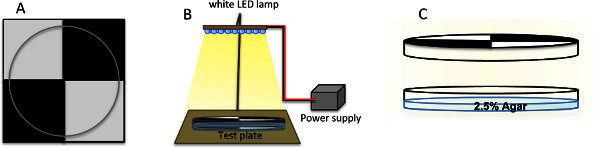 Figure 1.(A) Marking quadrants in Petri dish lids. Quadrants can be marked in the Petri dish lid with help of a printed quadrant used as canvas. Posteriorly the first layer of aluminum foil can be glued on the external surface of the plate and be covered with black tape in the two opposite quadrants for darkness. (B) Schematic set up for the light-dark preference test. (C) A Petri dish covered with dark quadrants and filled with 2.5% agar, ready to be used for experiments. Click here to view larger figure.
Figure 1.(A) Marking quadrants in Petri dish lids. Quadrants can be marked in the Petri dish lid with help of a printed quadrant used as canvas. Posteriorly the first layer of aluminum foil can be glued on the external surface of the plate and be covered with black tape in the two opposite quadrants for darkness. (B) Schematic set up for the light-dark preference test. (C) A Petri dish covered with dark quadrants and filled with 2.5% agar, ready to be used for experiments. Click here to view larger figure.
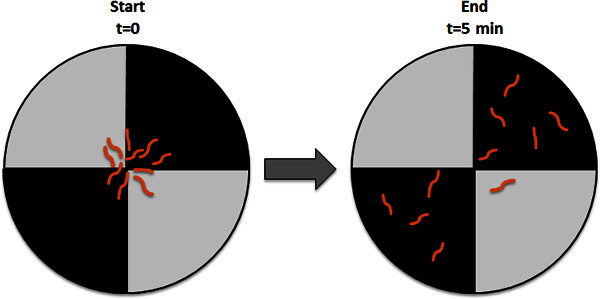 Figure 2. Light-dark preference test, example of a dish right after setting larva on the test plate (Start) and after 5 min (End). Typically, we use a group of 30 animals for each experiment.
Figure 2. Light-dark preference test, example of a dish right after setting larva on the test plate (Start) and after 5 min (End). Typically, we use a group of 30 animals for each experiment.
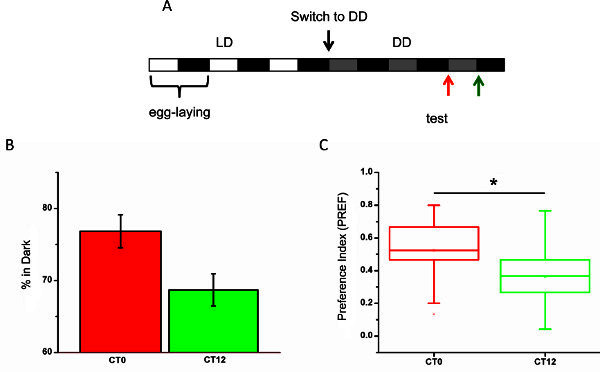 Figure 3.(A) Light regime followed to test light preference in feeding L3 larva. (B) Percentage of dark preference for both time points tested (CT0 and CT12). Percentage of larva that preferred darkness and light are counted for each repetition and all repetitions averaged. Larvae in the borders of the plate or digging on the agar are considered as neutral. (C) Dark preference index calculated for the same time points. Significant statistical difference between groups is shown by the Wilcoxon sum-rank test (p<0.05). (N=18 (540 larvae) per time point). Click here to view larger figure.
Figure 3.(A) Light regime followed to test light preference in feeding L3 larva. (B) Percentage of dark preference for both time points tested (CT0 and CT12). Percentage of larva that preferred darkness and light are counted for each repetition and all repetitions averaged. Larvae in the borders of the plate or digging on the agar are considered as neutral. (C) Dark preference index calculated for the same time points. Significant statistical difference between groups is shown by the Wilcoxon sum-rank test (p<0.05). (N=18 (540 larvae) per time point). Click here to view larger figure.
Discussion
The light preference test described takes advantage of the larval innate photobehavior. The assay is easy to establish, allows many repetitions at low cost and delivers valuable information about light sensing and processing. The experimental paradigm allows relatively quick quantification of how many individuals prefer light or dark. Such preference can be displayed as crude percentages or alternatively as Preference Index (PREF). The PREF is expressed as the difference of animals that preferred light and animals that preferred darkness divided by the total of animals.
A crucial point for the light preference test is the duration of the experiments. Here, we tested five minutes, however it is possible that other genotypes or under other light qualities differences are not so clear using this experiment duration. For certain stimuli (gustatory) we have realized that longer experiments, with 20 min of duration, deliver cleaner preferences with smaller variability. Testing different experimental durations could be fair especially when using genotypes where locomotion or sensitivity is affected and larvae require longer time to explore the testing plate. In that case, the time should be equally adjusted for controls and experimental genotypes.
The assay also allows detecting differences among time points of the day through the circadian cycle as shown here and in previous reports7,13. Light responses to photic stimuli are strongly regulated by the circadian clock, presumably by modulating the sensitivity of photoreceptors. Larval responses to light decrease during the first two hours of the day and become stable during the remaining ten hours of the light phase. To compare different groups in the course of the day, it is crucial to make experiments at equivalent or identical time points of the circadian cycle for each group. In addition, it is also important to avoid changes that can affect larval behavior, as thermal fluctuations or light inputs previous to experiments if working in darkness. For this, it is important to rear the larvae at the same temperature and humidity than the ones used for experimentation and be careful by keeping animals in darkness when needed.
At least three recently described pathways contribute to light sensing in Drosophila larva5,14: 1) the rhodopsin dependent system mediated by the larval eye; 2) the multidendritic neurons class IV, spread along the larval body and 3) clock intrinsic blue light sensors expressing Cry. Contribution of distinct light-sensing pathways for visual behavior can be addressed by the preference test described here. Different sources of illumination with specific intensities and wavelengths can be used to test different light qualities. This possibility can depend on details about the sensitivity of individual elements of the light sensing system and how such light inputs are processed. For a more detailed experimentation regarding light stimulation, the assay can be easily modified to test different light qualities. Increasing and decreasing light intensities, as well as testing different light wavelengths is possible by using self-made LED lamps with specific wavelengths (many possibilities are available in the market). Control of such lamps with power supplies offers a good range of intensities. These possibilities for manipulating light qualities provide a wide range of variables to be tested with the light preference test.
Moreover, Drosophila larvae are able to associate punishment or rewards with either light or darkness, modifying their native photobehavior15. For this, an adaptation of the protocol described was employed showing the versatility of the assay. In summary, the light-dark preference test is a valuable instrument contributing with a better understanding of rapid and circadian behaviors derived from light stimuli and which elements of the light sensitive system and the circadian clock of the Drosophila larva are involved in this process.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank our colleagues at the Department of Biology, University of Fribourg for fruitful discussions. We thank the Bloomington Stock Center for providing fly stocks. This work was financially supported by the Swiss National Science Foundation (PP00P3_123339) and the Velux Foundation to S.G.S.
References
- Sawin-McCormack EP, Sokolowski MB, Campos AR. Characterization and genetic analysis of Drosophila melanogaster photobehavior during larval development. J. Neurogenet. 1995;10:119–135. doi: 10.3109/01677069509083459. [DOI] [PubMed] [Google Scholar]
- Sprecher SG, Pichaud F, Desplan C. Adult and larval photoreceptors use different mechanisms to specify the same Rhodopsin fates. Genes Dev. 2007;21:2182–2195. doi: 10.1101/gad.1565407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher SG, Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz NN, Sprecher SG. Photoreceptors: unconventional ways of seeing. Curr. Biol. 2011;21:R25–R27. doi: 10.1016/j.cub.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Emery P, et al. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Keene AC, et al. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J. Neurosci. 2011;31:6527–6534. doi: 10.1523/JNEUROSCI.6165-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong ZF, et al. Two Pairs of Neurons in the Central Brain Control Drosophila Innate Light Preference. Science. 2010;330:499–502. doi: 10.1126/science.1195993. [DOI] [PubMed] [Google Scholar]
- Lilly M, Carlson J. smellblind: a gene required for Drosophila olfaction. Genetics. 1990;124:293–302. doi: 10.1093/genetics/124.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, editor. Biology of Drosophila. John Wiley & Sons; 1950. pp. 275–367. [Google Scholar]
- Pittendrigh CS. Biological Rhythms. 4 Handbook of Behavioral Neurobiology. Plenum; 1981. Circadian systems: Entrainment; pp. 95–124. [Google Scholar]
- Collins B, Kane EA, Reeves DC, Akabas MH, Blau J. Balance of Activity between LN(v)s and Glutamatergic Dorsal Clock Neurons Promotes Robust Circadian Rhythms in Drosophila. Neuron. 2012;74:706–718. doi: 10.1016/j.neuron.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Sprecher SG. Seeing the light: photobehavior in fruit fly larvae. Trends Neurosci. 2012;35:104–110. doi: 10.1016/j.tins.2011.11.003. [DOI] [PubMed] [Google Scholar]
- von Essen AM, Pauls D, Thum AS, Sprecher SG. Capacity of visual classical conditioning in Drosophila larvae. Behav. Neurosci. 2011;125:921–929. doi: 10.1037/a0025758. [DOI] [PubMed] [Google Scholar]


