Abstract
Endothelial outgrowth cells (EOCs) have garnered much attention as a potential autologous endothelial source for vascular implants or in tissue engineering applications due to their ease of isolation and proliferative ability; however, how these cells respond to different hemodynamic cues is ill-defined. This study investigates the inflammatory and thrombotic response of baboon EOCs (BaEOCs) to four hemodynamic conditions using the cone and plate shear apparatus: steady, laminar shear stress (SS); pulsatile, nonreversing laminar shear stress (PS); oscillatory, laminar shear stress (OS); and net positive, pulsatile, reversing laminar shear stress (RS). In summary, endothelial nitric oxide synthase (eNOS) mRNA was significantly upregulated by SS compared to OS. No differences were found in the mRNA levels of the inflammatory markers intercellular adhesion molecule-1 (ICAM-1), E-selectin, and vascular cell adhesion molecule-1 (VCAM-1) between the shear conditions; however, OS significantly increased the number of monocytes bound when compared to SS. Next, SS increased the anti-thrombogenic mRNA levels of CD39, thrombomodulin, and endothelial protein-C receptor (EPCR) compared to OS. SS also significantly increased CD39 and EPCR mRNA levels compared to RS. Finally, no significant differences were detected when comparing pro-thrombotic tissue factor mRNA or its activity levels. These results indicate that shear stress can have beneficial (SS) or adverse (OS, RS) effects on the inflammatory or thrombotic potential of EOCs. Further, these results suggest SS hemodynamic preconditioning may be optimal in increasing the efficacy of a vascular implant or in tissue-engineered applications that have incorporated EOCs.
Introduction
Endothelial outgrowth cells (EOCs), a subpopulation of endothelial progenitor cells, have been proposed as an autologous cell source for the endothelialization of a vascular implant including their use in tissue engineering applications. The interest in their use is due to (1) their relative ease of isolation compared to mature vascular endothelial cells that require harvesting tissue for isolation and (2) the ability for rapid expansion in vitro.1–4 Many studies on EOCs have centered on their origin and marker profile, their response to various cytokine exposures, their role in disease, as well as similarities and differences among mature endothelial cells and EOCs5–10; however, there are few studies that have investigated the effects of differing hemodynamic conditions on EOCs. Of these studies, most, such as Ensley et al., have focused on the use of steady, laminar shear stress to precondition a vascular graft before implant.11–17
The endothelium is exposed to various mechanical, biochemical, and extracellular cues that are critical in maintaining vascular homeostasis.18–21 Shear stress, the frictional force created by blood flow on the vascular endothelium, is a mechanical cue that mediates the phenotype of an endothelial cell.21,22 In the vasculature, areas that are exposed to oscillating and low mean shear stresses are prone to atherosclerotic development, such as the carotid sinus,23 while areas that are exposed to unidirectional, nonpathological high shear stresses, such as the common carotid, remain relatively healthy.18,24 Further in vitro studies show that oscillatory shear stresses increase the inflammatory and thrombotic potential of endothelial cells.18,21,22,25 Interestingly, EOCs have been shown to have distinct shear stress responses when compared to mature endothelial cells.12,13 Before EOCs can be used for a vascular implant or in tissue-engineered applications, their response to varying hemodynamic environments, such as shear stress, must be determined. Further, by determining how EOCs respond to differing fluid shear stresses, a set of hemodynamic conditions should be determined to optimally precondition EOCs for use on a vascular implant or in tissue engineered applications to increase their efficacy.
Two pathways that will be important in determining the efficacy of EOCs for a vascular implant are the inflammatory and thrombotic pathways. In this study, we used a cone and plate apparatus to study the effects of four different hemodynamic environments (steady, laminar shear stress [SS]; pulsatile, nonreversing laminar shear stress [PS]; oscillatory, laminar shear stress [OS]; and net positive, pulsatile, reversing laminar shear stress [RS]) on baboon EOCs (BaEOCs) in terms of the inflammatory and thrombotic pathway activation. Our objective was to better understand the following three questions: First, how does steady, laminar flow, classically used in endothelial shear stress studies, compare to pulsatile shear stress with the same average magnitude in BaEOCs? Second, does oscillatory shear stress change BaEOCs' inflammatory and thrombogenic profiles as compared to steady shear stress? Lastly, would BaEOCs exposed to a reversing flow, but with a net positive mean shear stress, behave similarly to a steady shear or an oscillatory shear condition? To answer these questions we chose to investigate well-known pro-inflammatory markers (intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 (VCAM-1), MCP-1, E-Selectin, P-Selectin26–28), pro-thrombogenic marker (tissue factor29) and anti-thrombogenic markers (thrombomodulin, endothelial protein-C receptor [EPCR], CD39, and TFPI15,16,30,31), as well as a well-known shear responsive gene important in atherosclerotic inhibition (endothelial nitric oxide synthase [eNOS]18,22,32).
Materials and Methods
Cell isolation
BaEOCs were isolated using fresh anti-coagulated blood drawn from male juvenile baboons.1,12 The blood was then subjected to a modified isolation procedure as shown previously.1,33 Briefly, using density centrifugation the mononuclear cells were isolated from the peripheral blood. The cells were then seeded on fibronectin-coated plates and cultured in endothelial cell growth media (EGM) media (Lonza) supplement with 18% fetal bovine serum (FBS). The media was exchanged daily for 7 days, after which it was replaced every other day. The endothelial colonies were then isolated and placed on collagen-coated plates and fed with EGM supplement with 8% FBS. The cells were passaged and at passage 2 the EOCs were isolated using a CD31 antibody and magnetic beads (Invitrogen).1 All experiments were completed using BaEOCs at passage 5. Cells were expanded in a modified EGM-2 bullet kit (Lonza) supplemented with 1% l-glutamine (Cellgro®), 1% penicillin–streptomycin (Cellgro), and 10% FBS.
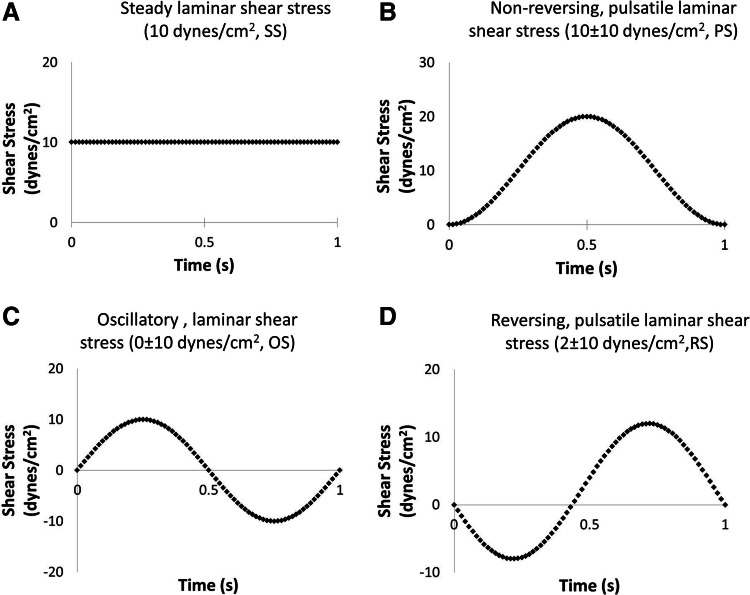
Shear stress application
BaEOCs were subjected to four different hemodynamic environments using the cone and plate shear apparatus as previously described.34,35 Briefly, shear stress waveforms were entered into a computer that controlled a servo motor to rotate the cone as previously described.34 A nondimensional parameter, R-tilde (R∼≡r2ωα2/12ν; where r is radius; ω, angular velocity of the cone; α, cone angle in radians; and ν, kinematic viscosity), was used to characterize the inertial and viscous forces of the fluid flow.35 Experimentally, Sdougos et al. found that R-tilde values less than 0.5 yielded concentric and laminar fluid flow while R-tilde values greater than 4 yielded fluid flow that was turbulent. For R-tilde values between 0.5 and 4.0, flow was laminar in nature but included secondary flow.35 In our system, maximum R-tilde values, at the outer radius of the cone were 0.1 and thus we classify all of our waveforms as laminar. The cells were exposed to one of the following conditions (Fig. 1): steady, laminar shear stress (SS, 10±0 dynes/cm2); pulsatile, nonreversing laminar shear stress (PS, 10±10 dynes/cm2); oscillatory, laminar shear stress (OS, 0±10 dynes/cm2); net positive, pulsatile, reversing oscillatory laminar shear stress (RS, 2±10 dynes/cm2); or static (no shear stress). Shear stress was applied to cells for 24 h. Before the application of shear stress, cells were seeded onto a tissue culture-treated 100 mm dish (Corning) coated with 3.6 μg/cm2 of collagen type I (rat tail; Millipore). Cells were allowed to grow to confluence under static conditions for 48 h. One hour before shear stress application, growth medium was replaced with shear medium (EBM-2, 5% FBS, EGF [0.0005 μg/mL], bFGF [0.002 μg/mL], 1% penicillin streptomycin [Cellgro], 1% l-glutamine [Cellgro], 13% Hyskon).
FIG. 1.
Shear stress waveforms. Four hemodynamic conditions were applied in this study: steady laminar shear stress (A, SS, 10±0 dynes/cm2), pulsatile, nonreversing laminar shear stress (B, PS, 10±10 dynes/cm2), oscillatory laminar shear stress (C, OS, 0±10 dynes/cm2), and net positive, pulsatile, reversing oscillatory laminar shear stress (D, RS, 2±10 dynes/cm2).
Immunocytochemistry
To determine the effects of shear on the elongation of BaEOCs, immunocytochemical staining for the junctional protein VE-Cadherin was performed following shear stress exposure. Briefly, BaEOCs were washed with HBSS with calcium and magnesium (Cellgro) and fixed in 4% formaldehyde. Following fixation, cells were blocked for 1 h with donkey serum, and then incubated with a VE-cadherin antibody (Santa Cruz) overnight. Following overnight primary staining, cells were washed four times with HBSS for 5 min, and then a secondary antibody Alexa Fluor 488 (Invitrogen) was incubated for 1 h. Cells were then counterstained for nuclei using DAPI (Invitrogen). Cells were then imaged using an epifluorescent microscope (Nikon Eclipse Ti). Cell perimeter and areas were then determined manually using ImageJ. Cell elongation was then calculated as previously described method, where a circle would have a value of 1, while a straight line would have a value of 0.36
Quantitative real-time polymerase chain reaction and analysis
Following shear exposure, BaEOCs were rinsed with HBSS with calcium and magnesium (Cellgro), and imaged. RNA was then isolated using a commercially available RNA isolation kit (Qiagen) and following the manufacturer's recommended protocol with DNase digestion (Qiagen). Following RNA isolation, RNA amount and quality were measured using the NanoDrop 1000 (Thermo Scientific). cDNA was then made from 1 μg of RNA using a commercially available SuperScript® III First-Strand Synthesis System (Invitrogen) for real-time polymerase chain reaction (RT-PCR). The cDNA was diluted 1:10 and RT-PCR was then run using an Applied Bioscience Step One Plus PCR machine. Primer pairs are described in Table 1. Furthermore, 18S, used as a housekeeping gene, was found not to be affected by shear stress, and due to its abundance, a 1:1000 dilution of the cDNA was used.
Table 1.
Quantitative Real-Time Polymerase Chain Reaction Primer Sequences
| Gene | Forward | Reverse |
|---|---|---|
| CD39 | AGTGATTCCAAGGTCCCAGCACC | TCCTGAGCAACCGCATGCCT |
| eNOS | ATCTCCGCCTCGCTCATG | AGCCATACAGGATTGTCGCCT |
| EPCR | CACCCTGCAGCAGCTCAATGC | ACATCGCCGTCCACCTGTGC |
| ICAM-1 | GCAGTCAACAGCTAAAACCTTCCT | GCAGCGTAGGGTAAGGTTCTTG |
| PECAM-1 | CAGCCTTCAACAGAGCCAACC | CACTCCGATGATAACCACTGC |
| Tissue factor | CACCGACGAGATTGTGAAGGAT | TTCCCTGCCGGGTAGGAG |
| TFPI | GACTCCGCAATCAACCAAGGT | TGCTGGAGTGAGACACCATGA |
| Thrombomodulin | GGTGGACGGCGAGTGTGTGG | GGTGTTGGGGTCGCAGTCGG |
| VCAM-1 | GGGAAGATGGTCGTGATCCTT | TGAGACGGAGTCACCAATCTG |
EPCR, endothelial protein-C receptor; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1.
The Δ-ΔCt method described by Pfaffl37 was used to determine fold regulation between different hemodynamic conditions. More specifically, this method uses the efficiency of the primer pair rather than using the idealized efficiency of 2. Efficiency values for each primer pair were determined by at least 3 standard curves.
Western blot analysis
Following shear stress application, cells were washed with phosphate-buffered saline and lysed in RIPA buffer containing phosphatase and proteinase inhibitors (Roche) and collected from the dish. Protein samples were sonicated and then a BCA protein assay was performed (Pierce). Cell lysates were then loaded and separated on a 10% SDS-PAGE. Protein was then transferred to a nitrocellulose membrane. Following a 1 h blocking period, membranes were incubated with the primary antibodies (ICAM-1 [Abcam], E-Selectin [Proteintec], VCAM-1 [Novus]) overnight. Following primary incubation, membranes were incubated with the corresponding secondary antibody (Li-Cor). Membranes were then imaged using an Li-Cor Odyssey® imaging system. Total protein concentration was then quantitated using Scion Image.
Monocyte adhesion
Following shear stress application, Tamm-Horsfall protein-1 (THP-1) monocytes were statically incubated with sheared cells using a protocol modified from Tsao et al.38 Briefly, monocytes were concentrated to 106 cells/mL and incubated with 5 μL/mL of 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester (BCECF-AM; Invitrogen) for 30 min in serum-free RPMI media to fluorescently label the monocytes. The shear-conditioned BaEOCs were washed three times with HBSS with calcium and magnesium, and then 6 mL of monocytes was incubated with the shear-conditioned BaEOCs at a final concentration of 0.5×106 monocytes/mL in serum-free RPMI media. After 30 min, the unbound monocytes were washed away four times with HBSS with calcium and magnesium and the BaEOCs with the adhered monocytes were fixed. The bound monocytes were then imaged using an epifluorescent microscope (Nikon Eclipse Ti). For each monocyte adherence study, eight random images were taken throughout the plate and averaged to obtain a value for the individual plate. For each experiment, there were two technical replicates (i.e., plates). The values for the two plates were then averaged to determine the value for number of the monocytes bound per condition per experiment. Five donors were used in these experiments.
Factor Xa activity assay
To quantify the effect of shear stress on tissue factor activity, a Factor Xa assay was performed. Following shear, a solution of Factor VIIa (20 nM; Enzyme Research Laboratories) and Factor X (200 nM; Enzyme Research Laboratories) was incubated with the BaEOCs for 1 h. Following the hour incubation, the sample activity levels were colorimetrically determined using Spectroenzyme FXa (0.667 nM; American Diagostica) and compared to a standard curve of Factor Xa (American Diagnostica). Samples were visualized every 31 s for 20 min at 37°C. EDTA was added during the 20 min incubation period to stop the conversion of Factor X to Factor Xa. Sample amounts were determined by the maximum slopes of the colorimetric curves. The samples were then normalized to total DNA content as determined by a PicoGreen® assay according to the manufacturer's specifications (Invitrogen).
To minimize the use of expensive enzymes, suspension cell culture 100-mm plates (Corning) were used with 9-mm-diameter collagen type I spots adsorbed to the plate surface for 1 h. BaEOCs were seeded on these collagen type I regions for 1 h. Growth medium was then placed in the dish, and BaEOCs were allowed to grow to confluency and sheared. Following shear, a 15.6-mm-diameter cylinder was placed around the cellular growth area to contain reagents of the Factor Xa assay.
Statistical analysis
All analysis was performed on experiments using the statistical analysis program JMP v.9. More specifically, analysis of variance and Tukey's post hoc testing were performed regarding a p-value<0.05 as significant. Additionally, all experiments were independently performed with an n-value of greater than or equal to three baboon donor cell lines.
Results
Cellular elongation of BaEOCs under shear stress
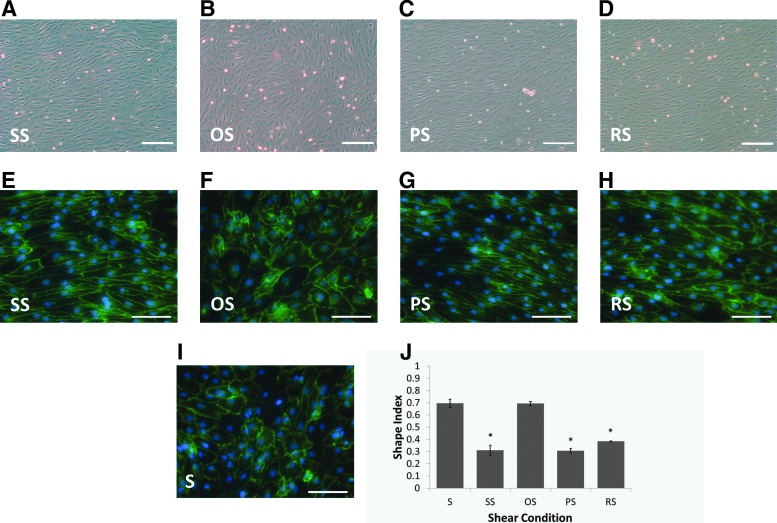
As seen in Figure 2, BaEOCs elongated under SS, i.e., steady laminar shear (Fig. 2A, E), and PS, that is, pulsatile shear (Fig. 2B, F), while maintaining their cobblestone morphology under OS, that is, oscillatory shear (Fig. 2C, G) conditions. Interestingly, even BaEOCs exposed to RS, that is, reversing shear (Fig. 2D, H), elongated. To quantify the elongation, BaEOCs were stained with an antibody against the junctional protein VE-Cadherin, and the perimeter and the area of the cell was calculated using ImageJ. We found that cells exposed to SS, PS, and RS were significantly more elongated than cells exposed to static or OS (Fig. 2I).
FIG. 2.
Baboon endothelial outgrowth cells (BaEOC) elongation under shear stress. Bright-field image at an original objective of 5×and immunocytochemistry staining of VE-Cadherin at an original objective of 10×of BaEOCs after exposure to SS (A, E), OS (B, F), PS (C, G), RS (D, H), and static (I). Cellular elongation of the cells was determined through immunocytochemical staining for VE-Cadherin (E–I) and quantification can be seen in (J). BaEOCs aligned in response to flow in SS, PS, and RS conditions, while OS maintains the cobblestone-like morphology of the static BaEOCs. S, static. Values are mean±standard error of the mean (SEM) with an ndonor=3. *p<0.05 in S and OS versus SS, PS, and RS. Scale bars represent 200 μm for (A–D) and 100 μm for (E–I).
Steady, laminar and pulsatile, laminar shear stress increased eNOS mRNA in BaEOCs while not affecting pro-inflammatory gene or protein levels
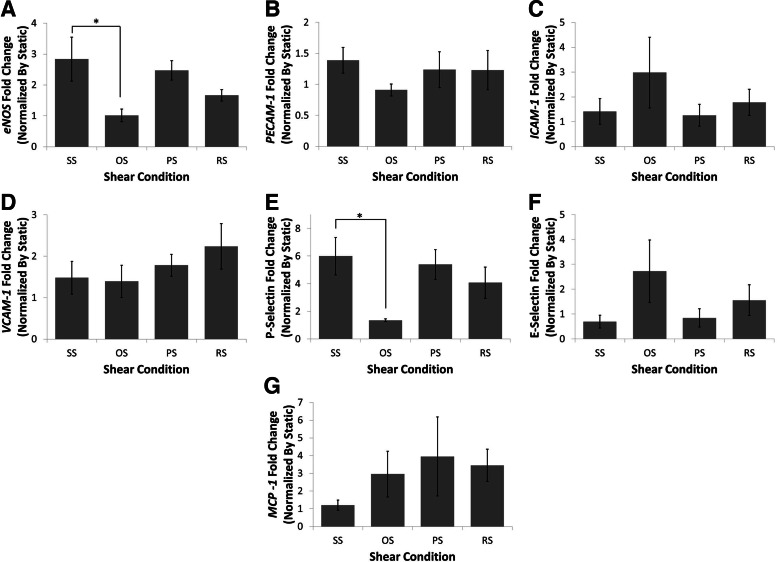
To determine if BaEOCs react in a similar manner to temporal varying shear stress waveforms as mature endothelial cells, eNOS, a well-known shear responsive gene,22 was analyzed (Fig. 3A). Exposure of EOCs to SS significantly increased eNOS mRNA over that of OS (2.5-fold). Interestingly, RS caused eNOS mRNA levels to fall in between SS and OS levels.
FIG. 3.
Laminar and pulsatile shear stress increased eNOS mRNA while not affecting inflammatory markers of BaEOCs. eNOS (A), PECAM-1 (B), intercellular adhesion molecule-1 (ICAM-1) (C), vascular cell adhesion molecule-1 (VCAM-1) (D), P-Selectin (E), E-Selectin (F), and MCP-1 (G) mRNA levels were determined by using quantitative real-time polymerase chain reaction (RT-PCR). mRNA was quantified and compared between shear conditions. Values are mean±SEM with an ndonor=5. *p<0.05 in OS versus SS.
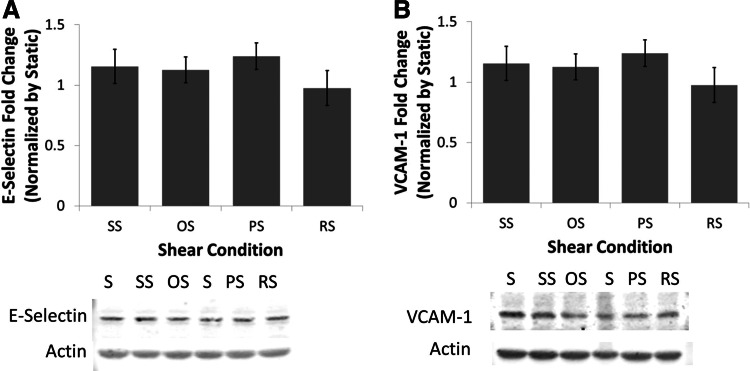
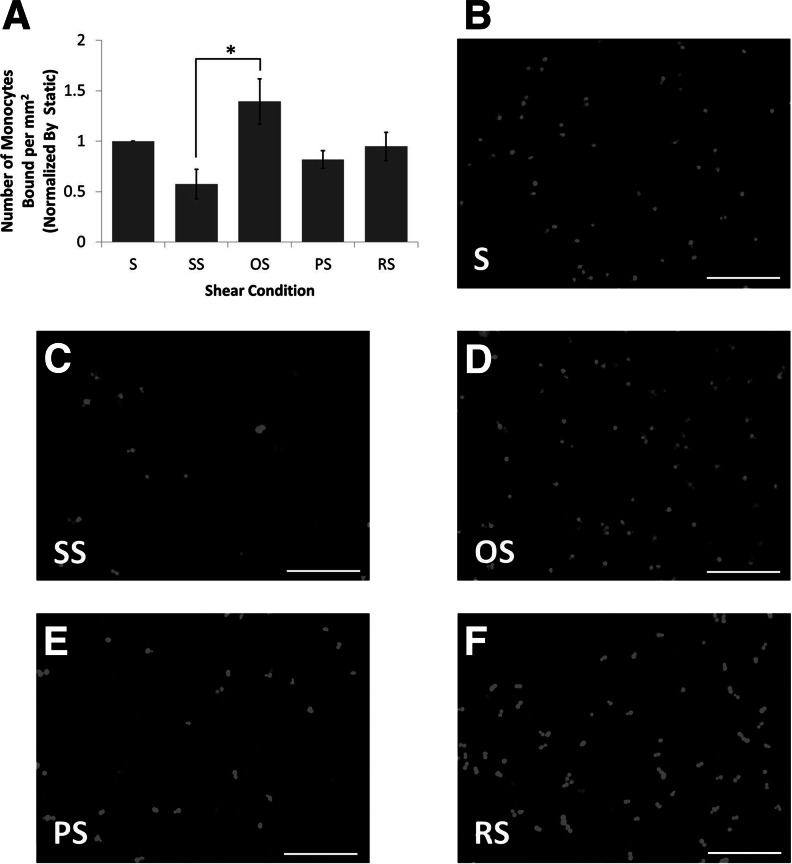
Next, the effect of shear stress on the pro-inflammatory molecules ICAM-1, PECAM-1 VCAM-1, E-Selectin, P-Selectin, and MCP-1 was determined (Fig. 3B–G). It was found that the differing hemodynamic conditions had no effect on the mRNA levels of ICAM-1, PECAM-1, VCAM-1, E-Selectin, or MCP-1. Interestingly, mRNA levels of P-Selectin were decreased by OS when compared to SS. To determine if the protein levels of the adhesion molecules changed with shear, western blot analysis was performed. The protein levels of VCAM-1 and E-Selectin, similar to the mRNA levels, were not affected by shear stress application (Fig. 4). ICAM-1 was also investigated, but the protein levels were below the level of detection by western blotting. Inflammation at the functional level was investigated using a monocyte adhesion assay. OS was found to significantly increase the number of monocytes bound to the endothelium when compared to SS (2.5-fold; Fig. 5A). The monocyte binding level in BaEOCs exposed to PS and RS fell between SS and OS levels.
FIG. 4.
Shear stress does not affect the protein levels of the inflammatory markers VCAM-1 and E-Selectin. VCAM-1 (A) and E-Selectin (B) were determined by western blotting. Actin was used as a loading control. Protein levels were determined by densitometry. n=4.
FIG. 5.
Laminar shear stress decreased the inflammatory potential of BaEOCs. Monocyte binding assay was performed and the number of bound monocytes was quantified (A) and normalized by static levels of monocyte binding. (B–F) are representative images of fluorescently labeled monocytes. Values are mean±SEM with an ndonor=5. S, static. *p<0.05 in SS versus OS. Original magnification of 10×. Scale bars represent 200 μm.
Laminar shear stress increased anti-thrombotic gene responses while not affecting pro-thrombotic responses
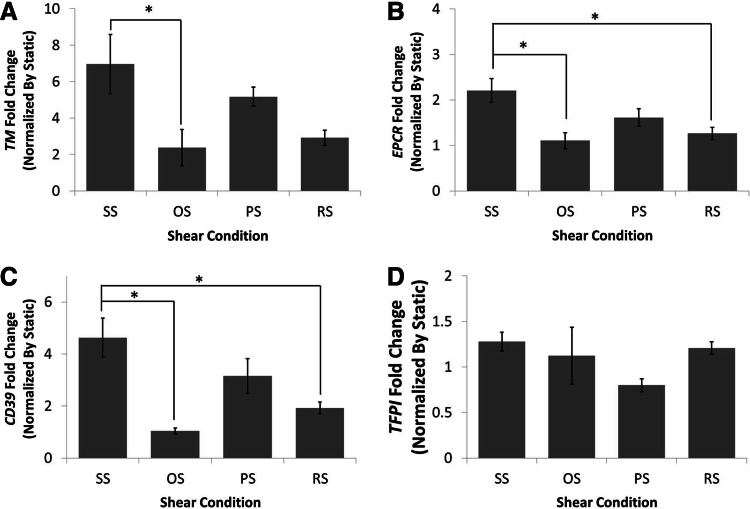
To determine how shear stress affected the thrombotic pathway activation the anti-thrombotic molecules thrombomodulin, CD39, tissue factor pathway inhibitor (TFPI), and EPCR were investigated. TFPI was found to be the only anti-thrombotic molecule that was not significantly affected by shear stress application (Fig. 6D). Thrombomodulin (Fig. 6A), EPCR (Fig. 6B) and CD39 (Fig. 6C) were all significantly upregulated when compared to OS (three-, two-, and fivefold, respectively). Furthermore, CD39 and EPCR levels under SS were significantly higher when compared to RS.
FIG. 6.
Laminar shear stress increased the anti-thrombogenicity of BaEOCs. Thrombomodulin (TM) (A), endothelial protein-C receptor (EPCR) (B), CD39 (C), and TFPI (D) mRNA levels were determined by using quantitative real-time PCR. mRNA was quantified and compared between shear conditions. Values are mean±SEM with an ndonor=5. *p<0.05.
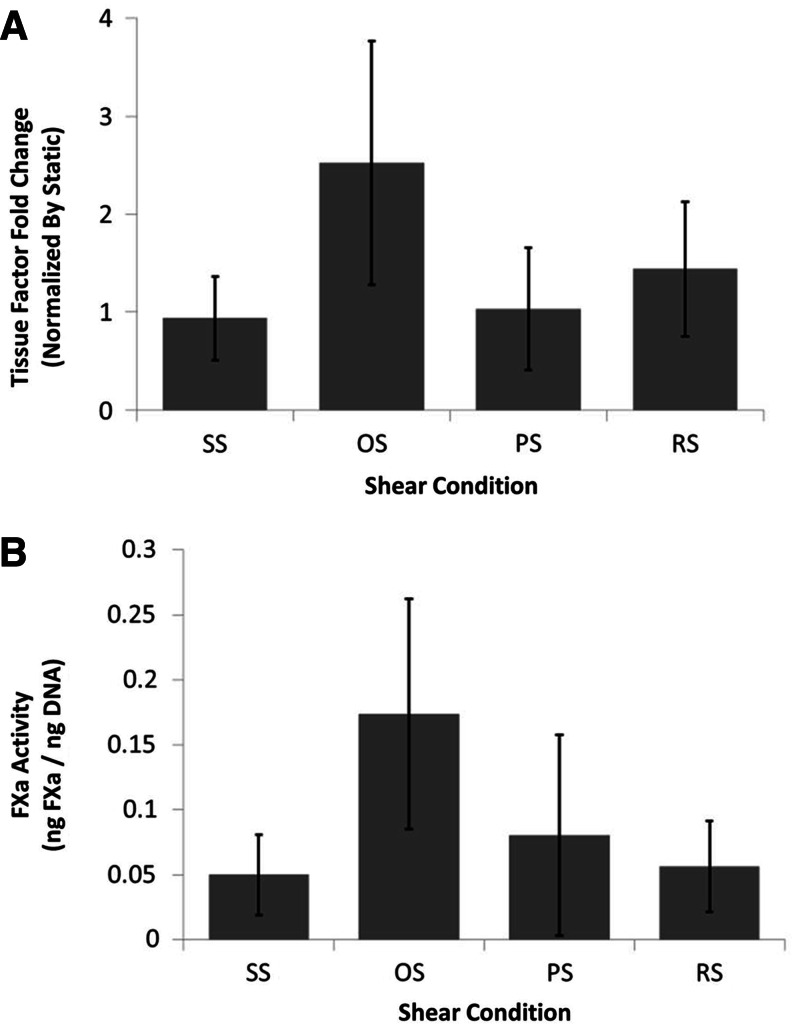
To examine how shear stress affected tissue factor, a marker of pro-thrombotic pathway activation, expression, and activity were measured (Fig. 7). No significant shear regulation at the mRNA level of tissue factor was observed (Fig. 7A). To verify this finding, an assay that measures tissue factor activity through the activation of Factor X to Factor Xa was used. Similar to the tissue factor mRNA, no significant shear regulation of the conversion of Factor X into Factor Xa was found (Fig. 7A). Interestingly, we found that static levels were higher than all shear conditions (data not shown).
FIG. 7.
Shear stress had no significant effect on tissue factor mRNA or activity in BaEOCs. Tissue factor mRNA (A) was determined by using quantitative real-time PCR. mRNA was quantified and compared between shear conditions. Tissue factor activity was determined by conversion of Factor X to Factor Xa through Spectroenzyme FXa and was normalized by total DNA by a PicoGreen® assay. Values are mean±SEM with an ndonor=5 (A) or ndonor=4 (B).
Discussion
In this study, the modulation of the inflammatory and thrombotic pathways in BaEOCs was investigated using four different hemodynamic environments. In summary, it was found that (1) there was an increase in monocyte adhesion in BaEOCs between oscillatory shear stress and steady shear stress; (2) steady, laminar shear stress increased eNOS mRNA levels when compared to oscillatory, shear stress; (3) the five inflammatory markers, ICAM-1, PECAM-1, VCAM-1, E-Selectin, and MCP-1, were not significantly affected by shear stress at the mRNA level, and, in additional, protein levels of E-Selectin and VCAM-1 were not altered by shear stress; (4) the anti-thrombotic markers CD39, EPCR, and thrombomodulin mRNAs were increased under laminar shear stress when compared to oscillatory shear; (5) tissue factor mRNA and activity was not significantly affected by shear stress. At this point, we are unable to explain the mechanism responsible for the increase in monocyte adhesion, but in what follows is a discussion of possible mechanisms and future research directions to explore.
The first marker to be investigated in this study was the well-known, shear-responsive mRNA eNOS.18,22 eNOS is typically present in healthy endothelial cells and produces the vasodilator molecule NO.31,39 eNOS has also been shown to play an important role in inhibiting platelet aggregation. NO produced in ECs can affect circulating platelets by activating protein kinase A, which then can diminish platelet aggregation.31,40–43 Additionally, NO affects leukocyte–endothelial interactions, causes vasodilation, and inhibits smooth muscle cell proliferation.44 eNOS knockout mice have increased risk for atherosclerosis, stroke, and thrombosis while also being hypertensive.44 It is believed that low levels of eNOS, and, in turn, low levels of NO cause endothelial dysfunction and eventual vascular pathology.44 It was found that eNOS (Fig. 3A) was significantly increased in cells exposed to SS when compared to OS. The response under RS was similar to that of OS. This result agrees with other findings in EOCs and mature endothelial cells where eNOS is significantly increased by laminar shear stress as compared to static conditions.3,4,12,13,17 Interestingly, Hinds et al. have shown that BaEOCs have decreased eNOS levels in comparison to mature baboon endothelial cells, but even though eNOS levels are low, the gene is still shear-responsive.1 This study agrees with previous shear studies published in mature endothelial cells looking at differences between SS and OS.45
The inflammatory markers ICAM-1, PECAM-1, VCAM-1, E-Selectin, P-Selectin, and MCP-1 were investigated in BaEOCs under the four shear stress conditions. Only P-Selectin mRNA was found to be changed by shear stress application (Fig. 3E), while the other inflammatory markers were not affected at the mRNA or protein level. This agrees with previous studies performed in human EOCs investigating VCAM-1 and ICAM-1 when comparing SS versus static conditions13,14,17,46 and in Mazzolai et al., which compares VCAM-1 in bidirectional flow versus static conditions.14 There is, however, one study that found an increase in ICAM-1 under laminar, shear stress compared to static conditions.13 In this study, E-selectin was not affected by shear stress application at mRNA or protein levels. Interestingly, E-selectin in EOCs has been shown to be increased by SS exposure in one study13 but not another.14 Finally, P-Selectin mRNA was down-regulated by OS when comparing to SS and PS. A previous study found that P/E-Selectin knockout mice have less atherosclerotic development.47
The monocyte adhesion assay was performed to determine the overall inflammation level of the BaEOCs (Fig. 4), and as noted earlier BaEOCs exposed to OS had more monocytes bound than SS (2.5-fold) agreeing with results seen in mature endothelial cells (Fig. 5A).25,48 Interestingly, one of the inflammatory makers shown here can have both inflammatory and anti-inflammatory actions. PECAM-1 has been found to have pro-inflammatory activities, such as facilitating the leukocyte transendothelial migration, and anti-inflammatory properties such as suppression of inflammatory cytokines and lowering leukocyte activation.49 The dual nature of this molecule may provide potential mechanism by which monocyte adhesion is occurs. Additionally, localization of inflammatory markers (VCAM-1, E-Selectin) may provide clues to why monocyte adhesion increases under OS when compared to SS. Finally, other molecules, such as ICAM-2, may mediate monocyte adhesion and should be investigated.
Endothelial markers for the thrombotic pathway were measured to understand the ability of the EOCs to mediate thrombus formation. The anti-thrombotic markers thrombomodulin, CD39, EPCR, and TFPI (Fig. 6) were examined. Here, no differences in the mRNA level of TFPI for any of the shear conditions were found (Fig. 6D); however, SS significantly increase thrombomodulin mRNA (threefold) over OS (Fig. 6A). This result agrees with previous findings showing thrombomodulin increased in EOCs; however, in those studies, SS values were compared only against the static level expression of the gene.11–13 Additionally, there was a trend of an increase in thrombomodulin (twofold, p<0.06) when comparing SS to RS. Similarly CD39 mRNA levels were significantly increased under SS conditions when compared to OS and RS (Fig. 6). Additionally, a trend for increased CD39 (p<0.1) was observed when comparing PS to OS. Finally, EPCR showed the same response as CD39, where SS was significantly higher than OS and RS (Fig. 5). Interestingly, in human umbilical vein endothelial cells, SS was shown to decrease EPCR, highlighting the need for more EOC specific shear studies.50
Although CD39, EPCR, and thrombomodulin are anti-thrombotic and membrane-bound, each acts through a distinct mechanism. CD39 is an ecto-nucleosidase51 that inhibits platelet activation by the hydrolysis of ADP and ATP to AMP.52 AMP is then degraded to adenosine by CD73.53 Adenosine is a strong inhibitor of platelet aggregation.31 Thrombomodulin is a cofactor for the activation of protein C by thrombin. When thrombin is bound to thrombomodulin, the activation of protein C is increased 20,000-fold compared to free thrombin-protein C activation.30,54 EPCR acts as an enhancer for the activation of protein C. It is located next to the thrombin-thrombomodulin complex on the cell membrane and binds protein C. When bound to EPCR, the activation for protein C by thrombin is increased 6-fold in vitro55 and 20-fold in vivo.56 Taken together, SS increases the anti-thrombogenic potential of the BaEOCs through cofactors of activation of protein C (EPCR, thrombomodulin), which agrees with previous work,12 and through molecules that help in the inhibition of platelet aggregation (eNOS, CD39). Additionally, a previous study has found other molecules, such as tissue plasminogen activator, to be increased by SS in human EOCs, which can further increase the EOC anti-thrombogenic potential.15 These results suggest that SS will increase activated protein C levels over that of OS, which warrants further investigation.
The pro-thrombotic activity of the BaEOCs was investigated by looking at tissue factor mRNA and activity (Fig. 7A, B). Mazzolai et al. have previously shown that tissue factor mRNA and activity are increased under bidirectional flow in human EOCs cultured in a tubular construct.14 Additionally, Lund et al. have shown that exposure to steady, laminar shear stress decreases tissue factor mRNA and activity, although not significantly, from static conditions in human EOCs.17 Here, similar results to Lund et al. were found where tissue factor mRNA and activity were decreased by SS when compared to OS; however, it was not significantly different.17 In addition, no changes were observed when investigating PS and RS conditions.
It is important to note that some of the differences seen between the results shown here and previous studies may be due to differences in species and cell source. Two of the studies cited use human umbilical cord blood for the isolation of the EPCs, while the study presented here used juvenile baboon blood for EOC isolation.13,14 Additionally, the extracellular matrix used can have impact on endothelial cell biology. Some of the previous studies used fibronectin as the extracellular matrix, while in this study collagen type I was used.13 Cells cultured on fibronectin and exposed to shear stress have different signaling mechanisms than cells exposed to other matrices, such as collagen. Specifically, endothelial cells cultured on fibronectin and exposed to shear stress showed increased NF-κB activation.19,57,58 Additionally, a limitation of this study is the simplified model system of shear stress; however, the shear stress magnitudes used in this study are on the same magnitude of order found in the human vasculature.59–61 While it would be interesting to determine the EOC response to physiological waveforms, the goal of this article is to determine the best and worst case shear condition for preconditioning of vascular grafts. Finally, because this is a simplified model system of shear stress, the manner in which the cells react in vivo in a vascular graft where they are subjected to complex shear stress waveforms, chemical cues, and cyclic stretch may be different. Interestingly, studies of a vascular graft lined with ovine endothelial progenitor cells resisted the attachment of blood elements (i.e., platelets, red blood cells) when preconditioned with cyclic pressure and steady shear stress (13 dynes/cm2).62 In this study, they found increased expression of eNOS and prostaglandin I synthase when steady shear and cyclic stress was applied when compared to steady shear stress alone.
Taken together, these results suggest that shear stress application can have beneficial or adverse effects on the inflammatory and thrombotic potential of BaEOCs. The results show that SS increases the anti-inflammatory and anti-thrombogenic environments in BaEOCs. Additionally, OS increases the inflammatory and decreases the anti-thrombogenic environment when compared to SS. Finally, RS decreased the anti-thrombogenic environment when compared to SS, and had few differences when compared to OS. These results suggest that SS may be the optimal shear condition for hemodynamic preconditioning of a vascular implant or in tissue-engineered applications with incorporated EOCs. Additionally, our results suggest that the in vivo hemodynamic environment should be taken into account before using EOCs, as it may have an adverse effect on the functional characteristics of a vascular implant endothelialized with EOCs.
Acknowledgments
This study was supported by the National Institutes of Health R01-HL095474 through a subcontract from Oregon Health and Sciences University. The authors would like to thank Alexander J. Kiener and Angela R. Kamino for help in performing experiments. The authors would also like to acknowledge the support of Drs. Stephen R. Hanson and Deirdre E.J. Anderson at Oregon Health & Science University.
Disclosure Statement
No competing financial interest exists.
References
- 1.Hinds M.T. Ma M. Tran N. Ensley A.E. Kladakis S.M. Vartanian K.B., et al. Potential of baboon endothelial progenitor cells for tissue engineered vascular grafts. J Biomed Mater Res Part A. 2008;86A:804. doi: 10.1002/jbm.a.31672. [DOI] [PubMed] [Google Scholar]
- 2.Griese D.P. Ehsan A. Melo L.G. Kong D. Zhang L. Mann M.J., et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts. Circulation. 2003;108:2710. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 3.Tao J. Yang Z. Wang J.M. Tu C. Pan S.R. Effects of fluid shear stress on eNOS mRNA expression and NO production in human endothelial progenitor cells. Cardiology. 2006;106:82. doi: 10.1159/000092636. [DOI] [PubMed] [Google Scholar]
- 4.Ahmann K.A. Johnson S.L. Hebbel R.P. Tranquillo R.T. Shear stress responses of adult blood outgrowth endothelial cells seeded on bioartificial tissue. Tissue Eng Part A. 2011;17:2511. doi: 10.1089/ten.tea.2011.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina R. O'Neill C. Sweeney M. Guduric-Fuchs J. Gardiner T. Simpson D., et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F., et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertolini F. Mancuso P. Braidotti P. Shaked Y. Kerbel R.S. The multiple personality disorder phenotype(s) of circulating endothelial cells in cancer. Biochim Biophys Acta (BBA) - Reviews on Cancer. 2009;1796:27. doi: 10.1016/j.bbcan.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Critser P.J. Voytik-Harbin S.L. Yoder M.C. Isolating and defining cells to engineer human blood vessels. Cell Prolif. 2011;44:15. doi: 10.1111/j.1365-2184.2010.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen S. McDonald S.P. Toby P. Coates H. Bonder C.S. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin Sci. 2010;120:263. doi: 10.1042/CS20100429. [DOI] [PubMed] [Google Scholar]
- 10.Timmermans F. Plum J. Yöder M.C. Ingram D.A. Vandekerckhove B. Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thébaud N.B. Bareille R. Daculsi R. Bourget C. Rémy M. Kerdjoudj H., et al. Polyelectrolyte multilayer films allow seeded human progenitor-derived endothelial cells to remain functional under shear stress in vitro. Acta Biomater. 2010;6:1437. doi: 10.1016/j.actbio.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Ensley A.E. Nerem R.M. Anderson D.E.J. Hanson S.R. Hinds M.T. Fluid shear stress alters the hemostatic properties of endothelial outgrowth cells. Tissue Eng Part A. 2012;18:127. doi: 10.1089/ten.tea.2010.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown M.A. Wallace C.S. Angelos M. Truskey G.A. Characterization of umbilical cord blood–derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15:3575. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzolai L. Bouzourene K. Hayoz D. Dignat-George F. Liu J.W. Bounameaux H., et al. Characterization of human late outgrowth endothelial progenitor-derived cells under various flow conditions. J Vasc Res. 2011;48:443. doi: 10.1159/000324844. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z. Tao J. Wang J-M. Tu C. Xu M-G. Wang Y., et al. Shear stress contributes to t-PA mRNA expression in human endothelial progenitor cells and nonthrombogenic potential of small diameter artificial vessels. Biochem Biophys Res Commun. 2006;342:577. doi: 10.1016/j.bbrc.2006.01.172. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z. Wang J.-M. Wang L.-C. Chen L. Tu C. Luo C.-F., et al. In vitro shear stress modulates antithrombogenic potentials of human endothelial progenitor cells. Eur J Clin Pharmacol. 2007;23:121. doi: 10.1007/s11239-006-9045-0. [DOI] [PubMed] [Google Scholar]
- 17.Lund T. Hermansen S.E. Andreasen T.V. Olsen J.O. Osterud B. Myrmel T., et al. Shear stress regulates inflammatory and thrombogenic gene transcripts in cultured human endothelial progenitor cells. Thromb Haemost. 2010;104:421. doi: 10.1160/TH09-12-0854. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham K.S. Gotlieb A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2004;85:9. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 19.Orr A.W. Sanders J.M. Bevard M. Coleman E. Sarembock I.J. Schwartz M.A. The subendothelial extracellular matrix modulates NF-κB activation by flow. J Cell Biol. 2005;169:191. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 21.Berk B.C. Min W. Yan C. Surapisitchat J. Liu Y. Hoefen R. Atheroprotective mechanisms activated by fluid shear stress in endothelial cells. Drug News Perspect. 2002;15:133. doi: 10.1358/dnp.2002.15.3.704684. [DOI] [PubMed] [Google Scholar]
- 22.Boon R.A. Horrevoets A.J.G. Key Transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39. [PubMed] [Google Scholar]
- 23.Glagov S. Zarins C.K. Giddens D.P. Ku D.N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018. [PubMed] [Google Scholar]
- 24.Zarins C. Giddens D. Bharadvaj B. Sottiurai V. Mabon R. Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 25.Sorescu G.P. Sykes M. Weiss D. Platt M.O. Saha A. Hwang J., et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 26.Davies M. Gordon J. Gearing A. Pigott R. Woolf N. Katz D., et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 27.Hsiai T.K. Cho S.K. Wong P.K. Ing M. Salazar A. Sevanian A., et al. Monocyte recruitment to endothelial cells in response to oscillatory shear stress. FASEB J. 2003;17:1648. doi: 10.1096/fj.02-1064com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson-Tidey R. McGregor J. Taylor P. Poston R. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952. [PMC free article] [PubMed] [Google Scholar]
- 29.Kretz C.A. Vaezzadeh N. Gross P.L. Tissue factor and thrombosis models. Arterioscler Thromb Vasc Biol. 2010;30:900. doi: 10.1161/ATVBAHA.108.177477. [DOI] [PubMed] [Google Scholar]
- 30.Esmon C.T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989;264:4743. [PubMed] [Google Scholar]
- 31.Jones C.I. Barrett N.E. Moraes L.A. Gibbins J.M. Jackson D.E. Endogenous inhibitory mechanisms and the regulation of platelet function. Methods Mol Biol. 2012;788:341. doi: 10.1007/978-1-61779-307-3_23. [DOI] [PubMed] [Google Scholar]
- 32.Tsao P.S. Buitrago R. Chan J.R. Cooke J.P. Fluid flow inhibits endothelial adhesiveness. Circulation. 1996;94:1682. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y. Weisdrof D.J. Solovey A. Hebbel R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sucosky P. Padala M. Elhammai A. Balachandran K. Jo H. Yoganathan A. Design of an ex vivo culture system to investigate the effects of shear stress on cardiovascular tissue. J Biomech Eng. 2008;130:035001. doi: 10.1115/1.2907753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sdougos H.P. Bussolari S.R. Dewey C.F. Secondary flow and turbulence in a cone-and-plate device. J Fluid Mech. 1984;138:379. [Google Scholar]
- 36.Levesque M. Nerem R.M. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107:341. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 37.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:2002. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsao P.S. Lewis N.P. Alpert S. Cooke J.P. Exposure to shear stress alters endothelial adhesiveness. Circulation. 1995;92:3513. doi: 10.1161/01.cir.92.12.3513. [DOI] [PubMed] [Google Scholar]
- 39.Ranjan V. Xiao Z. Diamond S.L. Constitutive NOS expression in cultured endothelial cells is elevated by fluid shear stress. Am J Physiol Heart Circ Physiol. 1995;269:H550. doi: 10.1152/ajpheart.1995.269.2.H550. [DOI] [PubMed] [Google Scholar]
- 40.Maurice D.H. Haslam R.J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990;37:671. [PubMed] [Google Scholar]
- 41.Nolte C. Eigenthaler M. Horstrup K. Honig-Liedl P. Walter U. Synergistic phosphorylation of the focal adhesion-associated vasodilator-stimulated phosphoprotein in intact human platelets in response to cGMP- and cAMP-elevating platelet inhibitors. Biochem Pharmacol. 1994;48:1569. doi: 10.1016/0006-2952(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 42.Jensen B.O. Selheim F. Døskeland S.O. Gear A.R.L. Holmsen H. Protein kinase A mediates inhibition of the thrombin-induced platelet shape change by nitric oxide. Blood. 2004;104:2775. doi: 10.1182/blood-2004-03-1058. [DOI] [PubMed] [Google Scholar]
- 43.Napoli C. de Nigris F. Williams-Ignarro S. Pignalosa O. Sica V. Ignarro L.J. Nitric oxide and atherosclerosis: An update. Nitric Oxide. 2006;15:265. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Atochin D. Huang P. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflügers Arch Eur J Physiol. 2010;460:965. doi: 10.1007/s00424-010-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holliday C.J. Ankeny R.F. Jo H. Nerem R.M. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. 2011;301:H856. doi: 10.1152/ajpheart.00117.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Egorova A. DeRuiter M. de Boer H. van de Pas S. Gittenberger-de Groot A. van Zonneveld A., et al. Endothelial colony-forming cells show a mature transcriptional response to shear stress. In Vitro Cell Dev Biol Anim. 2012;48:21. doi: 10.1007/s11626-011-9470-z. [DOI] [PubMed] [Google Scholar]
- 47.Dong Z. Chapman S. Brown A. Frenette P. Hynes R. Wagner D. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni C.-W. Qiu H. Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762. doi: 10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Privratsky J.R. Newman D.K. Newman P.J. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jun P. Huangqing C. Xiaoheng L. Ruheng L. Xiaohong Z. Effects of shear stress on protein C activation, EPCR expression and TM expression in endothelial cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009;26:303. [PubMed] [Google Scholar]
- 51.Robson S. Sévigny J. Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcus A.J. Safier L.B. Hajjar K.A. Ullman H.L. Islam N. Broekman M.J., et al. Inhibition of platelet function by an aspirin-insensitive endothelial cell ADPase. Thromboregulation by endothelial cells. J Clin Invest. 1991;88:1690. doi: 10.1172/JCI115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaczmarek E. Koziak K. Sévigny J. Siegel J.B. Anrather J. Beaudoin A.R., et al. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 54.Esmon C.T. Owen W.G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981;78:2249. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stearns-Kurosawa D.J. Kurosawa S. Mollica J.S. Ferrell G.L. Esmon C.T. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A. 1996;93:10212. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor F.B. Peer G.T. Lockhart M.S. Ferrell G. Esmon C.T. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood. 2001;97:1685. doi: 10.1182/blood.v97.6.1685. [DOI] [PubMed] [Google Scholar]
- 57.Orr A.W. Ginsberg M.H. Shattil S.J. Deckmyn H. Schwartz M.A. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17:4686. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orr A.W. Hahn C. Blackman B.R. Schwartz M.A. p21-activated kinase signaling regulates oxidant-dependent NF-κB activation by flow. Circ Res. 2008;103:671. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ku D.N. Giddens D.P. Zarins C.K. Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol. 1985;5:293. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 60.Soulis J.V. Farmakis T.M. Giannoglou G.D. Louridas G.E. Wall shear stress in normal left coronary artery tree. J Biomech. 2006;39:742. doi: 10.1016/j.jbiomech.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Sui B. Gao P. Lin Y. Gao B. Liu L. An J. Assessment of wall shear stress in the common carotid artery of healthy subjects using 3.0-tesla magnetic resonance. Acta Radiol. 2008;49:442. doi: 10.1080/02841850701877349. [DOI] [PubMed] [Google Scholar]
- 62.Yazdani S.K. Tillman B.W. Berry J.L. Soker S. Geary R.L. The fate of an endothelium layer after preconditioning. J Vasc Surg. 2010;51:174. doi: 10.1016/j.jvs.2009.08.074. [DOI] [PubMed] [Google Scholar]