Abstract
The health and quality-of-life implications of overweight and obesity span all ages in the United States. We investigated the association between dietary protein intake and loss of lean mass during weight loss in postmenopausal women through a retrospective analysis of a 20-week randomized, controlled diet and exercise intervention in women aged 50 to 70 years. Weight loss was achieved by differing levels of caloric restriction and exercise. The diet-only group reduced caloric intake by 2,800 kcal/week, and the exercise groups reduced caloric intake by 2,400 kcal/week and expended ~400 kcal/week through aerobic exercise. Total and appendicular lean mass was measured using dual energy x-ray absorptiometry. Linear regression analysis was used to examine the association between changes in lean mass and appendicular lean mass and dietary protein intake. Average weight loss was 10.8±4.0 kg, with an average of 32% of total weight lost as lean mass. Protein intake averaged 0.62 g/kg body weight/day (range=0.47 to 0.8 g/kg body weight/day). Participants who consumed higher amounts of dietary protein lost less lean mass and appendicular lean mass r(=0.3, P=0.01 and r=0.41, P<0.001, respectively). These associations remained significant after adjusting for intervention group and body size. Therefore, inadequate protein intake during caloric restriction may be associated with adverse body-composition changes in postmenopausal women.
In the United States, the prevalence of overweight and obesity is on the rise, even in older adults (1). Health complications and decreases in quality of life that are associated with overweight and obesity (2-6) indicate the need for immediate attention. However, clinicians are reluctant to recommend weight loss in overweight and obese older adults because aging is associated with a loss of lean mass (7,8), and weight loss can exacerbate this problem. Therefore, strategies to minimize lean mass losses during weight loss can be particularly beneficial in older adults.
Weight loss elicited through caloric restriction results in a loss of both fat mass and lean mass in middle-aged men and women (9-11). The magnitude of lean mass lost during caloric restriction has been associated with the macronutrient content of the diet (12); however, the effect of dietary intake on body composition changes during weight loss in older adults remains unclear. The Current Recommended Dietary Allowance (RDA) for protein (0.8 g/kg body weight/day) is based on data from adults in energy balance (13); however, the RDA may not be sufficient to meet the needs of older adults (14). In addition, protein needs may be increased during caloric restriction, particularly in older adults whose caloric needs are already decreased (15).
Diets higher in protein have been associated with greater fat-free mass retention than diets lower in protein during caloric restriction (12). In premenopausal women, loss of lean mass per kilogram of fat mass lost during a 10-week hypocaloric diet was reduced in those consuming a higher protein (1.6 vs 0.8 g/kg body weight/day) diet, suggesting an improvement in the use of fat for energy resulting in the sparing of lean mass (16). Additional studies also show that increasing dietary protein can preserve lean mass during weight loss (17,18). However, there is little information on whether the variation in protein intake (per kg body weight) using current macronutrient recommendations (as a percentage of energy) is predictive of the loss of lean mass in response to a hypocaloric diet, or whether these effects are also observed in postmenopausal women. Therefore, the purpose of this study was to determine whether dietary protein is associated with loss of lean mass in a retrospective analysis of a caloric restriction and exercise weight-loss intervention in postmenopausal women.
METHODS
Subjects and Study Design
Overweight and obese postmenopausal women (n=70) aged 50 to 70 years were recruited from Forsyth County, NC, to participate in a 20-week intervention that consisted of either diet-only, diet and low-intensity aerobic exercise, or diet and high-intensity aerobic exercise. Study inclusion/exclusion criteria have been described previously (19). All participants provided informed consent to participate in the study according to the guidelines set forth by the Wake Forest University Institutional Review Board for Human Research. Baseline measurements of dietary intake, body composition, and VO2max were performed prior to the beginning and after completion of the intervention.
Study Interventions
The hypocaloric diet and exercise interventions have been described in detail elsewhere (19). Briefly, diets were developed for each participant to elicit an approximate 2,800 kcal/week energy deficit using caloric restriction (340 to 400 kcal/day) and exercise energy expenditure (~400 kcal/week). Macronutrient composition was designed to include approximately 25% to 30% of energy from fat, 15% to 20% from protein, and 50% to 60% from carbohydrate. However, because of the moderate-to-severe obesity present in this population, coupled with low resting metabolic rates, the macronutrient prescription resulted in an absolute protein intake of 0.47 to 0.8 g/kg body weight/day. All women were provided lunch, dinner, and snacks daily from the Wake Forest University General Clinical Research Center metabolic kitchen. These meals were prepared based on each participant’s choices from a menu designed by a registered dietitian. Participants assigned to the exercise groups exercised 3 days per week under the supervision of an exercise physiologist.
Body Composition
Height and weight were measured without shoes or outer garments. Percent body fat, lean mass, and appendicular lean mass, and total mass were measured by dual energy x-ray absorptiometry (Hologic Delphi QDR, Bedford, MA, software version 11.2, 2002). Appendicular lean mass was calculated as the sum of nonbone lean mass in arms and legs.
Statistics
These retrospective analyses were performed using JMP software (version 4.0.4, 2001, SAS Institute, Cary, NC). Descriptive statistics were calculated and values reported as mean±standard deviation or frequencies. One-way analysis of variance was used to calculate the differences between the intervention groups. An α level of .05 was used as the nominal type I error rate. Linear regression analysis was performed to examine the association between lean mass and appendicular lean mass and dietary protein. Regression models were adjusted for intervention group, body size (height and baseline lean mass or appendicular lean mass), and change in fat mass.
RESULTS AND DISCUSSION
Subject Characteristics
Seventy women (diet only: n=24; diet and low-intensity aerobic exercise: n=24; diet and high-intensity aerobic exercise: n=22) completed this 20-week weight-loss intervention. Mean age of the population was 58 years and 33% were African American. Mean fitness capacity (VO2max) was 20.67 mg/kg/min. There were no significant differences in age, race, or fitness capacity at baseline between the three intervention groups.
Dietary Intake During the Hypocaloric Feeding Intervention
Mean caloric intake was 1,288±166 kcal/day. Average caloric restriction was 350 kcal/day (range=211 to 693 kcal/day). Mean caloric deficit was considerably greater in the diet-only group compared to the diet and low-intensity aerobic exercise and diet and high-intensity aerobic exercise groups; however, the caloric expenditure of exercise was similar between the exercising groups. To maintain the recommended protein intake (15% to 20% of energy), protein intakes averaged 0.62 g/kg body weight/day (range=0.47 to 0.80), but did not differ between intervention groups. Macronutrient intakes (52% of energy from carbohydrates, 27% from fat, and 17% from protein) were not substantially different between intervention groups.
The participants’ meals were provided by the Wake Forest University General Clinical Research Center metabolic kitchen. Participants were provided with choices within their meal plans, which added flexibility and improved both compliance (mean dietary compliance was 100.1%±0.4% of the provided calorie level) and satisfaction, factors necessary for long-term success of any diet modification. Moreover, providing meals to the participants alleviated the confounding associated with self-reporting of dietary intakes and provided a more accurate measure of protein and energy intakes. However, this highly controlled diet limits the translation of our caloric-restriction intervention plan to real-life, practical interventions.
Effects of Intervention Group on Changes in Body Composition
Baseline and postintervention body composition of the participants is shown in the Table. Average weight loss was 10.8±4.0 kg (−12.2%±4.2%) and was not significantly different between groups. Range of weight loss was −1.2 to −20.9 kg, with 94% of the participants losing ≥5% of initial body weight. Relative loss of lean mass was 33% for the total study population and there were no significant differences in lean mass by intervention group (34%, 32%, and 30% for diet-only, diet and low-intensity aerobic exercise, and diet and high-intensity aerobic exercise, respectively). As expected, the amount of lean mass lost increased as the amount of total weight lost increased (r=0.69, P<0.0001).
Table.
Body composition of participants at baseline and after a 20-week diet-only or diet-plus-exercise intervention for postmenopausal womena
| Total (n=70) | DIETb (n=24) | LOEXc (n=24) | HIEXd (n=22) | |
|---|---|---|---|---|
| mean±standard deviation | ||||
| Body mass index e | ||||
| Baseline | 33.0±3.6 | 33.2±3.8 | 33.3±3.2 | 32.5±4.0 |
| Postintervention | 29.0±3.4 | 29.3±3.5 | 28.9±3.2 | 28.7±3.5 |
| Total mass (kg) | ||||
| Baseline | 91.4 ± 11.1 | 92.9±10.2 | 90.6±8.8 | 90.8±14.2 |
| Postintervention | 79.9±10.4 | 81.3 ±10.1 | 78.3±8.9 | 79.5±12.2 |
| Total lean mass (kg) | ||||
| Baseline | 52.7±5.6 | 53.9±5.2 | 52.4±5.1 | 51.9±6.5 |
| Postintervention | 49.0±5.5 | 49.8±5.4 | 48.6±5.2 | 48.6±6.1 |
| Appendicular lean mass (kg) | ||||
| Baseline | 23.0±3.1 | 23.7±3.0 | 22.6±2.8 | 22.8±3.6 |
| Postintervention | 21.2±2.9 | 21.7±3.0 | 20.8±2.7 | 21.1 ±3.2 |
| Total fat mass (kg) | ||||
| Baseline | 38.7±6.7 | 39.0±6.7 | 38.2 ±5.3 | 38.9±8.3 |
| Postintervention | 30.7±6.3 | 31.4±6.6 | 29.7±5.8 | 30.9±6.8 |
| Body fat (%) | ||||
| Baseline | 42.1 ±3.3 | 41.8±3.7 | 42.1 ±3.1 | 42.6±3.0 |
| Postintervention | 38.2 ±4.1 | 38.4±4.5 | 37.7±4.5 | 38.6±3.4 |
No significant differences at P<0.05 across intervention groups.
DIET=hypocaloric diet only.
LOEX=hypocaloric diet plus low-intensity exercise.
HIEX=hypocaloric diet plus high-intensity exercise.
Calculated as kg/m2.
Effects of Protein Intake on Changes in Body Composition
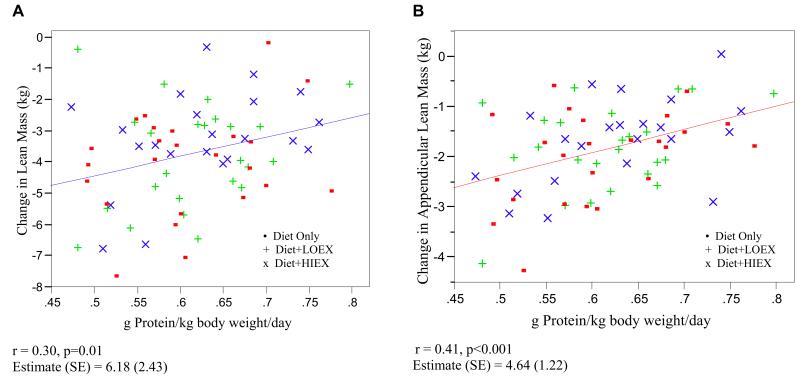
Because there were no statistically significant group differences in changes in body composition, the groups were combined for the analysis of protein intake. The Figure represents the relationship between loss of lean mass and appendicular lean mass by dietary protein (g/kg body weight/day) intake. Participants who consumed higher amounts of dietary protein lost less lean mass and appendicular lean mass (r=0.3, P=0.01 and r=0.41, P=0.001, respectively). There was also a significant correlation between protein intake (g/kg body weight/day) and absolute (kg) fat mass loss (r=0.37, P=0.001).
Figure.
Relationship of the loss of lean mass (A) and appendicular lean mass (B) with dietary protein (g/kg body weight/day) intake in postmenopausal women. LOEX=hypocaloric diet plus low-intensity exercise. HIEX=hypocaloric diet plus high-intensity exercise.
Dietary protein intake was associated with loss of lean mass and appendicular lean mass in regression analysis. In unadjusted models, participants who consumed higher amounts of dietary protein lost less lean mass and appendicular lean mass (β [standard error (SE)]: 0.62 [0.24], P=0.01, and β [SE]: 0.46 [0.12], P<0.001, respectively). Thus, for every 0.1 g/kg body weight/day increase in dietary protein, participants lost 0.62 kg less lean mass. The relationships remained significant after adjusting for intervention group (β [SE]: 0.59 (0.24), P=0.02, and β [SE]: 0.46 [0.12], P<0.001, for lean mass and appendicular lean mass, respectively) and body size (β [SE]: 0.68 [0.32], P=0.04, and β [SE]: 0.39 [0.17], P=0.02, for lean mass and appendicular lean mass, respectively).
In additional analyses, we excluded four participants who failed to lose at least 5% of their initial body weight in order to avoid the potential confounding effect of negligible weight loss on lean mass and three participants who were outliers (>2 standard deviations below the mean) for relative loss of lean mass. The relationship between dietary protein intake and loss of lean mass and appendicular lean mass remained significant (β [SE]: 0.91 [0.28] and 0.51 [0.17], respectively, P<0.01) after adjusting for intervention group and body size. In addition, protein intake remained a significant predictor of lean mass and appendicular lean-mass loss even after adjusting for change in fat mass (β [SE]: 0.54 [0.27], P=0.048, and 0.36 [0.17], P=0.04, respectively). We also stratified participants by percent weight lost (5% to <10%, 10% to <15%, and ≥15%) to determine whether the relationship between protein intake and lean-mass loss was consistent across weight-loss groups. Loss of lean mass was associated with protein intake in each group (5% to <10%, P=0.02; 10% to <15%, P=0.09; ≥15%, P=0.009).
Current National Institutes of Health guidelines for treating overweight and obese adults recommend low-calorie/low-fat diets for weight loss, with protein recommendations expressed as a percentage of total calories (20). People trying to lose weight by reducing calories may inadvertently consume very low levels of protein, possibly resulting in loss of lean mass, which can be especially detrimental in older adults (7). In a 9-week weight-maintenance study in older women, those consuming low dietary protein (0.45g/kg body weight/day) experienced substantial decreases in lean mass compared to those consuming adequate dietary protein (0.92 g/kg body weight/day) (21). In a meta-analysis by Krieger and colleagues (12), diets higher in protein (>1.05 to ≤1.20 g/kg body weight/day) were associated with greater fat-free mass retention than diets lower in protein (<0.7 g/kg body weight/day) during energy restriction. Thus, the RDA for protein may be inadequate for retaining fat-free mass during caloric restriction (12).
Our data suggest that there is a linear relationship between protein intake and loss of lean mass during periods of negative energy balance; therefore, efforts to identify weight-loss treatments that improve the quantity and quality of dietary protein are warranted. The results of this study support recommendations that protein needs should be based on body size and not on caloric intake. However, in a hypocaloric environment, planning meals with adequate protein is difficult, and increasing the ratio of protein to total calories cannot always be palatably met with food alone. We have recently shown that protein supplementation in this population is both feasible and palatable, in addition to being an effective strategy to attenuate lean-mass loss during caloric restriction (22).
This was a retrospective analysis of data previously collected in a randomized, controlled intervention. Although we found that protein intakes below the RDA are predictive of lean-mass loss during weight loss, our study was limited by a relatively small range of protein intakes—none of which was above the RDA. Thus, we cannot infer that this relationship exists at higher protein intakes. The conduct of a randomized, clinical weight-loss trial providing diets of varying and higher protein intake is needed to determine whether lean-mass loss is attenuated with protein intakes at or above the RDA.
CONCLUSIONS
As we have shown, adequate protein intake is a potentially modifiable component of a healthful lifestyle that may help maintain lean mass during voluntary dietary-induced weight loss in older adults. Considering the rise of overweight and obesity in the United States in all age populations, establishment of safe and healthful weight-loss guidelines should be a priority. To minimize the loss of lean mass associated with weight loss, adults should be educated on the benefits of balanced nutrition, and especially the need for adequate dietary protein. With the decline of lean mass with aging in adults (23), and the exacerbation of lean-mass loss as a result of low protein intake in hypocaloric diets, placing emphasis on the benefits of higher protein intakes may help attenuate age-related and diet-related losses of lean mass in older adults.
Acknowledgments
This study was supported by NIH Grant R01-AG/DK20583, Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332), and Wake Forest University General Clinical Research Center (M01-RR07122).
We are grateful to the study coordinators, bionutrition staff, exercise physiologists, nurses, and laboratory technicians of the Section on Gerontology and Geriatric Medicine, and the General Clinical Research Center at Wake Forest University School of Medicine for their assistance in the conduct of this study. We also thank all women who voluntarily participated in this study.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Harris TB, Launer LJ, Madans J, Feldman JJ. Cohort study of effect of being overweight and change in weight on risk of coronary heart disease in old age. BMJ. 1997;314:1791–1794. doi: 10.1136/bmj.314.7097.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahyoun NR, Hochberg MC, Helmick CG, Harris T, Pamuk ER. Body mass index, weight change, and incidence of self-reported physician-diagnosed arthritis among women. Am J Public Health. 1999;89:391–394. doi: 10.2105/ajph.89.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher S. Bariatrics: Considering Mobility, Patient Safety and Caregiver Injury. CRC Press; New York: 2004. [Google Scholar]
- 5.Goya WS, Gerald SA, Whincup PH, Walker M. Overweight and obesity and the burden of disease and disability in elderly men. Int J Obes Relat Metab Disord. 2004;28:1374–1382. doi: 10.1038/sj.ijo.0802775. [DOI] [PubMed] [Google Scholar]
- 6.Livingston EH, Ko CY. Use of the health and activities limitation index as a measure of quality of life in obesity. Obes Res. 2002;10:824–832. doi: 10.1038/oby.2002.111. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Ross R. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging. 2005;9:408–419. [PubMed] [Google Scholar]
- 8.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 9.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO. Washington University School of Medicine CALERIE Group. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care. 2002;25:431–438. doi: 10.2337/diacare.25.3.431. [DOI] [PubMed] [Google Scholar]
- 12.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression 1. Am J Clin Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine . Protein and Amino Acids. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. National Academies Press; Washington, DC: 2002. pp. 465–608. [Google Scholar]
- 14.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–M380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 15.Pellet PL, Young VR. The effects of different levels of energy intake on protein metabolism and of different levels of protein intake on energy metabolism: A statistical evaluation from the published literature. In: Scrimshaw NS, Schurch B, editors. Protein-Energy Interactions. International Dietary Energy Consultative Group; Lausanne, Switzerland: 1992. pp. 81–121. [Google Scholar]
- 16.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 17.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 18.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 19.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes (Lond) 2006;30:1211–1216. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]
- 20.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obesity Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 21.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr. 1995;62:30–39. doi: 10.1093/ajcn/62.1.30. [DOI] [PubMed] [Google Scholar]
- 22.Gordon MM, Bopp M, Easter L, Miller GD, Lyles MF, Houston DK, Nicklas BJ, Kritchevsky SB. Effects of dietary protein on the composition of weight loss in post-menopausal women. J Nutr Health Aging. doi: 10.1007/BF02983202. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierson RN, Jr, Lin DH, Phillips RA. Total-body potassium in health: Effects of age, sex, height, and fat. Am J Physiol. 1974;226:206–212. doi: 10.1152/ajplegacy.1974.226.1.206. [DOI] [PubMed] [Google Scholar]