Abstract
Ikaros encodes a zinc finger protein that is essential for hematopoiesis and that acts as a tumor suppressor in leukemia. Ikaros function depends on its ability to localize to pericentromeric heterochromatin (PC-HC). Ikaros protein binds to the upstream regulatory elements of target genes, aids in their recruitment to PC-HC, and regulates their transcription via chromatin remodeling. We identified four novel Ikaros phosphorylation sites that are phosphorylated by CK2 kinase. Using Ikaros phosphomimetic and phosphoresistant mutants of the CK2 phosphorylation sites we demonstrate that: 1) CK2-mediated phosphorylation inhibits Ikaros’ localization to PC-HC; 2) dephosphorylation of Ikaros at CK2 sites increases its binding to the promoter of the terminal deoxynucleotidetransferase (TdT) gene leading to TdT repression during thymocyte differentiation, and 3) hyperphosphorylation of Ikaros promotes its degradation by the ubiquitin/proteasome pathway. We show that Ikaros is dephosphorylated by Protein Phosphatase 1 (PP1) via interaction at a consensus PP1-binding motif, RVXF. Point mutations that abolish Ikaros-PP1 interaction result in functional changes in DNA-binding affinity and subcellular localization similar to those observed in hyperphosphorylated Ikaros and/or Ikaros phosphomimetic mutants. Phosphoresistant Ikaros mutations at CK2 sites restored Ikaros’ DNA-binding activity and localization to PC-HC and prevented accelerated Ikaros degradation. Results demonstrate the role of CK2 kinase in lymphocyte differentiation and in regulation of Ikaros’ function, and suggest that CK2 promotes leukemogenesis by inhibiting the tumor suppressor activity of Ikaros. We propose a model whereby the balance of CK2 kinase and PP1 phosphatase is essential for normal lymphocyte differentiation and for the prevention of malignant transformation.
Keywords: Ikaros, CK2 kinase, Protein Phosphatase 1, PP1, tumor suppression, lymphocyte differentiation, casein kinase II
Introduction
The Ikaros gene encodes a C2H2 zinc finger protein with expression that is restricted to hematopoietic cells and the pituitary gland [1, 2]. Proteins generated from the Ikaros gene contain two separate zinc finger domains. Four zinc fingers in the amino half of the protein take part in sequence-specific DNA binding [3]. The two zinc fingers at the C-terminus of the protein are responsible for protein-protein interaction [4], enabling Ikaros proteins to form dimers or multimers with different Ikaros isoforms or Ikaros family members. The association of full-length Ikaros with small Ikaros isoforms that lack DNA-binding zinc fingers results in a functionally inactive complex, thus small isoforms act as dominant inhibitors [3]. In humans, the function of the two largest Ikaros isoforms is regulated by their interactions with each other [5, 6]. In this review we summarize the evidence that CK2 kinase directly phosphorylates Ikaros and regulates its function as a tumor suppressor in leukemia.
Function of Ikaros
An essential requirement for Ikaros function is the ability to localize to pericentromeric heterochromatin (PC-HC) [7]. Ikaros associates with histone deacetylase (HDAC)-containing complexes (NuRD and Sin3A and Sin3B) [8], although HDAC-independent repression via binding to the transcriptional corepressor CtBP has been described [9]. Ikaros also associates with Brg-1, a catalytic subunit of the SWI/SNF nucleosome remodeling complex that acts as an activator of gene expression [10, 11]. The current hypothesis is that Ikaros binds the upstream region of target genes and aids in their recruitment to PC-HC, resulting in repression or activation of transcription of the gene [7, 12]. The ability to localize to PC-HC is essential for Ikaros’ function as an activator or repressor of transcription. Thus, Ikaros can act both as an activator and a repressor of target gene expression, depending on whether it associates with the NuRD, the CtBP or the SWI/SNF complex.
Ikaros is a tumor suppressor in leukemia
Homozygous dominant-negative (DN) Ikaros knock-out mice lack T, B, and NK cells and antigen-presenting dendritic cells [13]. Heterozygous Ikaros DN-knockout mice develop T cell leukemia with 100% penetrance. T cell leukemia in Ikaros knock-out mice is associated with clonal expansion of leukemic cells and the loss of the single wild type Ikaros allele [14]. These observations established Ikaros as a primary regulator of lymphoid development and a tumor suppressor. In human, Ikaros has been associated with a variety of malignancies including childhood ALL [15-19] infant T-cell ALL [20], adult B cell ALL [21], myelodysplastic syndrome [22], AML [23], and adult and juvenile CML [24]. Deletion of an Ikaros allele was detected in over 80% of BCR-ABL1 ALL and deletion or mutation of Ikaros has been identified as a poor prognostic marker for childhood ALL [15, 16, 25]. These data established Ikaros as a major tumor suppressor in human leukemia.
Previous studies demonstrated that Ikaros has essential roles in tumor suppression and in normal hematopoiesis. However, Ikaros is abundantly expressed in all hematopoietic cells and throughout the cell cycle, suggesting that its function might be regulated by post-translational modifications. Cell cycle-specific phosphorylation has been shown to regulate Ikaros’ function during mitosis [26], and sumoylation to regulate Ikaros function in transcriptional regulation [27]. Here, we summarize our findings that CK2 kinase-mediated phosphorylation regulates Ikaros function in T cell differentiation and transcriptional regulation, as well as its protein stability. Based on our results, we propose that increased CK2 kinase activity leads to hyperphosphorylation of Ikaros, loss of Ikaros’ tumor suppressor function, and the development of leukemia.
CK2-mediated phosphorylation controls Ikaros DNA-binding affinity for γ satellite DNA and its localization to pericentromeric heterochromatin
CK2 kinase has been shown to phosphorylate Ikaros at multiple sites, and indeed, CK2 kinase is responsible for the majority of Ikaros phosphorylation [28]. An early study demonstrated that some phosphorylation occurred in the region between amino acids 383 and 404 within exon 8, and at amino acid #63 [28], however the remaining phosphorylation sites were unidentified. We performed in vivo phosphopeptide mapping, as well as mass spectrometry of endogenous Ikaros in murine thymocytes, to identify amino acids that are phosphorylated in vivo. These experiments identified four novel, evolutionarily conserved amino acids – #13, #23, #101 and #294, in addition to the previously reported sites, that are phosphorylated in vivo.
To determine whether phosphorylation affects the ability of Ikaros to bind DNA, gel shift experiments were performed with nuclear extracts from 293T cells that expressed wild type Ikaros or Ikaros phosphomutants. NIH 3T3 cells do not express endogenous Ikaros and have previously been used to study the effects of Ikaros mutations on Ikaros DNA binding and subcellular localization [29]. Ikaros phosphomutants with mutation of specific phosphorylation sites to alanine are phosphoresistant and those where the phosphorylation site is mutated to aspartate are phosphomimetic. Our results showed that phosphomimetic substitution at amino acid #13 or #294 decreases the ability of Ikaros to bind a γ satellite DNA probe by 5-fold. Mutation of the same amino acids to alanine did not produce changes in DNA-binding affinity. These data demonstrate that phosphorylation of a single amino acid controls the DNA-binding affinity of Ikaros toward γ satellite repeats.
In hematopoietic cells, wild type Ikaros localizes in pericentromeric foci and produces a punctate pattern when visualized by confocal microscopy [7]. To determine if phosphorylation controls the localization of Ikaros to pericentromeric foci, we tested the effect of phosphorylation of individual amino acids on the subcellular localization of Ikaros. We expressed wild type and phosphomutant Ikaros in NIH 3T3 cells by retroviral transduction. Our results showed that a single phosphomimetic mutation at amino acid #13 or #294 caused the re-distribution of Ikaros protein in the nucleus from pericentromeric localization to a diffuse nuclear staining pattern, while phosphoresistant mutations produced no changes in the subcellular localization of Ikaros. These data suggest that targeting of Ikaros to PC-HC is regulated by its phosphorylation at specific amino acids.
Phosphorylation by CK2 controls Ikaros DNA-binding affinity for developmentally regulated Ikaros target genes
Our next question was whether phosphorylation of Ikaros regulates its DNA-binding affinity toward known in vivo Ikaros target genes. Ikaros has been shown to bind to the D’ regulatory sequence upstream of the TdT gene and to negatively regulate TdT expression [30]. Stimulation of thymocytes with PMA plus ionomycin induces thymocyte differentiation and leads to increased binding of Ikaros to the TdT D’ regulatory sequence and rapid downregulation of TdT expression [30]. The molecular mechanisms that result in increased binding of Ikaros to the TdT D’ regulatory sequence during thymocyte differentiation were unknown. We tested the ability of phosphomimetic Ikaros mutants to bind the TdT D’ regulatory sequence by gel shift assay. Results showed that phosphoresistant mutation at amino acid #13 or #294 increases Ikaros’ DNA-binding affinity toward the TdT D’ regulatory sequence, while phosphomimetic mutation of amino acid #294 decreases Ikaros’ ability to bind DNA by 3-fold as compared to wild type. These data suggest that phosphorylation of Ikaros controls its ability to bind to the regulatory sequence of the TdT gene.
We examined the ability of Ikaros to bind the TdT D’ regulatory element during differentiation of CD4+/CD8+ thymocytes. Electromobility shift assay was performed using nuclear extracts from unstimulated CD4+/CD8+ thymocytes and from thymocytes 24 hours following PMA plus ionomycin-induced differentiation. The results revealed that the affinity of Ikaros for the TdT D’ regulatory region was reduced by three-fold in unstimulated thymocytes as compared to PMA plus ionomycin-stimulated cells. Phosphatase treatment of nuclear extracts from unstimulated thymocytes increased the ability of Ikaros to bind the TdT D’ regulatory region to that observed in stimulated cells. Phosphatase treatment did not induce significant changes in cells stimulated with PMA plus ionomycin. These results suggest that the decreased affinity of Ikaros for the TdT D’ regulatory element in unstimulated thymocytes is due to direct phosphorylation.
To determine if CK2-mediated phosphorylation of Ikaros has a role in regulating gene expression during T cell development, we examined changes in the phosphorylation of endogenous Ikaros at CK2 phosphorylation sites during thymocyte differentiation. Two-dimensional phosphopeptide mapping of endogenous Ikaros, in unstimulated thymocytes and in thymocytes induced to differentiate, showed that Ikaros undergoes dephosphorylation of amino acids #13 and #294 following the induction of thymocyte differentiation. These data suggest that the DNA-binding affinity of Ikaros for the TdT D’ regulatory element during thymocyte differentiation is controlled by phosphorylation at specific amino acids. These results imply that the reversible phosphorylation of Ikaros at amino acids #13 and #294 are physiologically relevant in regulating expression of the TdT gene during thymocyte differentiation. Our studies suggest that one of the major factors that leads to increased Ikaros binding to the TdT D’ regulatory element and the initiation of TdT repression is the dephosphorylation of Ikaros.
The effects of CK2 kinase inhibitors on the phosphorylation of Ikaros during T cell differentiation were studied using the VL3-3M2 murine thymocyte cell line. Following stimulation with PMA plus ionomycin this cell line is induced to differentiate in the same manner as primary thymocytes [31]. We compared phosphopeptide maps from in vivo-labeled endogenous Ikaros in untreated VL3-3M2 cells and following partial inhibition of CK2 kinase with TBB (a specific CK2 inhibitor). Partial inhibition of CK2 kinase in VL3-3M2 cells caused a loss of phosphorylation at amino acids #13, #23, #101 and #294, and at several other amino acids, that were difficult to resolve. These findings suggest that phosphorylation at amino acids #13, #23, #101 and #294 is very sensitive to the fluctuation of CK2 kinase activity, and that high CK2 kinase activity is necessary for in vivo phosphorylation of these amino acids.
We verified the ability of CK2 kinase to phosphorylated Ikaros at CK2 consensus phosphorylation sites using in vitro assays. Phosphopeptide mapping of the recombinant Ikaros phosphorylated in vitro by CK2 kinase produced a similar phosphopeptide map to that obtained for endogenous, in vivophosphorylated Ikaros in VL3-3M2 cells. The phosphopeptides generated in vitro co-migrated with those from VL3-3M2 cells when VL3-3M2 samples were loaded together with in vitro CK2 kinase samples. These results suggest that CK2 kinase directly phosphorylates amino acids #13, #23, #63, #101 and #294 in vivo.
Our studies suggest that dephosphorylation of Ikaros is an integral part of normal thymocyte differentiation. These data reveal a novel role for CK2 kinase – the regulation of thymocyte differentiation by controlling Ikaros activity. These data provide evidence that alteration in CK2 kinase activity and/or hyperphosphorylation of Ikaros can impair T cell differentiation and potentially play a role in leukemogenesis.
PP1 interacts with Ikaros in vivo via a specific recognition motif
Screening to identify the potential phosphatase that dephosphorylates Ikaros identified the PP1 phosphatase has having high dephosphorylating activity toward Ikaros. The association of endogenous Ikaros with endogenous PP1 in vivo was confirmed by co-immunoprecipitation assay in various human and murine cells lines [32].
Sequence analysis showed that Ikaros contains an evolutionarily-conserved PP1 recognition motif R/K-X0-1-[V/I]-X-[F/W] [33-35] at amino acids 459-470. Mutation of valine and phenylalanine at positions 465 and 467, respectively (IK-A465/7) of the Ikaros protein results in the loss of interaction between Ikaros and PP1, and an inability of PP1 to dephosphorylate Ikaros. These results demonstrate that the presence of the PP1-recognition motif is essential for the interaction and dephosphorylation of Ikaros by PP1.
Dephosphorylation of Ikaros by PP1 regulates its DNA-binding ability and pericentromeric localization
DNA-binding assays showed that the wild type Ikaros expressed in 293T cells binds strongly to the γ satellite A probe, while the PP1-nonbinding Ikaros mutant (IK-A465/7) does not. Incubation with calf intestinal alkaline phosphatase (CIAP) during the DNA binding assay restored the DNA binding of the IKA465/7 mutant to that observed for wild type Ikaros. This suggested that the loss of DNA binding in the PP1-nonbinding Ikaros mutant was due to increased phosphorylation. Next we introduced phosphoresistant mutations in the PP1-nonbinding Ikaros mutant. The introduction of 11 phosphoresistant mutations at CK2 phosphorylation sites in the PP1-nonbinding Ikaros mutant (IK-A11+A465/7), restored its ability to bind the γ satellite A probe. These results provide evidence that a lack of dephosphorylation by PP1 leads to hyperphosphorylation of Ikaros by CK2 kinase, and a loss of DNA binding activity.
The PP1-nonbinding Ikaros mutant protein, IK-A465/7, showed a loss of pericentromeric localization. Introduction of phosphoresistant mutations at CK2-phosphorylated residues also restored PC-HC localization of this mutant.
The experiments with Ikaros mutants that are unable to interact with PP1, provide strong evidence that CK2-mediated phosphorylation is one of the major mechanisms regulating Ikaros function in DNA binding and chromatin remodeling. These results reveal that dephosphorylation of Ikaros is essential for preserving its function in hematopoietic cells.
Lack of dephosphorylation leads to increased degradation of Ikaros due to CK2-mediated phosphorylation at PEST regions
A comparison of mRNA and protein levels for wild type and PP1-nonbinding Ikaros mutants suggested that the inability of Ikaros to bind with PP1 regulated the expression of Ikaros at the post-translational level. When transfected into 293T cells which do not express endogenous Ikaros, the protein level of the PP1-nonbinding Ikaros mutant (Ikaros A465/7) was more than 5-fold decreased compared to wild type Ikaros, despite the presence of similar levels of Ikaros mRNA. This suggested that the decreased Ikaros protein level observed for the Ikaros A465/7 mutant was due to an increase in degradation.
Ikaros phosphorylation sites that are phosphorylated by CK2 kinase are located within two typical PEST sequences [28, 36]. The PEST motifs function as targets for phosphorylation-mediated protein degradation [37, 38]. We compared the half-life of wild type Ikaros to that of the PP1-nonbinding Ikaros mutant (A465/7), as well as the Ikaros A465/7 mutant that has phosphoresistant alanine mutations at eleven amino acids known to be phosphorylated by CK2 kinase in vivo (Ikaros-A11+A465/7) using a pulse-chase degradation assay. Our results showed that the loss of interaction with PP1 results in a severely shortened Ikaros half-life (8-fold). The presence of alanine mutations at CK2 phosphorylation sites stabilized the Ikaros A465/7 mutant protein and extended its half-life by 5-fold. These results suggest that the phosphorylation of Ikaros by CK2 kinase at PEST regions promotes its degradation while dephosphorylation by PP1 stabilizes the Ikaros protein and extends its half-life. These results also suggest that a large percentage of Ikaros protein undergoes phosphorylation/dephosphorylation in vivo, since the loss of interaction with PP1 decreases protein levels by 8-fold.
Degradation of Ikaros is mediated by the ubiquitin/proteasome pathway
The evidence that Ikaros degradation is regulated by its phosphorylation at PEST sequences suggested that degradation might involve the ubiquitin/proteasome pathway. To test this hypothesis, the human MOLT-4 T-cell leukemia line was treated with the proteasome inhibitor Cbz-LLL (MG132) [39, 40] or left untreated. Immunoprecipitation experiments with anti-Ikaros and anti-ubiquitin antibodies revealed the presence of high molecular weight ladder-like complexes corresponding to Ikaros/ubiquitin conjugates. Treatment with MG132 increased the levels of detected Ikaros/ubiquitin conjugates, which provided further evidence that this complex is degraded by the proteasome. These results demonstrate that Ikaros is ubiquitinated in vivo, which suggests that the mechanism of Ikaros degradation involves the ubiquitin/proteasome pathway.
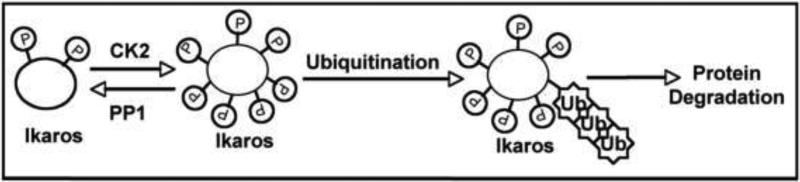
These data provide evidence that CK2-mediated phosphorylation of Ikaros regulates not only the function of the Ikaros protein, but also the Ikaros protein level in cells by inducing Ikaros degradation via the ubiquitin/proteosome pathway (summarized in Figure 1).
Fig. 1. A model for the regulation of Ikaros degradation by phosphorylation.
The phosphorylation of Ikaros by CK2 kinase promotes ubiquitin-mediated degradation. Dephosphorylation by PP1 prevents Ikaros degradation by the ubiquitin-proteosome pathway.
Conclusion
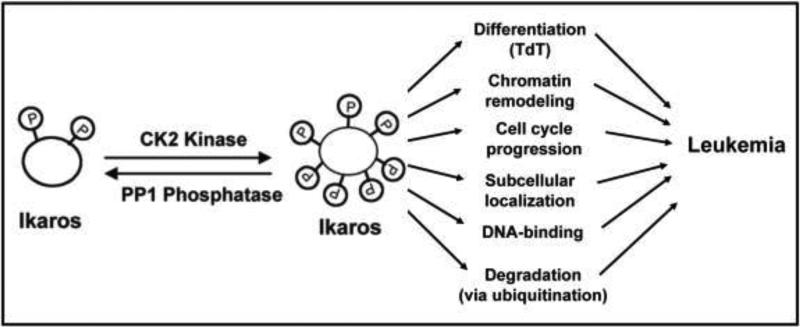
Overexpression of the catalytic subunit of CK2 kinase in transgenic mice contributes to the development of T cell leukemia and lymphoma [41-44]. Transgenic expression of the catalytic subunit of CK2 kinase also leads to lymphoproliferative and autoimmune disease [45]. Interestingly, all of these conditions have been observed in mice with impaired Ikaros function [14, 46-48]. Our studies demonstrate that CK2-mediated phosphorylation of Ikaros controls the essential functions of Ikaros including DNA-binding, subcellular localization, and chromatin remodeling, as well as the level of Ikaros protein in cells (via ubiquitination and degradation). Phosphorylation of Ikaros by CK2 kinase also regulates cell cycle progression and Ikaros function in T cell differentiation. Since the overexpression of CK2 kinase and the loss of Ikaros function have been strongly associated with leukemogenesis, we propose that increased CK2 kinase activity leads to impaired function and/or degradation of Ikaros which results in malignant transformation and the development of leukemia (summarized in Figure 2). Future research efforts will be directed toward testing this model and dissecting the molecular mechanisms by which the CK2 signal transduction pathway promotes leukemogenesis.
Fig. 2. Model of CK2 kinase and PP1 phosphatase regulation of Ikaros tumor suppressor activity.
Our model proposes that increased CK2 kinase activity leads to hyperphosphorylation of Ikaros, which impairs its function in transcriptional regulation, cell cycle progression, T cell differentiation, as well as its degradation. This leads to the loss of Ikaros tumor suppressor activity and the development of leukemia.
Acknowledgments
This work was supported in part by an R01 HL095120 grant, a St. Baldrick's Foundation Career Development Award, the Four Diamonds Fund of the Pennsylvania State University, College of Medicine, and the John Wawrynovic Leukemia Research Scholar Endowment (SD). This work was also supported by the Center for Health Disparities and Molecular Medicine and the Department of Pathology and Human Anatomy, Loma Linda University School of Medicine (KJP).
References
- 1.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258(5083):808–12. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 2.Yu S, Asa SL, Ezzat S. Fibroblast growth factor receptor 4 is a target for the zinc-finger transcription factor Ikaros in the pituitary. Mol Endocrinol. 2002;16(5):1069–78. doi: 10.1210/mend.16.5.0832. [DOI] [PubMed] [Google Scholar]
- 3.Molnár A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994;14(12):8292–303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. Embo J. 1996;15(19):5358–69. [PMC free article] [PubMed] [Google Scholar]
- 5.Ronni T, Payne KJ, Ho S, Bradley MN, Dorsam G, Dovat S. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282(4):2538–47. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Ebersole T, et al. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 2009;19(4):533–44. doi: 10.1101/gr.086496.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91(6):845–54. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 8.Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. Embo J. 1999;18(11):3090–100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem. 2000;275(26):19594–602. doi: 10.1074/jbc.M000254200. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill DW, Schoetz SS, et al. An ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol Cell Biol. 2000;20(20):7572–82. doi: 10.1128/mcb.20.20.7572-7582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Sif S, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10(3):345–55. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 12.Liberg D, Smale ST, Merkenschlager M. Upstream of Ikaros. Trends Immunol. 2003;24(11):567–70. doi: 10.1016/j.it.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Nichogiannopoulou A, Shortman K, Georogpoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7(4):483–92. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 14.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83(2):289–99. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 15.Mullighan CG, Gooha S, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–64. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 16.Mullighan CG, Miller CB, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–4. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper RP, Schoenmakers EF, et al. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–66. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- 18.Marcais A, Jeannet R, et al. Genetic inactivation of Ikaros is a rare event in human T-ALL. Leuk Res. 2010;34(4):426–9. doi: 10.1016/j.leukres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Dovat S, Payne KJ. Tumor suppression in T cell leukemia--the role of Ikaros. Leuk Res. 2010;34(4):416–7. doi: 10.1016/j.leukres.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L, Heerema N, et al. Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1999;96(2):680–5. doi: 10.1073/pnas.96.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakase K, Ishimaru F, et al. Dominant negative isoform of the Ikaros gene in patients with adult B- cell acute lymphoblastic leukemia. Cancer Res. 2000;60(15):4062–5. [PubMed] [Google Scholar]
- 22.Crescenzi B, La Starza R, et al. Submicroscopic deletions in 5q- associated malignancies. Haematologica. 2004;89(3):281–5. [PubMed] [Google Scholar]
- 23.Yagi T, Hibi S, et al. High frequency of Ikaros isoform 6 expression in acute myelomonocytic and monocytic leukemias: implications for up-regulation of the antiapoptotic protein Bcl-XL in leukemogenesis. Blood. 2002;99(4):1350–5. doi: 10.1182/blood.v99.4.1350. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama H, Ishimaru F, et al. Decreases in Ikaros activity correlate with blast crisis in patients with chronic myelogenous leukemia. Cancer Res. 1999;59(16):3931–4. [PubMed] [Google Scholar]
- 25.Mullighan CG, Su X, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dovat S, Ronni T, Russell D, Ferrini R, Cobb BS, Smale ST. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 2002;16(23):2985–90. doi: 10.1101/gad.1040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-del AP, Koipally J, Georgopoulos K. Ikaros SUMOylation: switching out of repression. Mol Cell Biol. 2005;25(7):2688–97. doi: 10.1128/MCB.25.7.2688-2697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-del AP, Maki K, Georgopoulos K. Phosphorylation controls Ikaros's ability to negatively regulate the G(1)-S transition. Mol Cell Biol. 2004;24(7):2797–807. doi: 10.1128/MCB.24.7.2797-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes and Development. 2000;14(17):2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinh LA, Ferrini R, et al. Down-regulation of TdT transcription in CD4+CD8+ thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes and Development. 2001;15(14):1817–1832. doi: 10.1101/gad.905601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groves T, Katis P, Madden Z, Manickam K, Ramsden D, Wu G, Guidos CJ. In vitro maturation of clonal CD4+CD8+ cell lines in response to TCR engagement. J Immunol. 1995;154(10):5011–22. [PubMed] [Google Scholar]
- 32.Popescu M, Gurel Z, Ronni T, Song C, Hung KY, Payne KJ, Dovat S. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284(20):13869–80. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakula P, Beullens M, Ceulemans H, Stalmans W, Bollen M. Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J Biol Chem. 2003;278(21):18817–23. doi: 10.1074/jbc.M300175200. [DOI] [PubMed] [Google Scholar]
- 34.Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115(Pt 2):241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 35.Zhao S, Lee EY. A protein phosphatase-1-binding motif identified by the panning of a random peptide display library. J Biol Chem. 1997;272(45):28368–72. doi: 10.1074/jbc.272.45.28368. [DOI] [PubMed] [Google Scholar]
- 36.Gurel Z, Ronni T, Ho S, Kuchar J, Payne KJ, Turk CW, Dovat S. Recruitment of ikaros to pericentromeric heterochromatin is regulated by phosphorylation. J Biol Chem. 2008;283(13):8291–300. doi: 10.1074/jbc.M707906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234(4774):364–8. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 38.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21(7):267–71. [PubMed] [Google Scholar]
- 39.Kim TK, Maniatis T. Regulation of interferon-gamma-activated STAT1 by the ubiquitin-proteasome pathway. Science. 1996;273(5282):1717–9. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- 40.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberhg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78(5):761–71. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 41.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21(34):5280–8. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 42.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. Embo J. 1996;15(19):5160–6. [PMC free article] [PubMed] [Google Scholar]
- 43.Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267(5199):894–7. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 44.Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2alpha collaborate in the development of thymic lymphomas in mice. Oncogene. 1998;16(23):2965–74. doi: 10.1038/sj.onc.1201854. [DOI] [PubMed] [Google Scholar]
- 45.Rifkin IR, Channavajhala PL, et al. Acceleration of lpr lymphoproliferative and autoimmune disease by transgenic protein kinase CK2 alpha. J Immunol. 1998;161(10):5164–70. [PubMed] [Google Scholar]
- 46.Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10(3):333–43. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- 47.Kathrein KL, Lorenz R, Innes AM, Griffiths E, Winandy S. Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol Cell Biol. 2005;25(5):1645–54. doi: 10.1128/MCB.25.5.1645-1654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojcik H, Griffiths E, Staggs S, Hagman J, Winandy S. Expression of a non-DNA-binding Ikaros isoform exclusively in B cells leads to autoimmunity but not leukemogenesis. Eur J Immunol. 2007;37(4):1022–32. doi: 10.1002/eji.200637026. [DOI] [PubMed] [Google Scholar]