Abstract
‘Amyloid binging proteins’ is a generic term used to designate proteins that interact with different forms of amyloidogenic peptides or proteins and that, as a result, may modulate their physiological and pathological functions by altering solubility, transport, clearance, degradation, and fibril formation. We describe a simple affinity chromatography protocol to isolate and characterize amyloid-binding proteins based on the use of sequential elution steps that may provide further information on the type of binding interaction. As an example, we depict the application of this protocol to the study of Alzheimer’s amyloid β (Aβ) peptide-binding proteins derived from human plasma. Biochemical analysis of the proteins eluted under different conditions identified serum amyloid P component (SAP) and apolipoprotein J (clusterin) as the main plasma Aβ-binding proteins while various apolipoproteins (apoA-IV, apoE, and apoA-I), as well as albumin (HSA) and fibulin were identified as minor contributors.
Keywords: Affinity chromatography, Alzheimer disease, Amyloid peptides, Amyloid-binding proteins
1. Introduction
Amyloid-binding protein is a generic term used to group together a heterogeneous collection of proteins that interact with different structural assemblies (monomeric, oligomeric, and fibrillar) of amyloidogenic peptides or proteins. These interactions have been described to modulate physiological and pathological functions of the respective amyloid subunits by altering their solubility, transport, clearance, degradation, and fibril formation.
Extensive immunohistochemical analysis have demonstrated that, in vivo, complex mixtures of unrelated molecules, collectively referred in the field as amyloid-associated proteins, colocalize with all types of amyloid deposits albeit not being a structural part of the final fibril (reviewed in ref. 1). Serum amyloid P component (SAP), α1-antichymotrypsin (ACT), apolipoprotein E (apoE), apolipoprotein J (apoJ) or clusterin, complement components, vitronectin, glycosaminoglycans, interleukins, and extracellular matrix proteins are among the many amyloid-associated components so far described coexisting with all forms of cerebral and systemic amyloidosis (2–11). To the moment, it is still unclear whether these molecules are innocent bystanders or whether their presence is related to the mechanism of amyloidogenesis. Several lines of investigation favor the latter notion, at least for some of them. For example, apoE and SAP have been found in all amyloid light chain (AL) fibrillar deposits, whereas their presence could not be demonstrated in the nonfibrillar Congo red negative immunoglobulin deposits in cases of light chain deposition disease (12, 13). Similar findings have been reported in cerebral forms of amyloidosis in which SAP and activation-derived products of the complement system are present in amyloid deposits but consistently absent in nonfibrillar preamyloid lesions (8, 12). Mice knockout for either SAP or apoE exhibit fewer amyloid lesions and delay in their onset although neither SAP nor apoE gene ablation prevents the formation of amyloid deposits (14, 15).
Most of the published reports dealing with amyloid-binding proteins are limited to those found associated with amyloid β (Aβ), the major constituent of the fibrils deposited into senile plaques and cerebral blood vessels of patients with Alzheimer’s disease (AD) (16). Aβ extracted from senile plaques of AD patients is mainly 42–43 amino acids long (17), while vascular amyloid is two residues shorter at the C-terminus (18). A soluble form of Aβ (sAβ), homologous to the amyloid protein extracted from cerebrovascular lesions (19), has also been identified in culture media supernatants from untransfected and β amyloid precursor protein (βAPP)-transfected cells, as well as in cerebrospinal fluid (CSF), plasma, urine and brain parenchyma from normal subjects and AD patients (19–23). Notably, several proteins have been shown to interact with sAβ in physiological fluids (24–26) and in vitro experiments have demonstrated that the presence of plasma or CSF prevents the fibril formation of synthetic peptides homologous to sAβ (27, 28), likely reflecting the result of the peptide’s interaction with the biological fluid components. In this sense, many of the amyloid-associated proteins have also the ability to modulate the formation of Aβ fibrils in vitro. Some of them (e.g., complement component C1q, apoE4, SAP, ACT) enhance Aβ fibril formation (27, 29–32), while others (e.g., apoJ) contribute to the peptide solubility precluding fibrillogenesis in vitro (11, 33). In the latter, this protecting effect has been proposed to contribute to the enhanced production of slowly sedimenting Aβ-derived diffusible ligands (ADDLs) highly toxic to neurons in culture at nanomolar concentrations (34).
The present work describes a simple methodology to identify amyloid-binding proteins by using affinity chromatography (see Note 1). This methodology is readily applicable to the study of different amyloidogenic proteins and their respective interactions with components of different physiological fluids. As an example, we describe the application of this protocol to the study of human plasma proteins with Aβ-binding properties by affinity chromatography using sequential elution with buffers of different characteristics. Detailed biochemical analysis and quantitation of the proteins eluted in the different fractions indicated that SAP and apoJ (clusterin) are the main plasma Aβ-binding proteins, while other minor components were identified as apoA-IV, apoE, and apoA-I, as well as HSA and fibulin (see Fig. 1). The distribution of these proteins within the different elution protocols is indicative of the distinct nature of the physicochemical interactions involved.
Fig. 1.
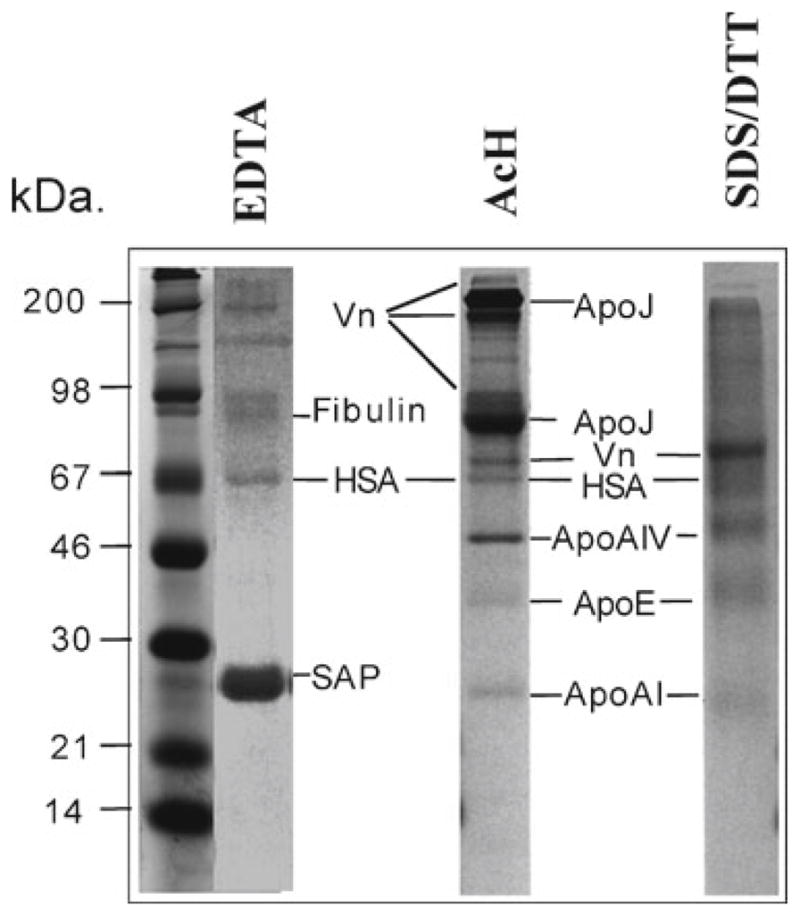
Binding of human plasma proteins to an Aβ-affinity matrix. Normal human plasma was incubated for 3 h at room temperature with Aβ1–40 affinity matrix equilibrated in TBS containing physiological concentrations of Ca2+. After extensive washing to eliminate non-specific binding, bound proteins were eluted from the Aβ-matrix sequentially with 10 mM EDTA (EDTA), 2 M NaCl, 1% (v/v) Triton X-100, 1 M acetic acid (AcH), and 5%/1 M SDS/DTT (SDS/DTT). Aliquots of the eluted fractions were separated on 10% tris-tricine SDS-PAGE, transferred to PVDF and the membranes stained with Coomassie Blue. The identity of the eluted components, determined by amino-terminal amino acid sequence, is indicated. Affinity chromatography of normal human serum revealed an array of Aβ-binding proteins that interact with immobilized Aβ. The major Ca2+-dependent binding component observed in the EDTA eluate corresponded to amyloid P component (SAP), while other minor bands were also observed and identified as albumin and fibulin. Under acidic conditions (AcH elution), we observed the presence of two major bands corresponding to apoJ (monomer and dimer), several vitronectin isoforms, as well as other minor bands identified as apolipoprotein A-IV, apolipoprotein A-I, apolipoprotein E, and albumin (see Note 11). Under denaturing conditions (SDS/DTT fraction), we observed the elution of vitronectin, apoE, apoA-IV, apoA-I, and albumin (HSA).
2. Materials
2.1. Reagents
Plasma samples were obtained from normal healthy subjects (age 25–40 years) after 12 h fast with adequate understanding and written consent of subjects.
Peptide DAEFRHDSGYEVHHQKLVFFAEDVGSNKGA IIGLMVVGGV (Aβ1–40), homologous to residues 672–711 of β PP770 was synthesized at W.M. Keck facility at Yale University using tBOC (N-tert-butyloxycarbonyl) chemistry.
All buffers and solutions were freshly prepared using water provided by a Milli-Q system (18 MΩ/cm at 25°C, Millipore Corp., Bedford, MA). All reagents were of the highest purity available and were purchased from Sigma-Aldrich, unless otherwise noted.
-
Specific buffers.
Tris-buffered saline (TBS):10 mM Tris–HCl, 150 mM NaCl, pH 7.4.
TBS-Ca: TBS containing 2.5 mM CaCl2.
TBS-E: TBS containing 10 mM EDTA.
TBS-HS: TBS containing 2 M NaCl.
TBS-T: TBS containing 1% Triton X-100 (v/v).
AcH: Acetic acid 1 M solution. Prepare by adding 57.5 ml of glacial acetic acid (Sigma-Aldrich, # 537020) to 942.5 ml of Milli-Q water to obtain 1 l of 1 M acetic acid solution, pH ~2.2. Do not adjust the pH.
SDS/DTT buffer: 5% SDS and 1 M DTT in TBS buffer.
-
Other reagents.
CNBr-activated Sepharose 4B (GE HealthCare, # 17-0430-01).
CAPS (3-cyclohexylamino-1-propanesulphonic acid) transfer buffer: 10 mM CAPS, 10% methanol. Prepare 1 l of 10× CAPS stock (100 mM CAPS) by adding 22.13 g of CAPS (Sigma-Aldrich, #C2632) to 950 ml of Milli-Q water, adjust to pH and complete volume to 1 l. Prepare CAPS transfer buffer (1×) with 100 ml of CAPS 10× solution, 100 ml of methanol, and 800 ml of Milli-Q water.
DC Protein Assay (Bio-Rad, #500-0111).
Tricine Sample Buffer (Bio-Rad #161-0739).
2.2. Instrumentation
Peristaltic pump. Single channel laboratory peristaltic pump for use in liquid chromatography (GE-Healthcare, #18-1110-91).
Fraction Collector (GE-Healthcare, #18-1177-40).
Spectrophotometer UV-Vis.
Polystyrene 20-ml volume chromatographic columns including upper bed supports, end caps, tip closures (Econo-Pac Chromatography Columns, Bio-Rad #732-1010).
Appropriate column holders and clamps for attaching columns and tubing to the system.
3. Methods
3.1. Immobilization of Amyloid β Peptides to Chromatography Medium
3.1.1. Selection of Affinity Matrix Support and Coupling Chemistry
The nature of the affinity matrix support (solid support onto which ligand is immobilized) is an important factor that has to be considered when planning an affinity chromatography experiment. Detailed discussion on how to select an appropriate matrix is discussed in depth elsewhere (35). For the particular case of coupling hydrophobic amyloidogenic peptides, it is very important to select a hydrophilic, non-charged supports that minimize nonspecific hydrophobic and ionic interactions (see Note 2).
Depending on the nature of the peptide or protein to be coupled (amino acid composition, presence of carbohydrates, sulphydril groups, etc.) and the aim of the experiment, different chemistries can be used for coupling the ligand to the chromatographic media (35). Activated chromatography media with carboxyl esters such as N-hydroxysuccinimide (NHS) or cyanogen bromide (CNBr) represent two of most convenient methods for covalent binding of short peptides. Both NHS or CNBr moieties react with free primary amino groups from peptides or proteins to form stable covalent links, most typically through lysine side chains. In this protocol, we used CNBr-activated Sepharose (GE HealthCare) for binding of Aβ peptides.
CNBr-activated Sepharose 4B is a commercially available preactivated medium for immobilization of ligands containing primary amines with a very high coupling efficiency. It provides a very convenient way to immobilize proteins and peptides. The coupling reaction is spontaneous, rapid and easy to carry out (see Note 3). Buffers containing primary amines (e.g., Tris and glycine) should not be used for coupling reactions.
3.1.2. Ligand Preparation and Coupling
Amyloid peptides have an intrinsic tendency to oligomerize and aggregate. Therefore, proper handling of peptide solutions is a key aspect to be controlled to obtain an affinity matrix with a defined ligand composition. For this protocol, we aimed at coupling to the matrix support Aβ1–40 peptide mainly as monomers or small oligomers. Coupling of the peptide to CNBr-activated Sepharose was performed following the manufacture’s instruction with one important exception regarding the coupling buffer where the ligand is dissolved. In brief, 2.3 μmol (10 mg) of Aβ1–40 peptide was slowly dissolved in 20 ml of 0.1 M carbonate/bicarbonate buffer, pH 9.0 without NaCl. After centrifugation at 16,000 × g at 4°C, this peptide solution was allowed to interact with 2 ml of activated Sepharose matrix for 16 h at 4°C on a rotating wheel. After washing, any remaining active groups were blocked by washing with 0.1 M Tris–HCl buffer, pH 8.0.
The Aβ1–40 peptide coupled affinity matrix (2 ml) was packed into a polystyrene chromatographic column (Bio-Rad #732-1010) and washed extensively by three cycles of alternating pH with 0.1 M acetic acid/sodium acetate, pH 4.0 containing 0.5 M NaCl, and 0.1 M Tris–HCl, pH 8 containing 0.5 M NaCl.
3.2. Affinity Chromatography with Sequential Elution
This procedure can be performed in an FPLC system, or conveniently in an in-house chromatography system composed of a column containing the packed affinity media, a peristaltic pump to provide flow circulation, a fraction collector, and a UV-Vis spectrophotometer for later analysis of fractions.
3.2.1. Protocol for Affinity Chromatography with Sequential Elution
Equilibrate the affinity matrix by flowing 10 volumes of TBS-Ca (see Note 4). For a bed volume of 2 ml, use at least 20 ml of TBS-Ca.
Drain remaining TBS-Ca buffer, stop the flow, and load the sample of interest into the column (see Notes 5 and 6). Connect the exit tubing to the top of the column and recirculate the sample through the column with a slow flow (0.2 ml/min) for 1 h at 37°C, 3 h at room temperature, 16 h at 4°C, or any other suitable setting (see Note 7).
Collect the pass-through sample in one single fraction that contains unbound proteins.
Remove additional unbound material by extensive washing with at least 10 bed volumes of TBS-Ca, or until the absorbance of the eluent at 280 nm is negligible.
Elute Ca2+-dependent binding proteins with TBS-E. Carefully add 5 volumes of TBS-E to the drained ligand matrix, restart the flow and take fractions of about one-forth of the bed volume (see Note 8).
Wash EDTA excess with 5 volumes of TBS.
Elute electrostatically bound proteins with TBS-HS. Carefully add to the drained matrix bed 5 volumes of TBS-HS, restart the flow and take fractions of about one-forth of the bed volume.
Wash salt excess with 5 volumes of TBS.
Elute hydrophobically bound proteins with TBS-T. Carefully add 5 volumes of TBS-T to the drained matrix bed, restart the flow and take fractions of about one-forth of the bed volume.
Wash Triton X-100 detergent with 5 volumes of TBS.
Elute pH-dependent binding proteins bound proteins with 1 M acetic acid (AcH). Carefully add 5 volumes of AcH to the drained matrix bed, restart the flow, and take fractions of about one-forth of the bed volume.
Wash AcH with 5 volumes of TBS.
Analyze remaining bound proteins by taking a small aliquot of affinity matrix and elute by boiling the sample for 5 min in the presence of 5% SDS and 1 M DTT in TBS (SDS/DTT buffer) (see Note 9).
To determine the fractions containing the eluted proteins at the different steps, read the absorbance at 280 nm of each fraction against a blank reference buffer, or by a suitable protein quantitation method such as the Bio-Rad DC Protein Assay (Bio-Rad, #500-0111) (see Note 8).
Pool fractions containing protein for each elution step, dialyze extensively against 50 mM ammonium bicarbonate, and store at −80°C until used.
3.3. Downstream Analysis
3.3.1. Identification
Proteins bound to the affinity column and eluted with different buffers could be easily identified via either amino acid sequence or mass spectrometry analysis. To obtain rapid N-terminal sequence data without the need of complex and time-consuming fractionation procedures (e.g., high performance liquid chromatography separation of the individual components of the eluate), electrophoretic methodology usually provides enough resolution and sufficient amount of material to successfully carry out protein identification. Samples are separated on 10% tris-tricine SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon P, Millipore, Mildford, MA) using CAPS (3-cyclohexylamino-1-propanesulphonic acid) pH 11, containing 10% methanol. After transfer, the membrane is stained with Coomassie Blue (see Note 10) to visualize the components of the mixture, procedure that in our example render a series of components of different molecular mass (see Fig. 1). The protein bands are excised from the membrane and used directly to obtain N-terminal sequence (in our example, proteins were identified on a 477 A protein sequencer, Applied Biosystems, Foster City, CA). An alternative identification methodology that has greater sensitivity is mass spectrometry, an approach highly recommended for samples with low abundance components. The technique, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), requires an additional step in the sample preparation; samples separated on SDS-PAGE are stained with mass spectrometry compatible Silver stain (SilverQuest, InVitrogen) following the manufacturer’s guidelines. Bands are excised from the gel, subjected to proteolytic degradation with TPCK-trypsin and the resulting peptide mix resolved in the mass spectrometer using the protocols recommended by the manufacturer. Searching the mass data against a database of tryptic peptides will allow the identification of the unknown components (36).
3.3.2. Data Validation
Further validation of the data obtained via N-terminal sequence or mass spectrometry can be obtained via Western blot analysis of the different eluates using specific antibodies for the identified proteins. The technique is highly sensitive and will provide direct visualization of each of the specific components of the sample. The use of complementary immunohistochemical techniques will provide additional confirmation of the topographical location of the identified amyloid-binding proteins in the tissue deposits.
Acknowledgments
This work was supported by grants from the Fundación CIEN-ISCIII (MPY 1308/08) and CIBERNED to MC and from the National Institutes of Health NS051715 to AR and AG030539 to JG.
Abbreviations
- ACT
α1-Antichymotrypsin
- AD
Alzheimer’s disease
- apoA-I
Apolipoprotein A-I
- apoA-IV
Apolipoprotein A-IV
- apoE
Apolipoprotein E
- apoJ
Apolipoprotein J (clusterin)
- CAPS
3-Cyclohexilamino-1-propanesulphonic acid
- CNBr
Cyanogen bromide
- ELISA
Enzyme-linked immunosorbent assay
- HSA
Human serum albumin
- NHS
N-hydroxysuccinimide
- SAP
Serum amyloid P component
- Vn
Vitronectin
Footnotes
Affinity chromatography is one of the most powerful chromatographic methods for purification of specific molecules from a complex mixture based on the interaction with the affinity matrix. Sequential elution may allow purifying proteins that interact by different mechanism, providing further information on the type of binding interaction.
Sepharose from GE-HealthCare and Affigel from Bio-Rad are agarose-derived matrices that fulfill these criteria. Cellulose or synthetic supports such as polyacrylamide beads, Sephacryl, or Ultragel are also good options. Hydrophobic polystyrene beads and negatively charged silica supports are not generally recommended for this purpose.
This type of attachment presents also some drawbacks, including partial leaking of ligands, and potential steric hindrance due to the absence of linker. Additionally, attachment of ligands through free primary amino groups may alter their interaction with other proteins.
A regular flow from 0.2 to 0.5 ml/min is recommended for most applications.
Do not allow to matrix to get dry at any point during the procedure, because this will adversely affect the performance of the column.
Viscous samples, such as plasma and serum, should be diluted at least 1:1 with TBS-Ca. Additionally, before loading the sample into the column, samples should be centrifuged for 15 min at >4,000 × g, or filtered through a 0.23-μm filter to remove debris.
Incubation at 37°C mimics physiological conditions; however, for labile proteins this setup may result unsuitable. Adjust temperature and time for binding according to experimental needs. In the experiment depicted in this work (see Fig. 1), 2 ml of the Aβ1–40 peptide matrix was allowed to interact with 10 ml of normal plasma for 3 h at room temperature with continuous recirculation.
For a ligand matrix bed volume of 2 ml, take 0.5 ml fractions. The bulk of protein should elute within the last two fractions of the first bed volume.
Since this procedure may render the affinity matrix unusable, it is important that you analyze only a small fraction (20–50 μl) by this procedure. The rest of the affinity matrix can be reused several times with optimal performance, provided proper care of column has been taken. For general cleaning and regeneration, wash the column with 3 volumes of alternating high pH (0.1 M Tris–HCl, 0.5 M NaCl, pH 8.5) and low pH (0.1 M sodium acetate, 0.5 M NaCl, pH 4.5) buffers. To remove precipitated or denatured substances, wash with 2 column volumes of 6 M guanidine hydrochloride, and wash immediately with 5 column volumes of TBS or other suitable buffer. To remove hydrophobically bound substances, wash with 3 column volumes of 70% ethanol or 1% Triton X-100, and immediately with 5 column volumes of TBS. For storage, keep the column at 4°C in 20% ethanol at neutral pH.
Use the high-quality reagents for the preparation of the Coomassie Blue staining and distaining solutions. Use 1% acetic acid as fixative in the solutions instead of 5–10% commonly used in Coomassie Blue staining standard protocols to facilitate downstream analysis by N-terminal sequencing.
In some Aβ-affinity chromatography experiments, we have found a significant presence of IgG (0–10%) in the Triton X-100 and acetic acid fractions. This fact may be associated to a specific interaction of bound apoJ with IgG molecules (37).
References
- 1.Ghiso J, Frangione B. Amyloidosis and Alzheimer’s disease. Adv Drug Delivery Rev. 2002;54:1539–1551. doi: 10.1016/s0169-409x(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 2.Coria F, Castano E, Prelli F, Larrondo-Lillo M, van Duinen S, Shelanski ML, et al. Isolation and characterization of amyloid P component from Alzheimer’s disease and other types of cerebral amyloidosis. Lab Invest. 1988;58:454–458. [PubMed] [Google Scholar]
- 3.Kalaria RN, Galloway PG, Perry G. Widespread serum amyloid P immunoreactivity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer’s disease and other degenerative disorders. Neuropathol Appl Neurobiol. 1991;17:189–201. doi: 10.1111/j.1365-2990.1991.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 4.Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991;541:163–166. doi: 10.1016/0006-8993(91)91092-f. [DOI] [PubMed] [Google Scholar]
- 5.Wisniewski T, Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992;135:235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- 6.Yamada T, Kakihara T, Gejyo F, Okada M. A monoclonal antibody recognizing apolipoprotein E peptides in systemic amyloid deposits. Ann Clin Lab Sci. 1994;24:243–249. [PubMed] [Google Scholar]
- 7.Kindy MS, King AR, Perry G, de Beer MC, de Beer FC. Association of apolipoprotein E with murine amyloid A protein amyloid. Lab Invest. 1995;73:469–475. [PubMed] [Google Scholar]
- 8.Rostagno A, Ghiso J. Amyloidosis. In: Aminoff M, Daroff R, editors. Encyclopedia of neurological sciences. Vol. 1. Academic Press; San Diego: 2003. pp. 129–135. [Google Scholar]
- 9.Ghiso J, Rostagno A, Tomidokoro Y, Lashley T, Holton J, et al. Familial British and Danish dementias. In: Sipe JD, editor. Amyloid proteins. The beta sheet conformation and disease. Vol. 2. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2005. pp. 515–526. [Google Scholar]
- 10.Rostagno A, Tomidokoro Y, Lashley T, Ng D, Plant G, et al. Chromosome 13 dementias. Cell Mol Life Sci. 2005;62:1814–25. doi: 10.1007/s00018-005-5092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veerhuis R, Boshuizen RS, Familian A. Amyloid associated proteins in Alzheimer’s and prion disease. Curr Drug Targets CNS & Neurological Dis. 2005;4:235–248. doi: 10.2174/1568007054038184. [DOI] [PubMed] [Google Scholar]
- 12.Gallo G, Picken M, Frangione B, Buxbaum JN. Nonamyloidotic monoclonal immunoglobulin deposits lack amyloid P component. Mod Pathol. 1988;1:453–456. [PubMed] [Google Scholar]
- 13.Gallo G, Wisniewski T, Choi-Miura NH, Ghiso J, Frangione B. Potential role of apolipoprotein-E in fibrillogenesis. Am J Pathology. 1994;145:526–530. [PMC free article] [PubMed] [Google Scholar]
- 14.Botto M, Hawkins PN, Bickerstaff MCM, Herbert J, Bygrave AE, et al. Amyloid deposition is delayed in mice with targeted deletion of serum amyloid P component. Nature Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 15.Holtzman DM, Bales KR, Wu S, Bhat P, Parsadanian M, et al. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 17.Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985;4:2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prelli F, Castano EM, Glenner GG, Frangione B. Differences between vascular and plaque core amyloid in Alzheimer’s disease. J Neurochem. 1988;51:648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 19.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, et al. Isolation and quantitation of soluble Alzheimer’s β-peptide from bilogical fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 20.Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, et al. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 21.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 22.Ghiso J, Calero M, Matsubara E, Governale S, Chuba J, Beavis R, et al. Alzheimer’s soluble amyloid beta is a normal component of human urine. FEBS Lett. 1997;408:105–108. doi: 10.1016/s0014-5793(97)00400-6. [DOI] [PubMed] [Google Scholar]
- 23.Tabaton M, Nunzi MG, Xue R, Usiak M, Autilio-Gambetti L, Gambetti P. Soluble amyloid beta-protein is a marker of Alzheimer amyloid in brain but not in cerebrospinal fluid. Biochem Biophys Res Commun. 1994;200:1598–1603. doi: 10.1006/bbrc.1994.1634. [DOI] [PubMed] [Google Scholar]
- 24.Golabek A, Marques MA, Lalowski M, Wisniewski T. Amyloid β binding proteins in vitro and in normal human cerebrospinal fluid. Neuroscience Lett. 1995;191:79–82. doi: 10.1016/0304-3940(95)11565-7. [DOI] [PubMed] [Google Scholar]
- 25.Ghiso J, Matsubara E, Koudinov A, Choi-Miura NH, Tomita M, Wisniewski, et al. The cerebrospinal-fluid soluble form of Alzheimer’s amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993;293:27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaDu MJ, Lukens JR, Reardon CA, Getz GS. Association of human, rat, and rabbit apolipoprotein E with beta-amyloid. J Neurosci Res. 1997;49:9–18. [PubMed] [Google Scholar]
- 27.Wisniewski T, Castaño EM, Golabek AA, Vogel T, Frangione B. Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am J Pathology. 1993;145:1030–1035. [PMC free article] [PubMed] [Google Scholar]
- 28.Wegiel J, Chauhan A, Wisniewski HM, Nowakowski J, Wang KC, Le Vine H. Promotion of synthetic amyloid beta-peptide fibrillization by cell culture media and cessation of fibrillization by serum. Neurosci Lett. 1996;211:151–154. doi: 10.1016/0304-3940(96)12739-7. [DOI] [PubMed] [Google Scholar]
- 29.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 30.Ma J, Yee A, Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;392:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 31.Webster S, O’Barr S, Rogers J. Enhanced aggregation and β structure of amyloid β peptide after coincubation with C1q. J Neurosci Res. 1994;39:448–456. doi: 10.1002/jnr.490390412. [DOI] [PubMed] [Google Scholar]
- 32.Tennent GA, Lovat LB, Pepys MB. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer’s disease and systemic amyloidosis. Proc Natl Acad Sci USA. 1995;92:4299–4303. doi: 10.1073/pnas.92.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsubara E, Soto C, Governale S, Frangione B, Ghiso J. Apolipoprotein J and Alzheimer’s amyloid beta solubility. Biochem J. 1996;316:671–679. doi: 10.1042/bj3160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urh M, Simpson D, Zhao K. Affinity chromatography: general methods. Methods Enzymol. 2009;463:417–438. doi: 10.1016/S0076-6879(09)63026-3. [DOI] [PubMed] [Google Scholar]
- 36.Ovodenko B, Rostagno A, Neubert TA, Shetty V, Thomas S, et al. Proteomic analysis of exfoliation deposits. Invest Ophthalmol Vis Sci. 2007;48:1447–1457. doi: 10.1167/iovs.06-0411. [DOI] [PubMed] [Google Scholar]
- 37.Wilson MR, Easterbrook-Smith SB. Clusterin binds by a multivalent mechanism to the Fc and Fab regions of IgG. Biochim Biophys Acta. 1992;1159:319–326. doi: 10.1016/0167-4838(92)90062-i. [DOI] [PubMed] [Google Scholar]


