Abstract
Background and aims
There are no data showing whether or not age-related declines in physical function are related to in vitro properties of human skeletal muscle. The purpose of this study was to determine whether physical function is independently associated with histologic and metabolic properties of skeletal muscle in elderly adults.
Methods
The study was a cross-sectional observational study of 39 sedentary, older (60–85 yrs) men and women. A needle biopsy of the vastus lateralis for assessment of muscle fiber type, fiber area, capillary density and citrate synthase and aldolase activities was performed. Physical function tests included the Short Physical Performance Battery (balance, walking speed, and chair rise time), as well as self-reported disability.
Results
Total fiber area (R=−0.41, p=0.02), number of Type II fibers (R=−0.33, p=0.05), and aldolase activity (R=−0.54, p=0.01) were inversely related to age. Persons who reported greater difficulty with daily activities had lower capillary density (R=−0.51, p=0.03) and lower citrate synthase activity (R=−0.66, p=0.03). Walking speed was directly related to fiber area (R=0.40, p=0.02), capillary density (R=0.39, p=0.03), citrate synthase (R=0.45, p=0.03) and aldolase (R=0.55, p<0.01) activities, even after adjustment for age, BMI and disease status.
Conclusions
In older adults, skeletal muscle capillary density and metabolic enzymatic activity are independent predictors of lower extremity physical function.
Keywords: Capillary density, enzyme activity, physical function, skeletal muscle
INTRODUCTION
Clinical measures of physical function decline with age and are highly predictive of the incidence of disability, institutionalization, and/or mortality (1–4). Although the specific biological mechanisms underlying aging-related declines in physical function are poorly understood, alterations in skeletal muscle are likely contributors. While loss of muscle mass and declines in strength are well documented with aging (5), they do not fully account for the extent of aging-related loss of function (6, 7). Therefore, adverse alterations of the intrinsic properties of muscle tissue itself are also potential mechanisms underlying the decline in physical abilities with age.
Results from a number of studies indicate that there are age differences in skeletal muscle histology and metabolic properties (8, 9). Although almost exclusively from cross-sectional studies, most data show that individual fiber size (10), the relative number of type II muscle fibers (11, 12), oxidative enzyme activity (9, 11, 13, 14), and capillary density (14–16) are reduced in older persons. Unfortunately, there are no data showing whether or not age-related declines in physical function are related to these alterations in skeletal muscle.
Age-related loss of function can result from several different chronic diseases. However, while the primary impairments in these diseases clearly differ, there are similarities in their systemic consequences (e.g., effects on skeletal muscle function). Yet, there is little understanding of the etiology and underlying biological factors for this common pathway towards reduced function. It is generally accepted that abnormalities in skeletal muscle function are likely to be causal factors in the progression towards physical disability in the elderly. However, there have been limited advances in testing this hypothesis for several reasons, including the lack of data on muscle properties from tissue samples of well-characterized persons with clinical measures of physical function. Therefore, the purpose of this cross-sectional study was to determine whether measures of physical function are independently associated with skeletal muscle fiber area, fiber type, capillary density, and activity of an oxidative (citrate synthase) and a glycolytic (aldolase) enzyme, in elderly men and women.
METHODS
Study participants
The participants included a sample of 39 older men and women who were originally recruited for clinical studies conducted at the Wake Forest University J. Paul Sticht Center on Aging, and who also agreed to undergo a muscle biopsy to determine the relationships between physical function and skeletal muscle characteristics. The data reported here are from the baseline assessments of individuals enrolled in 3 separate clinical studies - a randomized, clinical trial of angiotensin converting enzyme inhibitor usage in chronic heart failure (CHF) patients (n=22), and a pilot study of power training (n=8), and a cross-sectional blood flow pilot study (n=9) in healthy (no evidence of chronic disease) older men and women with mild self-reported disability. The CHF patients were all diagnosed with isolated diastolic heart failure, defined as heart failure with normal systolic function (left ventricular ejection fraction >50%) and no evidence of significant coronary or valvular heart disease or pulmonary disease.
All participants gave written informed consent to participate in the study according to the guidelines of the WFU Health Sciences Institutional Review Board. All participants were older (≥60 yrs), community-dwelling, sedentary (no regular exercise consisting of >15 min, 2 times/wk in the past 6 months), and non-smokers. They underwent a screening medical exam and were excluded if they reported: 1) recent (within 6 months) history of stroke or myocardial infarction, 2) inducible myocardial ischemia, 3) active treatment for cancer, 4) peripheral vascular disease, 5) renal insufficiency, 6) uncontrolled diabetes or hypertension, 7) chronic obstructive pulmonary disease, 8) severe anemia, 9) liver disease, 10) orthopedic impairment (presence of a prosthesis or prior knee/hip replacement), 11) presence of pacemaker or defibrillator, or 12) cognitive impairment (MMSE<24). Measures of physical function and a skeletal muscle biopsy were collected on all participants within a three week window as described below.
Study assessments
Short physical performance battery (SPPB)
The SPPB consists of a timed short distance walk, repeated chair stands and a balance test. Each of the performance measures is assigned a score ranging from 0 to 4, with 4 indicating the highest level of performance and 0 the inability to complete the test. A summary score ranging from 0 (worst performers) to 12 (best performers) is calculated by adding all scores. The reliability of the SPPB is good, with intra-class correlation coefficients above 0.88 for each of the individual components, as well as the summary score (17).
Usual walking speed is assessed by asking participants to walk at their usual pace over a 4 meter course. The faster of two walks is used to compute walking speed as follows: unable to perform the test=0; ≥8.7 sec=1; 6.2–8.6 sec=2; 4.8–6.1 sec=3; <4.8 sec=4. For the repeated chair stands test, participants are asked to stand from a sitting position without using their arms five times, as quickly as possible. The time to complete the task is scored as follows: unable to perform the task=0; ≥ 16.7 sec=1; 13.7–16.6 sec=2; 11.2–13.6 sec=3; ≤11.1 sec=4. For the test of standing balance, participants are asked to maintain balance in three positions: feet together (side by side), the heel of one foot beside the big toe of the other (semi-tandem), and the heel of one foot in front of and touching the toes of the other (full tandem). Participants are assigned a score of 1 if they can hold a side-by-side standing position for 10 seconds, but are unable to hold a semi-tandem position for 10 seconds; a score of 2 if they can hold a semi-tandem position for 10 seconds, but are unable to hold a full-tandem position for more than 2 seconds; a score of 3 if they can stand in a full-tandem position for 3 to 9 seconds; a score of 4 if they can stand in a full-tandem position for 10 seconds.
Disability questionnaire
Physical disability was measured using a 23-item, self-report questionnaire which inquires about perceived difficulties in general activities of daily living during the last month. Respondents answer whether they experience: 1) no difficulty, 2) a little difficulty, 3) some difficulty, 4) a lot of difficulty, 5) unable to do or, 6) did not do for other reasons, and answers are averaged across items.
Skeletal muscle biopsy
All biopsies were performed in the early morning after an overnight fast. Subjects were asked to refrain from taking aspirin, prescription and over-the-counter non-steroidal anti-inflammatory drugs, or other compounds that may affect bleeding, platelets, or bruising for the week prior to the biopsy, and to refrain from any strenuous activity for at least 36 hours prior to the biopsy. Muscle was obtained from the vastus lateralis using the percutaneous needle biopsy technique with a University College Hospital needle under local anesthesia with 1% lidocaine. There were no medical complications or other reported adverse events from the procedure.
Visible blood and connective tissue were removed from the muscle specimen, and portions for enzymatic analysis (20–30 mg), capillary density (15 mg), and fiber typing (15 mg) were partitioned. The enzymatic assay samples were snap frozen in liquid nitrogen and stored at −80°C until analyses. The muscle portion used for histochemical analysis was oriented such that the fibers ran longitudinally, mounted on cork in embedding medium (OCT compound, Miles Laboratory, Naperville, IL), and frozen in isopentane cooled to its freezing point with liquid nitrogen. The muscle portion for capillary density was placed in a histology cassette and quick frozen in isopentane, which was first pre-cooled in liquid nitrogen for 20–30 minutes. The cassette was stored at −80°C and transported along with the sample for enzymatic analyses on dry ice to the laboratories of Drs. Kraus and Annex.
The average yield for all biopsies was 150±20 mg; however, not all biopsies yielded a sufficient amount or quality of tissue to perform all planned assays. As such, sample sizes varied and were n=37 for fiber typing, n=34 for fiber area and capillary density, and n=23 for enzymatic analyses.
Fiber typing
Fiber type histological analyses were performed following published procedures (18). Monoclonal antibodies against myosin heavy chain (MHC) were used to identify muscle fiber subtypes. Muscle specimens were pinned to the bottom of a plastic box and covered with OCT (embedding medium) and rapidly frozen in a container with metylbutane on dry ice. Muscle sections of 10 μm thickness, obtained with a cryostat, were mounted on a glass slide (~5 sections/slide). Slides were exposed to the primary antibody (Novocastra, Newcastle, Tyne, UK; Alexis, San Diego, CA) at 1:20 dilution in PBS for 4-hr, rinsed in PBS and exposed for another 4-hr in the secondary antibody (FITC conjugated IgG rabbit anti-mouse, Sigma). Immunostained cross-sections were analyzed in an inverted Axiovert microscope (Zeiss) equipped with fluorescence filters. Images were digitized using a Photometric CCD camera and Isee software (Inovision, Durham, NC). The percent of Type I and Type II fibers reported is based on fiber number and the different fibers are counted using a code system. Results are from the mean of at least 10 cross-sections for the Type I, and at least 10 cross-sections for the Type II, antibodies. The variability in the number of a specific fiber subtype from section to section stained with the same antibody is <2%.
Fiber area and capillary density
Endothelial cells were identified using immunohistochemistry and cell specific monoclonal (CD31) antibodies. CD31 is a mouse monoclonal antibody (Biogenex, cat #AM232-5M), designed for the specific localization of human endothelium in normal and tumor tissue. Slides were brought to room temperature, placed in ice-cold acetone for 2 min and PBS for 5 min. Blocking solution (10% horse serum in PBS) was applied for 1 hr at room temperature. The primary antibodies were applied for 1 hr followed by sequential incubation with a biotinylated anti-mouse IgG and ABC reagent, according to the manufacturer’s specifications (Vectastain ABC kit, Vector Laboratories). Levamisole was added to block endogenous alkaline phosphatase activity and immune complexes were localized using the chromogenic alkaline phosphatase substrate Vector Red (Vector Laboratories). When counterstained with hematoxolin, dehydrated and mounted with Permount (Fisher Scientific), the antigen appears red and the nuclei blue. A murine IgG monoclonal antibody served as a negative control. The stained slide of human muscle was placed on the Olympus 1x70 microscope and transferred onto a computer screen at a magnification of 100x through an Optronics Engineering DEI-750 camera to the Adobe Premiere 4.2.1 program. Within this program the image of the muscle fiber was captured and saved to the Adobe Photoshop LE program, then opened into the NIH Image 1.6/ppc program. In the NIH Image program the individual muscle fiber was outlined and the total area was calculated. Capillary density (mean number of capillaries/muscle fiber) was measured by counting endothelial cells and muscle fibers in at least three random 100x magnification fields per sample. A minimum of 100 muscle fibers was counted. Intra-observer variability on samples chosen for blind repeat analysis was 5% (19).
Citrate synthase and aldolase enzyme activity
The citrate synthase and aldolase enzyme assays were performed as previously described (19). Briefly, frozen tissue samples (20–30 mg) were homogenized in a phosphate buffer (pH 7.4) containing 0.02% bovine serum albumin (BSA), 5 mmol/L B-mercaptoethanol, and 0.05 mmol/L EDTA and diluted (1:100) in 20 mmol/L imidazole buffer with 0.02% BSA (20). Citrate synthase activity was detected fluorometrically and aldolase was measured per established kinetic assays (19). In our laboratory, the coefficient of variation for each of these enzyme assays is <5%.
Statistical analyses
Statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC). All data are presented as mean ± standard deviation and the level for statistical significance was set at α=0.05. Pearson’s correlation coefficients were calculated to describe pair-wise relationships between variables. The Student’s t-test was used to compare between group differences (such as between men and women and between SPPB ≤9 and SPPB >10 groups). Linear regression analysis was used to examine the association between physical function and skeletal muscle characteristics after adjustment for age, BMI, and CHF status.
RESULTS
Relationships between demographic and skeletal muscle characteristics
Participant physical characteristics, co-morbidities, and physical function are shown in Table 1. There was a wide range in age, body mass, and physical function, especially in walking speed (nearly 3-fold difference) and chair rise time (nearly 5-fold difference). Table 2 shows the measures of vastus lateralis fiber area, capillary density, citrate synthase and aldolase enzyme activities, and fiber type.
Table 1.
Physical characteristics, comorbidity, and physical function of the study sample.
| Mean±SD | Range | n | |
|---|---|---|---|
| Age (yrs) | 71±7 | 60–85 | 39 |
| Gender (% Female) | 67% | 39 | |
| Comorbidity (%) | 39 | ||
| Chronic heart failure | 56% | ||
| CHD history | 10% | ||
| Diabetes | 23% | ||
| Cancer history | 7% | ||
| Body mass (kg) | 79.4±15.0 | 50.8–108.9 | 39 |
| BMI (kg/m2) | 28.9±5.4 | 19.4–39.3 | 39 |
| Self-reported disability* | 1.61±0.57 | 1.00–2.74 | 24 |
| Walking speed (m/s) | 1.14±0.23 | 0.63–1.83 | 38 |
| Chair rise test (s) | 15.1±6.3 | 8.0–39.6 | 36 |
| SPPB score (0–12) | 10.2±1.5 | 6–12 | 35 |
Self-reported disability scale from 1 (no difficulty) to 5 (unable to perform).
Table 2.
Intrinsic skeletal muscle (vastus lateralis) characteristics of the study sample.
| Mean±SD | Range | |
|---|---|---|
| Fiber area (μm2) | 5018±1259 | 2562–8372 |
| Capillary density (number/mm2) | 377±98 | 151–536 |
| Citrate synthase activity (μmol/min/μg protein) | 0.086±0.033 | 0.020–0.183 |
| Aldolase activity (μmol/min/mg protein) | 0.269±0.107 | 0.033–0.498 |
| Fiber type | ||
| % Type I, slow twitch | 42±12 | 20–74 |
| % Type II, fast twitch | 58±12 | 26–80 |
n=34 for fiber area and capillary density; n=22 for enzyme activities; n=37 for fiber type.
Older persons had lower fiber area (R=−0.41, p=0.02), lower percentage of Type II fibers (R=−0.33, p=0.05), and lower aldolase activity (R=−0.54, p=0.01). Only citrate synthase (R=0.54, p=0.01) and aldolase (R=0.51, p=0.01) activities were related to BMI such that more obese persons had higher activities of these enzymes. Women had smaller relative number of Type II fibers (0.56±0.09) compared with men (0.64±0.11, p=0.04). There were no gender differences in any other skeletal muscle characteristics.
Relationships between physical function and skeletal muscle characteristics
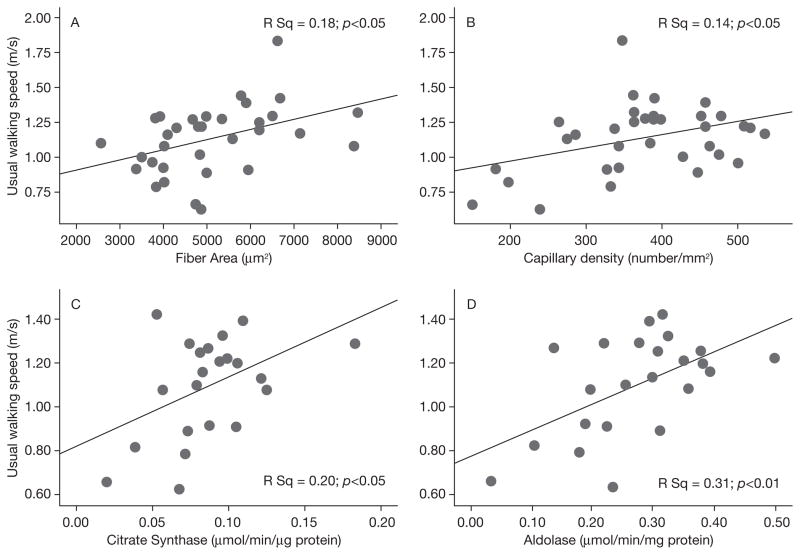
Table 3 shows pair-wise correlation coefficients between measures of physical function and skeletal muscle characteristics. Overall, better function was associated with higher fiber area, capillary density, and citrate synthase and aldolase activities. Specifically, persons who reported greater difficulty with daily activities (i.e., higher disability score) had lower capillary density (p=0.03) and lower citrate synthase activity (p=0.03). Walking speed was directly related to fiber area, capillary density, citrate synthase and aldolase activities (all p<0.05; Fig. 1a–d). The time to rise from a chair five times was inversely related to aldolase activity (p=0.03). There were no apparent sex interactions between muscle characteristics and physical function, and the direction of the relationships were consistent between men and women (data not shown).
Table 3.
Pearson correlation coefficients between skeletal muscle characteristics and physical function.
| Fiber area
|
Capillary density
|
Citrate synthase
|
Aldolase
|
% Type II fibers
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | p-value | R | p-value | R | p-value | R | p-value | R | p-value | |
| Self-reported disability | −0.20 | 0.41 | −0.51 | 0.03 | −0.66 | 0.03 | −0.30 | 0.36 | −0.13 | 0.55 |
| Usual walking speed | 0.40 | 0.002 | 0.39 | 0.03 | 0.45 | 0.04 | 0.55 | <0.01 | 0.25 | 0.14 |
| Chair rise test | −0.29 | 0.11 | −0.31 | 0.09 | −0.34 | 0.13 | −0.48 | 0.03 | −0.11 | 0.55 |
Fig. 1.
Fig. 1a–d - Scatterplots of the relationships between Walking Speed and Fiber Area (a), Capillary Density (b), Citrate Synthase (c), Aldolase Enzyme Activities (d).
We also analyzed whether skeletal muscle characteristics differed between participants with SPPB scores ≤9 (lower function, n=13) or ≥10 (higher function, n=21). Only capillary density was significantly different between these two groups (low SPPB: 329±111; high SPPB: 411±65; p=0.03).
Next, linear regression analyses were used to examine whether the relationships between usual walking speed and skeletal muscle characteristics were independent of age, BMI, and CHF status. Results showed that walking speed was not related to fiber area, aldolase activity or fiber type after adjusting for these confounding variables. However, walking speed remained significantly related to capillary density and citrate synthase activity independent of age, BMI and CHF status (Table 4).
Table 4.
Multiple regression analyses of associations between walking speed and capillary density, and walking speed and citrate synthase activity adjusting for age, BMI, and CHF status.
| Dependent variable: Walking speed | Parameter estimate | Standard Error | t Value | Pr > |t| | Parameter estimate | Standard Error | t Value | Pr > |t| | |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 2.030 | 0.429 | 4.73 | <0.0001 | Intercept | 1.612 | 0.603 | 2.67 | 0.02 |
| Age | −0.016 | 0.005 | −3.09 | <0.01 | Age | −0.011 | 0.007 | −1.49 | 0.15 |
| BMI | −0.005 | 0.007 | −0.68 | 0.50 | BMI | −0.003 | 0.008 | −0.40 | 0.70 |
| CHF status | −0.008 | 0.076 | −0.10 | 0.92 | CHF status | 0.146 | 0.095 | 1.53 | 0.14 |
| Capillary Density | 0.001 | 0.0004 | 2.73 | <0.01 | Citrate Synthase Activity | 3.025 | 1.395 | 2.17 | <0.05 |
Partial correlation coefficients were 0.46 (capillary density) and 0.47 (citrate synthase activity).
DISCUSSION
The purpose of this study was to examine whether clinical measures of physical function were related to metabolic and histologic properties of skeletal muscle in older individuals. Results showed that, overall, better physical function, including a self-report measure of disability, was associated with higher muscle fiber area, capillary density, and citrate synthase and aldolase activities measured in biopsy samples taken from the vastus lateralis of men and women aged 60–85 years old. Of the skeletal muscle properties we analyzed, capillary density and citrate synthase activity showed the strongest and most consistent associations with physical function in these individuals. In our adjusted analyses, both of these biological measures were independent predictors of usual walking speed, accounting for approximately 21% of the variance in walking speed after adjustment for age, BMI and CHF status. Whether this association is strong enough that increases in capillary density or citrate synthase activity would translate into a clinically relevant, or physiologically meaningful, increase in walking speed remains to be determined.
The current study is unique in its analyses of the relationship between clinically meaningful measures of physical function and intrinsic characteristics of skeletal muscle. There are only a few prior studies that report a relationship between physical abilities and skeletal muscle characteristics, and most of this work links peak aerobic capacity (VO2peak) and/or exercise tolerance with muscle fiber type and oxidative capacity. For example, exercise time and VO2peak correlated positively with capillary density and slow-twitch fiber area in elderly PAD patients (21), and VO2peak was related to muscle fiber area (22) and capillary density and oxidative enzyme activity (including citrate synthase) (19, 23, 24) in CHF patients. In addition, VO2peak was related to citrate synthase activity in young women (25), and in healthy men of a wide age range (26), although in the latter study, this relationship was only evident in the gastrocnemius, but not the vastus lateralis, muscle.
Previous studies have also shown that greater muscle strength is associated with larger overall and fast-twitch fiber area, and with a greater proportion of fast-twitch fibers (13, 27–29). Moreover, a more recent study showed that usual walking speed was inversely related to the percentage of fast-twitch muscle fibers in the paretic leg of stroke patients (30). The results of the current study extend these findings to show that usual walking speed, time to rise from a chair repeatedly, and even self-reported difficulty with daily tasks, are associated with skeletal muscle capillary density, and glycolytic and oxidative enzymes.
The inverse relationships we observed between age and total fiber area, relative number of fast-twitch fibers, and aldolase activity are consistent with existing data which show age-related differences in several properties of skeletal muscle. Type II fibers are those most consistently affected by age, with older persons observed to have a lower area and relative number of these fibers (both Type IIA and IIB) compared to younger persons (8, 11, 31, 32). Our data show that this relationship is still evident even within a group of older adults (i.e., ≥60 yrs). However, these results need to be interpreted in light of the fact that we did not discriminate between Type IIA and IIB fibers; and, in humans, Type IIA fibers can be more oxidative than Type I fibers (33).
Several enzymes (mainly those associated with aerobic energy production) are reported to be lower in skeletal muscle of older persons (9, 34). We observed only a trend for a relationship between citrate synthase and age, but other studies show that citrate synthase activity is lower in older compared to younger individuals (13, 14, 35–38). In addition, as with others (35–38), we saw large variability in the values we observed for citrate synthase (0.02–0.18 μmol/min/μg protein) in this sample of older individuals, suggesting that there are factors other than age that contribute to variability in oxidative capacity of skeletal muscle. For example, although Pastoris et al. observed a negative relationship between age and vastus lateralis citrate synthase, the range of citrate synthase within their 60–85 yr olds was quite similar to our data (0.02–0.12 μmol/min/μg protein) (37). Our values are also consistent with other reported citrate synthase values of older healthy persons (39) and those with heart failure (24) who are sedentary.
The mean (377±98 number/mm2) and range (151–536 number/mm2) of capillary density we observed is reflective of our combination of men and women, and healthy and heart failure older persons, and these values are quantitatively similar to that of the capillary density reported in other studies of older, sedentary persons (15, 16, 23, 39). For example, Magnusson et al. reported the vastus lateralis capillary density of older healthy persons and CHF patients to be 370±63 and 277±55 number/mm2, respectively (23). However, although other studies have shown that capillary density is inversely related to age among individuals of a wide age range (14–16, 40), our data did not confirm this relationship within our sample of 60–85 yr olds.
It is known that aerobic exercise training can affect the intrinsic properties of skeletal muscle (41). Therefore, one of the limitations of the current study was the lack of an objective or self-reported measure of physical activity and/or of aerobic fitness. However, our study included only sedentary individuals who reported no purposeful exercise, including walking, and did not include active older persons whereby fitness level would affect the associations. Moreover, while there may have been individual differences in daily physical activity (household chores, errands, caretaking), these differences are also unlikely to account for the relationships we observed between physical function and muscle characteristics, since these types of activity are not considered intense enough to elicit adaptations in physical function. Another limitation of the study is that the cross-sectional design prevents interpretation of the results regarding causality. Moreover, the nature of our study sample was such that over one-half of the participants had CHF. There are well-known effects of CHF on exercise intolerance/physical abilities (42) and there is emerging evidence that skeletal muscle characteristics, particularly capillary density, is also affected by CHF status (19). However, since walking speed remained significantly related to capillary density and citrate synthase activity after adjustment for CHF status, our data suggest that these associations were not confounded by the high prevalence of CHF in our sample.
CONCLUSIONS
This study reports novel data demonstrating that skeletal muscle capillary density and metabolic enzymatic activity are independently associated with clinical measures of physical function in the elderly. Although there is no way from our cross-sectional design to determine that the functional outcomes are a direct result of skeletal muscle properties, these observations, coupled with previously published data showing age differences in properties of muscle tissue, suggest that specific skeletal muscle abnormalities may constitute a biological pathway contributing to the onset of disability in the elderly.
Acknowledgments
This study was supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center from the National Institute of Aging (P30 AG21332), by the WFU General Clinical Research Center (M01-RR07122) and by National Institute of Aging grant R37 AG018915.
References
- 1.Onder G, Penninx BW, Lapuerta P, et al. Change in physical performance over time in older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002;57:M289–93. doi: 10.1093/gerona/57.5.m289. [DOI] [PubMed] [Google Scholar]
- 2.Inzitari M, Carlo A, Baldereschi M, et al. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2006;54:318–24. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–9. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 4.Sakari-Rantala R, Avlund K, Frandin K, Era P. The incidence of mobility restrictions among elderly people in two Nordic localities. A five-year follow-up. Aging Clin Exp Res. 2002;14 (Suppl 3):47–55. [PubMed] [Google Scholar]
- 5.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Guralnik JM, Buchner D, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M275–85. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–6. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams GN, Higgins MJ, Lewek MD. Aging skeletal muscle: physiologic changes and the effects of training. Phys Ther. 2002;82:62–8. doi: 10.1093/ptj/82.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Kaczor JJ, Ziolkowski W, Antosiewicz J, Hac S, Tarnopolsky MA, Popinigis J. The effect of aging on anaerobic and aerobic enzyme activities in human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:339–44. doi: 10.1093/gerona/61.4.339. [DOI] [PubMed] [Google Scholar]
- 10.Lexell J, Downham D. What is the effect of ageing on type 2 muscle fibres? J Neurol Sci. 1992;107:250–1. doi: 10.1016/0022-510x(92)90297-x. [DOI] [PubMed] [Google Scholar]
- 11.Orlander J, Kiessling KH, Larsson L, Karlsson J, Aniansson A. Skeletal muscle metabolism and ultrastructure in relation to age in sedentary men. Acta Physiol Scand. 1978;104:249–61. doi: 10.1111/j.1748-1716.1978.tb06277.x. [DOI] [PubMed] [Google Scholar]
- 12.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103:31–9. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 13.Borges O, Essen-Gustavsson B. Enzyme activities in type I and II muscle fibres of human skeletal muscle in relation to age and torque development. Acta Physiol Scand. 1989;136:29–36. doi: 10.1111/j.1748-1716.1989.tb08626.x. [DOI] [PubMed] [Google Scholar]
- 14.Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47:B71–6. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 15.Croley AN, Zwetsloot KA, Westerkamp LM, et al. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol. 2005;99:1872–9. doi: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- 16.Ryan NA, Zwetsloot KA, Westerkamp LM, et al. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol. 2006;100:178–85. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- 17.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol. 2002;55:916–21. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibres from transgenic mice. J Physiol. 2003;552:833–44. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duscha BD, Kraus WE, Keteyian SJ, et al. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–63. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 20.Henrikkson JM, Chi MY, Hintz CS. Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am J Physiol Cell Physiol. 1986;251:C614–32. doi: 10.1152/ajpcell.1986.251.4.C614. [DOI] [PubMed] [Google Scholar]
- 21.Askew CD, Green S, Walker PJ, et al. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802–7. doi: 10.1016/j.jvs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 22.Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27:140–5. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson G, Kaijser L, Rong H, Isberg B, Sylven C, Saltin B. Exercise capacity in heart failure patients: relative importance of heart and skeletal muscle. Clin Physiol. 1996;16:183–95. doi: 10.1111/j.1475-097x.1996.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 24.Duscha BD, Annex BH, Keteyian SJ, et al. Differences in skeletal muscle between men and women with chronic heart failure. J Appl Physiol. 2001;90:280–6. doi: 10.1152/jappl.2001.90.1.280. [DOI] [PubMed] [Google Scholar]
- 25.Hunter GR, Bamman MM, Larson-Meyer DE, et al. Inverse relationship between exercise economy and oxidative capacity in muscle. Eur J Appl Physiol. 2005;94:558–68. doi: 10.1007/s00421-005-1370-z. [DOI] [PubMed] [Google Scholar]
- 26.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85:1337–41. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- 27.Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand. 1978;104:129–36. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- 28.Grimby G, Danneskiold-Samsoe B, Hvid K, Saltin B. Morphology and enzymatic capacity in arm and leg muscles in 78–81 year old men and women. Acta Physiol Scand. 1982;115:125–34. doi: 10.1111/j.1748-1716.1982.tb07054.x. [DOI] [PubMed] [Google Scholar]
- 29.Holmback AM, Porter MM, Downham D, Andersen JL, Lexell J. Structure and function of the ankle dorsiflexor muscles in young and moderately active men and women. J Appl Physiol. 2003;95:2416–24. doi: 10.1152/japplphysiol.00517.2002. [DOI] [PubMed] [Google Scholar]
- 30.De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004;30:209–15. doi: 10.1002/mus.20085. [DOI] [PubMed] [Google Scholar]
- 31.Aniansson A, Hedberg M, Henning GB, Grimby G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: a follow-up study. Muscle Nerve. 1986;9:585–91. doi: 10.1002/mus.880090702. [DOI] [PubMed] [Google Scholar]
- 32.Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of Type IIA/B human skeletal muscle fibers. Clin Orthop Relat Res. 2006;450:231–7. doi: 10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- 33.Scott W, Stevens J, Binder-Macleod Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–6. [PubMed] [Google Scholar]
- 34.Steinhagen-Thiessen E, Hilz H. The age-dependent decrease in creatine kinase and aldolase activities in human striated muscle is not caused by an accumulation of faulty proteins. Mech Ageing Dev. 1976;5:447–57. doi: 10.1016/0047-6374(76)90043-9. [DOI] [PubMed] [Google Scholar]
- 35.Essen-Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126:107–14. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 36.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA. 1996;93:15364–9. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pastoris O, Boschi F, Verri M, et al. The effects of aging on enzyme activities and metabolite concentrations in skeletal muscle from sedentary male and female subjects. Exp Gerontol. 2000;35:95–104. doi: 10.1016/s0531-5565(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 38.Hunter GR, Newcomer BR, Weinsier RL, et al. Age is independently related to muscle metabolic capacity in premenopausal women. J Appl Physiol. 2002;93:70–6. doi: 10.1152/japplphysiol.01239.2001. [DOI] [PubMed] [Google Scholar]
- 39.Coggan AR, Spina RJ, King KS, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–6. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 40.Harris BA. The influence of endurance and resistance exercise on muscle capillarization in the elderly: a review. Acta Physiol Scand. 2005;185:89–97. doi: 10.1111/j.1365-201X.2005.01461.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton MT, Booth FW. Skeletal muscle adaptation to exercise: a century of progress. J Appl Physiol. 2000;88:327–31. doi: 10.1152/jappl.2000.88.1.327. [DOI] [PubMed] [Google Scholar]
- 42.Kitzman DW. Exercise intolerance. Prog Cardiovasc Dis. 2005;47:367–79. doi: 10.1016/j.pcad.2005.02.002. [DOI] [PubMed] [Google Scholar]