Abstract
The Genetic Absence Epilepsy Rat from Strasbourg (GAERS) is considered an isomorphic, predictive, and homologous model of typical childhood absence epilepsy. It is characterized by the expression of spike-and-wave discharges (SWDs) in the thalamus and cortex. The ketogenic diet is successfully used in humans and animals with various types of seizures, but was not effective in children with intractable atypical absence epilepsy. Here we studied its potential impact on the occurrence of SWDs in GAERS. Rats were fed the ketogenic diet for 3 weeks during which they were regularly subjected to the electroencephalographic recording of SWDs. The ketogenic diet did not influence the number and duration of SWDS despite a 15–22% decrease in plasma glucose levels and a large increase in β-hydroxybutyrate levels. Likewise, the ketogenic diet did not affect the level of expression of the blood-brain barrier glucose transporter GLUT1 or of the monocarboxylate transporters, MCT1 and MCT2. This report extends the observation in humans that the KD does not appear to show effectiveness in intractable atypical absence epilepsy to this model of typical childhood absence epilepsy which responds to specific antiepileptic drugs.
Keywords: Absence epilepsy, spike-and-wave discharges, ketogenic diet, β-hydroxyburate, glucose transporters, monocarboxylic acid transporters
INTRODUCTION
The ketogenic diet (KD) has been used successfully for several decades in the treatment of partial and generalized difficult-to-control epilepsies, and appears to be more effective than currently available antiepileptic drugs (for review, see Freeman et al., 1998; Hartman and Vining, 2007). The KD has been successfully used in patients of all ages suffering from a large variety of seizure syndromes and severities, linked to different etiologies (Freeman et al., 2007). It was also reported to be effective in the treatment of atypical absence epilepsy (Lennox-Gastaut syndrome) in children (Ross et al., 1985; Freeman et al., 2008) but to our knowledge, the KD was not tested in children with typical absence epilepsy. The efficacy of the KD was hypothesized to correlate with the circulating concentration of β-hydroxybutyrate (βHB) (Hawkins et al., 1971; Freeman et al., 1998), but this hypothesis has been challenged (Vining et al., 1998; Bough et al., 1999a). The antiepileptic effect of the diet starts after 5 days in the rat and is maximal after 12–14 days (Bough and Eagles, 1999), suggesting the development of some new “balance” to reduce brain excitability. However, the mechanisms of action underlying the efficacy of the KD have still been not been entirely clarified (Vamecq et al., 2005; Bough and Rho, 2007).
The KD has been shown to be highly effective, achieving more than 90% reduction in seizure frequency in about 40% of epileptic patients and reducing by more than 50% the extent and frequency of epileptic episodes in many types of symptomatic epilepsy, including intractable atypical absence epilepsy such as seen in Lennox-Gastaut syndrome (Freeman and Vining, 1999; Kang et al., 2005). However, in this syndrome, there is no clear correlation between electrical improvement and clinical improvement (Ross et al., 1985). Because of its obvious constraints and most likely because absence epilepsy is a benign, maturation-related form of idiopathic epilepsy that responds well to adapted antiepileptic medication and spontaneously remits at adolescence (Stefan et al., 2008), the KD has not been used in typical childhood absence epilepsy. Here, we used the KD in Genetic Absence Epilepsy Rats from Strasbourg (GAERS). This genetic model is considered an isomorphic, predictive, and homologous model of human generalized idiopathic absence epilepsy (Danober et al., 1998). In this strain, all animals express genetically determined spontaneous spike-and-wave discharges (SWDs) on the cortical electroencephalogram (EEG) concurrent with behavioral arrest. Bilateral SWDs take place within a thalamo-cortical loop and are recorded mainly from frontoparietal and sensorimotor cortex, and posterolateral thalamic relay nuclei (Danober et al., 1998). Absence seizures reflect transient disturbances of excitatory and/or inhibitory mechanisms in the thalamo-cortical circuit, mainly under the influence of the glutamatergic and/or the GABAergic system (Danober et al., 1998). Thus, absence seizures may be sensitive to the KD which might partly act on brain excitability via a change in glutamate and GABA ratios, mainly by accelerating the flux through glutamate decarboxylase hence increasing the concentration and rate of formation of GABA (Erecinska et al., 1996; Yudkoff et al., 2001). In the present study, we investigated the antiepileptic efficacy of the KD in GAERS using as a reference the typical and easily quantifiable expression of the syndrome, the expression of SWDs, along with the circulating levels of glucose and βHB. We also measured the expression of the glucose transporter protein at the blood-brain barrier, the 55 kD unit of GLUT1, and that of monocarboxylic acid transporter proteins, MCT1 and MCT2 that are responsible for the transfer of ketone bodies through the blood-brain barrier, and for their access to astroglial and neuronal cells, respectively (Simpson et al., 2007). We showed that despite a marked increase in βHB blood levels, the KD had no effect on the expression of SWDs, GLUT1 or MCTs in this model.
MATERIALS AND METHODS
Animals, surgery, recordings and diet
The experiments were performed on a total number of 48 adult male GAERS (49th generation) originating from our breeding colony and aged 5–6 months. Among the 48 GAERS used, 8 were used for EEG recording, 28 for assessment of plasma glucose and βHB levels and 12 for the measurement of the nutrient transporters. The animals were maintained at 22°C room temperature under a 12h/12h normal light/dark cycle (lights on at 7:00 a.m.) with food and water ad libitum. In those conditions, animals exhibit spontaneous SWDs alternating with periods of normal background EEG activity. All animal experimentation was performed in accordance with the rules of the European Communities Council Directive of November 24, 1986 (86/609/EEC), and the French Department of Agriculture (License N° 67–97). The rats received either a standard certified carbohydrate chow (A04C carbohydrate rodent diet, UAR, Villemoisson-sur-Orge, France), or a KD consisting of 91% fat and 9% protein (based on caloric content) balanced with essential salts and vitamins (Ketogenic Diet # TD96355, Harlan Teklad, Madison, WI, U.S.A.).
For EEG recordings, 8 GAERS were equipped with four single-contact electrodes over the frontoparietal cortex, two on each side. Animals were allowed a week recovery and handled twice a day. They first underwent an EEG recording session to get used to the recording cage and connection to electrode leads. Then, while they were all fed a normal carbohydrate diet, they underwent a second EEG recording which served as baseline. The baseline number and duration of SWDs was recorded twice in animals fed a normal carbohydrate chow. The rats were then switched to the KD for three weeks and the cumulated number and duration of SWDs over a period of one hour during each recording session were calculated. The animals subjected to the KD diet were recorded at 24, 48, 72 h, 4, 7, 10, 14 and 21 days after introduction of the KD.
Measurement of circulating glucose and β-hydroxybutyrate levels
Additional groups of animals were used for the measurement of circulating glucose and βHB levels. Blood freely flowing from the body was collected after decapitation and immediately centrifuged at 4000 g for 1 min. Measurements were immediately performed on the serum kept on ice in groups of 4 animals at each time (baseline, 24, 48, 72 h, 7, 14 and 21 days after introduction of the KD or maintenance of the carbohydrate chow). Concentrations of glucose and βHB in plasma were measured by enzymatic methods using test combination kits. The procedure for glucose measurement involved glucose oxidase and peroxidase (Sigma Diagnostics, St. Louis, MO, U.S.A.) and that for βHB involved βHB dehydrogenase and NAD (Sigma Diagnostics).
Measurement of glucose and monocarboxylic acid transporter proteins
The concentration of the glucose, GLUT1 and monocarboxylic acid transporter proteins, MCT1 and MCT2 was measured by Western blotting of extracts of the cortex and thalamus from 6 GAERS fed a normal carbohydrate diet and 6 GAERS fed a KD for three weeks. Brains were removed on ice, regionally dissected and kept at −80°C. Samples were homogenized in 5 volumes of TES (20 mM Tris, 1 mM EDTA, and 255 mM sucrose, pH 7.4, with the protease inhibitors aprotinin, leupeptin, phenylmethylsulfonyl fluoride, and pepstatin, 1 µg/ml each) and centrifuged at 150,000 g for 20 min at 4°C for the isolation of total membranes. For the analysis of the transporter proteins, tissue samples were solubilized in 1.5% SDS, 2.3 M urea, and 100 mM dithiothreitol, and aliquots of 15 µg of protein were separated on 10% SDS-polyacrylamide gels and transferred electrophoretically to nitrocellulose filters, and individual transporter proteins were analyzed by Western blot with appropriate antibodies as previously described (Vannucci and Simpson, 2003). All gels included an adult microsomal brain standard for purposes of normalization and quantitation. The reactive bands were revealed by chemiluminescence (Renaissance ECL, DuPont). The expression for each protein was quantified by measuring the appropriate integrated absorbance using a BiochemiSystem UVP Bioimaging System, C-80 and Labworks software (UVP, Inc., Upland, CA). The values for each experimental sample were expressed relative to the brain standard in arbitrary standard units, as previously described (Vannucci, 1994).
Statistical analysis
The number and duration of SWDs, plasma concentrations of glucose and βHB and expression of GLUT1 and MCTs in GAERS receiving a normal carbohydrate chow or a KD were analyzed by ANOVA followed by a post-hoc Scheffe’s test. The level of significance was set at p<0.05.
Results
Plasma concentrations of glucose and β-hydroxybutyrate
The KD induced a 15% decrease in the blood concentration of glucose which appeared as soon as 24 h, reached its maximum by 72 h (−22%) and went back to control levels by two weeks (Table 1).
Table 1.
Effects of the KD on plasma levels of glucose and βHB.
| Baseline | 24 H | 48 H | 72 H | 7 days | 14 days | 21 days | |
|---|---|---|---|---|---|---|---|
| Glucose (mmol/L) | 7.95 ± 0.75 | 6.74 ± 0.78* | 6.79 ± 0.83* | 6.24 ± 0.72** | 6.92 ± 0.84 | 7.55 ± 1.41 | 7.20 ± 1.05 |
| βHB (mmol/L) | 0.02 ± 0.03 | 0.98 ± 0.13** | 1.10 ± 0.29** | 0.93 ± 0.17** | 0.96 ± 0.10** | 0.98 ± 0.28** | 0.92 ± 0.18** |
The baseline value was assessed in a group of rats fed a normal carbohydrate diet and represents the reference control value for KD-exposed rats.
Values are expressed as mmol/mL and represent means ± S.D. of four animals at each time point.
p < 0.05, # p < 0.01, statistically significant differences from control levels
Blood levels of βHB were negligible in control animals fed a normal carbohydrate diet. They reached about 1 mmol/L as soon as 24 h after the onset of the KD and did not vary thereafter (Table 1).
Expression of spike-and-wave discharges
The total duration of SWDs reached 1249 s in GAERS fed a carbohydrate diet and recorded on the day before switching to the KD. This means that these animals experienced 21 s of SWDs per minute which is in the range of our previous observations for GAERS. The KD had no influence on the cumulative duration of SWDs in GAERS which ranged from 1064 s to 1314 s compared to the baseline of 1249 s/h (Table 2). The same was true for the total number of SWDs with values ranging from 62 to 79 episodes of SWDs in animals receiving the KD with a baseline of 73 episodes of SWDs per h (Table 2).
Table 2.
Effects of the KD on the cumulative duration and number of SWDs.
| Baseline | 24 H | 48 H | 72 H | 4 days | 7 days | 10 days | 14 days | 21 days | |
|---|---|---|---|---|---|---|---|---|---|
| Duration of SWDs (sec/hour) | 1249 ± 274 | 1164 ± 264 | 1314 ± 336 | 1094 ± 335 | 1064 ± 214 | 1065 ± 293 | 1262 ± 365 | 1189 ± 362 | 1089 ± 345 |
| Number of SWDs per hour | 73 ± 13 | 69 ± 10 | 74 ± 15 | 71 ± 20 | 62 ± 17 | 61 ± 16 | 79 ± 19 | 69 ± 16 | 72 ± 21 |
The baseline value was obtained in GAERS fed a normal carbohydrate diet on the day before switching to the KD.
Values are expressed as seconds or number of SWDs per hour and represent the mean ± S.D. of eight animals at each time point.
Glucose and monocarboxylic acid transporters
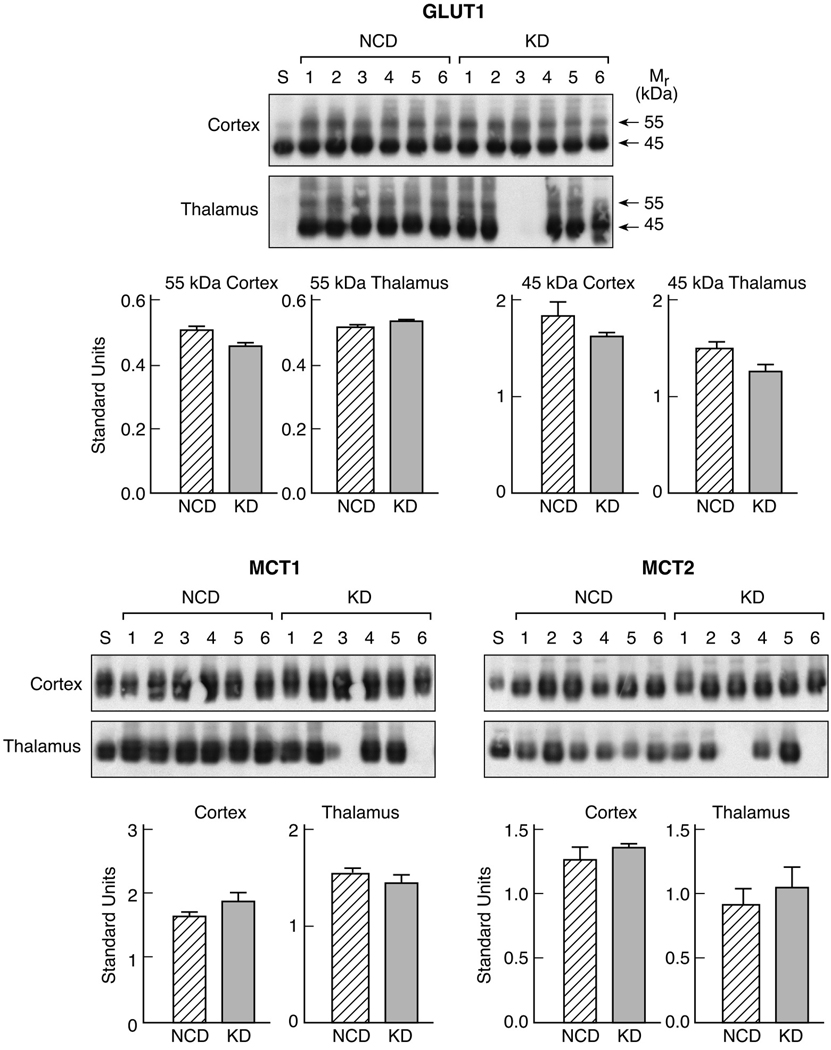
To determine whether 3 weeks of the KD altered the levels of either the glucose transporter protein GLUT1, or the monocarboxylic transporter proteins, MCT1 and MCT2, total membrane samples of cortex and thalamus were analyzed by Western blot. Figure 3 depicts the actual Western blots, as well as the quantitation of these proteins, relative to an internal brain standard (S). GLUT1 in whole brain membranes is detected as both a 55 kDa form, representative of the BBB GLUT1, and a 45 kDa form in the non-vascular brain, whereas the MCTs are detected as single bands at 45 kDa (MCT1) and 40 kDa (MCT2). Figure 1 demonstrates that the levels of the glucose or monocarboxylic acid transporter proteins measured in the cortex and the thalamus of GAERS rats fed a KD for 3 weeks were not different from those in rats fed the normal carbohydrate.
Figure 1.
Effects of the ketogenic diet on the level of expression of GLUT1, MCT1 and MCT2 in the cerebral cortex and thalamus of GAERS. Total membrane samples were prepared from cortex and thalamus of rats on the normal chow diet and rats on the KD. Equal levels of protein were analyzed by Western blot for GLUT1 (A) and MCT1 and MCT2 (B). Blots included an equal concentration of an internal brain standard for quantitation.
Values are expressed as arbitrary standard units which are the ratios of the absorbance value for the sample, relative to the standard, and represent means ± S.D. of six animals in each group.
Abbreviations: NCD: normal carbohydrate diet, KD: ketogenic diet
Discussion
The present data show that there was no effect of the KD on the expression, duration, and number of SWDs in GAERS, a genetic model of typical childhood absence epilepsy. This lack of effect occurred although the changes in the circulating concentration of metabolic substrates, glucose and βHB were in the range of those reported previously after exposure to the KD (Bough et al., 1999a; Raffo et al., 2008). The reduction in plasma glucose levels is paralleled by decreased glycolysis and oxidative metabolism of [1-13C]glucose (Yudkoff et al., 2005; Melø et al., 2006a) which most likely reflect adaptive changes of the brain switching from glucose to ketone bodies as the major substrate. The treatment performed here over 3 weeks was long enough to allow seeing an effect of the KD on SWDs since the antiepileptic effect of the KD has been reported to start after 5 days in the rat and to be maximal after 12–14 days (Bough and Eagles, 1999).
The lack of effect of the KD on this genetic model of typical childhood idiopathic absence epilepsy is in agreement with human data reporting no change in the number of electroencephalographic events induced by the use of the KD in children with intractable atypical absence epilepsy (Lennox-Gastaut syndrome) (Freeman et al., 2008). In contrast most animal studies report effectiveness of the KD in a large variety of acute and chronic models of epilepsy. In chronic models, the KD elevates kindled seizure threshold (Hori et al., 1997), reduces adverse neurologic effects (recurrent seizures, neuronal death, mossy fiber sprouting) consecutive to kainic acid-induced status epilepticus (Muller-Schwarze et al., 1999; Noh et al., 2003). In contrast, another group reported no efficacy of the KD on the afterdischarge threshold in rats subjected to full amygdala kindling (Nylen et al., 2006). In acute models, the KD elevates the threshold to seizures induced by electroshocks (Nakazawa et al., 1983) and the GABAA receptor antagonist pentylenetetrazol (PTZ) in rats (Bough et al., 2000; Nylen et al., 2005; Raffo et al., 2008). This is in line with other studies reporting that the KD increases the threshold for the appearance of absence-like seizures seen after low doses of PTZ and clonic seizures recorded after high doses of PTZ (André et al., 1998) but the KD does not prevent their occurrence (Raffo et al., 2008).
The age of the animals at the time of the study might also interfere with the outcome. GAERS were 5 month-old which is significantly older than in most studies in which young adult animals, usually about 2 month-old are used. The efficacy of a calorie-restricted KD against seizures induced by PTZ inversely relates to the age at which the KD is initiated, i.e. between 22 and 126 postnatal days. Moreover, in rats in which the KD was started at 126 days, plasma levels of βHB did not increase further than 1 mM, i.e. the levels reached in the present study vs 7.2 and 2.7 mM in 22- and 26-old rats, respectively and the KD did not significantly affect the threshold to PTZ-induced seizures in 4-month-old rats compared to large efficacy in young animals (Bough et al., 1999b). Therefore, the lack of efficacy of the diet in this study may partly relate to the age of GAERS. In order to make sure whether or not the KD may be effective on absence seizures, the present study should be repeated in young adult rats, with the limitation that the number/duration of SWDs increase over the first 3 months after their occurrence which may complicate the interpretation.
In GAERS, the occurrence of SWDs is related to the hypersynchronization of thalamo-cortical activity (Danober et al., 1998). Absence seizures reflect transient disturbances of excitatory and/or inhibitory mechanisms in the thalamo-cortical circuit (Snead, 1995), mainly under the influence of the glutamatergic and/or the GABAergic system (Danober et al., 1998). However, no significant change in the density and function of the glutamatergic or GABAergic receptors and transporters is underlying the occurrence of SWDs (Danober et al., 1998; Dutuit et al., 2002). Only a slight increase in extracellular GABA levels was reported in cortex and thalamus of GAERS (Richards et al., 1995, 2000). In GAERS, the occurrence of seizures is directly related to the intracerebral concentration of glutamate (Koerner et al., 1996) and to a moderate excess of GABA-mediated inhibition (Danober et al., 1998). In GAERS receiving a normal carbohydrate diet, the level of glutamate is higher while that of GABA is lower than in the control strain (Melø et al., 2006b). These changes appear critical for seizure expression since they were not observed in immature GAERS not expressing yet absence seizures (Melø et al., 2007). This increased glutamate content may reflect increased vesicular packing, since the expression of the vesicular glutamate transporter vGLUT2 is higher in the cortex of GAERS compared to control rats (Touret et al., 2007). The KD seems to partly act on brain excitability via a change in glutamate and GABA ratios, mainly by accelerating the flux through glutamate decarboxylase hence increasing the concentration and rate of formation of GABA (Erecinska et al., 1996; Yudkoff et al., 2001) on which the occurrence of absence seizures depends (Danober et al., 1998). On the reverse, the protein levels of the different neuronal and glial glutamate transporters (EEAC1, GLT-1, GLAST) were not affected by exposure to the KD (Bough et al., 2007). However, the effect of the KD on amino acid neurotransmitters is not the sole mechanism of action of the KD (Vamecq et al., 2005; Bough and Rho, 2007) and may not be so prominent in GAERS. Indeed, although glutamate was more actively converted to GABA in GAERS on the KD, the brain content of GABA was not affected (Melø et al., 2006a), hence allowing the continuous expression of the seizures. Indeed, in GAERS, the expression of SWDS strongly depends on the concentration of GABA. The injection of GABA-mimetics induces a dose-dependent increase in the number and duration of SWDs in GAERS (Marescaux et al., 1992) and the expression of seizures occurs only in a narrow range of glutamate and GABA levels (Marescaux et al., 1992; Dufour et al., 2001) in contrast with convulsive epilepsy characterized by a more marked imbalance between excitation and inhibition.
Likewise, there was no effect of the KD on the expression of the nutrient transporter proteins in the brain of GAERS. In earlier studies, a high-fat diet has been reported to increase the uptake of βHB (Moore et al., 1976) and diet-induced ketosis results in significant increases in blood-brain barrier MCT1 protein in both luminal and abluminal membranes of young Long-Evans rats (Leino et al., 2001). Increases in MCT1 gene expression were also detected by cDNA microarray analysis of hippocampal samples from juvenile Sprague-Dawley rats fed a KD for 4 weeks (Noh et al., 2004). Although the KD in the present study did induce changes in the circulating levels of glucose and βHB indicative of ketosis, there were no corresponding changes in the expression of the blood-brain barrier glucose transporter protein, the 55 kD unit of GLUT1 and of the two monocarboxylic acid transporter proteins, MCT1 and MCT2. The reasons for these differences are not clear but could clearly relate to strain differences, as well as the age of the animals at the start of the experiment. It has been long considered that a predominant factor in determining the rate at which the brain can use ketone bodies corresponds to the capacity of transport at the level of BBB endothelial cells. This capacity is dependent on both the concentration of transporter proteins and the plasma substrate concentration, which for β HB and MCT1 is well below the Km of the transporter, which is 10–12 mM (Halestrap and Meredith, 2004). In the rat, maximal rates are seen during suckling when the circulating level of ketones is high. After weaning, the rate of uptake of ketone bodies decreases rapidly to reach the adult level (Hawkins et al., 1971; Cremer, 1981), as does the level of MCT1 in the blood-brain barrier (Vannucci and Simpson, 2003).
Few studies have investigated the effect of seizures themselves on the glucose transporter proteins in brain. Cornford et al. (1998, 2000) reported an increase in BBB endothelial GLUT1 in a section of human brain with focal seizures. Experimental rat models of PTZ and kainic acid seizures were associated with increased GLUT1 as well as an increase in the neuronal glucose transporter isoform, GLUT3 (Gronlund et al., 1996) although in a more recent study of PTZ-induced epilepsy in juvenile rats we observed increases in mRNA for GLUT1 and GLUT3 without a corresponding increase in protein (Nehlig et al., 2005).
In conclusion, it appears that the KD did not increase to a large extent the circulating levels of βHB and did not affect the expression of SWDs or the protein levels of brain nutrient transporters. Whether or not these data are age- or epilepsy-related remains to be elucidated.
Acknowledgements
The present study was supported by the Institut de la Santé et de la Recherche Médicale (INSERM U398) and NIH.
References
- André V, Pineau N, Motte JE, Marescaux C, Nehlig A. Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. Eur. J. Neurosci. 1998;10:2094–2106. doi: 10.1046/j.1460-9568.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Eagles DA. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999;40:138–143. doi: 10.1111/j.1528-1157.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Chen RS, Eagles DA. Path analysis shows that increasing ketogenic ratio, but not β-hydroxybutyrate, elevates seizure threshold in the rat. Dev. Neurosci. 1999a;21:400–406. doi: 10.1159/000017390. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999b;35:21–28. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Matthews PJ, Eagles DA. A ketogenic diet has different effects upon seizures induced by maximal electroshock and by pentylenetetrazole infusion. Epilepsy Res. 2000;38:105–114. doi: 10.1016/s0920-1211(99)00079-0. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Paquet M, Paré JF, Hassel B, Smith Y, Hall RA, Dingledine R. Evidence against enhanced glutamate transport in the anticonvulsant mechanism of the ketogenic diet. Epilepsy Res. 2007;74:232–236. doi: 10.1016/j.eplepsyres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48:43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Cornford ME, Landaw EM, Delgado-Escueta AV. Interictal seizure resections show two configurations of endothelial Glut1 glucose transporter in the human blood-brain barrier. J. Cereb. Blood Flow Metab. 1998;18:26–42. doi: 10.1097/00004647-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Cornford EM, Nguyen EV, Landaw EM. Acute upregulation of blood-brain barrier glucose transporter activity in seizures. Am. J. Physiol. 2000;279:H1346–H1354. doi: 10.1152/ajpheart.2000.279.3.H1346. [DOI] [PubMed] [Google Scholar]
- Cremer JE. Nutrients for the brain: problems in supply. Early Hum. Dev. 1981;5:117–132. doi: 10.1016/0378-3782(81)90043-8. [DOI] [PubMed] [Google Scholar]
- Cullingford TE. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70:253–264. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Progr. Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Dufour F, Nalecz KA, Nalecz MJ, Nehlig A. Metabolic approach of absence seizures in a genetic model of absence epilepsy, the GAERS: study of the leucine-glutamate cycle. J. Neurosci. Res. 2001;66:923–930. doi: 10.1002/jnr.10086. [DOI] [PubMed] [Google Scholar]
- Dutuit M, Touret M, Szymocha R, Nehlig A, Belin MF, Didier-Bazès M. Decreased expression of glutamate transporter in genetic absence epilepsy rats before seizure occurrence. J. Neurochem. 2002;80:1029–1038. doi: 10.1046/j.0022-3042.2002.00768.x. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem. 1996;67:2325–2334. doi: 10.1046/j.1471-4159.1996.67062325.x. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Vining EP. Seizures decrease rapidly after fasting: preliminary studies of the ketogenic diet. Arch. Pediatr. Adolesc. Med. 1999;153:946–949. doi: 10.1001/archpedi.153.9.946. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Vining EPG, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet—1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102:1358–1363. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119:535–543. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Vining EP, Kossoff EH, Pyzik PL, Ye X, Goodman SN. A blinded, crossover study of the efficacy of the ketogenic diet. Epilepsia. 2008 Aug 19; doi: 10.1111/j.1528-1167.2008.01740.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gronlund KM, Gerhart DZ, Leino RL, McCall AL, Drewes LR. Chronic seizures increase glucose transporter abundance in rat brain. J. Neuropathol. Exp. Neurol. 1996;55:832–840. doi: 10.1097/00005072-199607000-00008. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Meredith D. The SLC16 gene family – from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Vining EP. Clinical aspects of the ketogenic diet. Epilepsia. 2007;48:31–42. doi: 10.1111/j.1528-1167.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Williamson DH, Krebs HA. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem. J. 1971;122:13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori A, Tandon P, Holmes GL, Stafstrom CE. Ketogenic diet: effects on expression of kindled seizures and behavior in adult rats. Epilepsia. 1997;38:750–758. doi: 10.1111/j.1528-1157.1997.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005;46:272–279. doi: 10.1111/j.0013-9580.2005.48504.x. [DOI] [PubMed] [Google Scholar]
- Koerner C, Danober L, Boehrer A, Marescaux C, Vergnes M. Thalamic NMDA-transmission in a genetic model of absence epilepsy in rats. Epilepsy Res. 1996;25:11–19. doi: 10.1016/0920-1211(96)00015-0. [DOI] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Marescaux C, Vergnes M, Depaulis A. Genetic absence epilepsy in rats from Strasbourg--a review. J Neural Transm Suppl. 1992;35:37–69. doi: 10.1007/978-3-7091-9206-1_4. [DOI] [PubMed] [Google Scholar]
- Melø TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem. Int. 2006a;48:498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Melø TM, Sonnewald U, Touret M, Nehlig A. Cortical glutamate metabolism is enhanced in a genetic model of absence epilepsy. J. Cereb. Blood Flow Metab. 2006b;26:1496–1506. doi: 10.1038/sj.jcbfm.9600300. [DOI] [PubMed] [Google Scholar]
- Melø TM, Sonnewald U, Bastholm IA, Nehlig A. Astrocytes may play a role in the etiology of absence epilepsy: a comparison between immature GAERS not yet expressing seizures and adults. Neurobiol. Dis. 2007;28:227–235. doi: 10.1016/j.nbd.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Moore TJ, Lione AP, Sugden MC, Regen DM. Beta-hydroxybutyrate transport in rat brain: developmental and dietary modulations. Am. J. Physiol. 1976;230:619–630. doi: 10.1152/ajplegacy.1976.230.3.619. [DOI] [PubMed] [Google Scholar]
- Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. NeuroReport. 1999;10:1517–1522. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–380. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Rudolf G, Leroy C, Rigoulot MA, Simpson IA, Vannucci SJ. Pentylenetetrazol-induced status epilepticus up-regulates the expression of glucose transporter mRNAs but not proteins in the immature rat brain. Brain Res. 2006;1082:32–42. doi: 10.1016/j.brainres.2006.01.078. [DOI] [PubMed] [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, Cho GJ, Choi WS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM, Choi WS. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res. Mol. Brain Res. 2004;129:80–87. doi: 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Nylen K, Likhodii S, Abdelmalik PA, Clarke J, Burnham WM. A comparison of the ability of a 4:1 ketogenic diet and a 6.3:1 ketogenic diet to elevate seizure thresholds in adult and young rats. Epilepsia. 2005;46:1198–1204. doi: 10.1111/j.1528-1167.2005.71204.x. [DOI] [PubMed] [Google Scholar]
- Raffo E, François J, Ferrandon A, Koning E, Nehlig A. Calorie-restricted ketogenic diet increases thresholds to all patterns of pentylenetetrazol-induced seizures: critical importance of electroclinical assessment. Epilepsia. 2008;49:320–328. doi: 10.1111/j.1528-1167.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- Richards DA, Lemos T, Whitton PS, Bowery NG. Extracellular GABA in the ventrolateral thalamus of rats exhibiting spontaneous absence epilepsy: a microdialysis study. J. Neurochem. 1995;65:1674–1680. doi: 10.1046/j.1471-4159.1995.65041674.x. [DOI] [PubMed] [Google Scholar]
- Richards DA, Morrone LA, Bowery NG. Hippocampal extracellular amino acids and EEG spectral analysis in a genetic rat model of absence epilepsy. Neuropharmacology. 2000;39:2433–2441. doi: 10.1016/s0028-3908(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Ross DL, Swaiman KF, Torres F, Hansen J. Early biochemical and EEG correlates of the ketogenic diet in children with atypical absence epilepsy. Pediatr. Neurol. 1985;1:104–108. doi: 10.1016/0887-8994(85)90045-1. [DOI] [PubMed] [Google Scholar]
- Stefan H, Snead OC, III, Eeg-Olofsson O. Typical and atypical absence seizures, myoclonic absences, and eyelid myoclonia, in Epilepsy. In: Engel J Jr, Pedley TA, Aicardi J, Moshé SL, Dichter MA, Trimble MR, Perucca E, editors. A comprehensive textbook. 2nd edn. Philadelphia: Wolters Kluver/ Lippincott Williams & Wilkins; 2008. pp. 573–584. [Google Scholar]
- Touret M, Parrot S, Denoroy L, Belin MF, Didier-Bazès M. Glutamatergic alterations in the cortex of genetic absence epilepsy rats. BMC Neurosci. 2007;8:69. doi: 10.1186/1471-2202-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamecq J, Vallée L, Lesage F, Gressens P, Stables JP. Antiepileptic popular ketogenic diet: emerging twists in an ancient story. Progr. Neurobiol. 2005;75:1–28. doi: 10.1016/j.pneurobio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1127–E1134. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch. Neurol. 1998;55:1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, amino acid metabolism, and seizure control. J. Neurosci. Res. 2001;66:931–940. doi: 10.1002/jnr.10083. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S, Nissim I. Response of brain amino acid metabolism to ketosis. Neurochem. Int. 2005;47:119–128. doi: 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]