Abstract
Nicotine and methamphetamine (METH) cause addiction by triggering neuroplastic changes in brain reward pathways though they each engage distinct molecular targets (nicotine receptors and dopamine transporters respectively). Addiction to both drugs is very prevalent, with the vast majority of METH users being also smokers of cigarettes. This co-morbid occurrence thus raised questions about potential synergistic rewarding effects of the drugs. However, few studies have investigated the chronic neurobiological changes associated with co-morbid nicotine and METH addiction. Here we investigated the effects of these two drugs alone and in combination on the expression of several immediate early genes (IEGs) that are sensitive to drug exposures. Chronic exposure to either nicotine or METH caused significant decreases in the expression of fosb, fra1, and fra2 in the nucleus accumbens (NAc) but not in the dorsal striatum whereas the drug combination increased fra2 expression in both structures. Except for junB mRNA levels that were decreased by the three drug treatments in the NAc, there were no significant changes in the Jun family members. Of the Egr family members, NAc egr2 expression was decreased after nicotine and the drug combination whereas NAc egr3 was decreased after METH and the drug combination. The drug combination also increased striatal egr3 expression. The Nr4a family member, nr4a2/nurr1, showed increased striatal expression after all 3 drug treatments, while striatal nr4a3/nor-1 expression was increased by the drug combination whereas NAc nr4a1/nurr77 was decreased by nicotine and the drug combination. These observations suggest that, when given in combination, the two drugs exert distinct effects on the expression of IEGs in dopaminergic projection areas from those elicited by each drug alone. The significance of these changes in IEG expression and in other molecular markers in fostering co-morbid METH and nicotine abuse needs to be further evaluated.
Keywords: nicotine, methamphetamine, striatum, nucleus accumbens, immediate early genes
Introduction
Illicit and licit drug use are major public health problems associated with serious adverse medical consequences and with criminal behaviors. Methamphetamine (METH) is an illicit psychostimulant drug (Meredith et al., 2005; Krasnova and Cadet, 2009) that exerts it rewarding effects via dopamine transporters through which it releases dopamine (DA) into the synaptic terminal causing molecular changes in post-synaptic cells (Cho and Melega, 2008; Cadet et al., 2010). Nicotine is a licit but highly addictive substance (Benowitz, 2010) that exerts its effects by interacting with nicotinic acetylcholine receptors (nAChR) that are abundantly found on midbrain dopaminergic cell bodies that project to the dorsal striatum and nucleus accumbens (NAc) (Livingstone and Wonnacott, 2009). Stimulation of midbrain nicotinic receptors causes DA release in projection areas (Livingstone and Wonnacott, 2009).
The co-abuse of nicotine and METH is very prevalent (Goldsamt et al., 2005; Yen and Chong, 2006). This phenomenon might reflect possible augmentation of the rewarding effects of METH by nicotine (Suemaru et al., 1993; Kuribara, 1999) or conditioning of one drug to the other, as exemplified by METH re-instatement after nicotine exposure (Neugebauer et al., 2010). Of interest, behavioral differences have been reported in animals co-exposed to nicotine and METH when compared to animals exposed to either drug alone (Gatch et al., 2008; Neugebauer et al., 2010). These findings suggest that distinct consequences can emerge with the drug combination. As a first step towards investigating the molecular effects of the co-administration of nicotine and METH, we compared mRNA expression of several immediate early genes (IEGs) in the dorsal striatum and the NAc as consequences of the administration of these drugs being given alone or in combination. We sought to investigate the changes in IEG expression because IEGs encode transcription factors that regulate neuronal responses to stimuli (Hughes and Dragunow, 1995; Hergden and Leah, 1998) and because they have been implicated in the neuronal responses that contribute to drug addiction and abuse (McClung et al., 2004; Nestler, 2008). We chose the dorsolateral striatum because it participates in automaticity in drug taking behaviors and reinstatement of drug seeking and the NAc because it is thought to be involved in many aspects of drug addiction including drug reward and conditioning (Fuchs et al., 2006; Bossert et al., 2009; Pickens et al., 2011).
Experimental Procedures
Animals
Male Sprague-Dawley rats ~76–100g (Taconic Farms Hudson, NY) were used in this study. Treatment began one week after arrival at 5 weeks of age. Rats were single housed in clear plexi-glass cages in an environmental controlled room (temperature 72 ± 2 ° F and humidity 40–60% with a 12 h light/dark cycle: lights off at 8 AM and lights on at 8 PM). Food and water was provided ad-lib. Body weight and food intake was measured daily.
Drug Administration
Rats were subcutaneously implanted with an osmotic mini-pump supplying nicotine (1.3 mg/kg/day) or an equivalent volume saline at a rate of 2.5 μl/hr. Subsequently, the rats received daily intraperitoneal (IP) injections of either (+)-methamphetamine (METH) hydrochloride (8mg/kg/day) or an equivalent volume of saline for 30 days. We chose to use the IP route for METH injections because, as reviewed previously, the most common route of METH administration, regardless of doses, is the IP injection (Krasnova and Cadet, 2009). METH was not delivered via the osmotic pumps as this would mean injecting METH via the subcutaneous route. Because of nicotine’s shorter half life, 20 minutes in the plasma of rats (Sastry et al., 1995; Ghosheh et al., 1999), the SC route is often the route of choice in these kinds of experiments (Benwell et al., 1995; Matta et al., 2007). The osmotic pump delivers a nicotine dose SC, at a controlled rate, the continuous flow of nicotine also keeps blood plasma concentrations of nicotine at a constant rate, similar to chronic human smokers. We did not mix the two drugs together as the pH of the nicotine solution needs to be adjusted to pH 7.4 and the addition of METH to a nicotine solution might have introduced additional confounds.
Four treatment groups were used: saline+saline (SS), nicotine+saline (NS), saline+METH (SM) or nicotine+METH (NM). Rats were euthanized 24 hours after the last drug injection. Striatal and NAc tissues were dissected out and analyzed separately. All experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals and Brookhaven National Laboratory Institutional Animal Care and Use Committee Protocols.
qRT-PCR
Total RNA was isolated from the striatal and NAc tissues by using RNeasy Micro Kit and Mini Kit, respectively (QIAGEN, Valencia, CA). The RNA concentration was determined using NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA). Unpooled total striatal RNA (1μg) obtained from 8 rats per group was reverse transcribed with oligo dT primers using Advantage RT for PCR kit (Takara Bio Inc., Mountain View, CA) while NAc RNA (50ng) was reverse transcribed with oligo dT primers using iScript Reverse Transcription Supermix for RT-qPCR (BioRad, Hercules, CA). PCR experiments were performed using Light Cycler RT-PCR machine (Roche, Indianapolis, IN) and iQ SYBR Green Supermix (BioRad). Primers were synthesized and HPLC-purified at John Hopkins University (Baltimore, MD) and were the same sequences used previously (see McCoy et al., 2011 for sequence data). Clathrin and 18S rRNA were used as an internal control for striatum and NAc, respectively.
Statistical Analysis
Data were analyzed with the statistical software StatView (version 4.02, SAS Institute, Cary, NC). Statistical analysis was done using ANOVA followed by Fisher’s protected least significant differences (PLSD). Differences were considered significant if the probability of error was less than 5%. Data are reported as fold changes (Means ± SEM).
Results
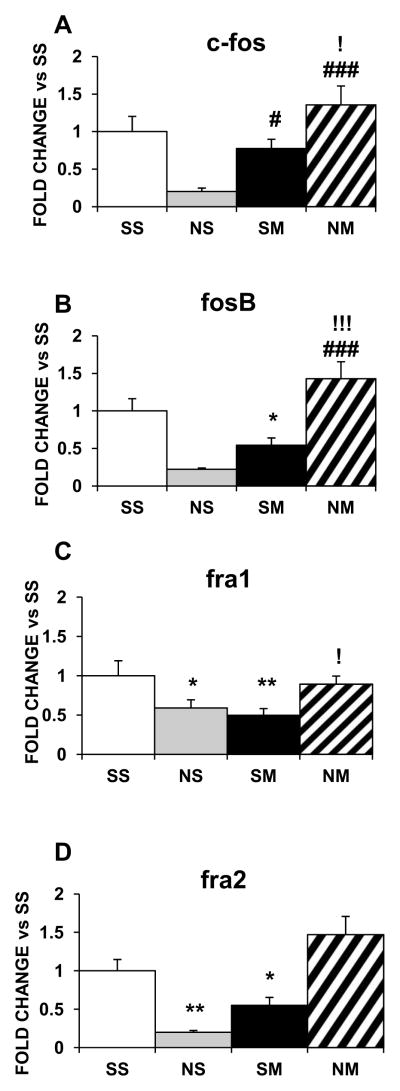
Figure 1 shows the effects of chronic METH and nicotine, separately and in combination, on the mRNA expression of the Fos family in the dorsal striatum. Nicotine or METH given alone caused no significant changes in their mRNAs (Fig. 1A–1D). However, co-administration of the two drugs increased striatal fosb (~1.6-fold, p<0.05; Fig. 1B) and fra2 (~2-fold, p<0.05; Fig. 1D) mRNA expression. In contrast, in the NAc, chronic nicotine decreased c-fos (−80%, p<0.05; Fig. 2A), fosb (−80%, p < 0.01; Fig. 2B), fra1 (−40%, p, 0.05; Fig. 2C), and fra2 (−80%, p < 0.01; Fig. 2D) and chronic METH also decreased fosb (−50%, p<0.05; Fig. 2B), fra1 (−50%, p<0.01; Fig. 2C), and fra2 (−40%, p<0.01; Fig. 2D). However, there were no changes in these IEGs in the NAc with the drug combination (Fig. 2).
Figure 1.
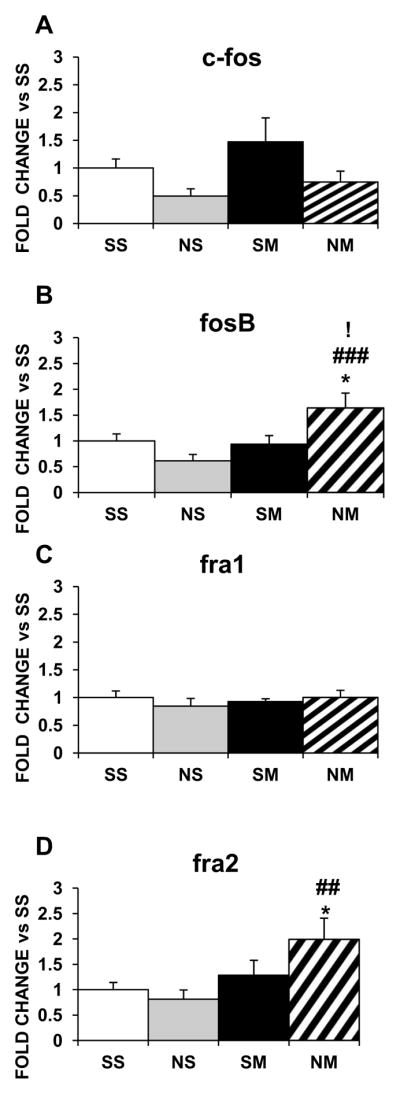
Effects of chronic nicotine, chronic METH and their combination on mRNA levels of Fos IEG family in the rat dorsal striatum. Rats were treated as described in the text. Results are presented as fold-changes SEM. The data were analyzed by one-way ANOVA followed by Fisher’s protected least significant difference (PLSD) post-hoc test. Key to statistics: * compared to SS; # compared to NS; ! compared to SM. Symbol guide: single symbol, p< 0.05; double symbol, p< 0.01, and triple symbol, p<0.001. n=7–8 rats per groups.
Figure 2.
Effects of chronic nicotine, chronic METH and their combination on mRNA levels of Fos IEG family in the rat NAc. Treatment is as described in the text. Results are presented as fold-changes ± SEM. The results were analyzed by one-way ANOVA followed by Fisher’s PLSD post-hoc test. Key to statistics: * compared to SS; # compared to NS; ! compared to SM. Symbol guide: single symbol, p< 0.05; double symbol, p< 0.01, and triple symbol, p<0.001. n=4–7 rats per groups.
The various treatments did not change mRNA expression of Jun family members in the dorsal striatum (Fig. 3A–3C), although they all decreased junB expression in the NAc (Fig 4B), without affecting c-jun (Fig. 4A) nor junD (Fig. 4C).
Figure 3.
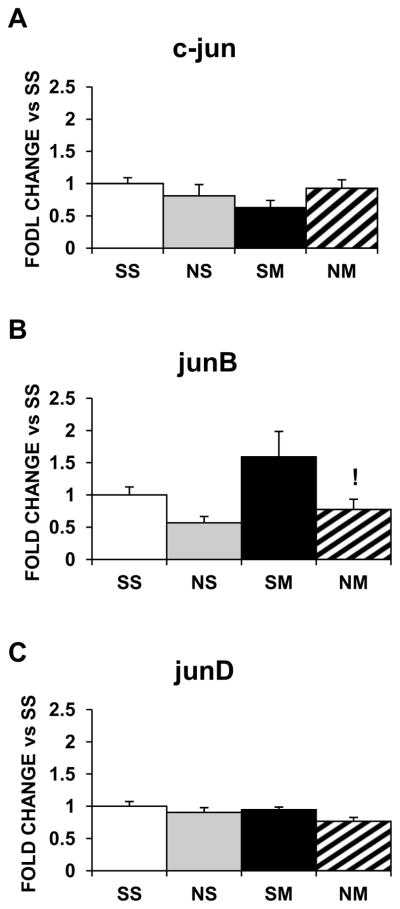
Effects of chronic nicotine, METH, and their combination on mRNA levels of mRNA levels of Jun IEG family members in the rat dorsal striatum. Treatment is as described in the text. Results are presented as described in figure 1. Key to statistics: !, p< 0.05, in comparison to the SM group. n=6–8 rats per groups
Figure 4.
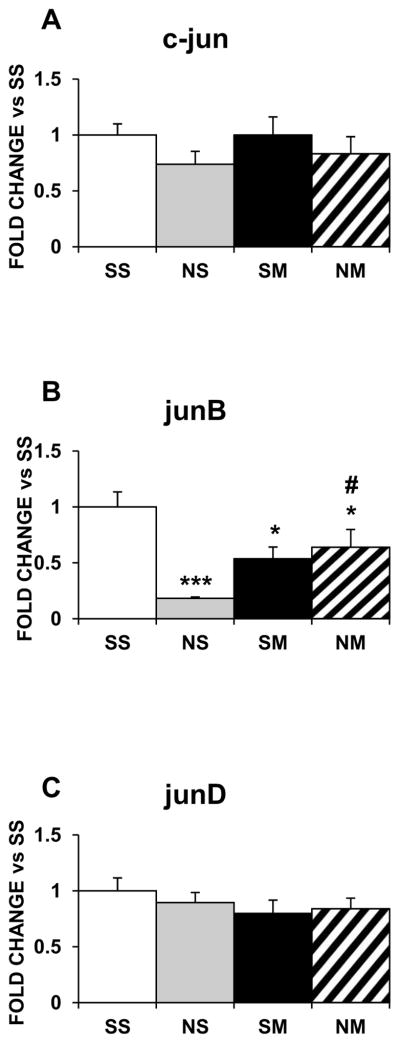
Effects of chronic nicotine, METH and their combination on the expression of Jun IEG family in the rat NAc. Results are presented as described in figure 1. Key to statistics: * compared to SS; # compared to NS. Symbol guide: single symbol, p< 0.05 and triple symbol, p<0.001. n=4–8 rats per groups.
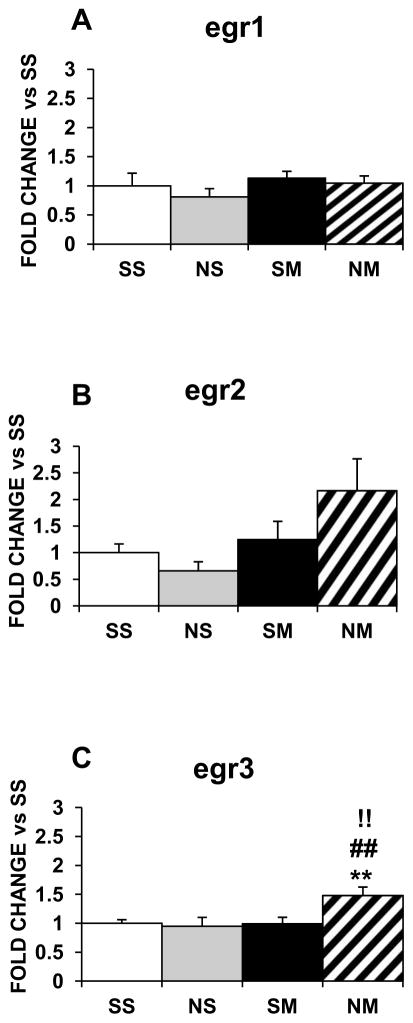
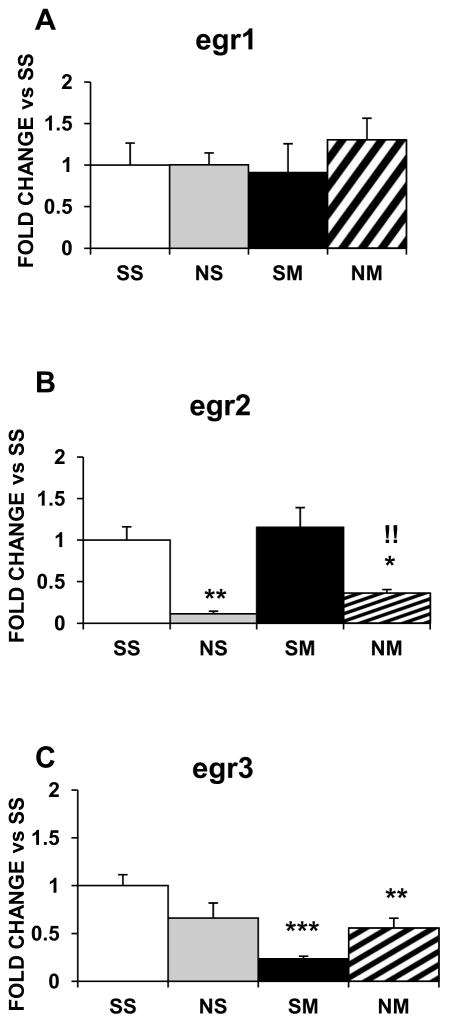
Nicotine or METH alone did not affect the expression of Egr family members in the striatum (Fig. 5) but the drug combination increased egr3 mRNA levels (1.5-fold, p<0.01; Fig. 5C). In the NAc, egr1 was not influenced by any of the treatments (Fig. 6A); egr2 was decreased by nicotine (−90%, p<0.01; Fig. 6B) and the drug combination (−60%, p<0.01; Fig. 6B) and egr3 was decreased by METH (−80%, p<0.001) and the drug combination (−60%, p < 0.01) (Fig. 6C).
Figure 5.
Effects of chronic nicotine, METH and their combination on mRNA levels of Egr IEG family members in the rat dorsal striatum. Results are presented as in figure 1. Key to statistics: * compared to SS; # compared to NS; ! compared to SM. Symbol guide: double symbol, p< 0.01. n=6–8 rats per groups.
Figure 6.
Effects of chronic nicotine, METH and their combination on the NAc expression of Egr IEG family members. Treatment is as described in the text. Key to statistics: * compared to SS; # compared to NS; ! compared to SM. Symbol guide: single symbol, p< 0.05; double symbol, p< 0.01, and triple symbol, p<0.001. n=4–8 rats per groups.
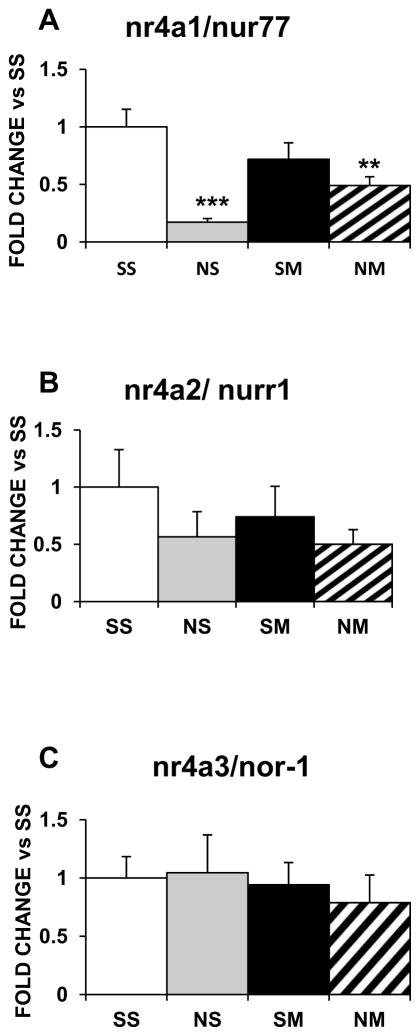
In the striatum, expression of nr4a1/nurr77 was not affected by any of the treatments (Fig. 7A); nr4a2/nurr1 was increased by nicotine (6.6-fold, p<0.01), METH (8.8-fold, p<0.001), and the drug combination (8.9-fold, p<0.001) (Fig. 7B); and nr4a3/nor-1 was not affected by nicotine or METH but was increased by the drug combination (1.8-fold, p<0.05; Fig. 7C). In the NAc, nr4a1/nur77 mRNA expression was decreased by nicotine (−80%, p<0.001) and the drug combination (−50%, p < 0.01) (Fig. 8A) but nr4a2/nurr1 (Fig. 8B) and nr4a3/nor-1 were not affected by any of the treatments (Fig. 8C).
Figure 7.
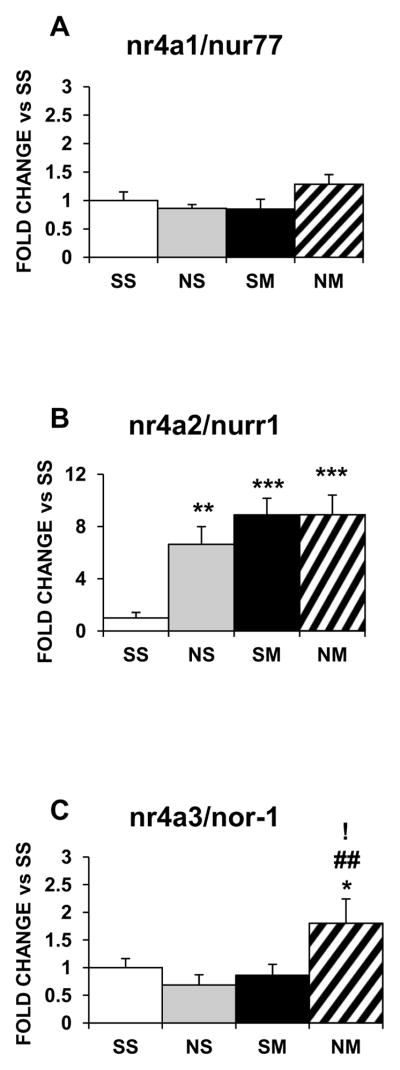
Effects of chronic nicotine, METH and their combination on mRNA levels of Nr4a IEG family members in the rat dorsal striatum. Treatment is as described in the text. Results are presented as above. Key to statistics: * compared to SS; # compared to NS; ! compared to SM. Symbol guide: single symbol, p< 0.05; double symbol, p< 0.01, and triple symbol, p <0.001. n=6–8 rats per groups.
Figure 8.
Effects of chronic nicotine, METH and their combination on the NAc expression of Nr4a IEG family members. Key to statistics: * compared to SS; # compared to NS. Symbol guide: double symbol, p< 0.01, and triple symbol, p<0.001. n=4–8 rats per groups.
Discussion
The major findings in this study are that chronic exposures to: (1) nicotine and METH, given alone, increased the striatal expression of nr4a2/nurr1; (2) nicotine decreased the expression of all members of the Fos family in the NAc; (3) METH also suppressed the expression of the Fos family members (except for cfos) in the NAc; and (4) the co-administration of the two drugs increased the expression of several IEGs in the dorsal striatum. The potential implications for these differential changes in gene expression are discussed below.
Chronic nicotine
Nicotine is thought to exert its addictive properties by enhancing dopamine (DA) release into the striatum and nucleus accumbens (NAc) through stimulation of nAChRs (Picciotto et al., 1998; Perez et al., 2009; Livingstone and Wonnacott, 2009). This is followed by activation of DA-related intracellular molecular signaling cascades (Hamada et al., 2004, 2005; Janhunen and Ahtee, 2007). In the current study, we show that chronic nicotine results in significant decreases in the expression of several IEGs in the NAc but not in the dorsal striatum. These observations are consistent with those of other studies that have reported that chronic administration of cocaine can suppress the expression of some IEGs in the NAc (Hope et al., 1992). These results are also consistent with the report that long-term nicotine exposure depresses dopamine release in the NAc (Perez et al., 2012) and decreases levels of phosphorylated CREB (pCREB) in the NAc (Brunzell et al., 2003). pCREB is known to control IEG expression in dopaminoceptive brain regions (Konradi et al., 1996; Hyman and Malenka, 2001). The lack of similar changes in the dorsal striatum might be related to the observations that nicotine preferentially affects DA release in the NAc in contrast to the dorsal striatum (Benwell and Balfour, 1997; Zhang et al., 2009).
Chronic METH
Previously, we have examined the effects of increasing METH doses on striatal IEG expression (McCoy et al., 2011). We found that METH caused no changes or decreased the expression of several IEGs in the dorsal striatum (McCoy et al., 2011). For example, using that paradigm, chronic METH was shown to cause significant decreases in the striatal expression of AP-1, Egr and Nr4a IEGs (McCoy et al., 2011) in contrast to acute METH that causes increased IEG expression (Cadet et al.,2010; McCoy et al., 2011). When taken together with the present findings, the consensus suggests that chronic METH administration might have caused tolerance to the acute effects of the drug. This argument is relevant for all the IEGs, except nr4a2/nurr1that showed increased gene expression in the dorsal striatum after chronic METH. In contrast to the observations in the striatum, chronic METH caused decreased expression of several IEGs in the NAc including fosB that has been implicated in the neurobiological processes of drug addiction (Nestler et al., 2001). These differential changes in the two structures further support the idea that the dorsal and ventral striatum play distinct roles in the long term effects of abused drugs. Our dichotomous findings also stress the fact that schedules of drug administration can result in differential molecular consequences. Our laboratory is actively investigating these issues further by using different models of METH intake.
Chronic nicotine and METH
To our knowledge, our study is the first investigation of the effects of nicotine and METH co-administration on IEG expression in the rodent forebrain. Previous studies have already examined the acute effects of nicotine (Kiba and Jayaraman, 1994; Pich et al., 1997) or METH (McCoy et al., 2011) on the expression of IEG’s in the striatum, with a general trend of increased IEG expression. In the current study, the expression of the majority of striatal and NAc IEG was not affected by chronic METH or nicotine given alone. These observations suggest that some degree of tolerance might have developed to the individual molecular effects of nicotine and METH. However, this tolerance might have been circumvented when the two drugs were given in combination. For example, co-administration of the two drugs increased fosb, fra2, egr3 and nr4a3/nor-1 expression in the striatum. Of note, when either nicotine or METH alone decreased IEG expression, the co-administration of the drugs either restored IEG expression to normal (i.e. c-fos, fosb and fra1) or increased their expression (fra2) in the NAc. These observations suggest that these two drugs, when given in combination, caused molecular changes in the brain that render it more permissible for the expression of certain genes. This idea is consistent with a recent report that suggested that administration of nicotine facilitated cocaine-induced molecular and behavioral effects in the brain, priming it to cocaine’s rewarding effects (Levine et al., 2011). This priming appears to depend on increased binding of acetylated histones on the fosB promoter region (Levine et al., 2011). Nevertheless, it is important to point out that the co-administration of METH and nicotine suppressed the expression of egr2, egr3 and nr4a1/nurr77 in the NAc. However, with regards to these IEGs, either nicotine (egr2, nr4a1/nurr77) or METH (egr3) was able to suppress their expression, suggesting that the suppression that we observed with the drug combination might be reflecting, in part, the effects of one of the drugs.
Summary
In summary, this is the first report to identify cross-talks between nicotine and METH on the transcriptional regulation of IEGs in the NAc and dorsal striatum, regions implicated in the neuroadaptive and reinforcing properties of drugs. The fact that the two drugs caused increased mRNA levels of certain IEGs that were not affected when the drugs were given alone supports the hypothesis that they might induce molecular alterations that lead to changes in gene expression and to the potential alteration of the rewarding effects of the individual drugs. It is also to be noted that the combination of METH and nicotine also caused additional changes in body weight, food intake, and locomotor activity in comparison to METH and nicotine alone (Thanos et al, in preparation). These observations from our behavioral studies suggest that the drug combination can potentiate decreased body weight and food intake while increasing locomotor activity caused by either drug alone (Thanos et al, in preparation). When taken together with these behavioral results, our molecular observations suggest a potential neurobiological substrate for the high prevalence of co-morbid METH and nicotine abuse and addiction.
Highlights.
Chronic nicotine and METH, alone, increased striatal expression of nr4a2/nurr1
Chronic nicotine decreased the expression of all members of the Fos family in the NAc
Chronic METH suppressed expression of Fos family members (except cfos) in the NAc
Co-administration increased the expression of several IEGs in the dorsal striatum
Acknowledgments
This research was supported by funds of the Intramural Research Program of the DHHS/NIH/NIDA.
We wish to thank Dr. Subramaniam Jayanthi and Michael T. McCoy for the critical reading and development of this manuscript. We also thank the comments of two reviewers who helped to improve the discussion in this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beauvais G, Jayanthi S, McCoy MT, Ladenheim B, Cadet JL. Differential effects of methamphetamine and SCH23390 on the expression of members of IEG families of transcription factors in the rat striatum. Brain Res. 2010;1318:1–10. doi: 10.1016/j.brainres.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz NL. Nicotine Addiction. The New England Journal of Medicine. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benwell MEM, Balfour DJK. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benwell ME, Balfour DJ, et al. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol. 1995;114(2):454–460. doi: 10.1111/j.1476-5381.1995.tb13248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benwell MEM, Balfour DJK. Regional variation in the effects of nicotine on catecholamine overflow in rat brain. Eur J Pharmacol. 1997;325:13–20. doi: 10.1016/s0014-2999(97)00101-5. [DOI] [PubMed] [Google Scholar]
- 6.Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology. 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57B1/6J mice. J Neurochem. 2003;84:1431–41. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 8.Cadet JL, Brannock C, Krasnova IN, Ladenheim B, McCoy MT, Chou J, Lehrmann E, Wood WH, Becker KG, Wang Y. Methamphetamine-induced dopamine-independent alterations in striatal gene expression in the 6-hydroxydopamine hemiparkinsonian rats. PLoS One. 2010 Dec 13;5(12):e15643. doi: 10.1371/journal.pone.0015643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One. 2009;4:e7812. doi: 10.1371/journal.pone.0007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B. Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: Evidence from cDNA array. Synapse. 2001;41:40–48. doi: 10.1002/syn.1058. [DOI] [PubMed] [Google Scholar]
- 11.Cho AK, Melega WP. Patterns of methamphetamine abuse and their consequences. J Addict Dis. 2002;21:21–34. doi: 10.1300/j069v21n01_03. [DOI] [PubMed] [Google Scholar]
- 12.Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010;13:1128–1136. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs RA, Branham RK, See RF. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatch MB, Flores E, Forster MJ. Nicotine and Methamphetamine Share Discriminative Stimulus Effects. Drug and Alcohol Dependence. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsamt LA, O’Brien J, Clatts MC, McGuire LS. The relationship between club drug use and other drug use: a survey of New York City middle school students. Subst Use Misuse. 2005;40:1539–1555. doi: 10.1081/JA-200066886. [DOI] [PubMed] [Google Scholar]
- 16.Ghosheh O, Dwoskin LP, et al. Residence Times and Half-Lives of Nicotine Metabolites in Rat Brain after Acute Peripheral Administration of [2′-14C]Nicotine. Drug Metabolism and Disposition. 1999;27(12): 1448–1455. [PubMed] [Google Scholar]
- 17.Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochem. 2004;90:1094–1103. doi: 10.1111/j.1471-4159.2004.02574.x. [DOI] [PubMed] [Google Scholar]
- 18.Hamada M, Hendrick JP, Ryan GR, Kuroiwa M, Higashi H, Tanaka M, Nairn AC, Greengard P, Nishi A. Nicotine regulates DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa) phosphorylation at multiple sites in neostriatal neurons. J Pharmacol Exp Ther. 2005;315:872–878. doi: 10.1124/jpet.105.090852. [DOI] [PubMed] [Google Scholar]
- 19.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 20.Hope B, Kosofsky B, Hyman SE, Nestler EJ. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes P, Dragunow M. Induction of immediate-early genes and the control of neurotransmitter-regulated gene expression within the nervous system. Pharmacol Rev. 1995;47:133–78. [PubMed] [Google Scholar]
- 22.Hyman SE, Malenka RC. Addiction and the Brain: The Neurobiology of Compulsion and Its Persistence. Nature Reviews Neuroscience. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 23.Janhunen S, Ahtee L. Differential Nicotinic Regulation of the Nigrostriatal and Mesolimbic Dopaminergic Pathways: Implications for Drug Development. Neuroscience & Biobehavioral Reviews. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Kiba H, Jayaraman A. Nicotine induced c-fos expression in the striatum is mediated mostly by dopaminergic D1 receptor and is dependent on NMDA stimulation. Mol Brain Res. 1994;23:1–13. doi: 10.1016/0169-328x(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 25.Knott V, Heenan A, Shah D, Bolton K, Fisher D, Villeneuve C. Electrophysiological evidence of nicotine’s distracter-filtering properties in non-smokers. J Psychopharmacol. 2011;25:239–248. doi: 10.1177/0269881109348158. [DOI] [PubMed] [Google Scholar]
- 26.Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–9. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krasnova IN, Cadet JL. Methamphetamine Toxicity and Messengers of Death. Brain Research Reviews. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuczenski R, Segal DS, et al. Human Methamphetamine Pharmacokinetics Simulated in the Rat: Behavioral and Neurochemical Effects of a 72-h Binge. Neuropsychopharmacology. 2009;34(11):2430–2441. doi: 10.1038/npp.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuribara H. Does nicotine modify the psychotoxic effect of methamphetamine? Assessment in terms of locomotor sensitization in mice. J Toxicol Sci. 1999;24:55–62. doi: 10.2131/jts.24.55. [DOI] [PubMed] [Google Scholar]
- 30.Levine A, Huang Y, Drisaldi B, Griffin EA, Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: Epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livingstone PD, Wonnacott S. Nicotinic Acetylcholine Receptors and the Ascending Dopamine Pathways. Biochemical Pharmacology. 2009;78:744–55. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. CBP in the Nucleus Accumbens Regulates Cocaine-Induced Histone Acetylation and Is Critical for Cocaine-Associated Behaviors. Journal of Neuroscience. 2011;31:16941–6948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, Cadet JL. Methamphetamine Causes Differential Alterations in Gene Expression and Patterns of Histone Acetylation/Hypoacetylation in the Rat Nucleus Accumbens. PLoS ONE. 2012;7:e34236. doi: 10.1371/journal.pone.0034236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matta SG, Balfour DJ, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190(3): 269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 35.McClung CA, Ulery PG, Perotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: A molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132: 146–154. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 36.McCoy MT, Jayanthi S, Wulu JA, Beauvais G, Ladenheim B, Martin TA, Krasnova IN, Hodges AB, Cadet JL. Chronic methamphetamine exposure suppresses the striatal expression of members of multiple families of immediate early genes (IEGs) in the rat: normalization by an acute methamphetamine injection. Psychopharmacology (Berl) 2011;215:353–365. doi: 10.1007/s00213-010-2146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melega WP, Williams AE, et al. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. Journal of Pharmacology and Experimental Therapeutics. 1995;274(1): 90–96. [PubMed] [Google Scholar]
- 38.Meredith CW, Jaffe C, Ang-Lee K, Saxon AJ. Implications of chronic methamphetamine use: a literature review. Harv Rev Psychiatry. 2005;13:141–154. doi: 10.1080/10673220591003605. [DOI] [PubMed] [Google Scholar]
- 39.National Research Council. Guide for the Care and Use of Laboratory Animals. 8 1996. [Google Scholar]
- 40.Nestler EJ. Review. Transcriptional mechanisms of addiction: Role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363: 3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neugebauer NM, Harrod SB, Bardo MT. Nicotine Elicits Methamphetamine-seeking in Rats Previously Administered Nicotine. Drug and Alcohol Dependence. 2010;106:72–78. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 44.Nijholt I, Blank T, Ahi J, Spiess J. In vivo CREB phosphorylation mediated by dopamine and NMDA receptor activation in mouse hippocampus and caudate nucleus. Brain Res Gene Expr Patterns. 2002;1:101–106. doi: 10.1016/s1567-133x(01)00020-5. [DOI] [PubMed] [Google Scholar]
- 45.Perez XA, O’Leary KT, Parameswaran N, McIntosh JM, Quik M. Prominent role of α3/α6β2* nAChRs in regulating evoked dopamine release in primate putamen: effect of long-term nicotine treatment. Mol Pharmacol. 2009;75:938–946. doi: 10.1124/mol.108.053801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez XA, Ly J, McIntosh JM, Quik M. Long-term Nicotine Exposure Depresses Dopamine Release in Nonhuman Primate Nucleus Accumbens. The Journal of Pharmacology and Experimental Therapeutics. 2012;342:335–44. doi: 10.1124/jpet.112.194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Persico AM, Schindler CW, O’Hara BF, Brannock MT, Uhl GR. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain Res Mol Brain Res. 1993;20:91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- 48.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 49.Pich EM, Pagliusi SR, Tessari M, Talbot-Ayer D, van Huijsduijen RH, Chiamulera C. Common neuronal substrate for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- 50.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robertson GS, Vincent SR, Fibiger HC. D1 and D2 Dopamine Receptors Differentially Regulate C-fos Expression in Striatonigral and Striatopallidal Neurons. Neuroscience. 1992;49:285–96. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 53.Sastry BV, Chance MB, et al. Distribution and retention of nicotine and its metabolite, cotinine, in the rat as a function of time. Pharmacology. 1995;50(2): 128–136. doi: 10.1159/000139274. [DOI] [PubMed] [Google Scholar]
- 54.Segal DS, Kuczenski R. Human Methamphetamine Pharmacokinetics Simulated in the Rat: Single Daily Intravenous Administration Reveals Elements of Sensitization and Tolerance. Neuropsychopharmacology. 2005;31(5):941–955. doi: 10.1038/sj.npp.1300865. [DOI] [PubMed] [Google Scholar]
- 55.Su D, Cha YM, West AE. Mutation of MeCP2 Alters Transcriptional Regulation of Select Immediate-early Genes. Epigenetics. 2012;7:146–54. doi: 10.4161/epi.7.2.18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suemaru K, Gomita Y, Furuno K, Araki Y. Chronic nicotine treatment potentiates behavioral responses to dopaminergic drugs in rats. Pharmacol Biochem Behav. 1993;46:135–139. doi: 10.1016/0091-3057(93)90329-r. [DOI] [PubMed] [Google Scholar]
- 57.Yen CF, Chong MY. Comorbid psychiatric disorders, sex, and methamphetamine adolescents: a case-control study. Comprehensive Psychiatry. 2006;47:215–220. doi: 10.1016/j.comppsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine Signaling Differences in the Nucleus accumbens and Dorsal Striatum Exploited by Nicotine. Journal of Neuroscience. 2009;29:4035–043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–69. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]