Abstract
The function of the sigma-1 receptor (S1R) has been implicated in modulating the activity of various ion channels. In the Central Nervous System (CNS) S1R is enriched in cholinergic postsynaptic densities in spinal cord motoneurons (MN). Mutations in S1R have been found in familial cases of Amyotrophic Lateral Sclerosis (ALS).
In this study we show that a knockout of S1R in the SOD1*G93A mouse model of ALS significantly reduces longevity (end stage).
Electrophysiological experiments demonstrate that MN of mice lacking S1R exhibit increased excitability. Taken together the data suggest the S1R acts as a brake on excitability, an effect that might enhance longevity in an ALS mouse model.
INTRODUCTION
S1R was originally characterized as an opioid receptor but further pharmacological evaluation showed it to be a unique non-opioid type of receptor (Quirion et al., 1992). S1R binds to a variety of psychoactive compounds such as cocaine (Sharkey et al., 1988), metamphetamine (Nguyen et al., 2005), neurosteroids (Su et al., 1988) and dimethyltryptamine (Fontanilla et al., 2009). S1R is highly expressed in many tissues, including the CNS (Langa et al., 2003), liver and lungs. Physiologically S1R has been shown to modulate the activity of various ion channels and to play a protective role during the propagation of neurodegenerative diseases (Maurice and Su, 2009, Su et al., 2010). Mutations in S1R were shown to result in onset of autosomal recessive form of Amyotrophic Lateral Sclerosis (ALS) (Al-Saif et al., 2011).
In the CNS, S1R is most abundant in motoneurons (MNs) in the brain stem and spinal cord (Gundlach et al., 1986, Mavlyutov et al., 2010). In MN the S1R is enriched in subsurface cisternae of the ER but is also expressed throughout the endoplasmic reticulum (ER) (Mavlyutov et al., 2010). The regions of MNs possessing subsurface cisternae are called C-terminals, where C stands for “cisternae” (Conradi, 1969). C-terminals are the postsynaptic components of cholinergic synapses, the functions of which were identified only recently (Zagoraiou et al., 2009). Presynaptic C-boutons originate from interneurons whose cell bodies are located close to the central canal of the spinal cord between laminae VII and X. Postsynaptically, the main receptor in C-terminals is known to be a type 2 muscarinic receptor (M2AChR)(Hellstrom et al., 2003). Activation of M2AChR in C-terminals results in inhibition of potassium channels (presumably SK types) and increased MN excitability (Miles et al., 2007).
The aim of the work reported here was to test the role of S1R in the SOD1*G93A mouse model of ALS. We generated ALS mice lacking S1R and found that the average longevity (determined by end-stage) of ALS S1R KO mice was significantly reduced. Electrophysiological experiments demonstrated that MN excitability increased in S1R KO mice indicating that sigma-1 receptor reduced MN excitability in WT mice. This reduced excitability may well serve as a mechanism that extends the lifespan of ALS mice.
RESULTS
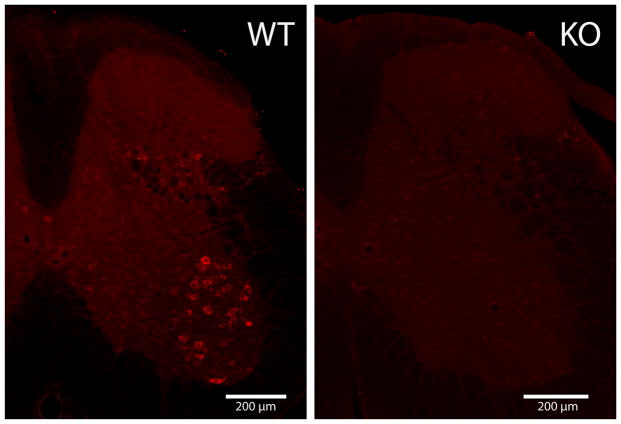
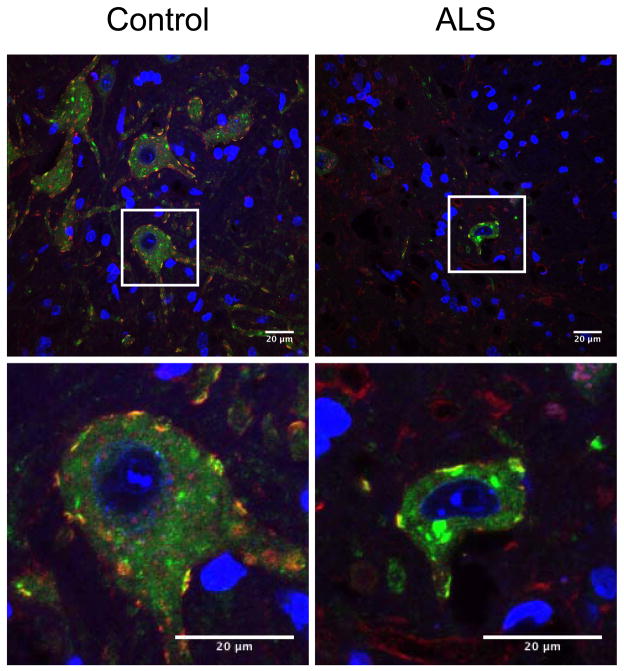
In the mouse spinal cord S1R is expressed at the highest level in the ventral horn MN (Fig. 1), see also (Mavlyutov et al., 2010). Previously we also have shown that in mouse MN, S1Rs are enriched in the postsynaptic sites of C-terminals (Mavlyutov et al., 2010, Mavlyutov et al., 2012). MN in terminally ill SOD*G93A mutant mice degenerate, lose their presynaptic coverage, shrink in size, and proteins such as SOD1 are mislocalized (Ilieva et al., 2009). Since evidence indicates that S1Rs are implicated in ALS (Al-Saif et al., 2011), we examined whether S1R could be localized differently in MN of ALS model animals. We observed that the distribution of S1R was not altered in C-terminals of degenerating SOD1*G93A MN (Fig 2). S1R persisted in C-terminals but may also be localized to intracellular inclusions in degenerating MNs.
Figure 1.
A crossection of the mouse lumbar spinal cord stained with sigma-1 receptor antibody. A section from the spinal cord of Sigma-1 receptor knockout mice (KO) was used as a negative control. The highest levels of sigma-1 receptor were detected in the ventral horn motoneurons.
Figure 2.
The Sigma-1 receptor (S1R) in degenerating motoneurons of SOD*G93A mice in the late disease stages. The S1R remained localized in C-terminals and is expressed at high levels. The Sigma-1 receptor is green and the Kv2.1 channel (marker of C-terminals) is red. Blue is DAPI nuclear stain. Single motoneurons in the white frame are shown at higher magnification in the lower panel. Scale bar= 20um.
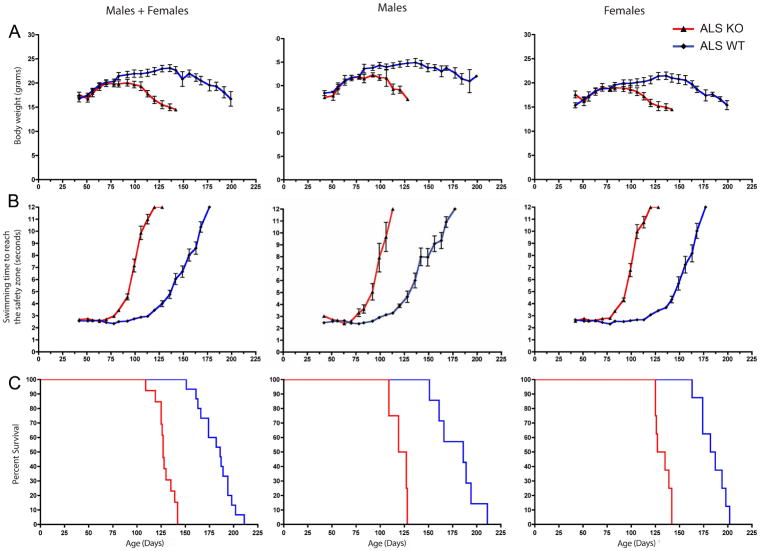
To determine whether S1R could ameliorate the establishment of ALS symptoms we generated S1R KO ALS mice and measured their average end stage and behavior. During the establishment and development of ALS, mice are known to lose weight and exhibit impaired motor performance (Ilieva et al., 2009). Initially we assessed motor performance by the rotorod assay, but results were inconsistent (data not shown). We found the swimming tank assay to be a more reliable method for evaluation of the disease progress. We observed that ALS S1R KO mice lose weight earlier (Fig 3A), exhibited an early decline in swimming performance (Fig 3B), and their average end stage was significantly reduced (Fig 3C) (Table).
Figure 3.
ALS mice without the Sigma-1 receptor (SOD1 ALS/S1R KO) exhibited earlier loss of body weight, earlier signs of motor decline and reduced longevity (terminal stage) compared to ALS mice with the Sigma-1 receptor (S1R WT). A. Body weight. B. Swimming time to reach the safety platform. Error bars represent standart error. C. Kaplan-Meyer end stage curve. Median survival of mice is 186.0 days for ALS S1R WT mice, and 127.0 days for ALS S1R KO mice. p<0.0001; χ2 =32.29.
Table.
Median end stage of Sod1*G93A ALS mice with and without S1R
| Median end-stage (days) | p-value | χ2 | |
|---|---|---|---|
|
| |||
| ALS S1R WT males (n=7) | 186.0 | =0.0004 | 12.35 |
| ALS S1R KO males (n=4) | 123.0 | ||
|
| |||
| ALS S1R WT females (n=8) | 184.5 | <0.0001 | 16.23 |
| ALS S1R KO females (n=8) | 131.0 | ||
|
| |||
| ALS S1R WT males+females (n=15) | 186 | <0.0001 | 32.29 |
| ALS S1R KO males+females (n=12) | 127 | ||
The average end stage of our S1R WT SOD1*G93A mice is longer than that found in mice with high copy number SOD1*G93A (Heiman-Patterson et al., 2011). It is known that the end stage of SOD1*G93A varies with the background of the mice. We measured the copy number of SOD1*G93A transgenes in our mice to determine if differences in copy number could account for the increased life span. Our qPCR results from two S1R WT and two S1R KO mice showed that they maintain the same copy number as high copy number SOD1*G93A mice from Jackson Laboratories. The ratio of the copy number for both S1R WT and KO SOD1*G93A to the copy number in SOD1*G93A mice from Jackson Laboratories is 1.02. Thus the extended longevity seen in our mice does not result from a change in copy number; we propose that differences in background underlie the increased longevity.
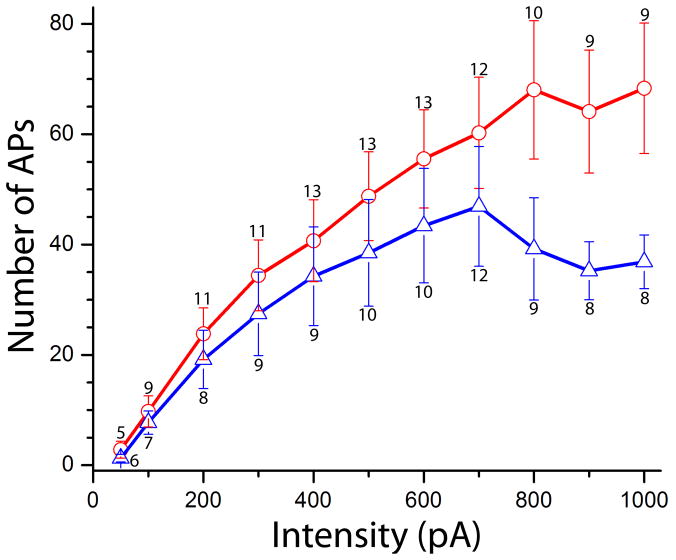
There is a growing body of evidence that S1R can modulate the activity of ion channels (Aydar et al., 2002, Renaudo et al., 2007, Su et al., 2010, Johannessen et al., 2011). Therefore whole-cell current clamp recordings were performed to determine whether S1R plays a role in regulating MN excitablity. We analyzed the MN electrical properties and firing rate in WT and S1R KO mice without the SOD1*G93A mutation. Repetitive firing was generated by intracellular injections of prolonged pulses (1 s) of positive currents of intensities that ranged from 50 to 1000 pA in 50 pA steps (Fig.4). Firing frequency was estimated as the number of action potentials generated during the 1 s current pulse. Plotting the frequency (F) as a function of current injected (I) demonstrated a relatively linear increase in F-I relationship in MN of SR1 KO mice with peak frequency of approximately 67 Hz at 1000 pA depolarizing current (n = 14). A linear increase was also apparent in MNs of WT mice but only up to an average frequency of approximately 45 Hz (n=13). The findings demonstrated that the firing frequency of MN of S1R KO juvenile mice was significantly higher than in S1R WT mice at current intensities > 700 pA. This was not associated with significant differences in the input resistance of KO and WT MNs that were estimated as 147 ± 32MΩ (s.d) and 172 ± 48MΩ respectively. The reduced firing rate at large depolarizing currents might result from the action of the S1R on potassium channels.
Fig 4.
Frequency-current relationships in motoneurons of SR1 KO (red) and WT (blue) mice. A significant increase in the slope of F-I relationship was apparent in S1R KO mice at current intensities > 700 pA (p < 0.05). Bars are ± standard errors. Number of recordings per intensity is shown above/below standart error bars.
DISCUSSION
Our study demonstrates for the first time that the lifespan of ALS mouse model decreases in the absence of S1R. Synaptic coverage of MN somas by C-terminals remained until death while most other synapses retracted (Pullen and Athanasiou, 2009). Our data showing that the S1R remains in C-terminals of degenerating MN suggest that the receptor plays an important role in halting the progression of ALS. We propose that the S1R acts as a brake on increased MN excitability, which is one of the hallmarks of the disease (Kuo et al., 2004, Kuo et al., 2005, Pambo-Pambo et al., 2009). The reduced excitability is likely to extend MN end stage and thus longevity. One of the mechanisms of increased excitability that has been identified is the blocking potassium channels in C-terminals where two different potassium channels exist (SK and Kv2.1). It has been shown that activation of the muscarinic receptor in C-terminals of MNs resulted in inhibition of potassium channels (presumably SK type) (Miles et al., 2007). This inhibition resulted in increased MN excitability. Furthermore, loss of Choline Acetyl Transferase (ChAT) in C-terminals resulted in decreased excitability that was revealed during the swimming assay but not during walking. Zagaroiu et al. further demonstrated that ablation of ChAT in PITX2 positive neurons, which give rise to C-terminals, decreases motor output under stressful conditions (2009). The EMGs during swimming but not walking were significantly weaker in mice with dysfunctional C-terminals (Zagoraiou et al., 2009).
Previous studies have shown that S1R modulates the activity of various ion channels. For example, S1R alters the activity of potassium channels in pituitary cells (Aydar et al., 2002). Thus, we propose that the S1R in C-terminals increases the activities of SK and/or Kv2.1 channels, resulting in membrane hyperpolarization and reduced MN excitability in wildtype mice but not in S1R KO mice.
The exact mechanism by which S1R action influences the activities of potassium channels in the plasma membrane of C-terminals is not understood at present. One possibility is that S1R directly interacts with these channels and thereby increases their activity thus increasing afterhyperpolarization. On the other hand, since S1R is localized in C-terminals in subsurface cisternae, a known site of calcium storage (Rosenbluth, 1962), it is also reasonable to speculate that S1R may function indirectly by mediating the release of calcium from subsurface cisternae. S1R was shown to interact with the IP3 type 3 receptor and this interaction amplifies calcium release (Hayashi and Su, 2007). This higher cytosolic calcium when released from the cisternae could thus activate calmodulin and produce a higher conductance of potassium channels (Mavlyutov et al., 2012) as was shown for SK type channels (Schumacher et al., 2001). In summary we propose, therefore, that S1R activates potassium channels in C-terminals and thereby decreases MN excitability in S1R WT animals but not in their KO counterparts. S1R is known to regulate a range of ion channels including the NMDA type (Martina et al., 2007), which may contribute to the mechanism of action of S1R. Recently it has been reported that application of S1R ligand PRE-084 significantly extended end stage in ALS model mice (Mancuso et al., 2012).
Interestingly, the loss of function mutation of S1R does not have a phenotype in the mouse as it does in the human. Al-Saif et al. showed that an autosomal-recessive mutation in S1R results in the establishment of juvenile ALS (Al-Saif et al., 2011). They showed that a mutation on one allele does not result in the disease phenotype, but mutations on both alleles do. These data indicate that this mutation results in a loss of function that was proposed to be the reason for the MN degeneration. However, in mice, the knockout of S1R produces slight motor abnormalities (Mavlyutov et al., 2010) but does not itself result in an ALS phenotype. Thus it remains unclear at present how loss of function produces these very different responses. One possibility is that mice can compensate for the lack of S1R. Multiple proteins involved in cytoprotective antioxidant function are upregulated in S1R KO mice (Pal et al., 2012) and in hippocampal cultures after knockdown of S1R by SiRNA (Tsai et al., 2012). Nevertheless, we showed that the SOD1 ALS/S1R KO mice lost weight, showed impaired motor performance, and ultimately died faster as shown by our data from end stage and behavioral experiments.
Therefore the data presented in this paper is consistent with S1R serving as a brake on MN excitability which can be a mechanism to resists ALS progression. Future behavioral and electrophysiological experiments with activation of S1R in ALS model animals will test this hypothesis further.
EXPERIMENTAL PROCEDURES
Animals
Oprs1 mutants (Sigma-1 receptor; +/−) PprsGT(IRESBetageo)33Lex litters on a C57BL/6Jx129s/SvEv mixed background were purchased from the Mutant Mouse Regional Resource Center, UC Davis, CA, USA. ALS model mice, SOD1*G93A were purchased from Jackson Laboratories. To generate Sigma-1 knockouts SOD1*G93A mice, a female Sigma-1 (−/−) on a C57BL/6Jx129s/SvEv were mated with a SOD1*G93A male on B6SJL background. The resulting Sigma-1 (+/−) SOD1*G93A males were backcrossed for 4 generations to female SOD1*G93A on C57BL/6Jx129s/SvEv to insure that animals have a homogenous background. The resulting mice were genotyped to select SOD1*G93A S1R WT and KO mice. To produce mice expressing GFP in MN for electrophysiological experiments, S1R KO mice were mated to Hb9::eGFP transgenic mice (Wichterle et al., 2002). The resulting S1R (+/−) mice were then mated to produce Hb9::eGFP S1R WT and KO mice. In the Hb9::eGFP mouse line, motoneurons, ventral interneurons and sympathetic neurons express the reporter gene eGFP (Wilson et al., 2005). All mice were maintained on a normal 12-h light/dark cycle and handled in accordance with animal care and use guidelines of the University of Wisconsin, Madison. Procedures were optimized to minimize suffering and to reduce the number of animals used.
Immunohistochemistry
For histological evaluation of sigma-1 receptor in MN of L3-L5 region of the mouse spinal cord we used three terminal ALS SOD1*G93A males and three age-matching (18-weeks old) normal males. Mice were anesthetized with Nembutal and perfused through the left ventricle with phosphate buffered saline (PBS) containing heparin followed by 4% paraformaldehyde for 30 min. The spinal cords were then dissected and postfixed with the same fixative overnight. The tissue was rinsed for 10 h in PBS, and cryoprotected in 30% sucrose in PBS for 48 h, all at 40C. The tissue was cut on a sliding freezing microtome at 60μm, collected in PBS, permeabilized with 1% Triton X-100 for 30 min, blocked with 10% normal goat serum and stained with rabbit anti-sigma serum (1/700) and with monoclonal anti Kv2.1 antibody (Neuromab# 75/014) (1/300).
Sections were rinsed and secondary antibodies of Alexa-488 conjugated goat-anti-rabbit and Alexa 568 conjugated goat-anti-mouse IgG at a concentration 2μg/ml were applied overnight. Sections were rinsed, counterstained with DAPI, embedded into Prolong Gold mounting media (Invitrogen), and coverslipped. Images were taken through a Apo60X VC oil-immersion objective on a Nikon A1R laser confocal microscope (Nikon, Tokyo, Japan) equipped with green 488 nm Argon and red 561 nm DPSS lasers and NIS elements software. Final figures were produced in FIJI software.
Swimming tank experiments
For swimming tank, body weight, and longevity (end stage) experiments we used 15 of SOD1*G93A S1R WT mice (7 males; 8 females) and 12 of SOD1*G93A S1R KO mice (4 males, 8 females). Mice were trained as described below to swim in a water tank from one end to another as described previously (Mavlyutov et al., 2010). Swimming tests were terminated after the mice swam for more than 12 seconds.
Determination of terminal stage of ALS mice
After difficulty in movements were first observed, mice were placed on their side daily to determine if they were at the terminal stage of ALS. Inability to right themselves within 20 sec was considered an indicator of having reached the terminal stage and mice were immediately euthanized by exposure to carbon dioxide.
Quantitative real time PCR and copy number variation
DNA was isolated from tails from our mice and from mice maintained in a colony that originated from Jackson Laboratory and was quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Pittsburgh, PA). To measure copy number of SOD1 gene we performed quantitative real-time PCR (qPCR) using the CFX96 instrument (Bio-Rad) and the SsoFast TM EvaGreen Supermix kit (Bio-Rad). Ten nanograms of DNA were used per qPCR reaction. All reactions were run in duplicate and the Ct values were averaged for each set of two reactions. We used the same primers and method mentioned previously to calculate the copy number of SOD1 gene in our mice (Henriques et al., 2010). Briefly, primers: were as follows: SOD1 forward 5′-GTGTGCGTGCTGAAGGGCGA -3′ and reverse 5′-GTGTGCGTGCTGAAGGGCGA -3′; Cyclophilin forward 5′-GTGTGCGTGCTGAAGGGC GA -3′ and reverse 5′-GTGTGCGTGCTGAAGGGCGA -3′. Cyclophilin was used as a control. Copy number of SOD-1 was calculated relative to cyclophilin (the average Ct value generated using SOD1 primers for a particular mouse / Ct value generated using Cyclophilin primers for the same mouse).
Whole-cell patch clamp recordings
Hb9::eGFP S1R WT and KO mice were used for electrophysiological experiments in methods detailed in previous studies of Hb9::eGFP transgenic mice (Hinckley et al., 2005, Ziskind-Conhaim and Hinckley, 2008, Ziskind-Conhaim et al., 2010). Juvenile mice, 12–15-day old (P12–15) were lightly anesthetized by isoflurane and following decapitation the spinal cord was extracted in ice-cold oxygenated solution. The isolated spinal cord was embedded in 3–4% agar or agarose and thick transverse sections (350 um) were obtained with a vibratome (Leica, VT100S). Prior to whole-cell recordings, slices were incubated in extracellular solution at room temperature for 30–60 min. The extracellular solution contained (in mM): 113 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 1 NaH2PO4, and 11 glucose. The solution was equilibrated with 95% O2-5% CO2 (pH 7.2–7.3 at 20–22°C). Slices were transferred to a recording chamber, which was mounted on the stage of an epi-fluorescence microscope (Olympus, BX50WI) equipped with a 475 nm excitation filter, a 505 nm dichroic mirror and a 535 nm emission filter (Omega Optics, Inc.). GFP+ motoneurons were identified and targeted for whole-cell recordings using patch electrodes pulled to tip resistances of 4–6 MΩ using a multi-stage puller (P-97, Sutter Instrument). Whole-cell patch clamp configuration was obtained using infrared DIC optics.
Intracellular potentials/currents were filtered at 3 kHz, sampled at 10–20 kHz (Multiclamp 700B amplifier, Molecular Devices) and recorded on a PC with Clampex software (v9.2). Membrane potentials were corrected for a 10 mV liquid junction potential (Gao et al., 2001). The whole-cell pipette solution contained (in mM): K gluconate 140, KCl 9, N-(2-Hydeoxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES) 10, Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) 0.2, Na-ATP 4, and Na-GTP 0.3. The solution was adjusted to pH 7.2 using KOH, and the osmolarity was 290–305 mOsm.
Knockout of S1R in the SOD1*G93A mouse model of ALS significantly reduces longevity.
MN of mice lacking S1R exhibit increased excitability.
S1R acts as a brake on excitability, an effect that might enhance longevity in an ALS mouse model.
Acknowledgments
This work was supported by NIH Grant Number 5 R21 NS075820 (AER), the UW McPherson Eye Research Institute Retina Research Foundation Edwin and Dorothy Gamewell Professorship (AER), NIH Grant RO1-DK081634 (MLE).
Abbreviations
- S1R
sigma-1 receptor
- CNS
central nervous system
- ALS
Amyotrophic Lateral Sclerosis
- MN
motoneurons
- WT
wildtype
- KO
knockout
- ER
endoplasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011 doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson TD, Sher RB, Blankenhorn EA, Alexander G, Deitch JS, Kunst CB, Maragakis N, Cox G. Effect of genetic background on phenotype variability in transgenic mouse models of amyotrophic lateral sclerosis: a window of opportunity in the search for genetic modifiers. Amyotroph Lateral Scler. 2011;12:79–86. doi: 10.3109/17482968.2010.550626. [DOI] [PubMed] [Google Scholar]
- Hellstrom J, Oliveira AL, Meister B, Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol. 2003;460:476–486. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Henriques A, Pitzer C, Schneider A. Characterization of a novel SOD-1(G93A) transgenic mouse line with very decelerated disease development. PLoS One. 2010;5:e15445. doi: 10.1371/journal.pone.0015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB. Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol. 2011;300:C328–337. doi: 10.1152/ajpcell.00383.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Schonewille M, Siddique T, Schults AN, Fu R, Bar PR, Anelli R, Heckman CJ, Kroese AB. Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J Neurophysiol. 2004;91:571–575. doi: 10.1152/jn.00665.2003. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol. 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa F, Codony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- Mancuso R, Olivan S, Rando A, Casas C, Osta R, Navarro X. Sigma-1R Agonist Improves Motor Function and Motoneuron Survival in ALS Mice. Neurotherapeutics. 2012;9:814–826. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neuroscience. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Liu P, Verbny YI, Ziskind-Conhaim L, Ruoho AE. Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N,N′-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience. 2012;206:60–68. doi: 10.1016/j.neuroscience.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pal A, Fontanilla D, Gopalakrishnan A, Chae YK, Markley JL, Ruoho AE. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur J Pharmacol. 2012;682:12–20. doi: 10.1016/j.ejphar.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G(93A-Low) mice. J Neurophysiol. 2009;102:3627–3642. doi: 10.1152/jn.00482.2009. [DOI] [PubMed] [Google Scholar]
- Pullen AH, Athanasiou D. Increase in presynaptic territory of C-terminals on lumbar motoneurons of G93A SOD1 mice during disease progression. Eur J Neurosci. 2009;29:551–561. doi: 10.1111/j.1460-9568.2008.06602.x. [DOI] [PubMed] [Google Scholar]
- Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Renaudo A, L’Hoste S, Guizouarn H, Borgese F, Soriani O. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl- channels. J Biol Chem. 2007;282:2259–2267. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Hinckley CA. Hb9 versus type 2 interneurons. J Neurophysiol. 2008;99:1044–1046. doi: 10.1152/jn.01163.2007. author reply 1047–1049. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Mentis GZ, Wiesner EP, Titus DJ. Synaptic integration of rhythmogenic neurons in the locomotor circuitry: the case of Hb9 interneurons. Ann N Y Acad Sci. 2010;1198:72–84. doi: 10.1111/j.1749-6632.2010.05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]