Abstract
Rats treated with three daily urocortin 1 (UCN) injections into the basolateral amygdala (BLA; i.e., UCN/BLA-primed rats) develop prolonged anxiety-associated behavior and vulnerability to panic-like physiological responses (i.e., tachycardia, hypertension and tachypnea) following intravenous infusions of 0.5 M sodium lactate (NaLac, an ordinarily mild interoceptive stressor). In these UCN-primed rats, the osmosensitive subfornical organ (SFO) may be a potential site that detects increases in plasma NaLac and mobilizes panic pathways since inhibiting the SFO blocks panic following NaLac in this model. Furthermore, since SFO neurons synthesize angiotensin II (A-II), we hypothesized that the SFO projects to the BLA and releases A-II to mobilizing panic responses in UCN/BLA-primed rats following NaLac infusions. To test this hypothesis, rats received daily bilateral injections of UCN or vehicle into the BLA daily for 3 days. Five to seven days following the intra-BLA injections, we microinjected either the nonspecific A-II type 1 (AT1r) and 2 (AT2r) receptor antagonist saralasin, or the AT2r-selective antagonist PD123319 into the BLA prior to the NaLac challenge. The UCN/BLA-primed rats pre-injected with saralasin, but not PD123319 or vehicle, had reduced NaLac-induced anxiety-associated behavior and panic-associated tachycardia and tachypnea responses. We then confirmed the presence of AT1rs in the BLA using immunohistochemistry which, combined with the previous data, suggest that A-II’s panicogenic effects in the BLA is AT1r dependent. Surprisingly, the SFO had almost no neurons that directly innervate the BLA, which suggests an indirect pathway for relaying the NaLac signal. Overall these results are the first to implicate A-II and AT1rs as putative neurotransmitter-receptors in NaLac induced panic-like responses in UCN/BLA-primed rats.
Keywords: Anxiety, panic, angiotensin, circumventricular organ, amygdala, GABA, saralasin
1. Introduction
Panic disorder is a severe anxiety disorder characterized by recurrent panic attacks, consisting of pronounced fear and heightened cardiorespiratory responses (DSM-IV, 1994). A unique characteristic of panic disorder patients is their sensitivity to ordinarily mild interoceptive stressors such as intravenous infusions of mildly hypertonic 0.5 M sodium lactate (NaLac) (Cowley et al., 1987, Lapierre et al., 1984, Liebowitz et al., 1985). The pathological sensitivity to interoceptive stressors appears to involve an alteration somewhere in the central neural pathways controlling normal panic responses. The amygdala may be part of this circuitry since abnormalities in this structure have been noted in panic patients (Reiman et al., 1984, Reiman et al., 1989, Wiest et al., 2006).
The amygdala plays a critical role in attention mechanisms that facilitate learning and survival in response to important sensory input [see review (LeDoux, 2000)]. Synaptic plasticity changes within the basolateral amygdala (BLA) parallel anxiety-like behavior and are believed to be important in fear-associated memories (Mitra et al., 2005). Preclinical data from our laboratory support the hypothesis that aberrant conditions within the amygdala underlie panic attacks in some cases. For instance, chronic loss of local inhibitory GABAergic tone (Sajdyk and Shekhar, 2000, Shekhar et al., 1999), or three daily injections of urocortin 1 (UCN, a potent corticotropin-releasing factor 1 and 2 receptor agonist) (Rainnie et al., 2004, Sajdyk et al., 1999a) within the BLA produces rats that display a chronic anxiety-like state and are also prone to panic-like responses following intravenous infusions of 0.5 M NaLac.
Previous clinical studies suggest that increased sodium, not lactate or osmotic stress, may be the critical factor for provoking panic attacks in panic disorder patients. For example, i.v. infusions of hypertonic (0.5 M) NaLac, sodium chloride (NaCl) (Peskind et al., 1998), or sodium bicarbonate (Gorman et al., 1989) provoke equivalent panic associated responses in panic disorder patients; and in a hypothalamic rat model of panic vulnerability, i.v. infusions of hypertonic (0.5 M) solutions of NaCl and NaLac resulted in equivalent panic-like behavioral and cardiovascular responses (Molosh et al., 2010). In rodents, the expression of the NaX channel protein, which is critical for changes in drinking behavior in response to increases in plasma concentrations in Na+ [i.e., dehydration (Watanabe et al., 2000)], is largely restricted to regions with a reduced blood-brain barrier called circumventricular organs (CVOs) (Hiyama et al., 2004, Watanabe, Fujikawa, 2000).
Circumventricular organs are believed to play a critical role in relaying osmotic-related signals (e.g., changes in Na+) from the periphery to the central nervous system (Hochstenbach and Ciriello, 1996). Previously, Shekhar and colleagues have demonstrated the importance of the forebrain subfornical organ (SFO) CVO in NaLac’s ability to elicit panic-like responses in UCN/BLA-primed rats (Shekhar, Sajdyk, 1999). The SFO may be especially important in relaying the NaLac signal to the BLA since blocking neuronal transmission in the SFO of UCN/BLA primed rats prevents NaLac-induced anxiety-like and cardiorespiratory responses (Shekhar, Sajdyk, 1999). The present study was designed to determine the neurochemical substrate underlying this NaLac-SFO-BLA circuit. Since the majority of SFO neurons produce angiotensin (A-II) (Lind et al., 1985a, Lind et al., 1985b, Saavedra and Chevillard, 1982, Saavedra et al., 1982), and centrally acting A-II type 1 receptor (AT1r) antagonists are anxiolytic (Saavedra et al., 2005, Saavedra et al., 2006), there is reason to believe that A-II may be the critical neuropeptide in this circuit. In order to test this hypothesis, rats were made panic-prone by receiving 3 daily bilateral injections of UCN into the BLA. After another 3–5 days, rats then received bilateral injections of saline vehicle, saralasin (a nonselective A-II receptor antagonist), or PD123319 [a selective A-II type 2 receptor (AT2r) antagonist] into the BLA 30 min prior to i.v. NaLac infusions. Furthermore, the presence of AT1Rs in the BLA was assessed using immunohistochemistry. In light of the presence of A-II-synthesizing neurons in the SFO, and the SFO’s ability to ‘sense’ the NaLac signal, we also determined if neurons in the SFO directly innervate the BLA region, using intra-BLA microinjections of retrograde tracer.
2. Methods and Materials
2.1 Animals
All experiments, except for the retrograde tracer experiment, were conducted on adult male Wistar rats (300–325 g), which were purchased from Harlan Laboratories and were housed individually in plastic cages under standard environmental conditions (22 °C; 12/12 light/dark cycle; lights on at 7:00 A.M.) for 7–10 days prior to the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80–23) revised 1996 and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
For the retrograde tracing experiment, adult male Wistar rats arrived from the vendor (Mølegaarden, Denmark; N = 34) weighing approximately 200 g and were kept in standard laboratory conditions with ad libitum tap water and standard rat chow (Altromin, Lage, Germany) and maintained under a photoperiod of 12-h light:12-h dark with lights on at 6:00 AM. The experiment was conducted under the authority of the Animal Core Facility of the Panum Institute, Department of Neuroscience and Pharmacology, The Panum Institute, University of Copenhagen, in accordance with and approved by The Animal Experiments Inspectorate, Ministry of Justice, Denmark and the European Communities Council Directive of 24 November 1986 (86/609/EEC). Care was taken to minimize the number of animals used and their suffering. Experimental data from these rats have been previously reported (Hale et al., 2008a, Hale et al., 2008b)
2.2 Venous catheterization for NaLac infusions
Prior to surgery, rats were anaesthetized by placing them in a closed Plexiglas® box that was connected to an isoflurane system (MGX Research Machine; Vetamic, Rossville IN) and then with a nose cone connected to the same system during the surgery. All rats were fitted with femoral arterial catheters for measurement of mean arterial blood pressure (MAP) and heart rate (HR) and with femoral venous catheters for i.v. infusions, as previously described (Shekhar et al., 1996). Briefly, MAP and HR were monitored by an arterial line attached to a pressure transducer connected to a Beckman R511 Dynograph (Beckman Instruments. Inc., Brea, CA). Windows based DSI dataquest software was used to monitor and record MAP and HR, whereas an indirect measurement of respiration rate (RR) was obtained from normal sinus arrhythmia. For the duration of each experiment, MAP, HR and RR were recorded continuously in freely moving conscious rats. Cardio-respiratory data are expressed as peak changes in MAP or HR. The peak for MAP and HR was defined as the highest value sustained for 1 min or longer.
2.3 Implantation of chronic microinjection cannulae into the BLA and drug injections
Immediately following venous and arterial catheterization, rats were placed into a stereotaxic instrument (Kopf Instruments, Tujunga, CA) with the incisor bar set at −3.3 mm and nose cone connected to the same system during the surgery. Two stainless steel guide cannulae (26 gauge, 10 mm length: Plastics One, Roanoke, VA) were situated into guide cannulae holders fixed onto the stereotaxic arms. The injector was lowered into position of the BLA using coordinates (anterior, −2.1 mm; lateral, ±5.0 mm; ventral, −8.5 mm) according to a standard stereotaxic atlas of the adult rat brain (Paxinos and Watson, 1986). The guide cannulae were secured into place using three 2.4 mm screws anchored into the skull along with cranioplastic cement. Following placement of dummy cannulae into the guide cannulae, rats were removed from the stereotaxic apparatus and allowed to recover for 72 hrs.
All injections of UCN into the BLA were conducted utilizing microinjection cannulae (33 gauge, Plastics One) that fit into and extended 1 mm beyond the guide cannulae. UCN was administered in 1% bovine serum albumin (BSA) in a total volume of 100 nl per/site. A 10-µl Hamilton syringe was situated on an infusion pump (Harvard Apparatus, Holliston, MA, model PHD 2000) and subsequently connected to the injection cannulae via polyethylene (PE 50) tubing (Fisher Scientific, Pittsburg, PA). Once the injection cannulae were securely placed into the rat, the infusion pump was turned on and set to automatically deliver 100 nl/site over 30 seconds. Following the injection, the cannulae remained in place for an additional min before being removed. Smooth flow of the solutions via the tip of the injection cannulae was verified before and after each injection to ensure proper drug delivery. UCN was generously provided by Dr. Nick Ling (Neurocrine, San Diego, CA).
2.4 Experimental Protocol
After 3 days of recovery from surgeries, baseline reactivity to i.v. NaLac infusions was determined [(i.e., cardiorespiratory responses and social interaction (SI) test immediately following the end of NaLac challenge]). Seventy-two hrs following BLA cannulations, 6 rats received bilateral injections of UCN (6 fmoles) daily for 3 days. On experimental days 5–11, (in a counter-balanced design with 2 days between injections) rats randomly received 100 nl injection of saline, 20 pmoles saralasin (a nonselective A-II receptor antagonist; cat. no. A2275, Sigma-Aldrich), 100 pmoles saralasin or 100 pmoles of PD123319 (PD 123,319 di(trifluoroacetate) salt; an A-II type 2 receptor antagonist; cat. no. P186 Sigma-Aldrich], bilaterally, into the BLA 30 min prior to NaLac challenge. Cardiorespiratory measures were recorded 5 min prior to (baseline) and 15 min post i.v. infusions of 0.5 M NaLac. Immediately following the end of NaLac infusions, rats were placed in the SI test for behavioral evaluation as described in following paragraph.
Anxiety-like behavior was measured utilizing the SI test, which is a validated measure of anxiety-like behavior (File, 1980). The apparatus, where the testing is done, consists of a solid wooden box with an open roof approximately 0.9 m long× 0.9 m wide with walls 0.3 m high. A video camera is fixed above the box, and all behavioral tests are videotaped under low red light conditions (approximately 100 lux) and in a familiar environment. The “experimental” rat and an unfamiliar “partner” rat are both placed individually in the center of the box and allowed to habituate to the environment for a 5 min period 24 hr prior to each SI test. During the SI test, the two rats are placed together in the center of the box, and the total duration (sec) of non-aggressive physical contact (grooming, sniffing, crawling over and under, etc.) initiated by the “experimental” rat is quantified over a 5 min duration. Videotaped sessions were scored at a later time by an investigator, who was blind to any drug treatment.
2.5 Histological verification of cannulae placements
After the final test in SI, all rats were anesthetized with isoflurane and decapitated; their brains were then removed and frozen. Four out of six brains were coronally sectioned at 60 µm on a Leica cryostat for verification of the cannulae placement. The sections were mounted onto slides and, once dry, were stained with cresyl violet to verify location of the cannulae. For the other three brains, confirmations of cannula placements in the BLA were done visually on coronal brain sections cut at 300 µm.
2.6 Statistical Analysis
A one-way ANOVA with a Newman-Keuls post hoc test was utilized for all data analysis (i.e., behavioral and cardiorespiratory data analysis). The significance level was set at p < 0.05 for all ANOVAs and posthoc tests. All statistical analyses and graphs were carried out using Graphpad Prizm 4.0 for Windows.
2.7 Immunohistochemistry for angiotensin II type 1 receptor (AT1r)
Three male Wistar rats (250–275 g; Harlan Laboratories, Indianapolis, IN) were group housed in conditions as previously described in methods. Rats were anesthetized with halothane then perfused transcardially with 250 ml of 0.1 M phosphate buffered saline (PBS), followed by 250 ml of 0.1 M sodium phosphate buffer (PB) containing 4% paraformaldehyde and 3% sucrose. Brains were removed and rinsed for 24 h in 0.1 M PB, then placed in cryoprotectant (30% sucrose in 0.1 M PB) for an additional 4–5 days. Brains were frozen by placing them in a glass beaker containing liquid isopentane that was previously cooled on dry ice. Serial coronal sections (30 µm) were cut using a freezing microtome and were immediately placed in cryoprotectant consisting of 27% ethylene glycol and 16% glycerol in 0.1 M PB to yield three alternate sets of sections. Sections were stored at −20 °C until immunohistochemical processing. All solutions had a pH of 7.4.
Sections were washed in 0.1 M PBS for 30 min, then incubated in 1% H2O2 in PBS for 20 min. Sections were then washed 10 min in PBS and 20 min in PBS with 0.3% Triton X-100 (PBST). Sections were incubated overnight (approximately 12–16 h) in PBST at room temperature with an affinity-purified rabbit anti-AT1R polyclonal antibody (1:250 to 1:500 dilution; Cat. no. AB-N27AP, Advanced Targeting Systems, San Diego, CA). Advanced Targeting Systems report the following: the AT1R antisera was generated in rabbits by immunization with the peptide PSDNMSSSAKKPASC [(amino acids 341–355 of A-II type 1A receptor (AT1Ar)] or with SSSAKKSASFFEVE [(amino acids 346-359 of A-II type 1B receptor (AT1Br)] conjugated to keyhole limpet hemocyanin (KLH); the AT1r antisera was then affinity-purified by passage through two affinity columns, one cross-linked to AT1Ar and the other with AT1Br, resulting in specificity to the sequence PSDNMSSSAKKPASCFEVE (341–359); this AT1r antibody recognizes the 1A and 1B isoforms of the AT1r in the rat. The AT1r antibody was originally generated by Dr. Robert Speth and has been previously characterized by preadsorping it with the AT1Ar and AT1Br peptides used to generate the AT1r antibody (Huang et al., 2003, Wang et al., 2004). We also conducted additional characterization for the batch of AT1r antibody that was used in this study (Shekhar et al., 2006). The following morning the sections were washed in PBST for 30 min, and then were incubated 2 hr in a biotinylated, affinity-purified, swine anti-rabbit IgG secondary antibody (1:200 dilution, cat. no. E0353, DAKO, Carpinteria CA) and then washed again for 30 min in PBST. At the beginning of the 30 min wash in PBST, an avidin-biotin complex solution was prepared from a Vector kit as recommended (cat. no. PK-6100, Vector Laboratories, Burlingame, CA). Briefly, the avidin and the biotin were added to PBST as a 1:140 dilution and placed on an orbital shaker for 30 min. Just prior to use, the avidin-biotin solution was further diluted to a 1:500 final concentration. The tissue was then incubated 1.5 h in the avidin-biotin complex solution. The tissue was then washed in PBST for 20 min, then PB for 10 min. The chromogen solution was prepared as recommended by Vector with SG (gray-blue; cat. no. SK-4700, Vector Laboratories) as the substrate. The sections were incubated with the chromogen solution for 5 min. All sections were mounted on glass slides, dried overnight, dehydrated and cover slipped using DPX mounting medium (Cat. no. 13512, Electron Microscopy Science, Ft. Washington PA).
2.8 Retrograde tracing and immunohistochemistry for CTb
In order to identify potential circumventricular organ projections to the BLA, we used cholera toxin B-subunit (CTb) as a retrograde tracer to identify neurons with direct afferent projections to the BLA. Basolateral amygdala injection sites were taken from a previous experiment examining activation of an anxiety-related neuronal circuit projecting to the BLA and thus the procedure for iontophoretic injection of CTb and exposure to an open-field was conducted as previously described (Hale, Hay-Schmidt, 2008a, Hale, Hay-Schmidt, 2008b). Briefly, prior to CTb injection rats were anesthetized between 4 and 6 h after light onset with Hypnorm-Dormicum mixture (3 ml/kg s.c.) (one part fentanyl citrate (0.315 mg/ml) and fluanisone (10 mg/ml): one part midazolam (5 mg/ml): and two parts sterile water) and placed in a stereotaxic frame (Kopf Instruments, Model 1404, Turjunga, CA, USA). A glass microelectrode was broken to a final tip diameter of 15–20 µm and a solution of dialyzed 4% CTb (Cat. No. 104, LIST Biological Laboratories, Campbell, CA) in PBS was applied iontophoretically using positive current pulses of 10 mA (7 s on; 7 s off) for 10 min. The coordinates used for the BLA were: posterior −2.8 mm; lateral 4.8 mm; ventral −8.5 mm with reference to bregma according to a standard rat brain stereotaxic atlas (Paxinos & Watson, 1998). Rats were housed individually (cage size: 45×25×20 cm) for 7–11 days after CTb injections and handled for 2 min each day. On the test day rats were either; 1) exposed to open-field in low-light conditions (8–13 lux) for 15 min 2) briefly handled in the testing room or 3) left undisturbed (control). Following open-field exposure or handling rats were immediately returned to the housing room. Two hours after the start of the open-field test or handling, rats were anesthetized with Hypnorm and midazolam and perfused transcardially with PBS for 3 min followed by a solution of 10% formalin (Merck, Germany) in 0.1 M phosphate buffer (pH 7.4) for 15 min. The brains were post-fixed in 10% formalin and 0.1 M phosphate buffer for 24 h, and stored in PBS + 0.1% sodium azide. Brains were cryoprotected for 3 days in 30% sucrose in PBS, frozen in dry ice and 40 µm sections were cut on a cryostat. Sections were divided into six series, and stored in PBS +/0.1% sodium azide until immunohistochemical procedures were performed.
One set of slices was used for double immunostaining using primary antibodies directed against CTb (goat anti-CTb, Cat No. 703, 1:3000 List Biological Laboratories, Campbell, CA) and c-Fos (rabbit anti-c-Fos polyclonal antibody, Cat No. PC38 (Ab-5), 1:3000; Oncogene Research Products, San Diego, CA).
Immunohistochemistry for CTb and c-Fos was conducted on free-floating tissue in 30 ml tubes and gently shaken on an orbital shaker throughout double immunostaining. Tissue was rinsed in 3% hydrogen peroxide (H2O2) in PBS for 30 min, followed by washing in PBS containing 0.1% Triton X-100 (PBST) for 3×10 min; tissue was then blocked in 1% human serum albumin (HSA) in 0.1% PBST for 30 min. Sections were incubated overnight at 4 °C with 1:3000 goat anti-CTb in 1% human serum albumin(HSA)/PBST. After 15 h, tissue was washed three times (for 10 min each) in 0.1% PBST followed by incubation with a biotinylated donkey anti-goat polyclonal antibody (Cat#705-096-147; 1:2000; Jackson Immuno Research Laboratories, PA) in 1% HSA/PBST for 60 min. Tissue was washed in 0.1% PBST for 3×10 min followed by incubation with an avidin-biotin complex (Elite ABC reagent; Cat. No. PK-6100, 1:200; Vector Laboratories, Peterborough, UK) in 0.1% PBST for 60 min. Tissue was washed in 0.1% PBST for 3×10 min then incubated in 0.05% 3-3’diaminobenzidine tetrahydrochloride (DAB) in PBS and 0.0066% H2O2 for 15 min. After the chromogen reaction, tissue was immediately washed in PBS (2×10 min), 3% H2O2 in PBS (30 min), 0.1% PBST respectively (3×10 min) and preincubated in 1% HSA in 0.1% PBST for 30 min. Slices were then incubated with a rabbit anti-c-Fos antibody (Cat. No. PC38 (Ab-5), 1:3000; Oncogene Research Products, San Diego, CA, USA) in 0.1% PBST overnight at room temperature. Tissue was then washed twice in 0.3% PBST for 15 min followed by incubation in biotinylated swine anti-rabbit secondary antibody (Cat. No. E0353, 1:200; DakoCytomation Ltd, Cambridgeshire, UK) in 0.1% PBST for 90 min. Following incubation with the secondary antibody, tissue was washed twice in 0.3% PBST for 15 min and incubated with Elite ABC reagent (as above). Tissue was washed in 0.3% PBST for 15 min and rinsed in 0.05 M PBS. Tissue was then placed in SG substrate (Vector Laboratories; Cat. No. SK4700; diluted as recommended by the vendor) in PBS for 15 min. Finally, sections were washed twice in PBS to stop the reaction. Brain sections were rinsed briefly in distilled water then mounted on SuperFrost Plus microscope slides (Fisher Scientific UK, Leicestershire, UK), dehydrated through an alcohol series and cleared with xylene. Slides were then mounted with coverslips using DPX mounting medium (RA Lamb, London, UK). The color reaction of the c-Fos immunostaining was blue-black and localized to the nucleus while CTb immunostaining was orange-brown and localized to the cytoplasm. In the present study, c-Fos immunoreactivity in the circumventricular organs was not quantified.
2.9 Photography
Photomicrographs were obtained using a Leica brightfield microscope using N plan 5x, 10x, 20x and 40x objective lenses (model DMLB, Leica Microsystems), a SPOT digital camera (RT color, Diagnostics Instruments Inc., Sterling Heights, MI) and SPOT 4.0.6 for Windows digital imaging software (Silicon Graphics, Mountain View, CA) or a Nikon 90i microscope and a Nikon DS-Fi1 digital camera with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA). Photographic plates were prepared in CorelDraw 11.633 for Windows (Eden Prairie, MN).
3. Results
3.1 Histological verification of cannula placements
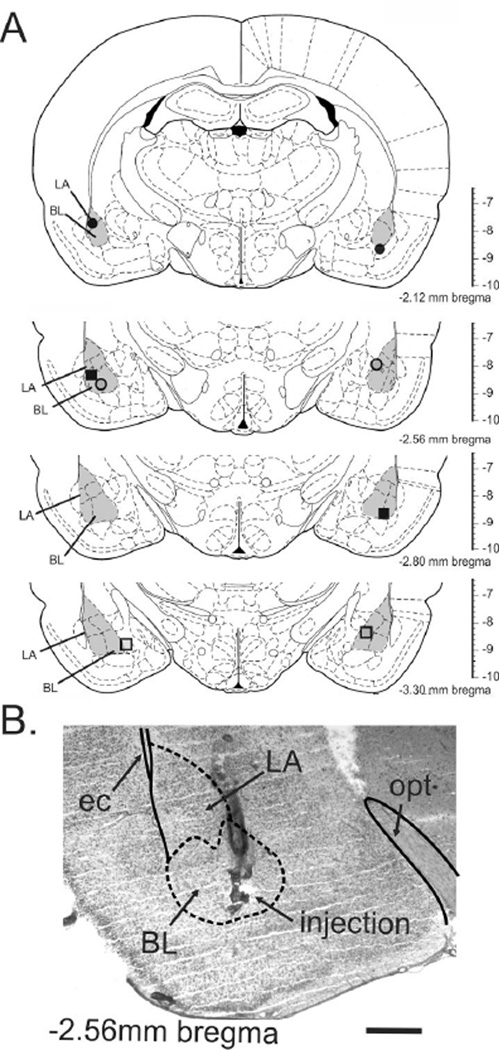
Post-mortem histological examination was utilized to confirm that the data were analyzed from only those rats with bilateral distribution of cannula in the BLA. The distribution of bilateral cannula placements for 4 rat brains stained with cresyl violet (represented by black circle) and 4 rat brains sectioned at 300 µm, visually confirmed (represented by gray shading) are depicted in Fig. 1A. A representative cannula placement is indicated in a photomicrograph in Fig. 1B.
Figure 1.
a) Schematic representation of the bilateral injection sites as determined by histology. Infusion cannulae placements are illustrated as symbols, where same symbols represent the bilateral injections for that individual rat. Illustrations of coronal brain sections are based on the rat brain atlas of Paxinos and Watson (1986). Numbers to bottom right of each section indicate the distance posterior from bregma; the vertical scale on the right of each section represents the distance ventral from bregma (in mm). b) Photomicrograph illustrates an example of histological verification of the injector tip placements for microinjections targeting the BL. The photomicrograph shows the injection site on the left side of a single rat through the basolateral amygdala (BLA: which is made up of the lateral amygdaloid nucleus (LA) and basolateral amygdaloid nucleus (BL)) at approximately –2.56 mm bregma. Solid lines represent white matter tracts and dashed lines illustrate subdivisions of the BLA. Abbreviations: BL, basolateral amygdaloid nucleus; ec, external capsule; LA, lateral amygdaloid nucleus; opt, optic tract. Scale bar = 800 µm.
3.2 Effects of microinjection of saralasin or PD123319 into the BLA on panic-like response evoked by i.v. NaLac
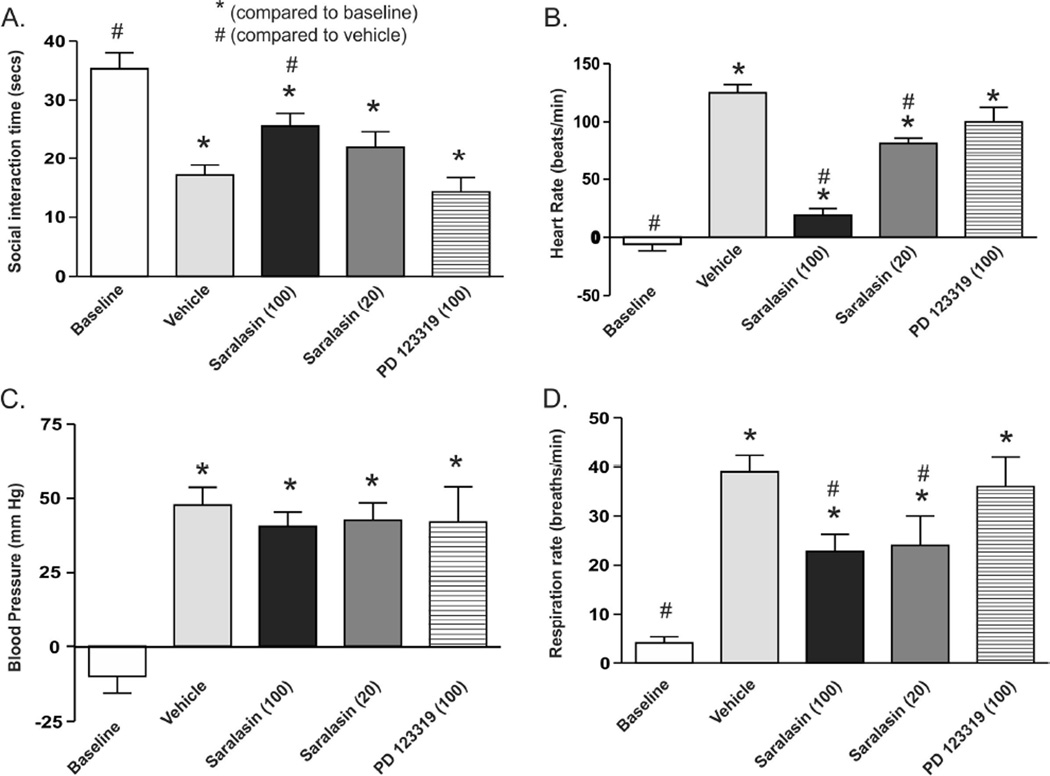
Intravenously infusing NaLac into UCN-primed rats that were pretreated with vehicle in the BLA elicited a robust panic-like response characterized by a significant decrease in SI duration (F(4,22) = 13.0, p < 0.001, Fig. 2A) and an increase in HR (F(4,22) = 70.5, p < 0.001, Fig. 2B), BP (F(4,22) = 16.6, p < 0.001, Fig. 2C) and RR (F(4,22) = 17.7, p < 0.001, Fig. 2D). Post hoc analyses revealed that prior microinjections of the highest dose of saralasin, a nonselective A-II receptor antagonist, into the BLA attenuated the decrease in SI duration, and increases in HR and RR, but had no effect on BP responses. Post hoc analyses revealed that vehicle, 20 pmoles of saralasin and 100 pmoles of PD123319 did not alter any of the lactate-induced panic-like responses in UCN-primed rats. The experimental design required 4 total days of a crossover with an expected n=6/group. However, problems with the patency of sodium lactate i.v. lines led to 1 less rat in the saralasin 100 pmole group on day 3, and 3 less rats in the saralasin 20 pmole group and PD123319 group which were to be run on day 4. Final n’s for each group were as follows due to attrition.
Figure 2.
Bar graphs illustrate A) social interaction (SI) time, B) heart rate (HR), C) mean arterial blood pressure (MAP) and D) respiration rate (RR) for each treatment group. Baseline bars represent reactivity (change in HR, MAP and RR following i.v. sodium lactate (NaLac) infusions in cannulated rats prior to urocortin 1 (UCN)/basolateral amygdala (BLA) priming. Five days following onset of UCN/BLA priming (in a counter-balanced design) rats randomly received 100 nl injection of saline vehicle, 20 pmoles or 100 pmoles saralasin (a nonselective A-II receptor antagonist), or 100 pmoles of PD123319 (a selective A-II type 2 receptor antagonist), bilaterally into the BLA 30 min prior to NaLac challenge. Data are presented as means ± SEM. * and #, respectively, represent significant difference from baseline or vehicle groups p < 0.05 (ANOVA with Newman-Keuls post hoc test).
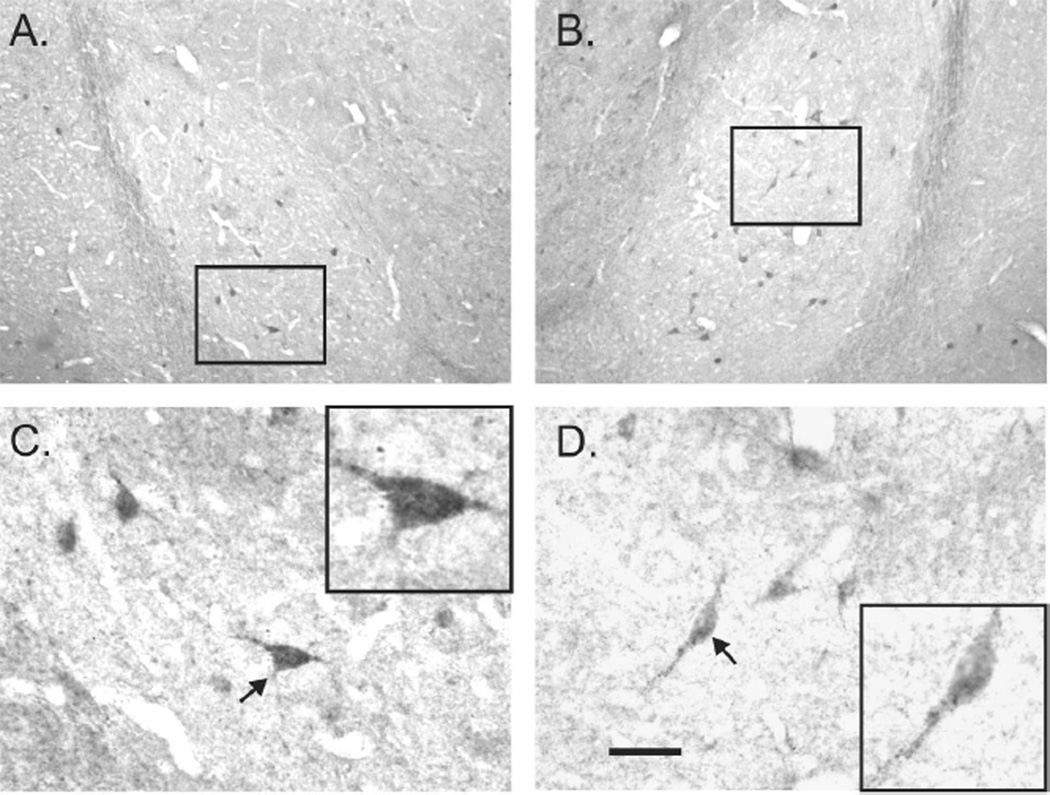
3.3 Immunohistochemical demonstration of AT1rs in the BLA
Immunostaining for AT1R proteins clearly demonstrated the presence of AT1rs on cell bodies and fibers in the BLA (Fig. 3A-D), which is consistent with previous data describing AT1r mRNA expression (Lenkei et al., 1998). In addition, we also detected AT1rs on cell bodies in CVOs such as the SFO (data not shown), which is also consistent with previous findings (Giles et al., 1999, Lenkei et al., 1997, Lenkei, Palkovits, 1998). Conducting the immunohistochemical protocol on brain sections in the absence of the primary AT1r antibody did not result in false positive immunostaining in analyzed brain regions, supporting the notion that the primary antibody used was immunostaining the AT1r antigens that it was raised against.
Figure 3.
Photomicrographs in A,B) and C,D) represent low and high magnification, respectively, images of immunohistochemical localization of the A-II type 1 receptor (AT1R) in the left and right basolateral amygdala (BLA: which is made up of the lateral amygdaloid nucleus (LA) and basolateral amygdaloid nucleus (BL)) at approximately –2.12 mm bregma) of an adult male rat. Black box inserts in A and B, represent high magnification photomicrographs shown in C and D, respectively. Black box inserts in C and D are higher magnification images of neurons in C and D that are indicated by arrows. Scale bars: 200 µm, A,B; 50 µm, C,D. Additional abbreviations: ec, external capsule; opt, optic tract.
3.4 Retrograde Tracing
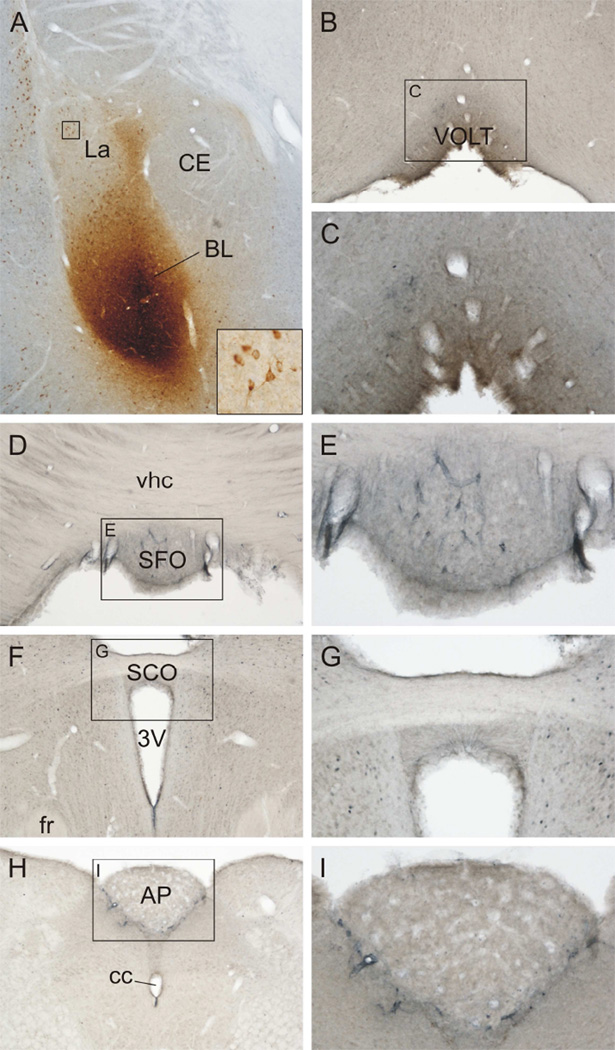
The distribution of CTb injection sites is summarized in Table 1 and a figure illustrating the injection sites has been previously published (Hale, Hay-Schmidt, 2008a, Hale, Hay-Schmidt, 2008b). Injection sites were often distributed across several subregions of the basolateral amygdaloid complex and six injections sites showed CTb-immunostaining in the ependyma of the lateral ventricles, suggesting that some of the retrograde tracer had been deposited into the ventricles as well as into the brain parenchyma. There were very few CVO projections to the BLA or surrounding amygdaloid subnuclei (Table 1 and Fig. 4). In the cases where CTb was partially injected into the ventricle, there were CTb-immunoreactive cells detected in the OVLT (2/4 cases) and the SFO (2/5 cases).
Table 1.
Circumventricular organ efferents to the basolateral amygdaloid complex
| Tracer | Injection site | OVLT | SFO | SCO | AP |
|---|---|---|---|---|---|
| T2794 | BLA/BLV | − | |||
| T2795 | BLP/BMP | − | − | − | |
| T2796 | BLA | − | − | − | |
| T2799 | BLA | − | − | − | |
| T2800 | BLV | − | − | − | |
| T2801 | BLA | − | − | − | − |
| T2802 | CE | − | − | ||
| T2803 | BMP | − | − | ||
| T2804 | BMP | − | − | ||
| T2805 | BMP | − | − | − | |
| T2807 | BMP | − | − | − | |
| T2808 | BMP | − | − | − | |
| T2810 | BMP | + | − | − | |
| T2811 | BLA | − | − | − | |
| T2814 | BMP | − | − | ||
| T2815 | BLA/BLP | − | − | ||
| T2816 | BLP/BLA | − | − | − | |
| T2818 | BLA/BLP | − | − | − | |
| T2819 | BLP/BMP | − | − | − | − |
| T2820 | BLA/Ce | + | − | − | |
| T2822 | BMP/BLA | + | − | − | |
| T2823 | B MA/BMP | + | + | − | |
| T2824 | BLA/BMP | − | − | − | |
| T2825 | BLA | − | − | ||
| T2826 | BLA/BLP | − | − | − |
represents the cells within the circumventricular organs that project to the basolateral amygdaloid complex and surrounding amygdala subdivisions. The table does not include cases where there was evidence of CTb injections hitting ventricles. Columns 1–2 respectively represent the rat identification number and site of amygdala injection, whereas columns 3–6 represent cholera toxin B subunit- (CTb-) labeled cells in circumventricular organs such as the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), subcommissural organ (SCO) and area postrema (AP). Symbols in columns 3–6 represent the following: − indicates 0 cells; + indicates 1–2 cells. Blank cells represent regions that were not available for analysis. For an illustration of the distribution of injection sites, see (Hale, Hay-Schmidt, 2008b) and also Figure 4A for a representative photomicrograph of an injection site. Abbreviations of amygdala subdivisions: BL, basolateral amygdaloid nucleus; BLA, basolateral amygdaloid nucleus, anterior part; BLP, basolateral amygdaloid nucleus, posterior part; BLV, basolateral amygdaloid nucleus, ventral part; BMA, basomedial amygdaloid nucleus, anterior part; BMP, basomedial amygdaloid nucleus, posterior part [see standard stereotaxic atlas of the rat brain for exact locations of these nuclei within the basolateral amygdaloid complex and adjacent amygdaloid nuclei (Paxinos and Watson, 1997)].
Figure 4.
Circumventricular organs including the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), subcommissural organ (SCO) and area postrema (AP) do not send afferent projections to the basolateral nucleus of the amygdala (BL). A) Photomicrograph showing the injection site from a rat (T2818) that received unilateral iontophoretic injection of the retrograde tracer, cholera toxin b subunit (CTb), into the BL. Black box in A is shown at higher magnification in the inset. The inset shows basolateral amygdala-projecting neurons in the lateral amygdala (La). B) Photomicrograph showing the OVLT. The black box in B represents the region that is shown at higher magnification in C. D) Photomicrograph showing the SFO. The black box in D represents the region that is shown at higher magnification in E. F) Photomicrograph showing the SCO. The black box in F represents the region that is shown at higher magnification in G. Photomicrograph showing the AP. The black box in H represents the region that is shown at higher magnification in I. Additional abbreviations: 3V, third ventricle; cc, central canal; Ce, central nucleus of the amygdala; vhc, ventral hippocampal commissure. Scale bar, 250 µm (A,B,D,F,H), 100 µm (C,E,G,I), 50 µm (inset).
4. Discussion
Consistent with previous data (Sajdyk et al., 1999b, Shekhar, Sajdyk, 1999) here we showed that bilateral injections of UCN into the BLA at a subthreshold dose (i.e., a dose that does not induce anxiety-like or cardiovascular responses acutely) daily for 3 consecutive days increased anxiety-like behavior (as measured by an SI test), and panic-associated cardiorespiratory responses following a NaLac challenge. The present study's main finding was that prior injections of saralasin (a nonselective A-II receptor antagonist), but not PD123319 (a selective AT2r antagonist, 100pmole/100nl) or vehicle, into the BLA of UCN/BLA-primed rats attenuated NaLac-induced anxiety-like behavior, tachycardia and tachypnea without altering pressor responses at a high 100pmol/100nl dose, and partially at a lower 20pmole/100nl dose. We then confirmed the presence of AT1r expression in the BLA using immunohistochemistry, which is consistent with previous data describing AT1r mRNA expression (Lenkei, Palkovits, 1998)] and a previous study showing that saralasin radioligand binding in the BLA is displaced by losartan (an AT1r selective antagonist), but not with WL 19 (an AT2r selective antagonist) (Gehlert et al., 1991). A final point is that A-II-mediated effects in the BLA can be altered with either AT1r (losartan) or AT2r (PD123319) antagonist, but there are some neurons that are only affected by the AT1r antagonist (Albrecht et al., 2000). Overall, this supports our hypothesis that the NaLac challenge increases A-II release in the BLA, which primarily binds to AT1rs to induce anxiety-like and panic-associated responses in UCN/BLA primed rats. The PD 123319 compound is a highly selective AT2 receptor antagonist and even at doses of 100 pmoles, which is equivalent to the highest doses of saralasin (the non-selective AT1 and AT2 receptor antagonist), it did not block the panic-like responses. Thus, it is highly unlikely that any of the effects of saralasin in blocking panic-response are mediated by the AT2 receptor. Many previous studies have used similar doses of PD 123319 to fully block the behavioral and cardiovascular effects of AT2 receptors (Chitravanshi and Sapru, 2011, da Silva et al., 2011, Kerr et al., 2005).
The secondary aim of this study was to determine if neurons in the SFO (which contains many A-II synthesizing cells) had direct projections to the BLA. Previously, Shekhar and colleagues demonstrated that the SFO plays a critical role in NaLac’s ability to induce panic-like responses in UCN/BLA-primed rats (Shekhar, Sajdyk, 1999). The majority of SFO neurons produce A-II (Lind, Swanson, 1985a, Lind, Swanson, 1985b, Saavedra and Chevillard, 1982, Saavedra, Fernandez-Pardal, 1982), but to our knowledge projections from the SFO to the BLA have never been specifically investigated. Contrary to our initial hypothesis, retrograde tracing from the BLA region (see Table 1 for details on amygdala nuclei labeled) led to very few CTb-positive neurons in the SFO in only one rat of 17 injected with CTb. Of the other 3 CVOs assessed, only the OVLT was identified as also receiving any significant retrograde tracing from the BLA region, but once again there were very few CTb-positive neurons in the SFO in only four of the 17 rats injected with CTb. Since we have previously shown that the SFO is critical for relaying the NaLac signal to the BLA (Shekhar, Sajdyk, 1999), this suggests that the SFO is relaying the signal to an alternative source of A-II neurons which then project to the BLA to initiate panic-like responses. A logical downstream, but not direct, projection site from the SFO is potentially the central nucleus of the amygdala (CeA), which contains a significant number of A-II-synthesizing neurons (Lind, Swanson, 1985b) and also projects to the BLA. The CeA is an important site for regulating Na+ intake and positively regulates water intake in response to increases in plasma Na+ concentrations. For example, lesioning the CeA blocks increases in water intake following deoxycorticosterone (DOCA)-salt treatment, or intake of hypertonic NaCl solution (Galaverna et al., 1992, Seeley et al., 1993). Our laboratory has previously demonstrated that UCN priming in the BLA reduces local spontaneous inhibitory postsynaptic synaptic potentials (Rainnie, Bergeron, 2004). Thus, a low NaLac signal, which does not normally provoke panic, is likely sensed by the SFO and relayed to an intermediate circuit such as the CeA A-II neurons, which in turn release A-II onto hypersensitive BLA neurons to elicit a panic response.
5. Conclusions
Overall, the data presented here suggest that loss of inhibitory tone in key panic-regulating brain regions such as the BLA lead to hypersentivity to NaLac, of which A-II and AT1rs appear to play a critical role. Consistent with this hypothesis is that A-II also appears to be a putative neurotransmitter in NaLac-induced panic-like responses in a hypothalamic model of panic vulnerability (Shekhar, Johnson, 2006, Shekhar and Keim, 1997). Furthermore, the postsynaptic AT1r may also a critical receptor component in this panic model. A previous study demonstrated that central injections of the AT1r antagonist losartan are anxiolytic and may represent a novel means of anxiety treatment (Kaiser et al., 1992), but did not elucidate the neuronal circuits through which losartan is producing anxiolytic-like effects. A recent article has shown that injecting losartan into the BLA and other surrounding amygdala nuclei led to anxiolytic-associated behaviors in stressed and non-stressed rats (Lopez et al., 2012), which further supports the hypothesis that A-II regulates anxiety and panic states by acting on AT1 receptors in the BLA.
Highlights.
We utilized the rodent amygdala CRF priming model of panic vulnerability to lactate infusions
The study determined the role of the angiotensin system in this model
Blocking A-II receptors in the amygdala attenuated panic responses
A local selective A-II 2 receptor antagonist had no effect on panic responses
The presence of A-II 1 receptor in the basolateral amygdala was confirmed
Acknowledgements
This work was supported by the following NIH grants, Supported by R01 MH 065702 and MH 052619 to AS and CAL.
Abbreviations
- UCN
Urocortin 1
- BLA
basolateral amygdala
- A-II
angiotensin
- AT1r
angiotensin type 1 receptor
- AT1r
angiotensin type 2 receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht D, Nitschke T, Von Bohlen Und Halbach O. Various effects of angiotensin II on amygdaloid neuronal activity in normotensive control and hypertensive transgenic [TGR(mREN-2)27] rats. FASEB Journal. 2000;14:925–931. doi: 10.1096/fasebj.14.7.925. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Cardiovascular responses elicited by a new endogenous angiotensin in the nucleus tractus solitarius of the rat. American journal of physiology. 2011;300:H230–H240. doi: 10.1152/ajpheart.00861.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley DS, Dager SR, Dunner DL. Lactate infusions in major depression without panic attacks. JPsychiatrRes. 1987;21:243–248. doi: 10.1016/0022-3956(87)90025-2. [DOI] [PubMed] [Google Scholar]
- da Silva AQ, Fontes MA, Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Res. 2011;1368:231–238. doi: 10.1016/j.brainres.2010.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-IV. Diagnostic and Statistical Manual - Fourth Edn (DSM - IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- Galaverna O, De Luca LA, Jr, Schulkin J, Yao SZ, Epstein AN. Deficits in NaCl ingestion after damage to the central nucleus of the amygdala in the rat. Brain Res Bull. 1992;28:89–98. doi: 10.1016/0361-9230(92)90234-o. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Schober DA. Autoradiographic localization of subtypes of angiotensin II antagonist binding in the rat brain. Neuroscience. 1991;44:501–514. doi: 10.1016/0306-4522(91)90073-w. [DOI] [PubMed] [Google Scholar]
- Giles ME, Fernley RT, Nakamura Y, Moeller I, Aldred GP, Ferraro T, et al. Characterization of a specific antibody to the rat angiotensin II AT1 receptor. J Histochem Cytochem. 1999;47:507–516. doi: 10.1177/002215549904700409. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Battista D, Goetz RR, Dillon DJ, Liebowitz MR, Fyer AJ, et al. A comparison of sodium bicarbonate and sodium lactate infusion in the induction of panic attacks. Arch Gen Psychiatry. 1989;46:145–150. doi: 10.1001/archpsyc.1989.01810020047008. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, et al. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience. 2008a;157:733–748. doi: 10.1016/j.neuroscience.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008b;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama TY, Watanabe E, Okado H, Noda M. The subfornical organ is the primary locus of sodium-level sensing by Na(x) sodium channels for the control of salt-intake behavior. J Neurosci. 2004;24:9276–9281. doi: 10.1523/JNEUROSCI.2795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach SL, Ciriello J. Effect of Lesions of forebrain circumventricular organs on c-fos expression in the central nervous system to plasma hypernatrmia. Brain Res. 1996;713:17–28. doi: 10.1016/0006-8993(95)01425-x. [DOI] [PubMed] [Google Scholar]
- Huang J, Hara Y, Anrather J, Speth RC, Iadecola C, Pickel VM. Angiotensin II subtype 1A (AT1A) receptors in the rat sensory vagal complex: subcellular localization and association with endogenous angiotensin. Neuroscience. 2003;122:21–36. doi: 10.1016/s0306-4522(03)00606-7. [DOI] [PubMed] [Google Scholar]
- Kaiser FC, Palmer GC, Wallace AV, Carr RD, Fraser-Rae L, Hallam C. Antianxiety properties of the angiotensin II antagonist, DUP 753, in the rat using the elevated plus-maze. Neuroreport. 1992;3:922–924. doi: 10.1097/00001756-199210000-00026. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Bevilaqua LR, Bonini JS, Rossato JI, Kohler CA, Medina JH, et al. Angiotensin II blocks memory consolidation through an AT2 receptor-dependent mechanism. Psychopharmacology (Berl) 2005;179:529–535. doi: 10.1007/s00213-004-2074-5. [DOI] [PubMed] [Google Scholar]
- Lapierre YD, Knott VJ, Gray R. Psychophysiological correlates of sodium lactate. Psychopharmacol Bull. 1984;20:50–57. [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type 1 (AT1) and type 2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82:827–841. doi: 10.1016/s0306-4522(97)00328-x. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Fyer AJ, Gorman JM, Dillon D, Davies S, Stein JM, et al. Specificity of lactate infusions in social phobia versus panic disorders. Am J Psychiatry. 1985;142:947–950. doi: 10.1176/ajp.142.8.947. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Bruhn TO, Ganten D. The distribution of angiotensin II-immunoreactive cells and fibers in the paraventriculo-hypophysial system of the rat. Brain Res. 1985a;338:81–89. doi: 10.1016/0006-8993(85)90250-1. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985b;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Lopez LH, Caif F, Garcia S, Fraile M, Landa AI, Baiardi G, et al. Anxiolytic-like effect of losartan injected into amygdala of the acutely stressed rats. Pharmacological reports : PR. 2012;64:54–63. doi: 10.1016/s1734-1140(12)70730-2. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molosh AI, Johnson PL, Fitz SD, Dimicco JA, Herman JP, Shekhar A. Changes in central sodium and not osmolarity or lactate induce panic-like responses in a model of panic disorder. Neuropsychopharmacology. 2010;35:1333–1347. doi: 10.1038/npp.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Peskind ER, Jensen CF, Pascualy M, Tsuang D, Cowley D, Martin DC, et al. Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biol Psychiatry. 1998;44:1007–1016. doi: 10.1016/s0006-3223(98)00053-5. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310:683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Robins E, Mintun MA, Fusselman MJ, Fox PT, et al. Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry. 1989;46:493–500. doi: 10.1001/archpsyc.1989.01810060013003. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, et al. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept. 2005;128:227–238. doi: 10.1016/j.regpep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, et al. A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharmacology. 2006;31:1123–1134. doi: 10.1038/sj.npp.1300921. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Chevillard C. Angiotensin-converting enzyme is present in the subfornical organ and other circumventricular organs of the rat. Neurosci Lett. 1982;29:123–127. doi: 10.1016/0304-3940(82)90340-8. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Fernandez-Pardal J, Chevillard C. Angiotensin-converting enzyme in discrete areas of the rat forebrain and pituitary gland. Brain Res. 1982;245:317–325. doi: 10.1016/0006-8993(82)90814-9. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999a;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. Eur J Pharmacol. 2000;394:265–273. doi: 10.1016/s0014-2999(00)00128-x. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999b;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Galaverna O, Schulkin J, Epstein AN, Grill HJ. Lesions of the central nucleus of the amygdale. II: Effects on intraoral NaCl intake. Behav Brain Res. 1993;59:19–25. doi: 10.1016/0166-4328(93)90147-i. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Sajdyk TJ, Fitz SD, Keim SR, Kelley PE, et al. Angiotensin-II is a putative neurotransmitter in lactate-induced panic-like responses in rats with disruption of GABAergic inhibition in the dorsomedial hypothalamus. J Neurosci. 2006;26:9205–9215. doi: 10.1523/JNEUROSCI.2491-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TS, Keim SR, Yoder KK, Sanders SK. Role of the basolateral amygdala in panic disorder. Ann N Y Acad Sci. 1999;877:747–750. doi: 10.1111/j.1749-6632.1999.tb09315.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E, Fujikawa A, Matsunaga H, Yasoshima Y, Sako N, Yamamoto T, et al. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci. 2000;20:7743–7751. doi: 10.1523/JNEUROSCI.20-20-07743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest G, Lehner-Baumgartner E, Baumgartner C. Panic attacks in an individual with bilateral selective lesions of the amygdala. Archives of neurology. 2006;63:1798–1801. doi: 10.1001/archneur.63.12.1798. [DOI] [PubMed] [Google Scholar]