Abstract
The glycine deportation system is an essential component of glycine catabolism in man whereby 400 to 800 mg glycine per day are deported into urine as hippuric acid. The molecular escort for this deportation is benzoic acid, which derives from the diet and from gut microbiota metabolism of dietary precursors. Three components of this system, involving hepatic and renal metabolism, and renal active tubular secretion help regulate systemic and central nervous system levels of glycine. When glycine levels are pathologically high, as in congenital nonketotic hyperglycinemia, the glycine deportation system can be upregulated with pharmacological doses of benzoic acid to assist in normalization of glycine homeostasis. In congenital urea cycle enzymopathies, similar activation of the glycine deportation system with benzoic acid is useful for the excretion of excess nitrogen in the form of glycine. Drugs which can substitute for benzoic acid as substrates for the glycine deportation system have adverse reactions that may involve perturbations of glycine homeostasis. The cancer chemotherapeutic agent ifosfamide has an unacceptably high incidence of encephalopathy. This would appear to arise as a result of the production of toxic aldehyde metabolites which deplete ATP production and sequester NADH in the mitochondrial matrix, thereby inhibiting the glycine deportation system and causing de novo glycine synthesis by the glycine cleavage system. We hypothesize that this would result in hyperglycinemia and encephalopathy. This understanding may lead to novel prophylactic strategies for ifosfamide encephalopathy. Thus, the glycine deportation system plays multiple key roles in physiological and neurotoxicological processes involving glycine.
Keywords: Glycine, Deportation, Catabolism, Homeostasis, Neuroregulation, Benzoic acid, Hippuric acid, Ifosfamide encephalopathy
1. Introduction
Glycine (GLY) is the smallest and only achiral α-amino acid with the simple formula H2NCH2COOH. The French chemist Henri Braconnot boiled several natural substances with sulfuric acid, isolating a sugar from wood and what he believed was “gelatin sugar” from gelatin in 1820 (Labrude & Becq, 2003). This product was originally called “glycocolle” or “glycocoll” (from Greek meaning “sweet glue”) and later renamed glycine. Despite its relatively recent discovery by man, GLY may perhaps possess the longest history of any biochemical entity. In an attempt to replicate how organic molecules might be formed in deep space, photolysis of mixtures of carbon monoxide, ammonia, and water has been performed, yielding several so-called “prebiotic” products in the laboratory, the only amino acid of which was GLY (Briggs et al., 1992). The impact of comets with the early earth was thought unlikely to deliver prebiotic molecules from space until computer simulations suggested that the shock compression of comet ice would likely yield GLY complexes (Goldman et al., 2010). Moreover, highly-sensitive chemical analysis of the type of rocks found on Mars, that can trap organic molecules in their lattices, demonstrated the presence of GLY in these rocks (Kotler et al., 2008). Thus, GLY may be an ancient molecule involved in the evolution of life on Earth some 3.8 billion years ago.
GLY has several physiological functions that can be subdivided into (1) its role as a biosynthetic precursor, and (2) as a neurotransmitter in the central nervous system (CNS). Regarding its first role, GLY is used for protein synthesis, where all four possible codons begin with GG and end with any one of the possible nucleotides U, G, A or C. However, the occurrence of GLY in proteins is relatively low at around 1–4%, but in collagen the proportion of GLY is much higher at ~30% (van der Rest & Fietzek, 1982). It was recognized very early (Csonka, 1924) that newborn babies thrive on a practically glycine-free diet (milk) and yet they increase their weight and the newly formed tissue comprises about 4% GLY, which is not supplied by the diet. A study in developing hen chicks was reported in which the GLY content of the chick embryos increased during development, suggesting de novo synthesis (Patton, 1937). GLY is therefore not an essential amino acid. In addition to being a component of proteins, GLY is also an important metabolic starting point. There are two major biochemical groups whose de novo synthesis employs GLY, the porphyrins and the purines. The synthesis of heme in animals and chlorophyll in plants requires porphyrins, macrocycles comprising four modified pyrrole rings, that complex Fe2+ in the case of heme and Mg2+ in the case of chlorophyll. The first reaction in the complex synthesis of heme is between GLY and succinyl-CoA to yield 5-aminolevulinic acid by ALA synthase (EC 2.3.1.37) in mitochondria (Shemin & Rittenberg, 1945; Shoolingin-Jordan et al., 2003). Additionally, the synthesis of purines, such as guanine and adenine, and therefore nucleic acids, also depends upon GLY, whereby atoms C4, C5, and N7 incorporate the GLY backbone. In fact, DNA synthesis rates in cells have been measured using 13C-labeled GLY with determination of 13C incorporation into dA and dG (Chen & Abramson, 1998).
The second major physiological function of GLY is as a neurotransmitter in the CNS. GLY is the major excitatory neurotransmitter in the brainstem and spinal cord, where it is a agonist of GLY receptors (GlyR) (Betz & Laube, 2006). It is also a co-agonist of glutamate at glutamatergic N-methyl-D-aspartate (NMDA) receptors (NMDAR) in the forebrain (Dannhardt & Kohl, 1998), thus serving both inhibitory and excitatory functions. These neurotransmitter functions of GLY will be discussed in more detail later.
2. Glycine homeostasis
2.1. Glycine biosynthesis and degradation
GLY is not an essential amino acid because it can readily be synthesized from two major sources. First, L-serine (SER) can be converted to GLY by serine hydroxymethyltransferase (SHMT) in a reversible reaction, thus:
The CH2OH α-substituent of SER yields a methylene group which is transferred to tetrahydrofolate (THF) and thus converted to 5,10-methylene-tetrahydrofolate (5,10-MeTHF) and water. Second, a complete de novo synthesis of GLY can occur from CO2 and , using 5,10-MeTHF as the source of the second carbon and generating H4folate in a reversible reaction, thus:
This reaction is NADH-dependent and is carried out by the glycine cleavage system, operating in reverse. The glycine cleavage system, sometimes referred to as the glycine decarboxylase complex, is found in animals, plants, and bacteria and comprises four main components, namely, the P-protein (pyridoxyl phosphate containing protein, glycine dehydrogenase (decarboxylating); EC 1.4.4.2), the T-protein (a protein required for the THF-dependent reaction, aminomethyltransferase; EC 2.1.2.10), the H-protein (a carrier protein for the aminomethyl intermediate and for hydrogen), and the L-protein (dihydrolipoyl dehydrogenase; EC 1.8.1.4) (Kikuchi et al., 2008). The glycine cleavage system, a loose association of three enzymes and a carrier protein, appears to be confined to mitochondria in liver, kidney, and brain (Kure et al., 1991), although the individual components of the system have a broad tissue distribution, with the T-protein expressed in 27 of 29 tissues studied, but the P-protein restricted to liver, kidney, brain, pituitary and thyroid glands (Kure etal., 2001). Because of a pool of soluble THF in mitochondria, the two reversible glycine synthesis systems, SHMT and the glycine cleavage system are linked (Douce et al., 2001), and thus the overall reversible reaction is:
Note that the overall synthesis of GLY is NADH-dependent. We will return to this point below.
Of interest is an early report where the potential GLY precursors, alanine, cysteine, leucine, norleucine, isovaline, aspartic acid, glycolic acid, glycolaldehyde, glucose, urea, and sodium acetate were all individually administered to rabbits. None of them proved to be a synthetic precursor of GLY (Griffith & Lewis, 1923).
The degradation of GLY is merely a reverse of the two reactions, singly or linked that generate . GLY degradation is thus NAD+-dependent and generates NADH. There exists a third metabolic degradation of GLY and that is conversion to glyoxylate by D-amino acid oxidase (DAAO; D-amino-acid: O2 oxidoreductase (deaminating), EC 1.4.3.3). This is an FAD-requiring peroxisomal reaction (Pollegioni et al., 2007) whose action in brain has been linked to schizophrenia (Madeira et al., 2008). Regarding hepatic mitochondria, this DAAO reaction is of no importance. The glyoxylate formed can be converted by lactate dehydrogenase to oxalate in an NAD+-dependent fashion, generating NADH (Banner & Rosalki, 1967; Mdluli et al., 2005). This reaction occurs mainly in hepatic mitochondria (Mdluli et al., 2005).
We have recently proposed that the principal means of GLY removal from its systemic pool is not by metabolic degradation but by scavenging by benzoic acid (BA), mainly in the liver, but to some extent in the kidney (Beyoğlu et al., 2011). This reaction, in contradistinction to the mitochondrial degradation by the glycine cleavage system and SHMT, is irreversible. The biosynthesis and degradation of GLY are depicted in Fig. 1.
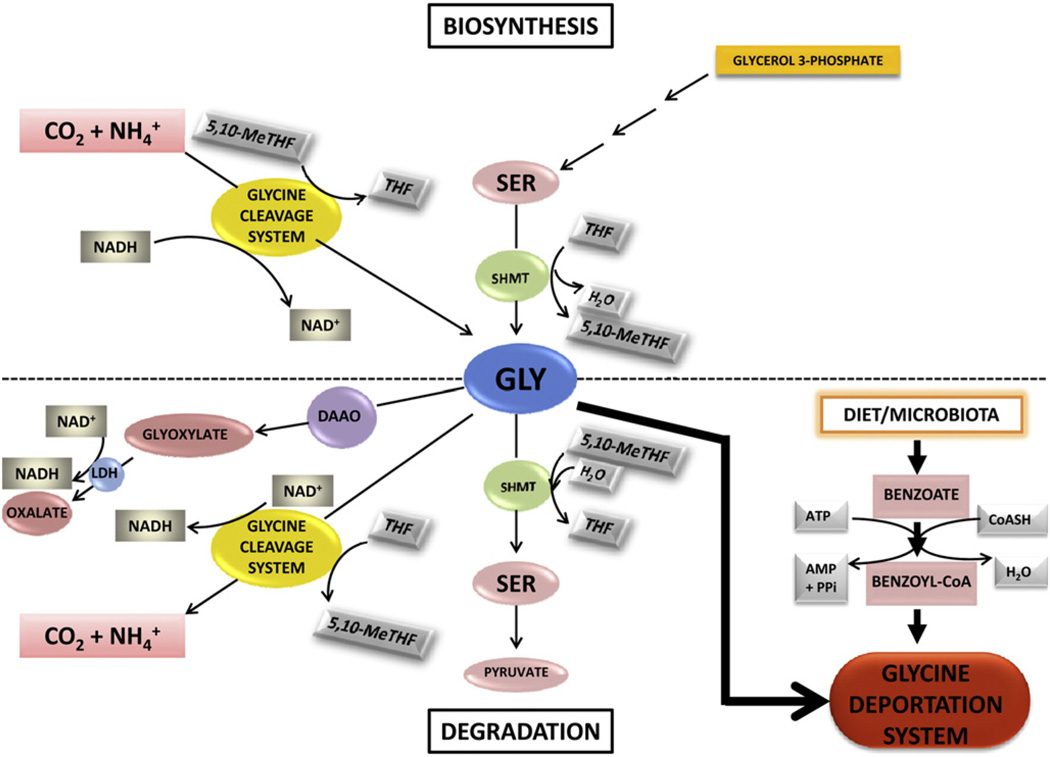
Fig. 1.
The biosynthesis and degradation of glycine. Glycine (GLY) is synthesized from serine (SER) by serine hydroxymethyltransferase (SHMT) using tetrahydrofolate (THF) as cofactor and generating 5,10-methylene-tetrahydrofolate (5,10-MeTHF) and water. GLY is also synthesized de novo from CO2 and by the glycine cleavage system that uses 5,10-MeTHF and NADH and generates THF and NAD+. GLY is metabolically removed by three pathways, specifically the SHMT-mediated synthesis of SER by SHMT, the reverse of the synthetic pathway; the conversion to CO2 and by the glycine cleavage system, utilizing NAD+ and generating NADH, the reverse of the synthetic pathway; and a minor pathway to glyoxylate catalyzed by D-amino acid oxidase (DAAO), and the resulting glyoxylate converted to oxalate by lactate dehydrogenase (LDH), using NAD+ and generating NADH. GLY is also removed by the glycine deportation system by acylation with benzoyl-CoA, yielding N-benzoylglycine (hippuric acid; HA), which is irreversibly excreted into urine. GLY deportation is the only truly irreversible reaction of GLY homeostasis.
2.2. Glycine transport
2.2.1. Glycine transport within the central nervous system
At synapses that contain GLY receptors in the CNS, GLY transporters (GlyT) remove GLY from the synaptic cleft of glycinergic inhibitory neurons and this process is also believed to keep extracellular concentrations of GLY below those required to saturate the GLY site on glutamatergic NMDA receptors (Cubelos et al., 2005; Huang et al., 2004). The human GlyTl transporter is Na+/Cl−-dependent and is encoded by the SLC6A9 gene and expressed in glia, while GlyT2 is encoded by the SLC6A5 gene, is expressed in neurons and recaptures released GLY from the synaptic cleft, where by its concentration in inhibitory neurons exceeds its concentration else where in the CNS by two to three orders of magnitude (Rousseau et al., 2008). The storage vesicles of these inhibitory neurons express an additional transporter, able to uptake both GLY and a second inhibitory amino acid neurotransmitter, gamma-aminobutyric acid, known as the vesicular inhibitory amino acid transporter, encoded by SLC32A1 (Juge et al., 2009). Glycinergic inhibitory neurotransmission is therefore terminated by uptake into glia by GlyTl and into neuronal terminals by GlyT2. Refilling of neuronal vesicles with GLY is dependent upon both GlyT2 and the vesicular inhibitory amino acid transporter (Juge et al., 2009; Rousseau et al., 2008). In addition, GlyTl prevents saturation of the GLY binding site on NMDAR (Betz et al., 2006). It is now possible to view the distribution of GlyTl in the brains of baboons and humans using the novel 11C-labeled PET radiotracer RO5013853 (Borroni et al., 2011; Wong et al., 2012).
2.2.2. Glycine transport between the central nervous system and the periphery
Early studies had suggested that all amino acids are transported into brain slices by active transport but in vivo studies in the rabbit established that GLY was extracted from blood into the CNS at a rate similar to that observed for fructose, a sugar that is not transported and crosses the blood-brain barrier (BBB) by simple diffusion (Pollay, 1976). Soon thereafter, a study in humans was reported in which the cerebrospinal fluid (CSF) and plasma concentrations of 18 common amino acids had been measured in fasted volunteers. Interestingly, GLY had the greatest plasma-CSF ratio, 45.4 ± 19.6 (mean± s.d.) (Iijima et al., 1978). There were no statistically significant alterations in the GLY plasma-CSF ratio in patients with infectious diseases (n = 60), spinal block (n = 12), cerebrovascular disease (n = 9), and degenerative diseases (n = 57) (Iijima et al., 1978). This suggests that factors distinct from the BBB may maintain homeostatic control over GLY plasma-CSF ratios.
The situation appeared to be different if plasma levels of GLY were artificially raised. Mice injected intraperitoneally with GLY showed no brain uptake, in contrast to other amino acids studied (Battistin et al., 1971). However, mice and rats fed a liquid diet fortified with 50 mg/ml GLY had plasma levels rise from 0.31 mM at time zero to 4.0 mM after 48 h on the fortified diet. Brain GLY levels also rose from a pretreatment value of 1.0 to 2.2 µmol/g tissue after 48 h on the diet (Toth & Lajtha, 1981). While the plasma level increased 13-fold, brain levels rose a mere 2.2-fold. For other amino acids studied, namely, taurine, aspartate, and glutamate, brain concentrations exceeded plasma concentrations on the fortified diets (Toth & Lajtha, 1981). It would appear that, unlike other amino acids, GLY does not readily cross the BBB or its plasma levels are homeostatically regulated by processes outside the CNS.
3. The glycine deportation system
3.1. Evidence for the glycine deportation system
We have recently argued that the conjugation of aromatic acids with the amino acids GLY, glutamine/glutamate, and taurine is not as hitherto believed a detoxication reaction for certain aromatic acids, such as BA and phenylacetic acid, but rather a homeostatic neuroregulatory mechanism for these same amino acids, that are CNS neurotransmitters (Beyoğlu et al., 2011). What follows from this is that benzoic acid should be viewed as a means of regulating GLY pools and not GLY a means of accelerating the excretion of benzoic acid. Here, we name this process the glycine deportation system. Molecular carrier systems can be subdivided into cellular export and import under the general heading of molecular transport. None of these processes adequately described the fate of GLY in the form of hippuric acid. The irreversible urinary excretion of GLY under the escort of benzoic acid in the form of the GLY adduct hippuric acid is best designated “deportation” because once hippuric acid is formed it does not again liberate GLY and is excreted into urine by the kidney from which there is no return. The analogy here is someone handcuffed to their police escort and taken away for deportation.
Various lines of evidence have been presented in support of this theory (Beyoğlu et al., 2011), and can be summarized as follows:
Compared with glucuronic acid conjugation, for example, amino acid conjugation does not render aromatic acids significantly more water soluble. In many cases, the converse is true. Thus, the detoxication value of amino acid conjugation is low.
The principal amino acid conjugations in vertebrates utilize GLY, glutamine, and taurine and GLY, glutamate, and taurine are CNS neurotransmitters.
The efflux of GLY and glutamate from the brain is a neuroprotective mechanism against elevated and neurotoxic concentrations of these amino acids.
Trafficked amino acids are scavenged by metabolic processes that lead to irreversible urinary excretion of amino acid conjugates of aromatic acids.
Experiments in rats demonstrate that scavenging of plasma glutamate by activation of blood transaminases also removes glutamate from the brain.
Both BA and phenylacetic acid are used clinically to scavenge GLY and glutamine for the purpose of excess nitrogen excretion in urea cycle defects.
Clinical use of phenylacetic acid and its metabolic precursors, which result in considerable glutamine scavenging, are commonly associated with CNS side-effects, a possible reflection of brain glutamate depletion.
Psychiatric conditions with reduced psychomotor activity display reduced GLY scavenging by BA, which can be reversed by GLY loading.
Psychiatric conditions with enhanced psychomotor activity display enhanced GLY scavenging by BA.
GLY scavenging by various aromatic acids is enhanced in autism.
GLY, glutamate, and arginine are scavenged by BA and its derivatives in certain species of insects and spiders that use these same amino acids as neurotransmitters in the CNS.
This review is concerned only with the disposition of GLY and not the other aforementioned amino acids.
3.2. Overview of the glycine deportation system
The overall scheme of GLY deportation is shown in Fig. 2. As can be seen, GLY is distributed throughout the tissues such as brain and muscle, for example, in which it occupies a large volume compartment, or GLY tissue pool, at high dilution. This includes small highly localized area of the CNS where GLY is concentrated in glycinergic neuronal vesicles and in glia (see above) at concentrations 100- to 1000-times its concentration in extraneuronal fluids (Huang et al., 2004; Rousseau et al., 2008). GLY freely diffuses from this compartment into a GLY central pool that includes the peripheral circulation. Regarding the brain, it is probable that GLY can exit across the BBB with the assistance of GlyTl (Huang et al., 2004). GLY molecules in the GLY central pool enter the liver where they are taken up by mitochondria by a rapid, respiration-independent and saturable transport with an apparent Km of 1.7 mM for rat liver mitochondria (Benavides et al., 1980). The nature of the mitochondrial GLY transporter is unclear at present, although GLY appears to be a low-affinity substrate for ASCT2 (SLC1A5) (Kanai & Hediger, 2004). The SCL25 genes encode the mitochondrial carriers that are located in the inner membrane of mitochondria where they shuttle a large number of substrates into the mitochondrial matrix (Palmieri, 2004). Mitochondrial transporters for many neutral amino acids have been characterized, but data for mitochondrial GLY transport is sketchy at best (Porter, 2000). Rat liver mitochondria swell rapidly when suspended in 150 mM solutions of GLY, ammoniumbenzoate or ammonium hippurate, demonstrating that all three compounds can freely cross the inner mitochondrial membrane. It has therefore been stated that there was no need to invoke specific carriers of GLY, BA or hippuric acid (HA) across the mitochondrial inner membrane (Gatley & Sherratt, 1976).
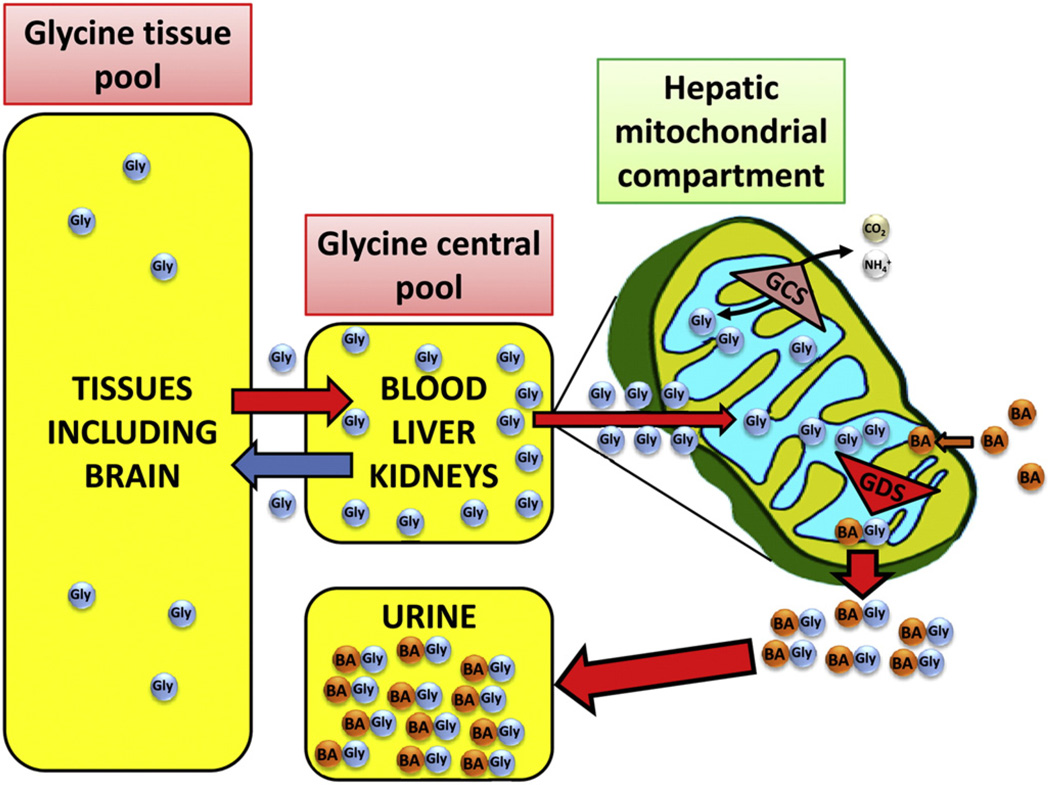
Fig. 2.
The glycine deportation system. Glycine (GLY) molecules in tissues such as brain and muscle form part of a large volume compartment which is in equilibrium with a smaller central compartment that comprises the blood, liver, and kidneys where GLY is both synthesized and removed, both by metabolism and deportation into urine. The glycine cleavage system (GCS) in mitochondria is a reversible process that can convert GLY to CO2 and (NAD+-dependent) or can resynthesize GLY from these same products (NADH-dependent). In contrast, the glycine deportation system (GDS) in the mitochondrial matrix uses predominantly benzoic acid (BA) as an escort molecule for GLY deportation through the formation of hippuric acid (N-benzoylglycine; ATP- and CoA-dependent). The GDS deporting GLY molecules from peripheral compartments, such as the central nervous system, into urine is depicted by the red arrows.
As shown in Fig. 2, BA enters the mitochondrial matrix and experiments using radiolabelled BA and rat liver mitochondria have shown that BA accumulates in the matrix ~50-fold over its extramitochondrial concentration, probably due to the pH gradient across the inner membrane, such that the higher matrix pH traps the BA (Gatley & Sherratt, 1976,1977). Two processes are shown in Fig. 2 that occur in the mitochondrial matrix, the glycine cleavage system (GCS) (Douce et al., 2001; Kikuchi et al., 2008) and the glycine deportation system (GDS). The former converts GLY into CO2 and under the influence of THF and NAD+ (see Fig. 1) and is reversible, while the latter escorts GLY out of mitochondria as a metabolic adduct with BA (HA, shown as BAGly in Fig. 2). The product HA is then irreversibly excreted into urine. We have termed this overall process the glycine deportation system.
3.3. Components of the glycine deportation system
In characterizing the GDS, an understanding of the disposition of both GLY and BA is required, together with a detailed description of HA synthesis in the mitochondrial matrix and the fate of HA so formed. These topics are dealt with in this section.
3.3.1. Glycine pools
The distribution and fate of GLY were investigated in the rat in early studies that utilized [15N]- and [α-14C]-GLY (Arnstein & Neuberger, 1951). These workers also administered unlabeled BA in different doses and at differing times relative to the GLY administration, and used the isotopic labeling of the formed HA to assist in calculating the size of the “first glycine pool”. This pool is conceptual and similar to our glycine central pool depicted in Fig. 2. Many assumptions about the movements of GLY and BA were made by these authors in order to interpret their findings. For example, they assumed that the reaction of GLY and BA was slow relative to the rate of diffusion of GLY within this pool, in other words, their administered labeled GLY mixed thoroughly with endogenous unlabeled GLY prior to HA formation commencing. However, the formation of HA was assumed to be fast compared with any other metabolic reaction of GLY or to its diffusion out of this central compartment. These investigators determined the isotope content of the hippuric acid produced and, using the “corrected dilution factor” (Bloch & Rittenberg, 1945), were able to estimate the amount of GLY present in the “first glycine pool” as 10mg/100g of rat (Arnstein & Neuberger, 1951). This compared well with the experimental determination of the total GLY content of rat internal organs of 5–7 mg/100g of rat (Arnstein & Neuberger, 1951).
Others have performed investigations with isolated perfused rat livers whose findings would question some of the key assumptions of Arnstein and Neuberger (1951), in particular, the relative rate of production of SER by the GCS. The production of 14CO2 from [1-14C]- and [2-14C]glycine has been determined in the isolated perfused rat liver (Hampson et al., 1984). The metabolism of GLY to CO2 is carried out by the GCS and it is the 1-carbon of GLY that yields the CO2 (see Section 2.1 and Fig. 2). The determination of 14CO2 in the liver perfusion studies was performed by trapping evolved CO2 in a well containing phenylethylamine (Hampson et al., 1983). These authors calculated that, at perfusion concentration of 1 mM, GLY is cleared by decarboxylation ~2% during each pass through the liver (Hampson et al., 1984). However, the only product of GLY metabolism that was determined was CO2. Although the isolated perfused rat liver may resemble in vivo conditions for some hepatic reactions, its disconnection from the gut and its portal supply to the liver means that the isolated liver is not continuously supplied with BA as it would be in vivo. Accordingly, the authors' statement that “the glycine cleavage systemis the predominant catabolic fate of glycine in the perfused rat liver” (Hampson et al., 1984) is only valid with respect to the isolated perfused liver and not to the liver in vivo that is supplied with BA and where HA formation is likely to be the dominant reaction.
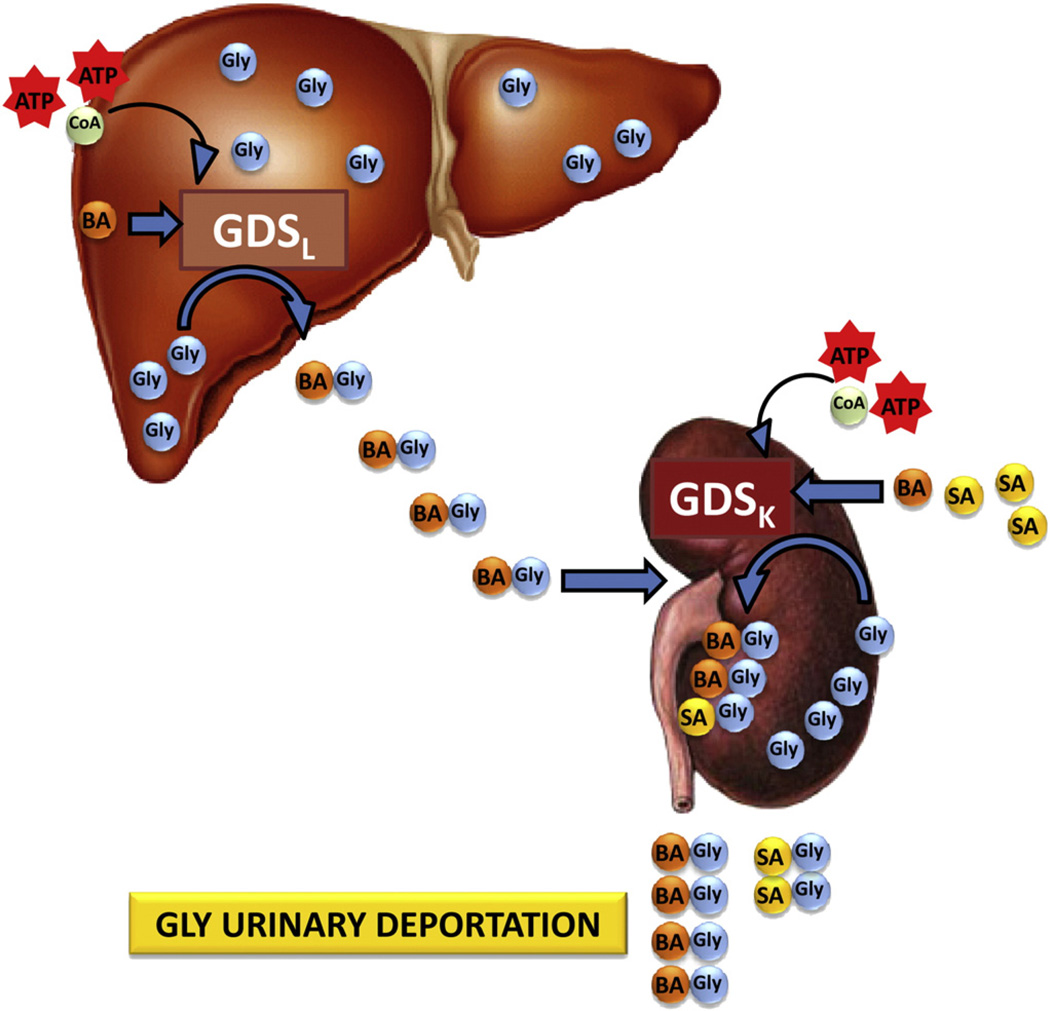
GLY is the most abundant free amino acid in the adult human liver (Ryan & Carver, 1966). The hepatic content of GLY is ~2.5 µmol/g wet weight (Marsh & Diffenderfer, 1991; Ryan & Carver, 1966). For a healthy adult liver of ~1.4 kg (Yoshizumi et al., 2003), the GLY content would be ~250 mg. In addition, GLY plasma concentrations have been reported to be ~250–500 µM (de Vries et al., 1948; Neeman et al., 2005), which would constitute a circulating amount of GLY of 650–1300 µmol, or ~50–100 mg (Fig. 3). GLY deportation can be calculated on the basis of the urinary excretion of ~1–2 g HA per day, equivalent to 400–800 mg/day deported GLY, escorted by HA that is largely produced by the gut microbiota (see below) at 700–1400 mg/day. The daily deportation of 400–800 mg GLY represents a considerable proportion of the GLY central pool (Fig. 3). This route of GLY catabolism appears to have been overlooked in much of the literature concerning GLY homeostasis.
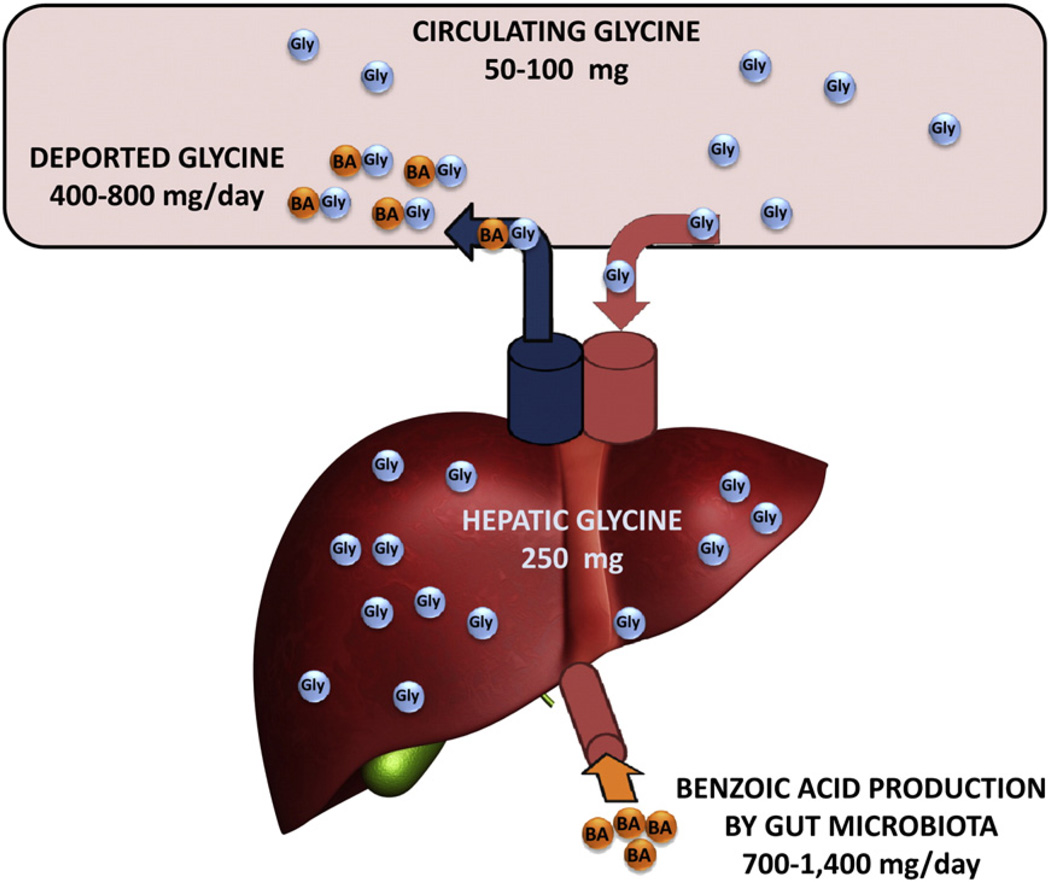
Fig. 3.
Quantitative aspects of the fate of glycine. This schematic shows that there is 50–100 mg of circulating GLY that enters the liver from the aorta into the hepatic artery, 250 mg of GLY in the liver and 400–800 mg GLY deported through the hepatic vein into the vena cava after reaction of GLY with BA in the liver. BA is produced by the gut microbiota at 700–1400 mg/day and enters the liver through the hepatic portal vein.
The question remains to what extent does the GDS influence GLY concentrations in the GLY tissue pool (Fig. 2), for example in the CNS? In answering this question, it is first important to understand the relationship between plasma GLY and CNS GLY concentrations. Firstly, how do elevations in plasma GLY influence GLY in the CNS? There have been studies that have determined GLY concentrations noninvasively in human brain and also in CSF. A recent investigation of the human brain in vivo using 1H magnetic resonance spectroscopy estimated brain GLY concentration to be 0.6±0.1 mM (mean ± s.d., n = 5) (Choi et al., 2011). Although GLY administration had been reported to produce clinical improvement of schizophrenia, no measurement of changes in brain GLY after GLY administration had been made. Therefore, a study was undertaken using 1H magnetic resonance spectroscopy in eleven schizophrenic men administered oral GLY over 14 days (Kaufman et al., 2009). In this time frame, plasma GLY rose from 240 ± 84 µM to 640 ± 340 µM and the occipital cortex GLY/creatine ratio rose from 0.022 ± 0.004 to 0.030±0.006 (P<0.002 by ANOVA) (Kaufman et al., 2009). An invasive investigation has also been reported in which CSF was sampled by lumbar puncture after the intravenous administration of GLY to 26 healthy human volunteers (13 female; 13 male) (D'Souza et al., 2000). Three i.v. doses of GLY were administered to each volunteer, 0, 100, and 200 mg/kg. After 45 min, the mean plasma GLY concentrations for each dose were 250 ± 89, 1352 ± 165, and 5093 ± 823 µM, respectively. Thus, 200 mg/kg i.v. GLY produced a 20-fold increase in peak GLY plasma concentration. The picture in CSF was somewhat different, with a GLY concentration for placebo, 100 mg/kg, and 200 mg/kg doses of 6.03 ± 2.77, 11.6±4.81, and 21.2±6.99 µM, a 3.5-fold increase in CSF at the highest administered GLY dose (D'Souza et al., 2000).
In summary therefore, brain sequesters GLY to a concentration of 600 µM (Choi et al., 2011), with a basal concentration in CSF of ~6 µM (D'Souza et al., 2000), compared to a plasma concentration of ~250 µM (D'Souza et al., 2000; Kaufman et al., 2009). There is a clear concentration gradient between the GLY brain pool and the GLY central pool (Fig. 2). To our knowledge, no investigations have been published in which plasma GLY concentration has been manipulated downwards (for example, by the administration of a large dose of BA (Quick, 1931)) and brain or CSF GLY concentration changes monitored. Nevertheless, it seems both plausible and highly likely that removal of GLY from the GLY central pool by either the GDS or GCS (Fig. 2) will lower levels of GLY in the CNS. This principle has already been reported for glutamate. When blood glutamate-pyruvate transaminase and glutamate-oxaloacetate transaminase in the rat were activated leading to a fall in plasma glutamate concentration, the brain efflux of radiolabeled glutamate was accelerated (Gottlieb et al., 2003).
Therefore, we propose here that the glycine deportation system is able to act as a homeostatic regulator of the GLY central pool and, by definition, also the GLY tissue pool. GLY can be deported from the circulation by the GDS in the case, for example, of elevated GLY efflux from the brain due to glycinergic hyperactivity. Of considerable interest also is the potential relationship between dietary sources of BA precursors, the gut microbiota, and the glycinergic neurophysiology of the CNS. This will be discussed below.
3.3.2. Origins and availability of benzoic acid
The first clues to the origin of BA came from a study conducted 150 years whereby Lautermann self-administered in the evening 8 g of quinic acid ((1S,3R,4S,5R)-1,3,4,5-tetrahydroxycyclohexane carboxylic acid) and isolated 2.2 g HA from his morning urine and a further 1.1 g from the afternoon urine. Two other volunteers similarly took quinic acid and their urines also contained large amounts of HA (Lautermann, 1863). (−)-Quinic acid is a ubiquitous cyclitol (cyclic polyol) found in numerous fruits and vegetables, together with tea and coffee. The observations of Lautermann were confirmed by many authors and in several species and it was proposed that dietary quinic acid in fruit such as cranberries and prunes was the precursor of urinary HA (Blatherwick & Long, 1923; Fellers et al., 1933). Studies in guinea pigs were conducted that showed that HA was produced fromorally, but not parenterally, administered quinic acid. These same authors treated guinea pigswith neomycin in doses sufficient to inhibit bacterial growth in the gut microbiota and reported that this maneuver prevented the conversion of administered quinic acid to urinary HA. They concluded that the aromatization of quinic acid in both man and guinea pig is performed by intestinal bacteria (Cotran et al., 1960). A comprehensive study of quinic acid aromatization was conducted in 22 animal species including man. In man and three species of Old World monkeys (rhesus monkey, baboon, and green monkey), (−)-quinic acid was extensively aromatized and excreted in urine as hippuric acid (20–60% of dose). In other species, including New World monkeys, lemurs, dog, cat, ferret, rabbit, rat, mouse, guinea pig, hamster, lemming, fruit bat, hedgehog, and pigeon, oral (−)-quinic acid was not extensively aromatized (0–5%). Neomycin pretreatment suppressed HA excretion in rhesus monkeys fed (−)-quinic acid. Administration of shikimic acid ((3R,4S,5R)-3,4,5-trihydroxycyclohex-1-ene-l-carboxylicacid; i.e. 1,2-dehydrated quinic acid) to rhesus monkeys was extensively excreted as HA (26–56%), but not in rats (Adamson etal., 1970).
Chlorogenic acid is an ester of caffeic acid with quinic acid and is one of the most abundant polyphenols in the human diet, deriving from coffee, fruits and vegetables. Groups of rats were fed diets supplemented with chlorogenic acid, caffeic acid or quinic acid (250 µmol/day) and 0–24 h urines analyzed. Urinary recovery of unchanged chlorogenic acid was 0.8% of dose and quinic acid formed from it <0.5% of dose. However, phenylpropionic acid, BA, and HA accounted for 57.4% of dose, demonstrating that chlorogenic acid is a major dietary precursor of BA that is metabolized by the gut microbiota (Gonthier et al., 2003). Studies using specific pathogen-free rats before and after inoculation with fecal microorganisms have demonstrated that several urinary acids in addition to BA derived from gut microbiota metabolism, including, phenylacetic acid, and 3- and 4-hydroxyphenylpropionic acid (Goodwin et al., 1994). A scheme representing the pathways that lead to BA and HA and with chemical structures is given in Fig. 4.
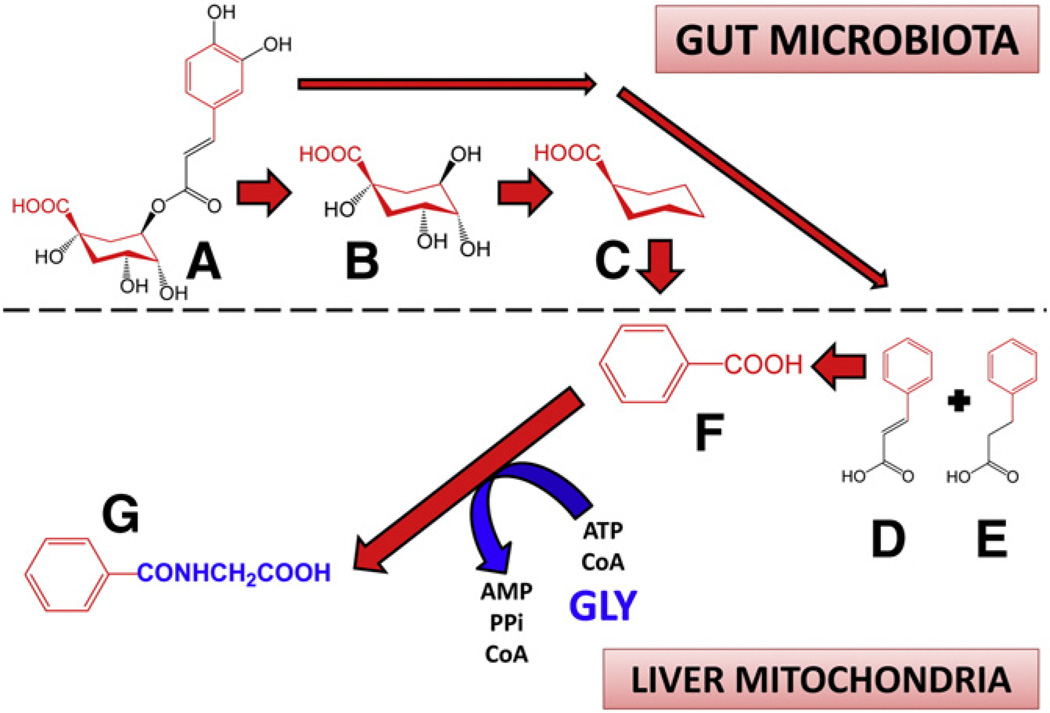
Fig. 4.
Dietary precursors of benzoic acid for the glycine deportation system. Ingested chlorogenic acid (3-caffeoylquinic acid; A) is hydrolyzed by gut microbiota to quinic acid (B) and caffeic acid (not shown). Quinic acid is metabolized to cyclohexane carboxylic acid (C) and this is aromatized by gut microorganisms to benzoic acid (F) which is absorbed into the liver by the hepatic portal vein. Caffeic acid can be metabolized by the microbiota to cinnamic acid (D) and phenylpropionic acid (E), which are also absorbed and taken up by the liver, whereby these are metabolized by β-oxidation to benzoic acid. The benzoic acid formed by these processes is utilized exclusively as the escort molecule for glycine in the glycine deportation system by conversion in hepatic mitochondria to hippuric acid (G). Chemical components of dietary precursors that become benzoic acid are shown in red. Glycine (GLY), both in its free and deported form, is shown in blue.
Finally, it should be recognized that we may be exposed directly to BA from our diets without the intervention of the gut microbiota. This is especially so for the consumption of foods and beverages in which BA has been added as a preservative. BA has been reported to occur in wines, fruit juices, fruit and natural yoghurts, butter, syrups, and various cheeses, but at levels generally <30 mg/1 (Vandevijvere et al., 2009). However, nonalcoholic sodas, such as Coca Cola, lemonade, iced tea and “aromatized water” had reported concentrations of BA of 112 to 146 mg/1, just under the regulatory level of 150 mg/1. Moreover, prepared salads, emulsified sauces, and dressings had BA levels of 500 to 850 mg/kg (Vandevijvere et al., 2009). The authors surveyed 3083 Belgian consumers and estimated that the mean daily intake of BA from the diet was 1.25 mg/kg b.w., which was 25% of the acceptable daily intake (Vandevijvere et al., 2009). This would be sufficient to escort ~50 mg GLY as HA in the GDS. Clearly, the major proportion of BA in the human diet comes through the gut microbiota.
3.3.3. Hippuric acid synthesis in the mitochondrial matrix
The conjugation of BA with GLY to yield HA was first localized to the mitochondrial matrix in rat liver (Gatley & Sherratt, 1976). These workers assumed that the conversion of BA to benzoyl-CoA was catalyzed by butyryl-CoA synthetase (EC 6.2.1.2), now known as butyrate-CoA ligase. A second enzyme system, also resident in the mitochondrial matrix, acyl-CoA-glycine N-acyltransferase (EC 2.3.1.13), now known as glycine N-acetyltransferase, was recognized by these authors as the second matrix component of HA formation (Gatley & Sherratt, 1977). Two distinct forms of the ligase have been isolated from human liver mitochondria and were termed xenobiotic/ medium-chain fatty acid:CoA ligases, HXM-A and HXM-B (Vessey et al., 1999). HXM-A comprised 60–80% of BA conjugation and BA was the best substrate tested with the highest Vmax/Km. In contrast, HXM-B had the majority of the hexanoic acid conjugating activity and hexanoic acid was the best substrate tested (Vessey et al., 1999). This same group cloned, sequenced, and expressed in COS-7 cells the cDNA corresponding to HXM-A. The heterologous expression had metabolic activity with respect to BA and phenylacetic acid of 0.91 and 0.71 pmol/min/mg protein, respectively, consistent with the known substrate preference of the native enzyme (Vessey et al., 2003). Regarding the N-acyltransferase that adds GLY to BA via benzoyl-CoA, this was purified and characterized metabolically from bovine kidney mitochondria (Kelley & Vessey, 1993). Benzoyl-CoA was the best substrate tested, with 5- to 300-fold greater activity than other acyl-CoAs, for example short-chain fatty acyl-CoAs. The enzyme also showed a clear preference for benzoyl-CoA in terms of the Km for GLY, which was 6 ± 1 mM for benzoyl-CoA and 79±11 mM for butyryl-CoA (Kelley & Vessey, 1993). This enzyme was also purified from human liver where the apparent Km value for benzoyl-CoA was lower than for short-chain fatty acyl-CoAs (Mawal & Qureshi, 1994). The specificity towards other amino acids has been tested. Bovine liver glycine N-acyltransferase showed a clear predilection (Km value) for GLY (6.2 mM) compared with asparagine (129mM), glutamine (353 mM), alanine (1573 mM), and glutamic acid (1148 mM). For the human liver enzyme, the Km values with respect to GLY and alanine were 6.4 and 997 mM, respectively (van der Westhuizen et al., 2000). Evidently, the GDS has little affinity for amino acids other than GLY and shows clear favoritism towards BA as the escort molecule. Overall, therefore, the reaction scheme of the glycine deportation system can be represented by the following sequential reactions in the mitochondrial matrix (φCOOH = BA):
The formation of hippuric acid does not appear to be restricted to the liver. HA formation was measured in homogenates of adult human liver, renal cortex, and renal medulla. Km values for these three tissues were 43.4±6.6 (n = 26), 33.3 ±6.1 (n = 5), and 34.7 ± 11.3 (n = 5) mM, respectively. Similarly, the Vmax values were 204 ± 47.8, 199 ±40.7, and 24.2 ± 16.9 pmol/min/mg tissue, respectively (Pacifici et al., 1991). This would suggest that the GDS also operates in the kidney, in particular, in the renal cortex. Experiments with the isolated perfused rat kidney confirmed this (Poon & Pang, 1995).
Under normal conditions, the glycine deportation system is believed to operate at submaximal rates. Experiments in rats determined that the normal endogenous GLY supply in the liver was about 1.7 mM and this permits the GDS to run at approximately half maximal rate (4.5 µmol/kg/min), the maximal rate having been determined in rat liver as 8.1 µmol/kg/min. These authors also concluded that the glycine cleavage system was the main source of GLY supply for HA formation (Gregus et al., 1993a). If that were true, the GCS would be feeding the GDS, in other words, GLY deportation would be a means by which excess GLY was removed from the liver when the retrograde GCS was operating, perhaps due to an imbalance between NADH and NAD+ (see Section 2.1). These workers also tested this hypothesis by administering cysteamine (200 mg/kg i.p.) to rats. Cysteamine is a powerful inhibitor of the GCS with an IC50 value of 80 µM (Jois et al., 1990). Cysteamine administration increased hepatic GLY concentration 2- to 3-fold, without affecting CoA and ATP concentrations. As a consequence, BA clearance from blood increased 50%, as did the appearance of HA in blood and its urinary elimination (Gregus et al., 1993a). Therefore, when the GCS failed, and GLY levels rose, the deportation of GLY was upregulated.
3.3.4. Metabolic stability of hippuric acid
The question arises, can HA, once formed, be hydrolyzed in the body back to BA and GLY? There is good evidence in the mammalian circulation that HA is metabolically stable. The tripeptides hippuryl-L-histidyl-L-leucine (van Platerink et al., 2007) and hippurylglycylglycine (Jafarian-Tehrani et al., 2000) are commonly employed as substrates for angiotensin converting enzyme (ACE; peptidyl-dipeptidase A; EC 3.4.15.1) and are hydrolyzed by dipeptidase activity leaving the stable HA. Circulating HAwill certainly be exposed to ACE since it is expressed mainly in lung, vascular endothelium and kidney (Femia et al., 2008). However, when HA is exposed to the gastrointestinal tract, it may not be stable. It was observed long ago that HA administered orally to rabbits was excreted in urine to a considerably greater extent when co-administered with GLY. The author interpreted these findings as meaning that HA was relatively poorly absorbed from the gut but was hydrolyzed to BA and GLY and the BA absorbed into the liver. When GLY was co-administered, the presumed absorbed BA was conjugated to HA and excreted in urine to a greater extent than when HA was administered alone (Griffith, 1925), since the same author had previously reported that co-administration of GLY with sodium benzoate enhanced the urinary excretion of HA in the rabbit (Griffith & Lewis, 1923). It had also been observed using rabbit intestinal loops that BA was absorbed across the intestine at a rate 3.5-times faster than HA (Griffith, 1925). Furthermore, there are a number of pathogenic bacteria that can inhabit the gut and that express the enzyme hippuricase that cleaves HA to BA plus GLY. Such bacteria include certain Campylobacter spp. (Caner et al., 2008), Legionella spp. (Marmet et al., 1990), and group B Streptococci, Gardnerella vaginalis, and Staphylococcus aureus (Lin et al., 1986). However, in a human investigation where the formation of HA was being studied, HA was administered by mouth at doses from 2.9 to 7.5 g to two volunteers after an overnight fast and with subsequent food restriction for 2 to 3 h (Amsel & Levy, 1969). Between 92 and 106% HA was recovered in urine with excretion rates of 1.4 to 2.6 g/h. unlike the study in rabbits, there was no evidence that HA was deconjugated in the gut and absorbed as BA. In conclusion, it can be stated with confidence that HA formed in the liver or kidney will be excreted into urine without hydrolysis back to BA plus GLY.
3.3.5. Urinary excretion of hippuric acid
As has been discussed above, the kidney would appear to contribute an additional component of the GDS. In addition, HA formed in the liver reaches the kidney and the hepatic plus renal components of the GDS present HA for urinary excretion. Experiments using the isolated rat kidney showed that exogenously administered HA had a renal clearance that was eight-times that of the glomerular filtration rate (Geng & Pang, 1999). Thus, HA underwent considerable renal tubular transport in the perfused isolated rat kidney. Moreover, when HA formed in the kidney was examined (using differential radiolabeling of BA and HA), endogenously formed HA had an even greater renal extraction than exogenously administered HA (Geng & Pang, 1999). The active tubular secretion of HA in rat kidney is performed by rat organic anion transporter 1 (SLC22A6) (Deguchi et al., 2005).
In summary, active renal tubular secretion by SLC22A6 represents a highly efficient and final stage of GLY deportation. Furthermore, the kidney itself possesses the GLY conjugation apparatus and so contributes to the hepatic effort in GLY deportation.
3.4. Factors affecting the glycine deportation system
3.4.1. Bile acid conjugation with glycine
The hepatic and renal mitochondrial GLY conjugating system adds GLY not just to BA, but also to short-chain fatty acids. As discussed above (Section 3.3.3), there is a CoA ligase with a clear preference for BA and a second ligase for which hexanoic acid was the preferred substrate. The so-formed CoA thioesters also appear to be handled by a glycine acyltransferase that shows a preference towards benzoyl-CoA and is rather specific for GLY. Nevertheless, in addition to xenobiotics such as BA and endogenous fatty acids, there is another group of compounds that undergo GLY conjugation in the liver via CoA intermediates, the bile acids. The question is, what is the effect of bile acids on the GDS and to what extent do the GLY conjugations of BA and bile acids overlap? That free bile acids appeared in bile as conjugates with GLY and taurine (bile salts) has long been recognized and moreover, major species differences were observed in utilization of either GLY or taurine. No GLY conjugates of bile acids were found in seven species of fish and eight species of birds, only taurine conjugates (Haslewood & Sjovall, 1954). Conversely, rabbit and pig bile contained almost exclusively GLY conjugates and rat bile exclusively taurine conjugates of bile acids. Inman and primates, both were found in variable proportions (Bremer, 1956; Haslewood & Sjovall, 1954). Importantly, the rat liver enzyme system that conjugated bile acids with taurine and GLY was located in the microsomal fraction of liver homogenates (Bremer & Gloor, 1955). Subsequent research has established that both human and rat bile acid-coenzyme A:amino acid N-acyltransferase, the sole enzyme responsible for GLY and taurine conjugation of bile acids, was localized exclusively in peroxisomes of human and rat hepatocytes, with undetectable amounts in the cytosol (Pellicoro et al., 2007). Localization to peroxisomes and cytosol had been reported in a previous study (Solaas et al., 2000).
Since the BA and bile acid conjugation systems use distinct enzymes in different subcellular compartments, it is extremely unlikely that there is any overlap between the two. However, both systems involve GLY and the question remains if there could be competition for GLY between the two systems. A study was reported in which 6 g of sodium benzoate was administered for 2 to 3 days to post-cholecystectomy patients with T-tube drainage from which bile could be sampled. The BA dosing was done in an attempt to deplete GLY in the liver and alter the bile salt GLY/taurine ratio, which is normally 3 or 4:1 in humans. No change in the GLY conjugated bile acids was observed (Dennis et al., 1975).
It would appear that there is no crosstalk between the mitochondrial GDS and the peroxisomal bile acid conjugating system.
4. Pharmacological consequences of the glycine deportation system
4.1. Glycine deportation system as a test of liver function
Armand J. Quick pioneered the use of BA as a test of liver function (Quick, 1936) and this test was soon to become eponymous with him. Patients were administered by mouth a solution of 6 g sodium benzoate in 30 ml water, followed by one-half glass of water. The patient immediately voided all urine and then collected his/her urine hourly for 4 h. Any specimen with a volume greater than 100 ml was concentrated on a water bath to about 50 ml. These four specimens were each acidified with 1 ml 11.6 M HCl and vigorously stirred until HA precipitated, which was filtered, washed with cold water, dried, and weighed. The normal adult was said to excrete ~3 g BA as HA in 0–4 h and this was taken as the normal value for the calculation of “the efficiency of the liver” (Quick, 1936). According to Quick's gravimetric analysis, normal subjects could deport ~1.8 g GLY during the first 4 h of Quick's test. In this report, Quick cites 100 assorted cases in which his test was applied (Quick, 1936). Many other workers followed suit and the literature is replete with reports of Quick's test in many different clinical circumstances, as we have previously reviewed (Beyoğlu et al., 2011).
Perhaps the most intriguing applications of Quick's test was in the realm of psychiatry, when schizophrenia was believed to have an organic, perhaps metabolic origin (Beyoğlu et al., 2011). At one extreme, cases of catatonia excreted significantly lower amounts of HA and, at the other, patients with “free anxiety”, displaying symptoms such as panic attacks, dilated pupils, tachycardia, and hyperpnoea, excreted significantly greater amounts of HA than normal (Beyoğlu et al., 2011). Since the rate limitation of HA synthesis by the GDS is dependent upon the supply of GLY (Beyoğlu et al., 2011; Gregus et al., 1993a), one can only assume that these early applications (1945–1950) of Quick's test in psychiatry produced, then unrecognized, evidence of abnormal GLY plasma levels in these conditions. Interestingly, when NMDA receptors are blocked in the CNS, for example using high-dose aptiganel (Cerestat) after stroke, catatonia is the principal side-effect (Lees, 1997), suggesting a relationship between low GLY and catatonia. These early observations also add force to our argument that BA conjugation with GLY, that is, the GDS, is a neuroregulatory mechanism (Beyoğlu et al., 2011).
4.2. Treatment of urea cycle enzymopathies
Urea cycle enzymopathies are a group of inborn errors of metabolism characterized by hyperammonemia, encephalopathy and respiratory alkalosis. Five disorders of urea synthesis have been described, specifically deficiencies of ornithine transcarbamylase (EC 2.1.3.3; mitochondrial matrix), carbamyl phosphate synthetase (EC 6.3.4.16; mitochondrial matrix), argininosuccinate synthetase (EC 6.3.4.5; cytosol), argininosuccinate lyase (EC 4.3.2.1; cytosol), and arginase (EC 3.5.3.1; cytosol). Failure to synthesize urea from the catabolism of protein is therefore due to inherited deficiencies in these urea cycle enzymes. Brusilow and his colleagues first proposed that alternative pathways of excess nitrogen excretion could be employed in the treatment of urea cycle enzymopathies (Brusilow et al., 1979). Among their various propositions was included the administration of BA in order to scavenge GLY and excrete nitrogen in the urine by this route. This was perhaps the first conceptualization of the GDS. The authors queried if this enzyme system was fully developed enough at birth to support HA formation and also if GLY synthesis would be sufficient to supply HA formation (Brusilow et al., 1979). Subsequently, they reported their first treatments of hyperammonemic coma due to congenital urea cycle enzymopathies. The administration of sodium benzoate (250–500 mg/kg/day i.v.) during eight episodes of coma in seven patients, six patients responded with a significant fall in plasma (Batshaw & Brusilow, 1980). Benzoate therapy was well-tolerated in such children and neonates who had been treated for 2 to 30 months (Batshaw & Brusilow, 1981). Phenylacetic acid was added to the regimen because this is conjugated by the GDS with glutamine, which contains two nitrogens compared to only one for GLY. With the dual therapy of BA and phenylacetic acid, it was reported that the deportation of these two amino acids accounted for 60% of the “effective urinary waste nitrogen” (Brusilow et al., 1984). Derivatives of phenylacetic acid have been developed, specifically sodium phenylbutyrate and glyceryltri(4-phenylbutyrate), and the advantage of the latter was that no sodium need be administered and each mol generates in situ 3 mol of phenylacetic acid (Beyoğlu et al., 2011). Ammonul® and Buphenyl® are the two FDA approved medicines for the treatment of urea cycle enzymopathies. Ammonul contains 10% sodium phenylacetate and 10% sodium benzoate. Buphenyl is sodium phenylbutyrate, also available in tablet formulation.
In this case, the GDS is utilized therapeutically for the treatment of an often fatal inherited group of diseases, hyperammonemia due to urea cycle enzymopathies.
4.3. Treatment of nonketotic hyperglycinemia (glycine encephalopathy)
Nonketotic hyperglycinemia (NKH) or glycine encephalopathy is a group of congenital enzymopathies that effect the GCS and its ability to catabolize GLY to CO2 and . Consequently, persons afflicted with NKH have elevated plasma GLY levels and, more importantly, significantly elevated CSF concentrations of GLY. The CSF/plasma ratio of GLY concentrations is usually diagnostic of this disease. The incidence of NKH has been estimated to be 1 in 55,000 in Finland and 1 in 63,000 in British Columbia, Canada. This is the second commonest inherited disease of amino acid metabolism after phenylketonuria, which affects phenylalanine metabolism. As discussed earlier, the GCS comprises three enzymes and a carrier protein. Mutations in the T-protein gene (aminomethyltransferase; AMT) and the P-protein gene (glycine dehydrogenase (decarboxylating); GLCD) comprise 10–15% and 80% of all the mutations, respectively. Mutations in the H-protein comprise <1% of the known mutations.
Most therapies of NKH involve attempts to reduce GLY concentrations in the plasma and thus in the brain. This is usually done through the administration of sodium benzoate to drive the GDS and remove excess GLY. In addition, antagonists of the NMDA receptor in the cerebral cortex, such as dextromethorphan or ketamine are also used (Applegarth & Toone, 2006; Suzuki et al., 2010). Some patients have been treated with considerable success, with the use of 500–750 mg/kg sodium benzoate and 5–7.5 mg/kg dextromethorphan. Treatment was continued from day 5 of life for one year, during which time, plasma GLY fell from 950 µM to around 100–150 µM until day 120 of life, after which it rose again to 400–700 µM. Most importantly, this therapy reduced the CSF GLY concentration from 150 µM to a trough of ~50 µM (normal values for CSF <20 µM) (Hamosh et al., 1992). There is no completely successful treatment and the long-term outcome is poor (Korman et al., 2006; Rossi et al., 2009). In all probability, GLY causes prenatal injury to the developing brain in utero, which is not reversible in the postnatal period (Korman et al., 2006).
Nevertheless, the GDS can be utilized by the supplementation of the molecular escort BA to treat two groups of congenital enzymopathies, those of the urea cycle and of the glycine cleavage system.
4.4. Adverse reactions to valproic acid
Sodium valproate is an anticonvulsant drug used to treat epilepsy and a number of other disorders including panic attacks, anxiety, and bipolar disorder. Chemically, valproate is 2-propylpentanoic acid and consequently is a branched medium-chain fatty acid. As such, valproate undergoes β-oxidation using the isoleucine catabolic pathway and enzymes (Luis et al., 2011), which requires formation of a CoA derivative. Not surprisingly, this metabolic pathway is inhibited by aspirin because salicylate competes for CoA during GLY conjugation (Abbott et al., 1986), that is, by using the GDS. Interestingly, valproate itself undergoes very little conjugation with GLY, the valproyl-CoA intermediate being used almost exclusively for β-oxidation. Three amino acid conjugates of valproic acid have been reported, with glutamate, glutamine, and GLY, and these three urinary metabolites accounted for only 1% of the dose (Gopaul et al., 2003). However, valproate has very subtle effects on the GDS. In normal rats, administration of valproate (2 to 3 mmol/ kgi.p.) 1 h prior to the i.v. injection of BA (1 mmol/kg) had little effect on the clearance of BA or the appearance of HA in urine. But, when rats were loaded with GLY (5 mmol/kg i.v.), valproate (2 and 3 mmol/ kgi.p.) reduced BA clearance by 34% and 59%, respectively and decreased the urinary excretion rate of HA by 28% and 66%, respectively, relative to controls loaded with GLY but injected with vehicle instead of valproate (Gregus et al., 1993b). Valproate therefore appeared to have an effect on the GDS. Although valproate did not inhibit the enzymes of the GDS, a metabolite, 2-propyl-4-pentenoic acid (the first β-oxidation metabolite of valproate), inhibited benzoyl-CoA synthetase (see Section 3.3.3) (Gregus et al., 1993b). Moreover, it has also been reported that chronic valproate administration to rats inhibits the GCS by both reducing the hepatic level of the P-protein (GLDC), the first step in glycine cleavage, and inhibiting its activity (Kochi et al., 1979).
In humans, the situation is much less clear. A single case report, however, is of interest. A 2.5-year-old girl with a history of developmental delay, failure to thrive, and a seizure disorder was administered valproate for her seizures and the seizure frequency significantly increased necessitating withdrawal of valproate. Detailed investigation of the underlying metabolic disorder revealed plasma and CSF GLY concentrations of 762 (normal range, 127–341) and 38 (normal range, 2–27) µM, respectively (Dhamija et al., 2011), diagnostic of NKH. She was started on sodium benzoate and dextromethorphan and her anticonvulsant therapy switched from valproate to carbmazepine. She remained seizure-free up to the time of the report (Dhamija et al., 2011). This patient presumably had congenital nonketotic hyperglycinemia due to mutations in the GLCD and/or AMT genes, which compromised her GCS. She had therefore relied on the GDS to remove excess GLY, but this was also compromised by the administration of valproate. Treatment involved accelerating GLY deportation with BA and blocking the effects of excess GLY in the cerebral cortex with the NMDA receptor antagonist dextromethorphan.
4.5. Adverse reactions to benzoic acid
There is some literature that points to an effect of BA on the CNS that might have its origins in the reduction of plasma and CSF GLY concentrations by excessive activity of the GDS. A study in weanling rats fed 3% BA in the diet for 5 days showed growth retardation and neurotoxicity manifested by irritability, ataxia, and tonic-clonic seizures. Feeding 1.1% BA for 35 days retarded growth but was not neurotoxic (Kreis et al., 1967). A significant number of patients with progressive encephalomyelitis with rigidity and myoclonus (PERM) have been reported to have anti-glycine receptor (GlyR) antibodies. PERM is associated with neuronal loss in the brainstem and spinal cord, exactly where GlyR are located (Mas et al, 2011). In neurophysiologic terms, one might equate PERM with hypoglycinemia, both conditions giving rise to similar symptoms. Therefore, the observations on the weanling rats fed 3% BA very likely were due to hypoglycinemia, that is, excessive working of the GDS. Weight can be added to this proposal from the field of strychnine toxicology. Strychnine binds to GlyR and inhibits the effects of GLY on its receptors in the brainstem and spinal cord. The pattern of early irritability followed by severe convulsions (Makarovsky et al., 2008) is very similar to the observations in the rat study with BA (Kreis et al., 1967).
It is extremely unlikely that human exposures to BA (see Section 3.3.2) in the diet or from exposure to environmental chemicals could lead to toxicity akin to the symptoms of PERM or strychnine poisoning. However, it is clear that there is a large range of daily intake from the diet in BA and, more importantly, BA precursors (Fig. 4). How these aspects of our diet influence plasma and CSF concentrations of GLY and its physiological effects on NMDAR and GlyR is completely unknown. One may only speculate at this time on the effects on mood, learning, and personality that might be mediated by long-term effects on the GDS by variable intake of BA and its precursors, and therefore variable GLY deportation.
4.6. Adverse reactions to salicylic acid
Salicylic acid (SA) is the active metabolite of the widely-used oral analgesic and anti-inflammatory drug aspirin (acetylsalicylic acid). Plant products containing SA have been used as remedies against pain and fever for at least 2500 years. Because salicylates are widely used and readily available as over-the-counter medications, they are often abused, with more than 10,000 toxic events per annum in the US (Pearlman & Gambhir, 2009). SA is conjugated with GLY forming salicylglycine, or salicyluric acid, which constitutes >70% urinary metabolites of SA or aspirin (Levy, 1965). This reaction was demonstrated to occur in bovine liver mitochondria and dependent upon CoA and ATP (Forman et al., 1971), analogous to the reaction used by the GDS with BA. A series of 2-substituted benzoic acids has been investigated in mouse liver and kidney mitochondria. The best substrate for both systems was BA itself, with activities of 25.1 and 69.3 nmol/mg for liver and kidney mitochondria, respectively (Kasuya et al., 2000). It was also reported that SA is not conjugated with GLY by liver mitochondria, but by kidney mitochondria only. Purified medium-chain acyl-CoA synthetases from mouse and bovine liver mitochondria showed the highest activity with hexanoic, octanoic, and decanoic acids, metabolized BA, but neither enzyme metabolized SA (Kasuya et al., 2006). These were virtually identical findings to those reported in the original paper on the bovine liver mitochondrial enzyme over 50 years previously, in which SA was also found not to be a substrate for the hepatic enzyme (Schachter & Taggart, 1954). There is clearly a species difference in SA conjugation with GLY between the ox (Forman et al., 1971) and the mouse (Kasuya et al., 2000).
Regarding humans, as discussed above (Section 3.3.3), the GDS operates well in kidney, especially in the cortex (Pacifici et al., 1991). In the intact human subject, it is generally very difficult to recognize that a metabolic reaction occurs in the kidney. It has been suggested that a metabolite with low plasma levels and an unexpectedly high urinary concentration is probably being produced by the kidney (Vree et al., 1992). Clearly, kidney is a major site of SA conjugation with GLY, although there are expected to be species differences in this regard. An interesting observation that appears to confirm that BA and SA may not overlap completely in how they scavenge GLY was a report that BA had a pronounced effect on the formation of salicyluric acid from SA in man, but SA had no effect upon the formation of HA from BA. In addition, HA formation could be enhanced by GLY administration, but salicyluric acid formation was not influenced by the administration of GLY (Amsel& Levy, 1969).
A study of aspirin overdose revealed that plasma GLY concentrations ~6 to 9 h after overdosing were 9.4 ± 0.5 mg/1 (125 ± 7 µM) compared with 15.7±1.1 mg/1 (209±15µM) for healthy volunteers who had not taken aspirin. Healthy volunteers who took a single 500 mg tablet of aspirin had intermediate values of 12.8±0.5 mg/l (171 ±7 µM) (Patel et al., 1990). Aspirin administration, in particular aspirin overdose, causes GLY deportation, presumably via the renal mitochondrial system. It is of note that aspirin intoxication causes multiple CNS effects, including confusion, coma, seizures, dizziness, lethargy, delirium, stupor, incoordination, restlessness, hallucinations, cerebral edema, agitation, encephalopathy, and psychosis (Pearlman & Gambhir, 2009), many of which might be attributed to hypoglycinemia effecting lower brain GLY concentrations. Here, the GDS, by accepting SA, a simple substituted BA, may play a role in the pattern of life-threatening CNS pathologies in aspirin poisoning.
4.7. Adverse reactions to nicotinic acid
Nicotinic acid (NA), also known as niacin or vitamin B3 is an essential human nutrient, which, when absent, causes a disease called pellagra. The recommended dietary allowance of NA is 14 mg for adult females and 16 mg for adult males, or the equivalent amount of dietary tryptophan, from which NA can be synthesized (60 mg tryptophan yields 1 mg NA) (IOM, 1998). In 1955, it was discovered that large doses of NA lowered serum cholesterol concentrations, both in healthy volunteers given 4g and in “patients with various diseases” (not specified) given 1 g. These authors also observed that all their healthy medical student volunteers (n= 11) experienced flushing and burning sensations in the skin (Altschul et al., 1955). NA was also used in the 1950s for the treatment of migraine at a dose of 50 mg four times a day, above which flushing sometimes occurred (Nelson, 1955).
Several investigators studied the effects of NA on lipid metabolism in vitro in an attempt to discover the mechanism by which NA lowered serum cholesterol. By feeding NA in the diet to rats at 1, 2, 3, and 4%, after first generating fatty liver with a “special hypolipotropic diet” (not specified), dose-dependent reduction in hepatic cholesterol content was reported with NA. This author proposed that the low availability of CoA was the cause of depressed cholesterol synthesis (Schön, 1958). At the same time, Merrill, who studied the incorporation of sodium [1-14C]acetate into cholesterol in rat liver slices, pointed out that metabolic requirements for NA excretion might alter cholesterol biosynthesis (Merrill, 1958). It was then reported that incubation of rat liver slices with NA led to an increase in fatty acid biosynthesis at the expense of the synthesis of sterols (Hardy et al., 1960). Again, this would suggest a competition for CoA resources within mitochondria. Because NA is conjugated with GLY (Ding et al., 1989) and therefore can be a molecular escort for GLY in the GDS, it is probable that the GDS utilizes extra CoA resources after the administration of large doses of NA. This may form the basis of these early theories of the mode of action of NA in lowering serum cholesterol by inhibiting its de novo synthesis in the liver. However, this may happen predominantly in the kidney and not the liver because administration of SA inhibits the conjugation of NA in the GDS in human volunteers (Ding et al., 1989).
It is recognized today that NA is a powerful lipid-altering drug that can lower levels of atherogenic apolipoprotein B-containing lipoproteins, such as LDL, VLDL, IDL, and Lp(a), while raising levels of atheroprotective HDL cholesterol. However, its use is limited by side-effects, especially flushing (incidence 25 to 40%), although gastrointestinal problems and metabolic effects may also present a problem (Creider et al., 2012). These authors also cite several studies that have reported that high-dose aspirin (325 mg) is the most effect means by which flushing could be prevented. This efficacy was not shared by ibuprofen, so it is unlikely that prevention of flushing is mediated by inhibition of prostaglandin synthesis, as various authors have suggested. Much more probable is an interaction between SA and NA at the level of the GDS, which is already known (Ding et al., 1989). In any case, the administration of aspirin will surely elevate plasma levels of NA and one must therefore propose that the flushing mechanism involves a metabolite of NA rather than NA itself. Nevertheless, ablation of the major limiting side-effect of this useful lipid-lowering drug certainly must contain a component of the GDS. Co-administration of NA and SA will elevate plasma GLY concentrations, and the role of GLY, either centrally or systemically, in the flushing response is completely unknown.
4.8. Ifosfamide encephalopathy
4.8.1. History of ifosfamide
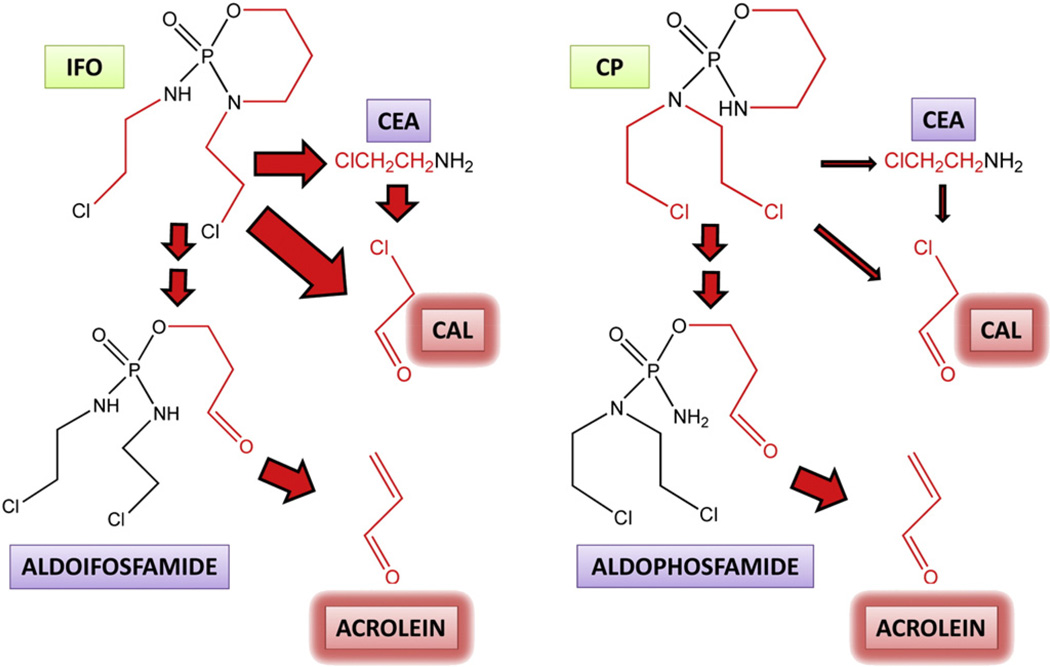
On May 8 1973, US Patent 3,732,340 was awarded to the German company Asta-Werke for “N′,O-propylene phosphoric acid ester diamides”, which contained the chemical structure of ifosfamide (IFO), whose features were described as “known to have the best cytostatic properties”. The company had earlier published data on the first such nitrogen mustard pro-drug, cyclophosphamide (CP), a structural isomer of IFO and a molecule specifically designed as inert but that might be converted into an active metabolite at its site of action (Arnold et al., 1958). The only known example of this cancer chemotherapy pro-drug principle at that time was stilboestrol diphosphate for the treatment of prostatic carcinoma (Druckrey & Raabe, 1952). The principle that was developed with respect to CP, IFO, and related drugs was that of the “transport form/active form” for these highly reactive nitrogen mustards whose “phosphamide and phosphoric ester bonds would be particularly susceptible to tumour-specific enzyme reactions” (Arnold et al., 1961). Fig. 5 shows the chemical structures of the oxazaphosphorines IFO and CP and their metabolism to the unwanted toxic aldehydes acrolein and chloroacetaldehyde (CAL). The formation of acrolein was said to be greater for CP than for IFO and acrolein was originally proposed to contribute to the cytotoxic activity of the drugs (Alarcon et al., 1967). There followed many metabolic studies of IFO and CP, both in vivo and in vitro, in an attempt to clarify the exact mode of cancer cytotoxicity of these drugs. An investigation of the in vitro metabolism of both drugs led to the finding that IFO underwent N-dechloroethylation (which yields CAL) while CP did not. Subsequently, it was reported that either of the IFO N-(2-chloroethyl) groups could be dealkylated in patients treated with IFO and that the resulting dealkylated compounds were major metabolites for IFO but not CP (Norpoth, 1976). Recently, a metabolomic study of IFO and CP metabolism has been conducted in mice, which reported 11 urinary IFO metabolites and 12 urinary CP metabolites. Comparison of normalized peak areas permitted estimation of N-dechloroethylated metabolites to represent ~40% of the dose for IFO and only ~10% dose for CP in the mouse (Li etal., 2010).
Fig. 5.
The metabolism of ifosfamide and cyclophosphamide to unwanted toxic intermediates. Ifosfamide (IFO) and its structural isomer cyclophosphamide (CP) are both metabolized by cytochrome P450 isozymes to their 4-hydroxy derivatives (not shown), which are ring-opened to the corresponding aldehydes, aldoifosfamide and aldophosphamide, respectively. These aldehyde metabolites both undergo a β-elimination of the highly toxic metabolite acrolein. Cytochrome P450-mediated metabolism of the nitrogen mustard side-chains also occurs leading to the toxic metabolite chloroacetaldehyde (CAL), either directly, or via 2-chloroethylamine (CEA), with the participation of amine oxidases. Acrolein is believed to cause urotoxicity and CAL has been proposed to cause neurotoxicity. Arrow size indicates the relative flux down different pathways and therefore that CAL formation is considerably greater with IFO than with CP. Chemical components of IFO and CP that become the toxic aldehydes acrolein and CAL are shown in red.
Historically, CP has been perhaps the most widely used cancer chemotherapeutic agent. Additionally, its isomer IFO has also become widely used in the treatment of a range of solid tumors, both in adults and children (Misiura, 2006). Both drugs have the propensity to cause unwanted urotoxicity, due to the production of acrolein (Brock et al., 1979) (Fig. 5), most commonly, hemorrhagic cystitis, which can be fatal, but this can be successfully managed by the co-administration of mesna (mercaptoethylsulfonate Na+ salt) (Lawson et al., 2008), which forms an excretable adduct with acrolein (Brock et al., 1981). What emerged soon after the introduction of IFO into clinical oncology was the appearance of neurotoxicity, in particular, so-called “ifosfamide encephalopathy”, which was not a feature of CP treatment regimens.
4.8.2. Occurrence of ifosfamide encephalopathy
The occurrence of encephalopathy associated with IFO (and mesna) treatment was first reported by groups in Birmingham and London, UK in 1985 when 5 of 31 patients with cervical and endometrial carcinoma had clinical and electroencephalographic features of severe encephalopathy. The dose employed was 5 g/m2 IFO and 8.2 g/m2 mesna over 36 h. The death of one patient was attributed to these untoward CNS effects (Meanwell et al., 1985). Soon thereafter a few further cases were reported for the IFO/mesna combination (Cantwell & Harris, 1985). There quickly followed a rebuttal of the notion that the encephalopathy might be due to mesna, from another group in London who had administered 7 g/m2 CP over 15 h and 10.5 g/m2 mesna over 33 h, without signs of encephalopathy (Osborne & Slevin, 1985). In the intervening years, there have been numerous reports of ifosfamide encephalopathy. This life-threatening drug effect has been estimated to occur with an incidence from <5% to >70% (Tajino et al., 2010), with a typical occurrence of 10–30% in adult patients (Liu et al., 2010; Ryan et al., 2008; Savica et al., 2011; Tajino et al., 2010) and 3–19% in pediatric patients (Ames et al., 2010; Kerdudo et al., 2006). Other types of neurotoxicity, particularly transient seizures, have been reported in ~5% children receiving IFO for solid tumors or leukemia (Di Cataldo et al., 2009). What is certain today is that ifosfamide encephalopathy has an unfortunate and unacceptably high incidence in cancer patients receiving IFO. This occurs particularly in adult patients, some of whom die as a result.
4.8.3. Theories of ifosfamide encephalopathy
Clinical commentators were quick to propose the source of ifosfamide encephalopathy soon after it was first noted. In the first report, Meanwell and his colleagues suggested that exceptional fluxes of iron or copper were a possible cause, by direct analogy with the encephalopathy seen in rheumatoid arthritis patients receiving desferrioxamine (Meanwell et al., 1985). Later, this same group reported a discriminant analysis on 77 patients that had received IFO, of which seven developed encephalopathy. This analysis showed that low plasma albumin, high plasma creatinine concentration, and the presence of pelvic malignancy were all risk factors for ifosfamide encephalopathy (Meanwell et al., 1986). A center in Newcastle, UK had treated 119 patients with 314 cycles of IFO and mesna. They proposed that women seemed to be more susceptible and that the encephalopathy was caused by a “direct effect of ifosfamide/mesna or one of their metabolites on the central nervous system” (Cantwell & Harris, 1985). Goren and his colleagues reasoned that CAL (Fig. 5) was the culprit. They were aware that little CP was dechloroethylated to CAL, but up to 50% of a dose of IFO may pass down this pathway, added to which, IFO doses were typically ten-times greater than CP doses, meaning that an IFO patient may be exposed to 100-times the quantity of CAL as a CP patient (Goren et al., 1986). These investigators determined blood and urine concentrations of CAL in six children who had received 1.6 g/m2 IFO and 400 mg/m2 mesna. Urinary CAL concentrations were as high as 221 µM. In two children with neurotoxicity, characterized by somnolence, urinary incontinence, and inappropriate behavior, blood concentrations of CAL were 88 µM at 6 h and 109 µM at 24 h after IFO administration, compared with 10 µM and 22 µM for the same times, respectively, in four patients without neurotoxicity. They proposed a causative role for CAL (Goren et al., 1986).
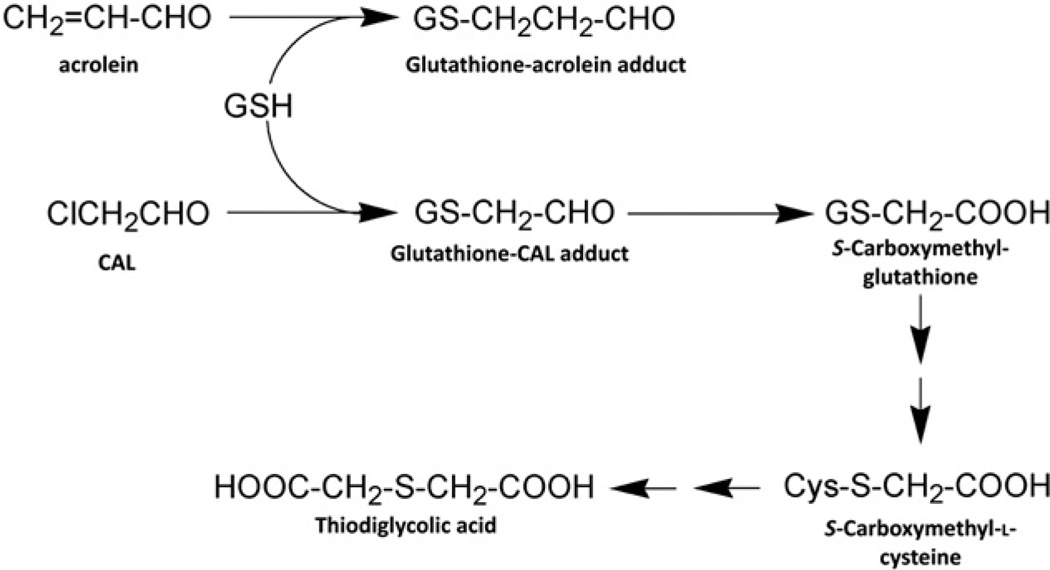
A detailed investigation of a single patient with ifosfamide encephalopathy after 25 g IFO and 20 g mesna within 24 h by Küpfer and his colleagues in our laboratories led to the observation that urinary glutaric acid was raised from a normal value of <0.02 mmol/day to 7.4 mmol on day 1 and 6.6 mmol on day 2, a >370-fold increase. In addition, they observed that urinary sarcosine output was elevated from a normal value of <0.03 mmol/day to 0.78 and 0.45 mmol on these same two days, a >26-fold increase (Küpfer et al., 1994). They proposed that ifosfamide encephalopathy was mimicking glutaric aciduria type II, an effect on glutaryl-CoA dehydrogenase (EC 1.3.99.7), an enzyme in the mitochondrial matrix (Koeller et al., 1995). As methylene blue (MB) had been used successfully to treat glutaric acidurias, Küpfer and his colleagues evaluated MB successfully in both the treatment and prophylaxis of ifosfamide encephalopathy (Küpfer et al., 1994). Many clinical groups have since investigated MB as a prophylaxis or therapy for ifosfamide encephalopathy, with variable results. Nevertheless, MB is still widely employed in this regard (Oz et al., 2011). The CAL liberated by IFO in particular can be further metabolized by adduct formation with cellular thiols leading to S-carboxymethyl-L-cysteine (SCMC) and the depletion of total cysteine, glutathione and homocysteine (Lauterburg et al., 1994). Transamination and decarboxylation of SCMC leads to thiodiglycolic acid (TDGA), which was reported to inhibit mitochondrial function in rats, specifically palmitic acid β-oxidation (Visarius et al., 1998). Of interest with respect to encephalopathy is that SCMC is structurally closely-related to glutamate. SCMC, but not TDGA, was reported to activate AMPA/kainate receptors (non-NMDA glutamate receptors) on single mouse cortical neurons in vitro and to induce cellular acidification. These properties were believed to provide a basis for ifosfamide encephalopathy (Chatton et al., 2001). Subsequently, it was reported that SCMC was formed directly in mouse brain after the administration of IFO, lending support to the SCMC theory of ifosfamide encephalopathy (Lerch et al., 2006).
Finally, a clinical perspective stated that previous cisplatin administration or the administration of IFO at doses >9 g/m2 are risk factors for ifosfamide encephalopathy (Tajino et al., 2010). However, one must distinguish between clinical risk factors for, and biochemical mechanisms of, ifosfamide encephalopathy. To date, here has been no consensus regarding the mechanism of ifosfamide encephalopathy.
4.8.4. Ifosfamide encephalopathy as a failure of the glycine deportation system
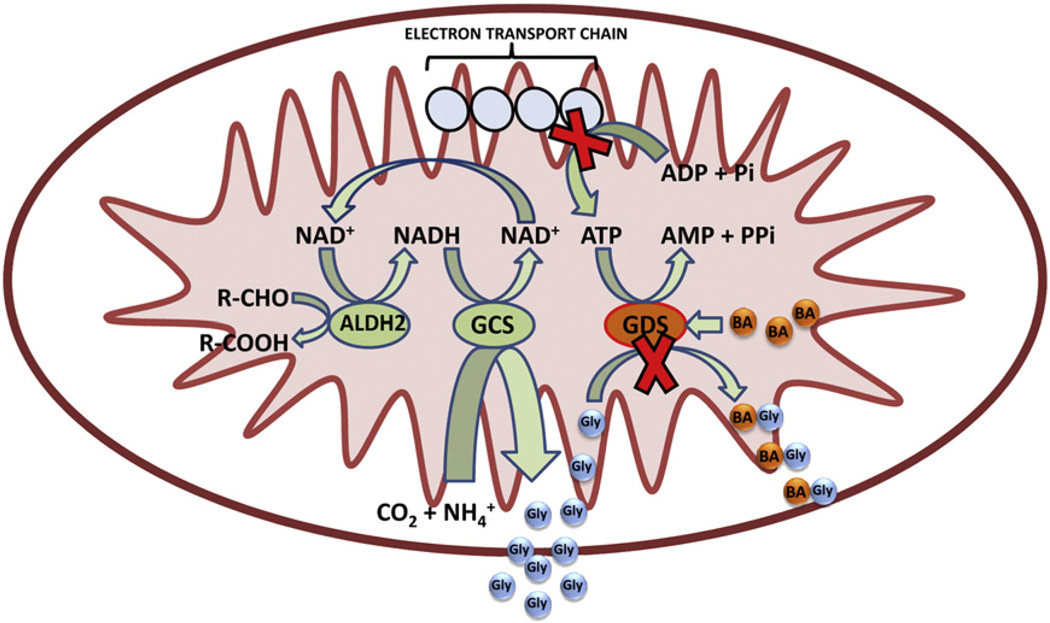
We wish to review the evidence that ifosfamide encephalopathy is due to a failure of the GDS. For GLY plasma levels to rise significantly, without the administration of GLY, not only must the glycine cleavage system be attenuated, but also the glycine deportation system. Is it possible to envisage a situation where both GLY clearance pathways are compromised? It has been stated above that the GCS is believed to be the main source of GLY for the GDS (Gregus et al., 1993a). Under these circumstances, the GCS would be synthesizing GLY de novo and driven by NADH (see Fig. 1). With the GCS powered by NADH and synthesizing GLY, the only route for removal of GLY would be the GDS.
As depicted in Fig. 5, the hepatic metabolism of IFO produces copious quantities of toxic aldehydes, acrolein and CAL. Acrolein is well-known to react rapidly with reduced glutathione (GSH) to form a stable adduct (Esterbauer et al., 1975) and to deplete GSH in rat hepatocytes in a dose-dependent manner (Zitting & Heinonen, 1980). Experiments with P388 cells in vitro and lymphocytes from an IFO patient ex vivo suggested that the decline in GSH observed in these cells could be accounted for by the metabolism of IFO to CAL (Lind et al., 1989). Additionally, CAL may reduce cerebral GSH levels in mice (Sood & O'Brien, 1996), although the role of this in ifosfamide encephalopathy is unclear. Furthermore, acrolein is a potent inhibitor of cytosolic aldehyde dehydrogenase 1 (ALDH1) with a Ki of 0.65 µM (Ren et al., 1999). By using the pharmacokinetic parameters for CP metabolites and the inhibition kinetics of acrolein, it has been calculated that ALDH1 is inhibited in vivo by 85–91% after CP administration in patients (Ren et al., 1999). The inhibition of ALDH1 by acrolein in the cytosol of liver cells will encourage formation of the glutathione-acrolein adduct as shown in Fig. 6. The metabolism by ALDH isozymes of this adduct has been investigated and reported to occur most efficiently by mitochondrial aldehyde dehydrogenase 2 (ALDH2), which was 3.5- to 175-times more active than other ALDH isozymes (Mitchell & Petersen, 1989). This is surely also be the case for the GSH adduct of CAL, which merely possesses one methylene group less than the acrolein adduct (see Fig. 6). These now stable aldehydes on penetration to the mitochondrial matrix, where ALDH2 is expressed at high levels in human liver (Hempel et al., 1985; Stewart et al., 1996), will be converted to their corresponding carboxylate metabolites (Fig. 6) with the generation of NADH from NAD+. The same will occur for any free aldehydes that can access this compartment, that is, acrolein and CAL themselves. Doses of IFO are of the order of 2.5 to 8.5 g per day (Boddy et al., 1995; Lind et al., 1990), which corresponds to ~10–30 mmol IFO. It has been estimated that as much as 50% of an administered dose of IFO may be converted to CAL (Goren et al., 1986), which would be equivalent to 5–15 mmol CAL (~400 to 1200 mg per day) .This, together with acrolein produced also from IFO, leads to what has been described as “hepatic aldehyde overload” (Adrian Küpfer, personal communication). These huge quantities of toxic aldehydes in the livers of ifosfamide patients have two major effects of relevance to ifosfamide encephalopathy.
Fig. 6.
Adduct formation and subsequent metabolism of ifosfamide toxic aldehydes. Acrolein and chloracetaldehyde (CAL) react readily with glutathione (GSH) to form adducts through the cysteine sulfur. Both adducts are metabolized by mitochondrial ALDH to the corresponding S-carboxymethyl- (for CAL) and S-carboxyethyl- (for acrolein; not shown) glutathione adducts. These undergo metabolism to S-carboxymethyl-L-cysteine, which in turn, is transaminated and decarboxylated to yield thiodiglycolic acid.
First, acrolein has been reported to diminish mitochondrial complexes I,II, and III (Jia et al., 2007). This may be the mechanism underlying the reported glutaric aciduria in IFO patients with encephalopathy that was assumed to be due to an inhibition of mitochondrial electron transport (Küpfer et al., 1994). Weight has been added to this theory by more recent studies in rats treated with IFO in which oxidative phosphorylation was inhibited at the level of complex I, with a significant elevation in NADH and depletion of NAD + (Nissim et al., 2006). These authors believed this effect to be due to CAL Reduction of electron flow in oxidative phosphorylation with reduce ATP formation. This has been reported in LSI 74T colon cancer cells where addition of 50 µM CAL reduced ATP synthesis (Motrescu et al., 2005). This is the first major effect of the aldehyde overload, the reduction of ATP synthesis by acrolein and CAL.
Second, the metabolism of toxic aldehydes and their adducts by ALDH2 in the mitochondrial matrix causes a sequestration of NADH in the matrix, at the expense of NAD+. This may be exacerbated by the inhibition of oxidative phosphorylation at complex I referred to above. Overall, therefore, a shortfall in mitochondrial matrix ATP production and sequestration of NADH are the hallmarks of IFO administration.
Fig. 7 illustrates the chain of reactions that we propose leads to ifosfamide encephalopathy. The high throughput of aldehyde oxidation by ALDH2 leads to NADH sequestration. This in turn drives the GCS towards GLY synthesis, oxidizing NADH to NAD+ (see Fig. 1). The NAD+ produced by the GCS is seen as fuelling ALDH2. Excess GLY produced under the influence of NADH in the mitochondrial matrix would normally be scavenged by the GDS, which, as was stated earlier, usually operates at only half-capacity. But the GDS requires 2 mol of ATP for each mol of GLY and BA converted to HA (BAGly in Fig. 7) (Gatley & Sherratt, 1977) and is extremely sensitive to ATP depletion (Gregus et al., 1996). Given the shortfall in ATP, the GDS will fail to dispose of the excess GLY, which is now free to leave the mitochondrion and the liver, and develops hyperglycinemia.
Fig. 7.
The effect of ifosfamide toxic aldehydes on the glycine cleavage system and glycine deportation system in the mitochondrial matrix. In this representation of the mitochondrion, showing the outer and inner membranes and the mitochondrial matrix, the aldehyde metabolites of ifosfamide (IFO), chloracetaldehyde and acrolein (represented by R-CHO), inhibit ATP production by the electron transport chain (shown by red X). They are also metabolized by aldehyde dehydrogenase 2 (ALDH2), thus converting NAD+ to NADH. The aldehyde overload in the liver from IFO therapy thus causes NADH sequestration in the mitochondrial matrix (shown in pink). This excess NADH drives the glycine cleavage system towards the de novo synthesis of glycine (GLY). The shortfall in ATP production attenuates the glycine deportation system (shown by red X) and GLY is no longer scavenged by benzoic acid (BA) and converted into hippuric acid (BAGly) for deportation and thus plasma levels of GLY rise.
What experimental evidence exists in support of the proposition that ifosfamide encephalopathy arises due to a failure of the glycine deportation system? As has been mentioned previously, Goren and colleagues determined CAL levels in children treated with IFO, including those with neurotoxicity and reported considerably elevated concentrations of CAL in the neurotoxic patients (Goren et al., 1986). In order to explain why IFO was neurotoxic and CP not, these authors calculated that IFO patients may be exposed to 100-fold greater levels of CAL than CP patients and furthermore proposed that CAL was the cause of the encephalopathy (Goren et al., 1986). Küpfer and his colleagues demonstrated that both glutaric acid and sarcosine were considerably raised (>370-fold and >26-fold, respectively) in the urine of a patient with ifosfamide encephalopathy and proposed that this was symptomatic of an effect of IFO administration on oxidative phosphorylation. Sarcosine is N-methylglycine and is the only alternative metabolite of GLY possible when the GCS and GDS are compromised. Küpfer and colleagues also successfully tested the efficacy of methylene blue (MB) in the therapy and prophylaxis of the encephalopathy, demonstrating that IFO administration had a negative impact on mitochondrial electron transport that could be reversed with the redox acceptor MB (Küpfer et al., 1994). Finally, using 1H nuclear magnetic resonance spectroscopy on the urine of both rats and humans treated with IFO, it was reported that many analytes were increased or decreased in urine. Of particular interest was elevated GLY and reduced HA (Foxall et al., 1996). A more detailed study by the same group was reported in which patients were investigated using high resolution 1H nuclear magnetic resonance spectroscopy (Foxall et al., 1997). In a group of ten patients, urinary GLY was increased by 0.35 mmol/mmol creatinine and urinary HA was decreased by 0.33 mmol/mmol creatinine during the first cycle of IFO treatment. In the second cycle, GLY was increased by 0.63 mmol/mmol creatinine and HA by 0.66 mmol/mmol creatinine. GLY was elevated and HA reduced in the urine of all ten patients (Foxall et al., 1997). Although the authors ascribe transient damage to the renal cortex as an explanation for these findings, the molar equivalence in the changes in GLY and HA suggests that these findings may be due to a failure of the GDS. Sadly, there has been to date no investigation of the effect of IFO administration on the GCS and GDS, neither in cancer patients nor in laboratory animals.
Our proposition also states that ifosfamide encephalopathy mimics nonketotic hyperglycinemia and its resultant encephalopathy (Hall & Ringel, 2004; Suzuki et al., 2010), where GLY concentrations in blood may be only modestly elevated but increased GLY concentrations in CSF is diagnostic of the disease (Applegarth & Toone, 2006).
Finally, it is of interest to note here that GLY has been administered in 3 g doses to persons who had complained about sleep quality and performed statistically significantly better than placebo in improving subjective sleep quality (Inagawa et al., 2006). Using polysomnography, these workers established that this night-time dose of GLY improved objective measures of sleep quality (Yamadera et al., 2007).
5. Conclusions
It is concluded that the glycine deportation system is responsible for the removal of 400 to 800 mg per day of glycine in the form of urinary hippuric acid. Therefore, the glycine deportation system is an essential component of glycine homeostasis and works in concert with the glycine cleavage system. The bolus administration of pharmaceutical doses of benzoic acid upregulates the glycine deportation system and this is a useful therapeutic maneuver in cases of congenital urea cycle enzymopathies and nonketotic hyperglycinemia. Aspirin overdose and adverse drug reactions to nicotinic acid and ifosfamide appear to involve perturbations of the glycine deportation system. Therefore, the glycine deportation system is an vital homeostatic mechanism that helps regulate glycine concentrations in the central nervous system and can be harnessed to remove both excess glycine and excess nitrogen in the form of glycine (Fig. 8).
Fig. 8.
The three components of the glycine deportation system. 1. Glycine (Gly) is deported from the liver as hippuric acid (BAGly) by the hepatic glycine deportation system (GDSL) that uses benzoic acid (BA), coenzyme A (CoA) and 2 mol ATP. 2. Gly is also deported from the kidney as BAGly by the kidney glycine deportation system (GDSK) that also requires BA, CoA, and 2 mol ATP. This system can also use salicylic acid (SA), deporting Gly as salicyluric acid (SAGly). 3. BAGly (and SAGly) are subjected to active tubular secretion in the final stage of glycine urinary deportation.
The importance of the concept of the glycine deportation system is that it may lead to new therapeutic and prophylactic strategies for the common adverse drug reactions that limit the use of at least two valuable drugs, nicotinic acid and ifosfamide.
Acknowledgments
JR1 wishes to acknowledge NIH (NIAID) grant U19 A1067773-07 and the Hassan Badawi Foundation Against Liver Cancer for financial support. We wish to acknowledge the encouragement of our colleague Professor Jean-François Dufour. Numerous stimulating conversations with our colleagues Professor Adrian Küpfer (Bern) and Professor Robert L Smith (London) over many years have helped sharpen some of the concepts contained herein.
Abbreviations
- GLY
glycine
- CNS
central nervous system
- GlyR
glycine receptor
- NMDA
N-methyl-D-aspartate
- NMDAR
N-methyl-D-aspartate receptor
- SER
L-serine
- SHMT
serine hydroxymethyltransferase
- THF
tetrahydrofolate
- 5,10-MeTHF
5,10-methylene-tetrahydrofolate
- DAAO
D-amino acid oxidase
- BA
benzoic acid
- GlyT
glycine transporter
- BBB
blood-brain barrier
- CSF
cerebrospinal fluid
- HA
hippuric acid
- GCS
glycine cleavage system
- GDS
glycine deportation system
- NKH
nonketotic hyperglycinemia
- PERM
progressive encephalomyelitis with rigidity and myoclonus
- SA
salicylic acid
- NA
nicotinic acid
- IFO
ifosfamide
- CP
cyclophosphamide
- CAL
chloracetaldehyde
- mesna
sodium 2-mercaptoethylsulfonate
- MB
methylene blue
- SCMC
S-carboxymethyl-L-cysteine
- TDGA
thiodiglycolic acid
- ALDH1
cytosolic aldehyde dehydrogenase 1
- ALDH2
mitochondrial aldehyde dehydrogenase 2
Footnotes
This manuscript has not been published elsewhere nor is it under consideration for publication elsewhere. Our sponsors played no role in the preparation of this manuscript.
References
- Abbott FS, Kassam J, Orr JM, Farrell K. The effect of aspirin on valproic acid metabolism. Clinical Pharmacology and Therapeutics. 1986;40:94–100. doi: 10.1038/clpt.1986.144. [DOI] [PubMed] [Google Scholar]
- Adamson RH, Bridges JW, Evans ME, Williams RT. Species differences in the aromatization of quinic acid in vivo and the role of gut bacteria. The Biochemical Journal. 1970;116:437–443. doi: 10.1042/bj1160437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon RA, Meienhofer J, Atherton E. Isophosphamide as a new acrolein-producing antineoplastic isomer of cyclophosphamide. Cancer Research. 1972;32:2519–2523. [PubMed] [Google Scholar]
- Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Archives of Biochemistry and Biophysics. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- Ames B, Lewis LD, Chaffee S, Kim J, Morse R. Ifosfamide-induced encephalopathy and movement disorder. Pediatric Blood & Cancer. 2010;54:624–626. doi: 10.1002/pbc.22361. [DOI] [PubMed] [Google Scholar]
- Amsel LP, Levy G. Drug biotransformation interactions in man. II. A pharmacokinetic study of the simultaneous conjugation of benzoic and salicylic acids with glycine. Journal of Pharmaceutical Sciences. 1969;58:321–326. doi: 10.1002/jps.2600580307. [DOI] [PubMed] [Google Scholar]
- Applegarth DA, Toone JR. Glycine encephalopathy (nonketotic hyperglycinemia): comments and speculations. American journal of Medical Genetics. 2006;140:186–188. doi: 10.1002/ajmg.a.31030. Part A. [DOI] [PubMed] [Google Scholar]
- Arnold H, Bourseaux F, Brock N. Chemotherapeutic action of a cyclic nitrogen mustard phosphamide ester (B 518-ASTA) in experimental tumours of the rat. Nature. 1958;181:931. doi: 10.1038/181931a0. [DOI] [PubMed] [Google Scholar]
- Arnold H, Bourseaux F, Brock N. On the relation between the chemical composition and the cancerotoxic effect in a series of phosphamide esters of bis-(beta-chloroethyl)-a mine. Arzneimittel-Forschung. 1961;11:143–158. [PubMed] [Google Scholar]
- Arnstein HR, Neuberger A. Hippuric acid synthesis in the rat. The Biochemical Journal. 1951;50:154–162. doi: 10.1042/bj0500154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner MR, Rosalki SB. Glyoxylate as a substrate for lactate dehydrogenase. Nature. 1967;213:726–727. doi: 10.1038/213726a0. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Brusilow SW. Treatment of hyperammonemic coma caused by inborn errors of urea synthesis. The journal of Pediatrics. 1980;97:893–900. doi: 10.1016/s0022-3476(80)80416-1. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Brusilow SW. Evidence of lack of toxicity of sodium phenylacetate and sodium benzoate in treating urea cycle enzymopathies. Journal of Inherited Metabolic Disease. 1981;4:231. doi: 10.1007/BF02263659. [DOI] [PubMed] [Google Scholar]
- Battistin L, Grynbaum A, Lajtha A. The uptake of various amino acids by the mouse brain in vivo. Brain Research. 1971;29:85–99. doi: 10.1016/0006-8993(71)90419-7. [DOI] [PubMed] [Google Scholar]
- Benavides J, Garcia ML, Lopez-Lahoya J, Ugarte M, Valdivieso F. Glycine transport in rat brain and liver mitochondria. Biochimica et Biophysica Acta. 1980;598:588–594. doi: 10.1016/0005-2736(80)90038-3. [DOI] [PubMed] [Google Scholar]
- Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V. Glycine transporters: essential regulators of synaptic transmission. Biochemical Society Transactions. 2006;34:55–58. doi: 10.1042/BST0340055. [DOI] [PubMed] [Google Scholar]
- Betz H, Laube B. Glycine receptors: recent insights into their structural organization and functional diversity. Journal of Neurochemistry. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- Beyoğlu D, Smith RL, Idle JR. Dog bites man or man bites dog? The enigma of the amino acid conjugations. Biochemical Pharmacology. 2011;83:1331–1339. doi: 10.1016/j.bcp.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatherwick NR, Long ML. Studies on urinary acidity. II. The increased acidity produced by eating prunes and cranberries. The Journal of Biological Chemistry. 1923;57:815–818. [Google Scholar]
- Bloch K, Rittenberg D. An estimation of acetic acid formation in the rat. The Journal of Biological Chemistry. 1945;159:45–58. [PubMed] [Google Scholar]
- Boddy AV, Proctor M, Simmonds D, Lind MJ, Idle JR. Pharmacokinetics, metabolism and clinical effect of ifosfamide in breast cancer patients. European Journal of Cancer. 1995;31A:69–76. doi: 10.1016/0959-8049(94)00300-t. [DOI] [PubMed] [Google Scholar]
- Borroni E, Zhou Y, Ostrowitzki S, Alberati D, Kumar A, Hainzl D, et al. Preclinical characterization of [(11)C]R05013853 as a novel radiotracer for imaging of the glycine transporter type 1 by positron emission tomography. Neurolmage. 2011 doi: 10.1016/j.neuroimage.2011.11.090. http://dx.doi.org/l0.1016/j.neuroimage.2011.11.090. [DOI] [PubMed]
- Bremer J. Species differences in the conjugation of free bile acids with taurine and glycine. The Biochemical Journal. 1956;63:507–513. doi: 10.1042/bj0630507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J, Gloor U. Studies on the conjugation of cholic acid with taurine in cell subfractions. Bile acids and steroids 23. Acta Chemica Scandinavica. 1955;9:689–698. [Google Scholar]
- Briggs R, Ertem G, Ferris JP, Greenberg JM, McCain PJ, Mendoza-Gomez CX, et al. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Origins of Life and Evolution of the Biosphere. 1992;22:287–307. doi: 10.1007/BF01810858. [DOI] [PubMed] [Google Scholar]
- Brock N, Hohorst HJ. Metabolism of cyclophosphamide. Cancer. 1967;20:900–904. doi: 10.1002/1097-0142(1967)20:5<900::aid-cncr2820200552>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Brock N, Pohl J, Stekar J. Detoxification of urotoxic oxazaphosphorines by sulfhydryl compounds. Journal of Cancer Research and Clinical Oncology. 1981;100:311–320. doi: 10.1007/BF00410691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock N, Stekar J, Pohl J, Niemeyer U, Scheffler G. Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide and sufosfamide. Arzneimittel-Forschung. 1979;29:659–661. [PubMed] [Google Scholar]
- Brusilow SW, Danney M, Waber LJ, Batehaw M, Burton B, Levitsky L, et al. Treatment of episodic hyperammonemia in children with inborn errors of urea synthesis. The New England Journal of Medicine. 1984;310:1630–1634. doi: 10.1056/NEJM198406213102503. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Valle DL, Batshaw M. New pathways of nitrogen excretion in inborn errors of urea synthesis. Lancet. 1979;2:452–454. doi: 10.1016/s0140-6736(79)91503-4. [DOI] [PubMed] [Google Scholar]
- Caner V, Cokal Y, Cetin C, Sen A, Karagenc N. The detection of hipO gene by real-time PCR in thermophilic Campylobacter spp. with very weak and negative reaction of hippurate hydrolysis. Antonie Van Leeuwenhoek. 2008;94:527–532. doi: 10.1007/s10482-008-9269-4. [DOI] [PubMed] [Google Scholar]
- Cantwell BM, Harris AL. Ifosfamide/mesa and encephalopathy. Lancet. 1985;1:752. doi: 10.1016/s0140-6736(85)91288-7. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Idle JR, Vagbo CB, Magistretti PJ. Insights into the mechanisms of ifosfamide encephalopathy: drug metabolites have agonistic effects on alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptors and induce cellular acidification in mouse cortical neurons. The Journal of Pharmacology and Experimental Therapeutics. 2001;299:1161–1168. [PubMed] [Google Scholar]
- Chen P, Abramson FP. Measuring DNA synthesis rates with [l-13C]glycine. Analytical Chemistry. 1998;70:1664–1669. doi: 10.1021/ac971294i. [DOI] [PubMed] [Google Scholar]
- Choi C, Ganji SK, DeBerardinis RJ, Dimitrov IE, Pascual JM, Bachoo R, et al. Measurement of glycine in the human brain in vivo by 1H-MRS at 3 T: application in brain tumors. Magnetic Resonance in Medicine. 2011;66:609–618. doi: 10.1002/mrm.22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R, Kendrick MI, Kass EH. Role of intestinal bacteria in aromatization of quinic acid in man and guinea pig. Proceedings of the Society for Experimental Biology and Medicine. 1960;104:424–426. doi: 10.3181/00379727-104-25860. [DOI] [PubMed] [Google Scholar]
- Creider JC, Hegele RA, Joy TR. Niacin: another look at an underutilized lipid-lowering medication. Nature Reviews. Endocrinology. 2012 doi: 10.1038/nrendo.2012.22. http://dx.doi.org/10.1038/nrendo.2012.22. [DOI] [PubMed]
- Csonka FA. On the administration of various proteins with benzoic acid to a pig. The Journal of Biological Chemistry. 1924;60:545–582. [Google Scholar]
- Cubelos B, Gimenez C, Zafra F. Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cerebral Cortex. 2005;15:448–459. doi: 10.1093/cercor/bhh147. [DOI] [PubMed] [Google Scholar]
- Dannhardt G, Kohl BK. The glycine site on the NMDA receptor: structure-activity relationships and possible therapeutic applications. Current Medicinal Chemistry. 1998;5:253–263. [PubMed] [Google Scholar]
- de Vries A, Alexander B, Quamo Y. Studies on amino acid metabolism. III. Plasma glycine concentration and hippuric acid formation following the ingestion of benzoate. The Journal of Clinical Investigation. 1948;27:665–668. doi: 10.1172/JCI102014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T, Takemoto M, Uehara N, Lindup WE, Suenaga A, Otagiri M. Renal clearance of endogenous hippurate correlates with expression levels of renal organic anion transporters in uremic rats. The Journal of Pharmacology and Experimental Therapeutics. 2005;314:932–938. doi: 10.1124/jpet.105.085613. [DOI] [PubMed] [Google Scholar]
- Dennis C, Gardner B, Parti J, Chenouda M. The effect of sodium benzoate and taurocholic acid feeding on human bile composition. Proceedings of the Society for Experimental Biology and Medicine. 1975;148:601–605. doi: 10.3181/00379727-148-38592. [DOI] [PubMed] [Google Scholar]
- Dhamija R, Gavrilova RH, Wirrell EC. Valproate-induced worsening of seizures: clue to underlying diagnosis. Journal of Child Neurology. 2011;26:1319–1321. doi: 10.1177/0883073811402204. [DOI] [PubMed] [Google Scholar]
- Di Cataldo A, Astuto M, Rizzo G, Bertuna G, Russo G, Incorpora G. Neurotoxicity during ifosfamide treatment in children. Medical Science Monitor. 2009;15:CS22–CS25. [PubMed] [Google Scholar]
- Ding RW, Kolbe K, Merz B, de Vries J, Weber E, Benet LZ. Pharmacokinetics of nicotinic acid-salicylic acid interaction. Clinical Pharmacology and Therapeutics. 1989;46:642–647. doi: 10.1038/clpt.1989.200. [DOI] [PubMed] [Google Scholar]
- Douce R, Bourguignon J, Neuburger M, Rebeille F. The glycine decarboxylase system: a fascinating complex. Trends in Plant Science. 2001;6:167–176. doi: 10.1016/s1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- Druckrey H, Raabe S. Specific chemotherapy of carcinoma of the prostate. Klinische Wochenschrift. 1952;30:882–884. doi: 10.1007/BF01471475. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, et al. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biological Psychiatry. 2000;47:450–462. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Zollner H, Scholz N. Reaction of glutathione with conjugated carbonyls. Zeitschrift für Naturforschung C, Journal of Biosciences. 1975;30:466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- Fellers CR, Redmon BC, Parrott EM. Effect of cranberries on urinary acidity and blood alkali reserve. The Journal of Nutrition. 1933;6:455–463. [Google Scholar]
- Femia FJ, Maresca KP, Hillier SM, Zimmerman CN, Joyal JL, Barrett JA, et al. Synthesis and evaluation of a series of 99mTc(CO)3+ lisinopril complexes for in vivo imaging of angiotensin-converting enzyme expression. Journal of Nuclear Medicine. 2008;49:970–977. doi: 10.2967/jnumed.107.049064. [DOI] [PubMed] [Google Scholar]
- Forman WB, Davidson ED, Webster LT., Jr Enzymatic conversion of salicylate to salicylurate. Molecular Pharmacology. 1971;7:247–259. [PubMed] [Google Scholar]
- Foxall PJ, Lenz EM, Lindon JC, Neild GH, Wilson ID, Nicholson JK. Nuclear magnetic resonance and high-performance liquid chromatography-nuclear magnetic resonance studies on the toxicity and metabolism of ifosfamide. Therapeutic Drug Monitoring. 1996;18:498–505. doi: 10.1097/00007691-199608000-00032. [DOI] [PubMed] [Google Scholar]
- Foxall PJ, Singer JM, Hartley JM, Neild GH, Lapsley M, Nicholson JK, et al. Urinary proton magnetic resonance studies of early ifosfamide-induced nephrotoxicity and encephalopathy. Clinical Cancer Research. 1997;3:1507–1518. [PubMed] [Google Scholar]
- Gatley SJ, Sherratt HSA. The localization of hippurate synthesis in the matrix of rat liver mitochondria. Biochemical Society Transactions. 1976;4:525–526. doi: 10.1042/bst0040525. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Sherratt HSA. The synthesis of hippurate from benzoate and glycine by rat liver mitochondria. Submitochondrial localization and kinetics. The Biochemical Journal. 1977;166:39–47. doi: 10.1042/bj1660039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W, Pang KS. Differences in excretion of hippurate, as a metabolite of benzoate and as an administered species, in the single-pass isolated perfused rat kidney explained. The Journal of Pharmacology and Experimental Therapeutics. 1999;288:597–606. [PubMed] [Google Scholar]
- Goldman N, Reed EJ, Fried LE, William Kuo IE, Maiti A. Synthesis of glycine-containing complexes in impacts of comets on early Earth. Nature Chemistry. 2010;2:949–954. doi: 10.1038/nchem.827. [DOI] [PubMed] [Google Scholar]
- Gonthier MP, Verny MA, Besson C, Remesy C, Scalbert A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. The Journal of Nutrition. 2003;133:1853–1859. doi: 10.1093/jn/133.6.1853. [DOI] [PubMed] [Google Scholar]
- Goodwin BL, Ruthven CR, Sandler M. Gut flora and the origin of some urinary aromatic phenolic compounds. Biochemical Pharmacology. 1994;47:2294–2297. doi: 10.1016/0006-2952(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Gopaul VS, Tang W, Farrell K, Abbott FS. Amino acid conjugates: metabolites of 2-propylpentanoic acid (valproic acid) in epileptic patients. Drug Metabolism and Disposition. 2003;31:114–121. doi: 10.1124/dmd.31.1.114. [DOI] [PubMed] [Google Scholar]
- Goren MP, Wright RK, Pratt CB, Pell FE. Dechloroethylation of ifosfamide and neurotoxicity. Lancet. 1986;2:1219–1220. doi: 10.1016/s0140-6736(86)92227-0. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Wang Y, Teichberg VI. Blood-mediated scavenging of cerebrospinal fluid glutamate. journal of Neurochemistry. 2003;87:119–126. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- Gregus Z, Fekete T, Halaszi E, Klaassen CD. Does hepatic ATP depletion impair glycine conjugation in vivo? Drug Metabolism and Disposition. 1996;24:1347–1354. [PubMed] [Google Scholar]
- Gregus Z, Fekete T, Varga F, Klaassen CD. Dependence of glycine conjugation on availability of glycine: role of the glycine cleavage system. Xenobiotica. 1993;23:141–153. doi: 10.3109/00498259309059370. [DOI] [PubMed] [Google Scholar]
- Gregus Z, Fekete T, Varga F, Klaassen CD. Effect of valproic acid on glycine conjugation of benzoic acid. The journal of Pharmacology and Experimental Therapeutics. 1993;267:1068–1075. [PubMed] [Google Scholar]
- Griffith WH. Benzoylated amino acids in the animal organism. I. The behavior of hippuric acid following its oral administration. The journal of Biological Chemistry. 1925;66:671–681. [Google Scholar]
- Griffith WH, Lewis HB. Studies in the synthesis of hippuric acid in the animal organism. V. The influence of amino acids and related substances on the synthesis and rate of elimination of hippuric acid after the administration of benzoate. The journal of Biological Chemistry. 1923;57:1–24. [Google Scholar]
- Hall DA, Ringel SP. Adult nonketotic hyperglycinemia (NKH) crisis presenting as severe chorea and encephalopathy. Movement Disorders. 2004;19:485–486. doi: 10.1002/mds.10681. [DOI] [PubMed] [Google Scholar]
- Hamosh A, McDonald JW, Valle D, Francomano CA, Niedermeyer E, Johnston MV. Dextromethorphan and high-dose benzoate therapy for nonketotic hyperglycinemia in an infant. The journal of Pediatrics. 1992;121:131–135. doi: 10.1016/s0022-3476(05)82559-4. [DOI] [PubMed] [Google Scholar]
- Hampson RK, Barron LL, Olson MS. Regulation of the glycine cleavage system in isolated rat liver mitochondria. The journal of Biological Chemistry. 1983;258:2993–2999. [PubMed] [Google Scholar]
- Hampson RK, Taylor MK, Olson MS. Regulation of the glycine cleavage system in the isolated perfused rat liver. The journal of Biological Chemistry. 1984;259:1180–1185. [PubMed] [Google Scholar]
- Hardy RW, Gaylor JL, Baumann CA. Biosynthesis of sterols and fatty acids as affected by nicotinic acid and related compounds. The journal of Nutrition. 1960;71:159–167. doi: 10.1093/jn/71.2.159. [DOI] [PubMed] [Google Scholar]
- Haslewood GA, Sjovall J. Comparative studies of bile salts. 8. Preliminary examination of bile salts by paper chromatography. The Biochemical journal. 1954;57:126–130. doi: 10.1042/bj0570126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel J, Kaiser R, Jornvall H. Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations. European journal of Biochemistry. 1985;153:13–28. doi: 10.1111/j.1432-1033.1985.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Barakat L, Wang D, Bordey A. Bergmann glial GlyTl mediates glycine uptake and release in mouse cerebellar slices. The journal of Physiology. 2004;560:721–736. doi: 10.1113/jphysiol.2004.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K, Takase S, Tsumuraya K, Endo M, Itahara K. Changes in free amino acids of cerebrospinal fluid and plasma in various neurological diseases. The Tohoku journal of Experimental Medicine. 1978;126:133–150. doi: 10.1620/tjem.126.133. [DOI] [PubMed] [Google Scholar]
- Inagawa K, Hiraoka T, Kohda T, Yamadera W, Takahashi M. Subjective effects of glycine ingestion before bedtime on sleep quality. Sleep and Biological Rhythms. 2006;4:75–77. [Google Scholar]
- IOM. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, D.C: National Academy Press; 1998. [PubMed] [Google Scholar]
- Jafarian-Tehrani M, Listwak S, Barrientos RM, Michaud A, Corvol P, Sternberg EM. Exclusion of angiotensin I-converting enzyme as a candidate gene involved in exudative inflammatory resistance in F344/N rats. Molecular Medicine. 2000;6:319–331. [PMC free article] [PubMed] [Google Scholar]
- Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, et al. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-alpha-lipoic acid. Investigative Ophthalmology & Visual Science. 2007;48:339–348. doi: 10.1167/iovs.06-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jois M, Hall B, Brosnan JT. Stimulation of glycine catabolism in isolated perfused rat liver by calcium mobilizing hormones and in isolated rat liver mitochondria by submicromolar concentrations of calcium. The journal of Biological Chemistry. 1990;265:1246–1248. [PubMed] [Google Scholar]
- Juge N, Hiasa M, Omoto H, Moriyama Y. Vesicular inhibitory amino acid transporter is a Cl-/gamma-aminobutyrate co-transporter. The journal of Biological Chemistry. 2009;284:35073–35078. doi: 10.1074/jbc.M109.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pfliigers Archiv. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- Kasuya F, Tatsuki T, Ohta M, Kawai Y, Igarashi K. Purification, characterization, and mass spectrometric sequencing of a medium chain acyl-CoA synthetase from mouse liver mitochondria and comparisons with the homologues of rat and bovine. Protein Expression and Purification. 2006;47:405–414. doi: 10.1016/j.pep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kasuya F, Yamaoka Y, Osawa E, Igarashi K, Fukui M. Difference of the liver and kidney in glycine conjugation of ortho-substituted benzoic acids. Chemico-Biological Interactions. 2000;125:39–50. doi: 10.1016/s0009-2797(99)00163-5. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Prescot AP, Ongur D, Evins AE, Barros TL, Medeiros CL, et al. Oral glycine administration increases brain glycine/creatine ratios in men: a proton magnetic resonance spectroscopy study. Psychiatry Research. 2009;173:143–149. doi: 10.1016/j.pscychresns.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley M, Vessey DA. Isolation and characterization of mitochondrial acyl-CoA: glycine N-acyltransferases from kidney. Journal of Biochemical Toxicology. 1993;8:63–69. doi: 10.1002/jbt.2570080203. [DOI] [PubMed] [Google Scholar]
- Kerdudo C, Orbach D, Sarradet JL, Doz F. Ifosfamide neurotoxicity: an atypical presentation with psychiatric manifestations. Pediatric Blood & Cancer. 2006;47:100–102. doi: 10.1002/pbc.20665. [DOI] [PubMed] [Google Scholar]
- Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proceedings of the japan Academy Series B, Physical and Biological Sciences. 2008;84:246–263. doi: 10.2183/pjab/84.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi H, Hayasaka K, Hiraga K, Kikuchi G. Reduction of the level of the glycine cleavage system in the rat liver resulting from administration of dipropylacetic acid: an experimental approach to hyperglycinemia. Archives of Biochemistry and Biophysics. 1979;198:589–597. doi: 10.1016/0003-9861(79)90535-6. [DOI] [PubMed] [Google Scholar]
- Koeller DM, DiGiulio KA, Angeloni SV, Dowler LL, Frerman FE, White RA, et al. Cloning, structure, and chromosome localization of the mouse glutaryl-CoA dehydrogenase gene. Genomics. 1995;28:508–512. doi: 10.1006/geno.1995.1182. [DOI] [PubMed] [Google Scholar]
- Korman SH, Wexler ID, Gutman A, Rolland MO, Kanno J, Kure S. Treatment from birth of nonketotic hyperglycinemia due to a novel GLDC mutation. Annals of Neurology. 2006;59:411–415. doi: 10.1002/ana.20759. [DOI] [PubMed] [Google Scholar]
- Kotler JM, Hinman NW, Yan B, Stoner DL, Scott JR. Glycine identification in natural jarosites using laser desorption Fourier transform mass spectrometry: implications for the search for life on Mars. Astrobiology. 2008;8:253–266. doi: 10.1089/ast.2006.0102. [DOI] [PubMed] [Google Scholar]
- Kreis H, Frese K, Wilmes G. Physiological and histological changes in rats fed benzoic acid. Food and Cosmetics Toxicology. 1967;5:505–511. doi: 10.1016/s0015-6264(67)83152-3. [DOI] [PubMed] [Google Scholar]
- Küpfer A, Aeschlimann C, Wermuth B, Cerny T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet. 1994;343:763–764. doi: 10.1016/s0140-6736(94)91839-2. [DOI] [PubMed] [Google Scholar]
- Kure S, Kojima K, Kudo T, Kanno K, Aoki Y, Suzuki Y, et al. Chromosomal localization, structure, single-nucleotide polymorphisms, and expression of the human H-protein gene of the glycine cleavage system (GCSH), a candidate gene for nonketotic hyperglycinemia. Journal of Human Genetics. 2001;46:378–384. doi: 10.1007/s100380170057. [DOI] [PubMed] [Google Scholar]
- Kure S, Koyata H, Kume A, Ishiguro Y, Hiraga K. The glycine cleavage system. The coupled expression of the glycine decarboxylase gene and the H-protein gene in the chicken. The journal of Biological Chemistry. 1991;266:3330–3334. [PubMed] [Google Scholar]
- Labrude P, Becq C. Pharmacist and chemist Henri Braconnot. Revue d'Histoire de la Pharmacie. 2003;51:61–78. [PubMed] [Google Scholar]
- Lauterburg BH, Nguyen T, Hartmann B, Junker E, Kupfer A, Cerny T. Depletion of total cysteine, glutathione, and homocysteine in plasma by ifosfamide/mesna therapy. Cancer Chemotherapy and Pharmacology. 1994;35:132–136. doi: 10.1007/BF00686635. [DOI] [PubMed] [Google Scholar]
- Lautermann E. XXV. Ueber die Reduction der Chinasaure zu Benzoësäure und die Verwandlung derselben in Hippursäure im thierischen Organismus. European journal of Organic Chemistry. 1863;125:9–13. [Google Scholar]
- Lawson M, Vasilaras A, De Vries A, Mactaggart P, Nicol D. Urological implications of cyclophosphamide and ifosfamide. Scandinavian journal of Urology and Nephrology. 2008;42:309–317. doi: 10.1080/00365590701570953. [DOI] [PubMed] [Google Scholar]
- Lees KR. Cerestat and other NMDA antagonists in ischemic stroke. Neurology. 1997;49:S66–S69. doi: 10.1212/wnl.49.5_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- Lerch S, Kupfer A, Idle JR, Lauterburg BH. Cerebral formation in situ of S-carboxymethylcysteine after ifosfamide administration to mice: a further clue to the mechanism of ifosfamide encephalopathy. Toxicology Letters. 2006;161:188–194. doi: 10.1016/j.toxlet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Levy G. Pharmacokinetics of salicylate elimination in man. Journal of Pharmaceutical Sciences. 1965;54:959–967. doi: 10.1002/jps.2600540703. [DOI] [PubMed] [Google Scholar]
- Li E, Patterson AD, Hofer CC, Krausz KW, Gonzalez FJ, Idle JR. Comparative metabolism of cyclophosphamide and ifosfamide in the mouse using UPLC-ESI-QTOFMS-based metabolomics. Biochemical Pharmacology. 2010;80:1063–1074. doi: 10.1016/j.bcp.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Chen KC, Hale J, Totten PA, Holmes KK. Two-dimensional thin-layer chromatography for the specific detection of hippurate hydrolysis by microorganisms. Journal of Clinical Microbiology. 1986;23:118–123. doi: 10.1128/jcm.23.1.118-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind MJ, McGown AT, Hadfield JA, Thatcher N, Crowther D, Fox BW. The effect of ifosfamide and its metabolites on intracellular glutathione levels in vitro and in vivo. Biochemical Pharmacology. 1989;38:1835–1840. doi: 10.1016/0006-2952(89)90419-x. [DOI] [PubMed] [Google Scholar]
- Lind MJ, Roberts HL, Thatcher N, Idle JR. The effect of route of administration and fractionation of dose on the metabolism of ifosfamide. Cancer Chemotherapy and Pharmacology. 1990;26:105–111. doi: 10.1007/BF02897254. [DOI] [PubMed] [Google Scholar]
- Liu YL, Tsai SH, Chang FW, Yu MH. Ifosfamide-induced encephalopathy in patients with uterine sarcoma. Taiwanese journal of Obstetrics & Gynecology. 2010;49:77–80. doi: 10.1016/S1028-4559(10)60014-9. [DOI] [PubMed] [Google Scholar]
- Luis PB, Ruiter JP, Ofman R, Ijlst L, Moedas M, Diogo L, et al. Valproic acid utilizes the isoleucine breakdown pathway for its complete beta-oxidation. Biochemical Pharmacology. 2011;82:1740–1746. doi: 10.1016/j.bcp.2011.07.103. [DOI] [PubMed] [Google Scholar]
- Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophrenia Research. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Makarovsky I, Markel G, Hoffman A, Schein O, Brosh-Nissimov T, Tashma Z, et al. Strychnine-a killer from the past. The Israel Medical Association journal. 2008;10:142–145. [PubMed] [Google Scholar]
- Marmet D, Bornstein N, Fleurette J. Detection of hippurate hydrolysis by Legionella spp. by using a rapid high-performance liquid chromatography method. Journal of Clinical Microbiology. 1990;28:2828–2829. doi: 10.1128/jcm.28.12.2828-2829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JB, Diffenderfer MR. Use of [15N]glycine in the measurement of apolipoprotein B synthesis in perfused rat liver. journal of Lipid Research. 1991;32:2019–2024. [PubMed] [Google Scholar]
- Mas N, Saiz A, Leite MI, Waters P, Baron M, Castano D, et al. Antiglycine-receptor encephalomyelitis with rigidity. Journal of Neurology, Neurosurgery, and Psychiatry. 2011;82:1399–1401. doi: 10.1136/jnnp.2010.229104. [DOI] [PubMed] [Google Scholar]
- Mawal YR, Qureshi IA. Purification to homogeneity of mitochondrial acyl coa:glycine n-acyltransferase from human liver. Biochemical and Biophysical Research Communications. 1994;205:1373–1379. doi: 10.1006/bbrc.1994.2817. [DOI] [PubMed] [Google Scholar]
- Mdluli K, Booth MP, Brady RL, Rumsby G. A preliminary account of the properties of recombinant human Glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochirnica et Bio-physica Acta. 2005;1753:209–216. doi: 10.1016/j.bbapap.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Meanwell CA, Blake AE, Kelly KA, Honigsberger L, Blackledge G. Prediction of ifosfamide/mesna associated encephalopathy. European journal of Cancer & Clinical Oncology. 1986;22:815–819. doi: 10.1016/0277-5379(86)90368-8. [DOI] [PubMed] [Google Scholar]
- Meanwell CA, Blake AE, Latief TN, Blackledge G, Mould JJ, Blake DR, et al. Encephalopathy associated with ifosfamide/mesna therapy. Lancet. 1985;i(8425):406–407. doi: 10.1016/s0140-6736(85)91432-1. [DOI] [PubMed] [Google Scholar]
- Merrill JM. Effect of nicotinic acid on the incorporation of radiocarbon into cholesterol. Circulation Research. 1958;6:482–484. doi: 10.1161/01.res.6.4.482. [DOI] [PubMed] [Google Scholar]
- Misiura K. Ifosfamide. Metabolic studies, new therapeutic approaches and new analogs. Mini Reviews in Medicinal Chemistry. 2006;6:395–400. doi: 10.2174/138955706776361385. [DOI] [PubMed] [Google Scholar]
- Mitchell DY, Petersen DR. Metabolism of the glutathione-acrolein adduct, S-(2-aldehydo-ethyl)glutathione, by rat liver alcohol and aldehyde dehydrogenase. The journal of Pharmacology and Experimental Therapeutics. 1989;251:193–198. [PubMed] [Google Scholar]
- Motrescu ER, Otto AM, Brischwein M, Zahler S, Wolf B. Dynamic analysis of metabolic effects of chloroacetaldehyde and cytochalasin B on tumor cells using bioelectronic sensor chips. journal of Cancer Research and Clinical Oncology. 2005;131:683–691. doi: 10.1007/s00432-005-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman G, Blanaru M, Bloch B, Kremer I, Ermilov M, Javitt DC, et al. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. The American journal of Psychiatry. 2005;162:1738–1740. doi: 10.1176/appi.ajp.162.9.1738. [DOI] [PubMed] [Google Scholar]
- Nelson LM. Fixed drug eruptions; a report of two cases, one caused by niacin, the other by cocaine. California Medicine. 1955;82:127–128. [PMC free article] [PubMed] [Google Scholar]
- Nissim I, Horyn O, Daikhin Y, Luhovyy B, Phillips PC, Yudkoff M. Ifosfamide-induced nephrotoxicity: mechanism and prevention. Cancer Research. 2006;66:7824–7831. doi: 10.1158/0008-5472.CAN-06-1043. [DOI] [PubMed] [Google Scholar]
- Norpoth K. Studies on the metabolism of isophosphamide (NSC-109724) in man. Cancer Treatment Reports. 1976;60:437–443. [PubMed] [Google Scholar]
- Osborne RJ, Slevin ML. Ifosfamide, mesna, and encephalopathy. Lancet. 1985;i(8442):1398–1399. [PubMed] [Google Scholar]
- Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Medicinal Research Reviews. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici GM, Mogavero S, Giuliani L, Rane A. Conjugation of benzoic acid with glycine in the human fetal and adult liver and kidney. Developmental Pharmacology and Therapeutics. 1991;17:52–62. doi: 10.1159/000457499. [DOI] [PubMed] [Google Scholar]
- Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflügers Archiv. 2004;447:689–709. doi: 10.1007/s00424-003-1099-7. [DOI] [PubMed] [Google Scholar]
- Patel DK, Ogunbona A, Notarianni LJ, Bennett PN. Depletion of plasma glycine and effect of glycine by mouth on salicylate metabolism during aspirin overdose. Human & Experimental Toxicology. 1990;9:389–395. doi: 10.1177/096032719000900606. [DOI] [PubMed] [Google Scholar]
- Patton AR. A comparison of the glycine contents of the proteins of normal and chondrodystrophic chick embryos at different stages of development. The journal of Nutrition. 1937;13:123–126. [Google Scholar]
- Pearlman BL, Gambhir R. Salicylate intoxication: a clinical review. Postgraduate Medicine. 2009;121:162–168. doi: 10.3810/pgm.2009.07.2041. [DOI] [PubMed] [Google Scholar]
- Pellicoro A, van den Heuvel FA, Geuken M, Moshage H, Jansen PL, Faber KN. Human and rat bile acid-CoA:amino acid N-acyltransferase are liver-specific peroxisomal enzymes: implications for intracellular bile salt transport. Hepatology. 2007;45:340–348. doi: 10.1002/hep.21528. [DOI] [PubMed] [Google Scholar]
- Pollay M. Movement of glycine across the blood-brain barrier of the rabbit. Journal of Neurobiology. 1976;7:123–128. doi: 10.1002/neu.480070205. [DOI] [PubMed] [Google Scholar]
- Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G. Physiological functions of D-amino acid oxidases: from yeast to humans. Cellular and Molecular Life Sciences. 2007;64:1373–1394. doi: 10.1007/s00018-007-6558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Pang KS. Benzoic acid glycine conjugation in the isolated perfused rat kidney. Drug Metabolism and Disposition. 1995;23:255–260. [PubMed] [Google Scholar]
- Porter RK. Mammalian mitochondrial inner membrane cationic and neutral amino acid carriers. Biochirnica et Biophysica Acta. 2000;1459:356–362. doi: 10.1016/s0005-2728(00)00172-9. [DOI] [PubMed] [Google Scholar]
- Quick AJ. The conjugation of benzoic acid in man. The journal of Biological Chemistry. 1931;92:65–85. [Google Scholar]
- Quick AJ. Clinical value of the test for hippuric acid in cases of disease of the liver. Archives of Internal Medicine. 1936;57:544–556. [Google Scholar]
- Ren S, Kalhorn TE, Slattery JT. Inhibition of human aldehyde dehydrogenase 1 by the 4-hydroxycyclophosphamide degradation product acrolein. Drug Metabolism and Disposition. 1999;27:133–137. [PubMed] [Google Scholar]
- Rossi S, Daniele I, Bastrenta P, Mastrangelo M, Lista G. Early myoclonic encephalopathy and nonketotic hyperglycinemia. Pediatric Neurology. 2009;41:371–374. doi: 10.1016/j.pediatrneurol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Aubrey KR, Supplisson S. The glycine transporter GlyT2 controls the dynamics of synaptic vesicle refilling in inhibitory spinal cord neurons. The journal of Neuroscience. 2008;28:9755–9768. doi: 10.1523/JNEUROSCI.0509-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan WL, Carver MJ. Free amino acids of human foetal and adult liver. Nature. 1966;212:292–293. doi: 10.1038/212292a0. [DOI] [PubMed] [Google Scholar]
- Ryan CW, Montag AG, Hosenpud JR, Samuels B, Hayden JB, Hung AY, et al. Histologic response of dose-intense chemotherapy with preoperative hypofractionated radiotherapy for patients with high-risk soft tissue sarcomas. Cancer. 2008;112:2432–2439. doi: 10.1002/cncr.23478. [DOI] [PubMed] [Google Scholar]
- Savica R, Rabinstein AA, Josephs KA. Ifosfamide associated myoclonus-encephalopathy syndrome. Journal of Neurology. 2011;258:1729–1731. doi: 10.1007/s00415-011-5990-4. [DOI] [PubMed] [Google Scholar]
- Schachter D, &Taggart JV. Glycine N-acylase: purification and properties. The journal of Biological Chemistry. 1954;208:263–275. [PubMed] [Google Scholar]
- Schon H. Effect of nicotinic acid on the cholesterol contents of rat-livers. Nature. 1958;182:534. doi: 10.1038/182534a0. [DOI] [PubMed] [Google Scholar]
- Shemin D, Rittenberg D. The utilization of glycine for the synthesis of a porphyrin. The journal of Biological Chemistry. 1945;159:567–568. [Google Scholar]
- Shoolingin-Jordan PM, Al-Daihan S, Alexeev D, Baxter RL, Bottomley SS, Kahari ID, et al. 5-Aminolevulinic acid synthase: mechanism, mutations and medicine. Biochirnica et Biophysica Acta. 2003;1647:361–366. doi: 10.1016/s1570-9639(03)00095-5. [DOI] [PubMed] [Google Scholar]
- Solaas K, Ulvestad A, Soreide O, Kase BF. Subcellular organization of bile acid amidation in human liver: a key issue in regulating the biosynthesis of bile salts. Journal of Lipid Research. 2000;41:1154–1162. [PubMed] [Google Scholar]
- Sood C, O'Brien PJ. 2-Chloroacetaldehyde-induced cerebral glutathione depletion and neurotoxicity. The British journal of Cancer. 1996;27(Supplement):S287–S293. [PMC free article] [PubMed] [Google Scholar]
- Stewart MJ, Malek K, Crabb DW. Distribution of messenger RNAs for aldehyde dehydrogenase 1, aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human tissues. Journal of Investigative Medicine. 1996;44:42–46. [PubMed] [Google Scholar]
- Suzuki Y, Kure S, Oota M, Hino H, Fukuda M. Nonketotic hyperglycinemia: proposal of a diagnostic and treatment strategy. Pediatric Neurology. 2010;43:221–224. doi: 10.1016/j.pediatrneurol.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Tajino T, Kikuchi S, Yamada H, Takeda A, Konno S. Ifosfamide encephalopathy associated with chemotherapy for musculoskeletal sarcomas: incidence, severity, and risk factors. Journal of Orthopaedic Science. 2010;15:104–111. doi: 10.1007/s00776-009-1431-y. [DOI] [PubMed] [Google Scholar]
- Toth E, Lajtha A. Elevation of cerebral levels of nonessential amino acids in vivo by administration of large doses. Neurochemical Research. 1981;6:1309–1317. doi: 10.1007/BF00964352. [DOI] [PubMed] [Google Scholar]
- van der Rest M, Fietzek PP. A comprehensive approach to the study of collagen primary structure based on high-performance liquid chromatography. European journal of Biochemistry. 1982;125:491–496. doi: 10.1111/j.1432-1033.1982.tb06709.x. [DOI] [PubMed] [Google Scholar]
- van der Westhuizen FH, Pretorius PJ, Erasmus E. The utilization of alanine, glutamic acid, and serine as amino acid substrates for glycine N-acyltransferase. Journal of Biochemical and Molecular Toxicology. 2000;14:102–109. doi: 10.1002/(sici)1099-0461(2000)14:2<102::aid-jbt6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- van Platerink CJ, Janssen HG, Haverkamp J. Development of an at-line method for the identification of angiotensin-I inhibiting peptides in protein hydro-lysates. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 2007;846:147–154. doi: 10.1016/j.jchromb.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Vandevijvere S, Andjelkovic M, De Wil M, Vinkx C, Huybrechts I, Van Loco J, et al. Estimate of intake of benzoic acid in the Belgian adult population. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment. 2009;26:958–968. doi: 10.1080/02652030902858939. [DOI] [PubMed] [Google Scholar]
- Vessey DA, Kelley M, Warren RS. Characterization of the CoA ligases of human liver mitochondria catalyzing the activation of short- and medium-chain fatty acids and xenobiotic carboxylic acids. Biochirnica et Biophysica Acta. 1999;1428:455–462. doi: 10.1016/s0304-4165(99)00088-4. [DOI] [PubMed] [Google Scholar]
- Vessey DA, Lau E, Kelley M, Warren RS. Isolation, sequencing, and expression of a cDNA for the HXM-A form of xenobiotic/medium-chain fatty acid: CoA ligase from human liver mitochondria. Journal of Biochemical and Molecular Toxicology. 2003;17:1–6. doi: 10.1002/jbt.10056. [DOI] [PubMed] [Google Scholar]
- Visarius TM, Bahler H, Kupfer A, Cerny T, Lauterburg BH. Thiodiglycolic acid is excreted by humans receiving ifosfamide and inhibits mitochondrial function in rats. Drug Metabolism and Disposition. 1998;26:193–196. [PubMed] [Google Scholar]
- Vree TB, Hekster YA, Anderson PG. Contribution of the human kidney to the metabolic clearance of drugs. The Annals of Pharmacotherapy. 1992;26:1421–1428. doi: 10.1177/106002809202601116. [DOI] [PubMed] [Google Scholar]
- Wong DF, Ostrowitzki S, Zhou Y, Raymont V, Hofmann C, Borroni E, et al. Characterization of [(ll)C]RO5013853, a novel PET tracer for the glycine transporter type 1 (GlyTl) in humans. Neurolmage. 2012 doi: 10.1016/j.neuroimage.2011.11.052. http://dx.doi.org/10.1016/j.neuroimage.2011.11.052. [DOI] [PubMed]
- Yamadera W, Inagawa K, Chiba S, Bannai M, Takahashi M, Nakayama K. Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep and Biological Rhythms. 2007;5:126–131. [Google Scholar]
- Yoshizumi T, Gondolesi GE, Bodian CA, Jeon H, Schwartz ME, Fishbein TM, et al. A simple new formula to assess liver weight. Transplantation Proceedings. 2003;35:1415–1420. doi: 10.1016/s0041-1345(03)00482-2. [DOI] [PubMed] [Google Scholar]
- Zitting A, Heinonen T. Decrease of reduced glutathione in isolated rat hepatocytes caused by acrolein, acrylonitrile, and the thermal degradation products of styrene copolymers. Toxicology. 1980;17:333–341. doi: 10.1016/0300-483x(80)90014-1. [DOI] [PubMed] [Google Scholar]