Abstract
Tumor angiogenesis plays an important role in the development of solid tumors, and targeting the tumor vasculature has emerged as a strategy to prevent growth and progression of solid tumors. In this study, we show that murine tumor vasculature express Rae1, a ligand for a stimulatory natural killer receptor NKG2D. By genetic modification of T cells with a NKG2D-based chimeric antigen receptor (CAR), referred to as chNKG2D in which the NKG2D receptor is fused to the signaling domain of CD3ζ chain, T cells were capable of targeting tumor vasculature leading to reduced tumor angiogenesis and tumor growth. This occurred, even in tumors where the tumor cells themselves did not express NKG2D ligands. H5V, an endothelial cell line, expresses Rae1 and was lysed by chNKG2D-bearing T cells in a perforin-dependent manner. In vitro capillary tube formation was inhibited by chNKG2D T cells through IFN-γ and cell-cell contact mechanisms. The in vivo anti-angiogenesis effects mediated by chNKG2D-bearing T cells at the tumor site were dependent on IFN-γ and perforin. These results provide a novel mechanism for NKG2D-based targeting of solid tumors.
Keywords: Cancer immunotherapy, NKG2D, Rae-1, chimeric antigen receptor, tumor angiogenesis, tumor microenvironment, endothelium
Introduction
Tumor-associated endothelial cells are one of the key determinants of the tumor microenvironment, and they play a critical role in tumor development and progression (1). In response to angiogenesis factors, such as VEGF, these endothelial cells form tumor-associated vasculature, providing nutrients to tumor cells and surrounding stroma (1, 2). At the same time, they form an interface between blood, tumor cells and extracellular matrix (1, 2).
The immune system can play a role in the inhibition of angiogenesis during tumorigenesis (3). A recent study has shown that combination therapy of active vaccination using autologous tumor cells expressing GM-CSF and antibody blockade of CTLA-4 led to the generation of antibodies against multiple angiogenic cytokines, such as VEGFs and angiopoietins, triggering disrupted tumor blood vessels in association with lymphocyte and granulocyte infiltrates in some long-term responding patients (4). Administration of Avastin (a humanized mAb that neutralizes VEGF-A) has been shown effective in promoting survival in colon cancer patients (5). Since VEGF mainly uses VEGFR-2 to mediate angiogenesis (2), several anti-tumor strategies targeting VEGFR-2 have been developed. Chinnasamy et al. designed a series of chimeric antigen receptors (CARs) consisting of a single chain anti-VEGFR-2 antibody linked to T cell intracellular signaling domains (6). After genetically modifying T cells with these CARs, the T cells had the ability to recognize tumor vessels that had high expression of VEGFR-2 (6). Adoptive transfer of VEGFR-2 CAR-modified T cells significantly inhibited tumor growth and prolonged survival and was accompanied by a persistent and increased T-cell infiltrate in several murine tumor models (6).
Natural killer (NK) cells are a first line of defense against infections and tumors through cytokine production and cytolytic activity. NK cell function is balanced by both inhibitory and activating receptors. One important activating receptor is NKG2D. The ligands for NKG2D receptor in mice include Rae-1, Mult-1, and H60, and in humans NKG2D binds to MICA/B and RAET1 proteins (also called UL-16 binding proteins), which are preferentially expressed on tumor cells but not on the surface of most normal tissues (7). In addition to tumor cells, some tumor-associated immunosuppressive cells, such as myeloid-derived suppressor cells (MDSCc) and regulatory T cells (Tregs), express NKG2D ligands (8-10). This suggests that the tumor microenvironment may play a role in inducing local Rae1 expression. Therefore, we hypothesized that blood vessels may also express a NKG2D ligand within the tumor microenvironment. Human umbilical cord vascular endothelial cells (HUVEC) express MICB, a human NKG2D ligand (11). In an autoimmune disease, Takayasu’s arteritis (TA), MICA is highly expressed in the aortic tissue accompanied by an infiltration of NKG2D+ cells, which are likely γδ T cells (12).
We have described a strategy to genetically modify T cells with a NK cell receptor-based CAR that consists of an NK activating receptor, NKG2D, linked to a gene encoding the cytoplasmic signaling domains of CD3ζ (13, 14). Murine or human T cells expressing such a chimeric receptor (chNKG2D) can kill NKG2D ligand-positive cells (including tumor cells, Tregs and MDSCs) and produce proinflammatory cytokines. In this study, we investigated NKG2D ligand expression in tumor vasculature in the MC-38 tumor model (colon cancer) and in the B16F10 (melanoma) tumor model. We demonstrate that tumor vessels express the NKG2D ligand Rae1 and investigated the mechanisms that NKG2D CAR T cells use to prevent tumor angiogenesis.
MATERIALS AND METHODS
Mice and cell lines
C57BL/6 (B6, wildtype, wt) were purchased from NCI (Frederick, MD). Interferon-γ deficient mice B6.129S7-Ifngtm1Ts/J (IFN-γ−/−) and perforin deficient mice C57BL/6-Prf1tm1Sdz/J (Pfp−/−) were obtained from the Jackson Laboratory (Bar Harbor, ME). Animals used in experiments were between 7 and 12 weeks of age. All experiments were conducted according to protocols approved by Dartmouth College’s Institutional Animal Care and Use Committee. B6-derived endothelial cell line H5V was obtained from Dr. José R. Conejo-Garcia (The Wistar Institute). Murine colon cancer MC-38 cells (H-2b) were obtained from Dr. Richard J. Barth (Dartmouth’s Geisel School of Medicine). Mouse melanoma cell line B16F10 has been described previously (9). B16F10 cells are NKG2D ligand-negative, whereas MC-38 cells are NKG2D ligand-positive. H5V cells were grown in Dulbecco’s modified Eagle medium (DMEM) with a high glucose concentration (4.5 g/liter) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM pyruvate, 10 mM Hepes, 0.1 mM non-essential amino acids and 50 μM 2-mercaptoethanol. All other cell lines were cultured in RPMI plus the same supplements as in DMEM.
Flow cytometry
For determination of Rae1 expression in tumor vasculature, established B16F10 and MC-38 tumors (~12mm in diameter) were excised, digested using a cocktail of DNAse and collagenase, according to the previously described protocol (15), and stained with APC-labeled anti-pan Rae1 (R&D Systems (Minneapolis, MN), PE-labeled anti-CD45 and FITC-labeled anti-CD31 mAbs (Biolegend). All samples were pre-incubated with FcR block antibodies (anti-mouse CD16/CD32, clone 93, eBioscience) to reduce non-specific staining. Cell fluorescence was monitored using an Accuri C6 cytometer (Ann Arbor, MI). Flow cytometry analysis was performed using either Accuri or FlowJo software (Ashland, OR).
In vitro drug treatment of H5V cells
Regulation of NKG2D ligand expression on H5V endothelial cells was examined in response to H2O2-induced oxidative stress. H5V cells (4×104) were plated in six-well plates in 2 ml complete media for 18-20 h before H2O2 treatment. Two hours before treatment, cells were given with fresh media. Either H2O2 (0.3mM) or PBS was added to culture for 48 h to mimic oxidative stress. For studies examining whether ATM (ataxia telangiectasia mutated) and ATR (ATM- and Rad3-related) protein kinase-mediated pathways were involved, caffeine (1mM, Sigma-Aldrich, St Louis, MO), an ATM and ATR inhibitor, or KU-55933 (10μM, Sigma-Aldrich), a specific inhibitor of ATM, were added to H5V cultures 2 h before H2O2 exposure.
Retroviral transduction of primary T cells
Retroviral transduction of murine primary T cells was performed using ecotropic retroviruses, as previously described (14, 16). After transduction and expansion, 80 to 90% of the T cells are CD8+ T cells and the remaining cells are CD4+ T cells. Because few murine CD4+ T cells express DAP10, the expression of NKG2D on the CD4+ T cells is low (14). The chNKG2D gene consists of the full length NKG2D fused with the cytoplasmic portion of CD3 ζ , while wild-type NKG2D (wtNKG2D) is the full length endogenous NKG2D gene. Both of these NKG2D proteins associate with DAP10 in the transmembrane, but only chNKG2D can induce a primary signal in T cells via the CD3ζ cytoplasmic domain. Thus, wtNKG2D T cells serve as control T cells for the transduction process, infusion of activated T cells, and bind to NKG2D ligands, but the wtNKG2D receptor only signals through Dap10 and does not induce a primary signal through CD3ζ. Genetically modified effector T cells (6–7 days post-transduction) were harvested, washed, and resuspended in cold HBSS before injection.
Cytotoxicity assays
Cytotoxicity of T cells was determined by a LDH release assay using the CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI). Specific lysis was determined by the following equation.
Tumor inoculation and T-cell treatment
B16F10 (5×105) tumor cells were injected s.c. into the shaved right flank of C57BL/6 mice. For treatment with T cells, wtNKG2D- or chNKG2D-modified T cells (2×106 cells) were administered intratumorally into mice 7 and 9 days post-tumor injection. Tumors were measured every two days using a caliper starting on day 7, and tumor areas calculated. To quantify tumor-associated vessels, the tumor-inoculated skin was separated from the underlying tissues, and only the vessels directly supplying the tumor were counted.
In vitro tube formation assay
The in vitro tube formation by mouse endothelial H5V cells was determined using an in vitro angiogenesis assay kit (Millipore, Billerica, MA). In brief, pre-chilled (4°C) 48-well tissue culture plates were coated with growth factor–reduced Matrigel (100μL/well; Becton Dickinson, Bedford, MA) and were incubated at 37°C for 1 h to allow the Matrigel to solidify. In control wells, H5V cells (4×104/well) were suspended in 300 μl complete DMEM and gently added to the Matrigel-coated wells. Conditional media (CM) from activated T cells were collected as supernatants from overnight culture of either wtNKG2D- or chNKG2D-modified T cells (106) in anti-NKG2D mAb (4μg/ml)-coated 24-well non-tissue culture-treated plates. To determine whether soluble factors from activated T cells affected H5V tumor formation, CM at different dilutions was added to the H5V cultures in a total volume of 300μl. Similarly, T cells were mixed with H5V cells at ratios ranging from 0.1:1 to 1:1 before addition to Matrigel-coated wells to determine the effects of T cells on H5V cell tube formation. After 6 hours, media was removed, and cells were fixed with cold PBS-buffered 2% paraformaldehyde. Images were captured under phase contrast microscopy at 40x magnifications using a Dino-Eye eyepiece digital camera (Microsope.com, Roanoke, VA).
In vivo angiogenesis assay
Growth factor–reduced Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) mixed with an equal volume of B16F10 cells in a final volume of 500 μl was injected s.c. into wild-type C57BL/6 mice. T cells (2×106, either wtNKG2D- or chNKG2D-modified T cells in 100μl of HBSS) were inoculated into Matrigel plugs on days 5 and 7 post-implantation. Two days after the final T cell injection, Matrigel plugs were isolated and hemoglobin content determined using Drabkin’s reagent (D5941, Sigma-Aldrich, M O ) according to the manufacturer’s instruction. Experiments were done twice.
Isolation of tumor-derived endothelial cells and MDSCs
Established B16F10 and MC-38 tumors (~12mm in diameter) were excised, digested using cocktails of DNAse and collagenase, according to the previously described protocol (15). The liberated cells were filtered through 70μm nylon mesh (BD Falcon, Bedford, MA), followed by a density gradient centrifugation over Histopaque-1083 (Sigma) to remove dead cells. CD45+ cells were depleted from the cell samples using magnetic cell separation (MACS) with anti-CD45 antibodies and LS columns (Miltenyi Biotec). The negative fraction was collected and purified using CD31+ magnetic beads (Miltenyi Biotec). The purity of CD45−CD31+ cells was >85%. Tumor-derived MDSCs were sorted using MACS columns with anti-F4/80 mAbs. The purity of F4/80+CD11b+ cells was >95%.
Cytokine production by T cells
To determine whether chNKG2D T cells responded to tumor-derived endothelial cells, T cells (105) were co-cultured with purified CD31+ cells (104) in 96-well plates for 24 h. Cell-free supernatants were assayed for IFN-γ by ELISA using Duoset ELISA kits (R&D systems).
Statistical analysis
Differences between groups were analyzed using a Student’s t-test or ANOVA. P values < 0.05 were considered significant.
Results
Treatment with chimeric NKG2D T cells inhibits subcutaneous NKG2D ligand-negative B16F10 tumor growth and tumor-induced angiogenesis
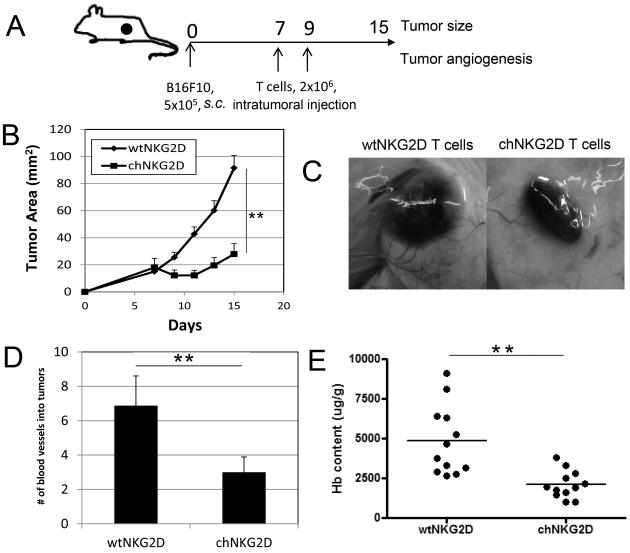
To determine whether the treatment with chNKG2D T cells has any effects on tumor angiogenesis, we chose the mouse B16F10 melanoma model because this tumor cell does not express NKG2D ligands either in vitro or in vivo (9), so we could evaluate the non-tumor targeted mechanisms of chNKG2D T cells in vivo. T-cell transfer was performed intratumorally (on days 7 and 9 post-tumor inoculation) to minimize systemic effects of T cell infusion (Fig. 1A). As shown in Fig. 1B, intratumoral injection of chNKG2D T cells significantly inhibited B16F10 tumor growth (p<0.01 from day 9 to day 15). In addition, we observed a reduced number of vessels around the tumor mass in the chNKG2D T cell-treated group, as shown in Fig. 1C & D. To further confirm the results, we performed an in vivo Matrigel assay. Intratumoral injection of chNKG2D T cells significantly reduced the hemoglobin content in the Matrigel compared to wtNKG2D T cells (Fig. 1E). Although the full length NKG2D receptor transduced into wtNKG2D T cells can recognize ligands similarly to the chNKG2D receptor, NKG2D alone does not induce a primary activation signal nor trigger effector functions in T cells. These results suggest that chNKG2D T cell-treatment inhibited tumor-induced angiogenesis when given at the early stage of tumor growth.
Figure 1. Intratumoral injection of chNKG2D T cells inhibits the growth of B16F10 tumors and tumor-associated angiogenesis.
A schematic diagram of tumor inoculation and treatment is shown in (A). B16F10 tumor cells (5×105) were given subcutaneously to B6 mice. Either wtNKG2D- or chNKG2D-modified T cells (2×106) were intratumorally injected on days 7 and 9 post-tumor inoculations. (B) Tumor diameters were measured every 2 days starting 7 days post-tumor injection (n = 10 mice per group). The tumor areas are represented as Mean + SEM. ** p<0.01 (from day 9 to 15). (C) The tumor-inoculated sites in wtNKG2D T cell- or chNKG2D T cell–treated mice on day 15. (D) Tumor-inoculated sites were isolated from wtNKG2D T cell- or chNKG2D T cell–treated mice on day 15 and tumor-supplying vessels were counted. Data are represented as the mean + SEM of 10 mice in each group pooled from 2 independent experiments. ** p<0.01 (E) Matrigel (250 μl) was mixed with an equal volume of B16F10 cells (5×105) before subcutaneous injection into the flanks of B6 mice. Either wtNKG2D or chNKG2D T cells (2×106) were injected in matrigel plugs 5 and 7 days after Matrigel implantation. On day 9, Matrigel plugs were isolated and the hemoglobin content was determined, as a measure of the degree of vascularization of the Matrigel explants. Results are pooled data from two independent experiments. ** p<0.01
CD31+ endothelial cells isolated from B16F10 and MC-38 tumors express Rae-1 and can stimulate NKG2D CAR T cells to produce IFN-γ
Because B16F10 cells do not express NKG2D ligands both in vivo and in vitro, the data indicated that tumor-associated cells, rather than tumor cells themselves, were targeted by NKG2D CAR T cells. Previous studies have shown that tumor infiltrating cells, such as Tregs and MDSCs, can express NKG2D ligands in tumors (7, 8, 10). These findings prompted us to hypothesize that the tumor microenvironment may be involved in the induction of NKG2D ligands and the tumor vasculature may also express NKG2D ligands. Therefore, we analyzed samples from well-established B16F10 tumors (diameters ~12 mm) for the expression of NKG2D ligands. Tumor-derived, but not normal tissue (heart endothelium)-derived CD31+ cells, expressed Rae-1, an NKG2D ligand (Fig. 2A, 2C). The result was confirmed in the mouse MC-38 colon cancer model. MC-38 tumor-derived CD31+ endothelial cells also expressed Rae1 (Fig. 2B). To determine whether chNKG2D T cells recognized these tumor vasculature cells, CD31+ cells were purified from either B16F10 or MC-38 tumors and co-cultured with chNKG2D T cells. As shown in Fig. 2D, chNKG2D T cells produced significant amounts of IFN-γ compared to wtNKG2D T cells when co-cultured with tumor-derived endothelial cells. To assess Rae1 expression on other cells within these tumors, we determined Rae1 expression on infiltrating leukocytes (CD45+, CD31−) and non-endothelium tumor and stromal cells (CD45−CD31−) (see Supplemental Fig. 1). These data show that the leukocytes had no or very low expression, while the non-leukocytes had no expression of Rae1.
Figure 2. Tumor vasculature expresses Rae1 and can stimulate chNKG2D T cells to produce IFN-γ.
Large (~12mm diameter) B16F10 tumors (A) or MC-38 tumors (B) were dissected and digested with collagenase and DNAse I. Viable cells were collected from interfaces by Histopaque-density gradient centrifugation. Cells were stained with anti-CD45-PE, anti-CD31-FITC, and anti-Rae1-APC mAbs (or isotype controls). Rae1 expression on tumor vessels (CD45−CD31+) is shown in histograms. The results shown are representative one of 4 independent experiments. Isotype control is shown in dashed lines, and anti-Rae1 staining is shown in solid lines. (C) Primary endothelial cells derived from B6 heart endothelial and aortas were isolated by digestion with collagenase and DNAse I, followed by density gradient. Cells were stained with anti-CD31-FITC mAbs and NKG2D-IgG-APC (or isotype controls). The results shown are representative one of 2 independent experiments. The isotype control is shown as a dashed line. NKG2D-IgG-APC staining is shown as a solid line. (D) B16F10 and MC-38 tumor-associated endothelial cells (CD31+) were isolated using MACS sorting. Endothelial cells (104) were co-cultured with either wtNKG2D (grey bar) or chNKG2D T cells (black bar) in 96-well plates for 24h. Endothelial cells alone (unfilled) were used as negative controls. The results shown are representative from three independent experiments and are shown as mean + SD. ** p<0.01
The ATM/ATR pathway is involved in the up-regulation of Rae1 on endothelial cells
DNA damage by genotoxic stress can activate a pathway initiated by ATM (ataxia telangiectasia mutated) and ATR (ATM- and RAD3-related), which has been shown to play a critical role in NKG2D ligand induction (17). Within tumor microenvironments, the ATM/ATR pathway is often activated due to hypoxia and reoxygenation in solid tumors (18-20). To determine whether this pathway was involved in the regulation of Rae1 expression on tumor-associated endothelial cells, a B6-derived endothelial cell line H5V was used. H5V cells are derived from the heart tissue of B6 mice and have been immortalized by expression of the large T antigen of the SV40 virus (21). As shown in Fig. 3A, H5V cells expressed Rae1. To mimic oxidative stress, H5V cells were treated with 0.3mM H2O2 in the presence or absence of ATM/ATR pathway inhibitors, caffeine and KU55933 for 48 h. As shown in Fig. 3A & 3B, H2O2 significantly increased Rae1 expression on H5V cells. Caffeine and KU55933 completely blocked H2O2-induced up-regulation of Rae1 expression, suggesting that ATM/ATR pathway was regulating Rae1 expression on endothelial cells.
Figure 3. Oxidative stress induces the expression of Rae1 on mouse endothelial cells.
(A) B6 mouse-derived endothelial cell line H5V cells were treated with 0.3 mM H2O2 or PBS for 48 h. Rae1 and CD31 cell surface expression were assessed by flow cytometry. Shaded histograms represent isotype controls, solid-line histograms represent PBS-treated cells, and dashed-line histograms represent H2O2-treated cells. Histograms are representative of at least 3 independent experiments. (B) H5V cells were incubated with 0.3 mM H2O2 in the presence of either 1mM caffeine (an inhibitor of ATM and ATR) or 10μM KU55933 (an ATM inhibitor) for 48 h. DMSO (0.02%) was used as a vehicle control. Surface expression of Rae1 and CD31 was analyzed by flow cytometry. The relative MFI values of Rae1 and CD31 expression were set as 100 in the vehicle control group. The results shown are pooled data from 3 independent experiments, and they are represented as mean + SD. * p<0.05, compared with DMSO controls or H2O2 plus ATM/ATR inhibitors. (C) ChNKG2D T cells use perforin to kill Rae1-positive H5V in vitro. Effector T cells derived from WT B6 or Pfp−/− mice that were modified with either wtNKG2D (open bars) or chNKG2D (filled bars) were cocultured with H5V cells at an E:T ratio of 5:1 in 5-hr LDH release assays. The data are presented as mean + SD of triplicates and are representative from 2 independent experiments. ** p<0.01
Both IFN-γ and perforin are important in NKG2D CAR T cell-mediated inhibition of in vitro angiogenesis
Next, we determined whether NKG2D CAR T cells recognized and responded to H5V cells. After overnight co-culture of H5V cells with chNKG2D-modified T cells, significantly higher IFN-γ was produced as compared co-culture with wtNKG2D T cells. In addition, chNKG2D T cells efficiently lysed H5V cells (Fig 3C). To determine whether perforin was involved in chNKG2D T cell-mediated killing of H5V cells, T cells generated from perforin deficient (Pfp−/−) mice were used. Pfp−/− chNKG2D T cells were unable to kill H5V cells, indicating its critical role in the killing process.
Similar to HUVEC cells, H5V cells can also form tube-like structures on extracellular matrix (Matrigel) and can be used to evaluate in vitro angiogenesis. Since chNKG2D T cells directly responded to H5V cells, we reasoned that in vitro tube formation by H5V cells may be disrupted by interaction with chNKG2D T cells. First, the effects of soluble factors from activated chNKG2D T cells were determined by mixing diluted T cell-derived conditional media (CM) with H5V cells before plating on Matrigel. T cell CM was prepared from chNKG2D or wtNKG2D T cells after cross-linking with plate-bound anti-NKG2D mAbs. Compared with control media or wtNKG2D T cell-derived CM, the CM from activated chNKG2D T cells significantly inhibited H5V tube formation in a dose-dependent manner (Fig. 4A-C). Co-culture of chNKG2D T cells and H5V cells at a low cell ratio (1:10) significantly reduces H5V tube formation (Fig 4D). To understand the molecular mechanisms involved, the effects of chNKG2D T cells to mediate anti-angiogenesis were determined using T cells derived from IFN-γ−/− or perforin–deficient (Pfp−/−) mice, respectively. As shown in Fig. 5, the CM from IFN-γ−/− chNKG2D T cells had a reduced ability to inhibit in vitro H5V tube formation. Furthermore, in vitro culture of chNKG2D T cells with H5V cells resulted in significant IFN-γ production, while co-culture of wtNKG2D T cells with H5V cells produced little IFN-γ(Fig. 5D). Less disruption of H5V tube formation was also observed when Pfp−/− chNKG2D T cells were used. The in vitro angiogenesis assays indicated that both IFN-γ and perforin were involved in chNKG2D T cell-mediated inhibition of endothelial cell tube formation.
Figure 4. ChNKG2D T cells inhibit H5V tube formation through both cell contact and soluble factors.
(A) H5V cells (4 × 104) were cultured on Matrigel-coated plates in the presence of 1:2 diluted condition media (CM) derived from immobilized anti-NKG2D mAb-stimulated T cells (either wtNKG2D or chNKG2D T cells). H5V cells alone were used as positive controls. The images shown are representative of 3 independent experiments. (B) H5V cells (4 × 104) were co-cultured with equal numbers of T cells (either wtNKG2D T cells or chNKG2D T cells) on Matrigel-coated plates for 6 hr. The images shown are representative of 2 independent experiments. (C & D) Scoring of H5V tube formation after addition of CM (C) or co-culture with T cells (D). The results shown are representative from two or three independent experiments, and they are shown as mean + SD. ** p<0.01, compared with the treatment with B6 wtNKG2D T cells (D) or its CM (C).
Figure 5. Perforin and IFN-γ are involved in chNKG2D T cell-mediated inhibition of H5V tube formation.
(A) H5V cells (4 × 104) were co-cultured with either T cells (wtNKG2D, chNKG2D, or Pfp−/− chNKG2D T cells at T: H5V ratios 1:1) or diluted CM (1:4) derived from immobilized anti-NKG2D mAb-stimulated T cells on Matrigel-coated plates for 6 hr. Representative images of H5V tube formation are shown. (B) H5V cells were co-cultured with either wtNKG2D T cells (◇), chNKG2D T cells (▲), or Pfp−/− chNKG2D T cells (■) at T: H5V ratios from 0.1:1 to 1:1. H5V cells alone were used as controls. H5V tube formation was scored and set to 100 for H5V cells alone. The mean value of H5V tube formation in the absence of T cells is set to 100. Cumulative data from two independent experiments are shown as mean + SD. ** p<0.01, compared with wtNKG2D T cells; # p<0.01, compared with chNKG2D T cells. (C) H5V cells were cultured in the presence of diluted CM (1:4) on Matrigel-coated plates. The mean value of tube formation of H5V cells in the absence of T cells was set to 100. Data from two independent experiments are shown as mean + SD. ** p<0.01 (D) ChNKG2D T cells (105) or wtNKG2D T cells were cultured with H5V cells (4:1, filled bars) or with media alone (open bars), and IFN-g production was measured after 24h cultures by ELISA. Data are shown as IFN-γ(pg/ml) + SD, and they are representative of three experiments.
Inhibition of B16F10 tumor growth and angiogenesis by NKG2D CAR T-cell treatment are IFN-γ and perforin-dependent
IFN-γ has been shown to inhibit tumor angiogenesis (22). One mechanism of IFN-γ-mediated anti-angiogenesis is induction of two potent anti-angiogenesis CXC chemokines, CXCL9 and CXCL10 (23). In a colon cancer (MC-38) lung metastasis model, treatment of tumor-bearing mice with chNKG2D T cells led to elevation of IFN-γ in serum (32-470 pg/ml) and prolonged survival (Supplemental Fig. 2).
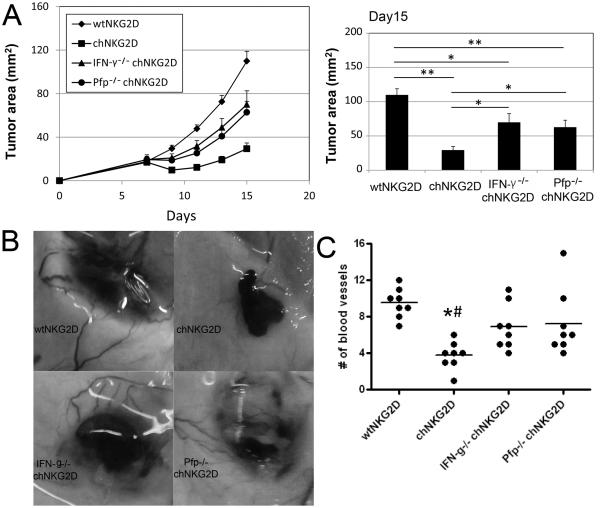
To determine the extent to which IFN-γ from chNKG2D T cells was involved in their in vivo anti-tumor effects, we used T cells derived from IFN-γ-deficient mice (IFN-γ−/−). As shown in Fig. 6, intratumoral administration of IFN-γ-deficient chNKG2D T cells into B16F10 tumors had an impaired ability to reduce tumor burden and angiogenesis as demonstrated by tumor growth and the numbers of blood vessels growing into tumors compared with chNKG2D T cells generated from WT mice, indicating the critical role for IFN-γ production by these transferred T cells in vivo. Thus, the production of cytokines was critical for the anti-tumor responses mediated by chNKG2D T cells in vivo. Considering the critical roles of perforin in chNKG2D T cell-mediated in vitro cytotoxicity against H5V cells, we tested whether perforin was also involved in chNKG2D T cell-mediated therapeutic efficacy in vivo. After intratumoral injection, Pfp−/− chNKG2D T cells resulted in a reduced capacity reduce tumor burden and angiogenesis (Fig. 6A), indicating that perforin was also required for in vivo therapeutic effects.
Figure 6. IFN-γ and perforin are involved in chNKG2D T cell-mediated anti-angiogenesis in vivo.
(A) Tumor-bearing mice that were inoculated s.c. with 5×105 B16F10 cells on day 0 were treated with two doses of chNKG2D T cells (2×106) derived from either WT B6 (■), IFN-γ−/−(▲) and Pfp−/−(•) mice on days 7 and 9. WtNKG2D transduced T cells (◇) from B6 mice were used as negative controls. Tumor diameters were measured every 2 days starting 7 days post-tumor injection. The results shown are pooled data from 2 independent experiments. The tumor areas are represented as Mean + SEM. (B) Representative images of tumor-inoculated sites in T cell–treated mice on day 15. (C) Tumor-inoculated sites were isolated from T cell–treated mice at day 15 and tumor-supplying vessels were counted. Data are shown as the individual values of 8 mice in each group, pooled from 2 independent experiments.
Discussion
Angiogenesis is an important hallmark of solid tumors and plays very important role in tumor development and metastasis (2). Anti-angiogenesis drugs, such as Avastin (anti-VEGF mAb), can significantly promote survival in some cancer patients (2). Besides antibodies, small molecule inhibitors that target tyrosine kinase receptors have also been used for anti-angiogenesis therapy. These tyrosine kinase targets include VEGF-R, FGFR, PDGFR, and Tie-2 that are known to play crucial roles in the angiogenesis in tumors (2, 24).
Adoptive cell therapy (ACT) with tumor antigen-specific T cells is a promising strategy to treat cancer and evidence suggests that T-cell immunity can be used to control tumor growth (25, 26). However, clinical efficacy of ACT is often negatively affected by an immunosuppressive tumor microenvironment (27-29). Aberrant tumor vasculature and limited expression of chemokines and adhesion molecules can lead to poor penetration of effector T cells into tumor stroma (27-30). In addition, effector functions of tumor-specific T cells can be impaired by immunosuppressive molecules, such as VEGF, TGF-β and IL-10, and interaction with suppressor cells (i.e., MDSCs and Tregs) (31-33). Hypoxia-HIF1α-VEGF axis plays critical roles in creating and maintaining the immunosuppressive tumor microenvironment (1). Combination therapy of anti-VEGF mAbs with ACT increases T cell-infiltration into tumors and can improve therapeutic efficacy (34). Active immunization with a soluble VEGF-R2-pulsed dendritic cell vaccine can elicit VEGF-R2-specific neutralizing antibodies as well as CD8+ cytotoxic T cell responses and break tolerance to VEGF-R2, leading to reduced tumor angiogenesis and growth in tumor-challenged mice (35). These results suggest that combination of anti-angiogenic agents with ACT may be a promising approach as cancer treatment. For conventional tumor-specific T cells to mediate anti-tumor efficacy in vivo, these cells must extravasate into the tumor sites. However, abnormal tumor vasculature with a disorganized structure and high interstitial fluid pressure remains a significant barrier for effector T cells to infiltrate tumor stroma and to mediate tumor destruction. ChNKG2D T cells may have significant advantages over conventional T cells due to their potential ability to mediate tumor disruption without penetrating into tumors through recognition of NKG2D ligands on the tumor vasculature.
Expression of NKG2D ligands is not restricted to tumor cells. Tumor-associated immunosuppressors including MDSCs and Tregs can also express NKG2D ligands and be targeted by NKG2D-based strategies (8, 36). In addition, MDSCs and Tregs have been shown to promote tumor angiogenesis (37, 38). In vitro, MDSCs can be targeted by chNKG2D T cells, leading to reduced production of a pro-angiogenic factor VEGF (Supplemental Fig. 3). A new finding in this study is that tumor vessels express NKG2D ligands, and therefore, it may be possible to target the tumor vasculature with NKG2D CAR T cells. Because NKG2D CAR T cells can recognize all cells that express NKG2D ligands (e.g. tumor cells, tumor endothelium, and immunosuppressive cells), it is likely that the CAR T cells are targeting all of these cells to some extent. Thus, NKG2D CAR T cells can directly inhibit tumor angiogenesis through recognition of ligands on tumor endothelium and indirectly through production of cytokines within the local microenvironment and by attacking ligand-expressing tumor cells and immunosuppressive cells that promote angiogenesis.
DNA pathways initiated by ATM (ataxia telangiectasia, mutated) or ATR (ATM- and Rad3-related) protein kinases have been shown to play important roles in up-regulation of NKG2D ligands (17). Hammond et al. showed that hypoxia and reperfusion in solid tumors activates the ATR/ATM pathway via inducing DNA damage (19, 39). Our results demonstrate that ATM/ATR pathway inhibitors KU55933 and caffeine inhibited H2O2-induced Rae1 up-regulation, indicating a possible role of the ATM/ATR pathway in NKG2D ligand expression on tumor blood vessels. In addition, Hamerman et al. showed that Rae1 expression on macrophages can be induced by LPS, suggesting that NF-κB pathway is involved in Rae1 regulation (40). It has been shown that the NF-κB signaling pathway is important in cancer-related inflammation and malignant progression (41, 42). Therefore, it is possible that induction of Rae1 expression is related to the damage and inflammation within the tumor microenvironment.
NK cells have shown to inhibit tumor angiogenesis, especially during IL-12 treatment (43, 44). In a xenograft mouse model, local treatment with IL-12 induced tumor necrosis, vascular damage and NK cell infiltration surrounding small vessels (43). In addition, depletion of NK cells during IL-12 treatment reduced IL-12-mediated anti-angiogenesis.
T cells can use multiple effector molecules. Previous results have shown that chNKG2D-derived IFN-γ, GM-CSF as well as cytotoxic mechanisms (perforin and FasL) are critical for their anti-tumor therapeutic efficacy (16, 45). IFN-γ is well-known to inhibit tumor angiogenesis. The mechanisms include reducing activation of a pro-angiogenesis adhesion molecule αVβ3 and inhibiting VEGF production via a post-transcriptional pathway (46, 47). IFN-γ also induces the production of two potent anti-angiogenic factors, CXCL9 and CXCL10 (23, 48). Using murine lymphoma and ovarian cancer models, we have determined that IFN-γ plays critical roles in chNKG2D T cell-meditated therapeutic efficacy. In this study, reduced anti-angiogenesis effects were observed both in vitro and in vivo when IFN-γ−/− chNKG2D T cells were used, indicating a critical role for IFN-γ in chNKG2D T cell-mediated anti-angiogenesis. Under some pathological conditions, such as cerebral malaria, acute coronary syndromes, Takayasu’s arteritis and transplant vascular diseases, perforin and granzymes B have been shown to play critical roles in mediating endothelial damage (49-52). The role of NKG2D ligands, if any, in these conditions of endothelial damage is unknown. There is a potential concern that chNKG2D T cells may interfere with normal angiogenesis. However, we did not detect NKG2D ligand expression on normal vasculature. In addition, we have not observed any significant side effects after i.v. or i.p. injection chNKG2D T cells in mice at therapeutic effective doses. As discussed above, up-regulation of NKG2D ligands on tumor vasculature is likely due to the conditions within the tumor microenvironment.
Taken together, there are several pathways by which chNKG2D T cells can target a tumor and its microenvironment besides a direct attack of NKG2D ligand-positive tumor cells (Fig. 7). First, chNKG2D T cells may directly target tumor vasculature due to their up-regulation of NKG2D ligand expression. The death of endothelial cells may directly cause intravascular blood coagulation, thus reducing the local blood flow to the tumor (53). Perivascular T-cell infiltration may increase after endothelial cell damage, resulting in enhanced tumor destruction (53-55). Second, angiogenesis-promoting cells, such as MDSCs and Tregs, may express NKG2D ligands within tumor microenvironment, and therefore, become targets of chNKG2D T cells (Fig. 7). Third, anti-angiogenesis factors (i.e., IFN-γ, CXCL9 and CXCL10) generated during the interaction between chNKG2D T cells and tumors (both tumor and stromal cells) may further inhibit tumor angiogenesis (56). In summary, the data from this study provides a novel mechanism for the efficacy of chNKG2D transduced T cells as an effective treatment for cancer.
Figure 7. Summary of mechanisms of chNKG2D T cell-mediated anti-tumor angiogenesis.
Inside the tumor, chNKG2D T cells can directly target NKG2D ligand-positive tumor cells, tumor vasculature as well as angiogenesis-promoting MDSC and Treg, leading to less angiogenesis and reduced tumor growth. Both chNKG2D T cell-derived IFN-γ and perforin are involved in the inhibition. See text for details.
Supplementary Material
Acknowledgements
We thank the NCI Biological Resource Branch for providing recombinant human IL-2 and the staff of the Dartmouth College Center for Comparative Medicine and Research for assistance with animal care.
Footnotes
This work was supported in part by grants from the Hitchcock Foundation (250-4032), NIH (CA130911, P20RR16437 COBRE and T32AR007576), and support from the Department of Microbiology & Immunology and Norris Cotton Cancer Center.
REFERENCES
- 1.Chouaib S, Kieda C, Benlalam H, Noman MZ, Mami-Chouaib F, Ruegg C. Endothelial cells as key determinants of the tumor microenvironment: interaction with tumor cells, extracellular matrix and immune killer cells. Crit Rev Immunol. 30:529–545. doi: 10.1615/critrevimmunol.v30.i6.30. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A, Fan AC, Yang Q, Braunstein L, Crosby E, Ryeom S, Felsher DW. CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation. Cancer Cell. 18:485–498. doi: 10.1016/j.ccr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenfeld J, Jinushi M, Nakazaki Y, Wiener D, Park J, Soiffer R, Neuberg D, Mihm M, Hodi FS, Dranoff G. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 70:10150–10160. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Los M, Roodhart JM, Voest EE. Target practice: lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist. 2007;12:443–450. doi: 10.1634/theoncologist.12-4-443. [DOI] [PubMed] [Google Scholar]
- 6.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, Feldman SA, Restifo NP, Rosenberg SA. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 8.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang T, Sentman CL. Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res. 2011;71:2066–2076. doi: 10.1158/0008-5472.CAN-10-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–6947. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern-Ginossar N, Elefant N, Zimmermann A, Wolf DG, Saleh N, Biton M, Horwitz E, Prokocimer Z, Prichard M, Hahn G, Goldman-Wohl D, Greenfield C, Yagel S, Hengel H, Altuvia Y, Margalit H, Mandelboim O. Host Immune System Gene Targeting by a Viral miRNA. Science. 2007;317:376–381. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: A 2011 update. Autoimmun Rev. doi: 10.1016/j.autrev.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–5933. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson M, Meadows SK, Basu S, Mselle TF, Wira CR, Sentman CL. TLRs mediate IFN-gamma production by human uterine NK cells in endometrium. J Immunol. 2006;176:6219–6224. doi: 10.4049/jimmunol.176.10.6219. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–11036. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 17.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ. Hypoxia links ATR and p53 through replication arrest. Mol Cell Biol. 2002;22:1834–1843. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- 20.Hammond EM, Dorie MJ, Giaccia AJ. Inhibition of ATR leads to increased sensitivity to hypoxia/reoxygenation. Cancer Res. 2004;64:6556–6562. doi: 10.1158/0008-5472.CAN-04-1520. [DOI] [PubMed] [Google Scholar]
- 21.Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Carozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci U S A. 1994;91:7291–7295. doi: 10.1073/pnas.91.15.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 23.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 24.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 25.Rossig C, Brenner MK. Genetic modification of T lymphocytes for adoptive immunotherapy. Mol Ther. 2004;10:5–18. doi: 10.1016/j.ymthe.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 27.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 28.Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 29.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 30.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan JK, Rosenzweig SA, Young MR. Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2. J Immunother. 2010;33:126–135. doi: 10.1097/CJI.0b013e3181b91c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 34.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Wang MN, Li H, King KD, Bassi R, Sun H, Santiago A, Hooper AT, Bohlen P, Hicklin DJ. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med. 2002;195:1575–1584. doi: 10.1084/jem.20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180:1729–1736. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 37.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 38.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 39.Hammond EM, Giaccia AJ. Antiangiogenic therapy and p53. Science. 2002;297:471. doi: 10.1126/science.297.5581.471a. discussion 471. [DOI] [PubMed] [Google Scholar]
- 40.Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- 41.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao L, Sgadari C, Furuke K, Bloom ET, Teruya-Feldstein J, Tosato G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood. 1999;93:1612–1621. [PubMed] [Google Scholar]
- 44.Strasly M, Cavallo F, Geuna M, Mitola S, Colombo MP, Forni G, Bussolino F. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J Immunol. 2001;166:3890–3899. doi: 10.4049/jimmunol.166.6.3890. [DOI] [PubMed] [Google Scholar]
- 45.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 46.Ruegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med. 1998;4:408–414. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- 47.Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 2007;26:3360–3372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, Farber JM, Liao F, Liu L, Tosato G. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J Leukoc Biol. 1998;64:384–392. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- 49.Potter S, Chan-Ling T, Ball HJ, Mansour H, Mitchell A, Maluish L, Hunt NH. Perforin mediated apoptosis of cerebral microvascular endothelial cells during experimental cerebral malaria. Int J Parasitol. 2006;36:485–496. doi: 10.1016/j.ijpara.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 51.Choy JC, Kerjner A, Wong BW, McManus BM, Granville DJ. Perforin mediates endothelial cell death and resultant transplant vascular disease in cardiac allografts. Am J Pathol. 2004;165:127–133. doi: 10.1016/S0002-9440(10)63281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seko Y, Minota S, Kawasaki A, Shinkai Y, Maeda K, Yagita H, Okumura K, Sato O, Takagi A, Tada Y, et al. Perforin-secreting killer cell infiltration and expression of a 65-kD heat-shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest. 1994;93:750–758. doi: 10.1172/JCI117029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Q, Tong S, Dewhirst MW, Yuan F. Targeting tumor microvessels using doxorubicin encapsulated in a novel thermosensitive liposome. Mol Cancer Ther. 2004;3:1311–1317. [PubMed] [Google Scholar]
- 54.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuttica MJ, Langenickel T, Noguchi A, Machado RF, Gladwin MT, Boehm M. Perivascular T-cell infiltration leads to sustained pulmonary artery remodeling after endothelial cell damage. Am J Respir Cell Mol Biol. 2011;45:62–71. doi: 10.1165/rcmb.2009-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.