Abstract
Metastasis is the major cause of death from cancer, yet the optimal strategy against it remains uncertain. The pathogenesis of hematogenous metastasis is dynamic and consists of the following steps: 1) detachment of tumor cells from the primary site, 2) invasion into the host’s blood vessels, 3) migration in the host’s blood stream, 4) transport along the circulation, 5) arrest in or adhesion to the capillary in a distant organ, 6) extravasation, and 7) proliferation within the foreign tissues. A key to successful hematogenous metastasis is tumor survival in the bloodstream because most circulating tumor cells are rapidly destroyed by the shear forces or are attacked by the immune system. Less than 0.01% of these cells result in metastasis. Tumor cell–induced platelet aggregation has been reported to facilitate hematogenous metastasis by increasing the arrest of tumor cell emboli in the microcirculation. Platelet aggregation is also believed to protect tumor cells from immunological assault in the circulation. We have identified Aggrus as a platelet–aggregating factor expressed on a number of human cancers. Because hematogenous metastasis is reduced when neutralizing antibodies or eliminating carbohydrates attenuates Aggrus function, Aggrus’s main contribution to hematogenous metastasis of Aggrus–expressing cells, then, is by promoting platelet aggregation. Aggrus could serve as an ideal target for drug development to block metastasis.
Keywords: tumor metastasis, platelet aggregation, Aggrus/podoplanin, O-glycan
Introduction
Specific proteins expressed on the surface of platelets enable the platelets to adhere to their receptors exposed in areas of vascular damage.1) The process of adhesion activates the platelets to aggregate, leading to the formation of a platelet plug in the vessel wall. Activated platelets also induce the formation of a fibrin clot by carrying coagulation factors (i.e., Fibrinogen, Factor V, etc.) and providing a catalytic surface for the major interactions of the coagulation cascade. Because there exists a clear link between atherosclerotic vascular disease, inflammation, tumor metastasis, and thrombosis,1)–3) it is important to identify the mechanisms of platelet aggregation that have pathobiologic, prognostic, and treatment–related relevance.
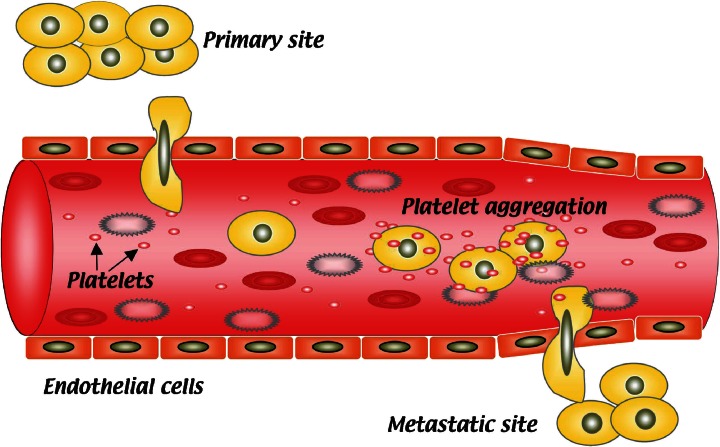
Studies on cancer metastasis have shown that some metastatic human and animal tumor cells possess platelet aggregation–inducing abilities that correlate with their metastatic potential.2),3) Interactions between tumor cells and platelets have been considered to facilitate tumor cell cluster arrest in the microvasculature, with the subsequent formation of experimental metastasis (Fig. 1).4) Ultrastructural studies have also demonstrated that platelets appear to enhance the development of arrested tumor emboli into a secondary metastatic colony.5),6) Several platelet aggregation inhibitors have been reported to retard tumor metastasis in certain animal models.7)–9) However, the molecules associated with the tumor–induced platelet aggregation had not been identified.
Fig. 1.
Schematic representation of platelet aggregation during hematogenous metastasis formation. After migrating through the host’s blood stream, tumor cells induce platelet aggregation, and the platelet–coated tumor cells are then protected from immunological assault in the circulation. Platelets also protect tumors from tumor necrosis factor α–mediated cytotoxicity. Another survival advantage is the tendency for the large tumor–platelet aggregate to embolize the microvasculature at a new extravasation site. Then, tumor cells extravasate and proliferate within the foreign tissue (metastatic site).
Establishment of highly and poorly metastatic sublines of mouse colon adenocarcinoma 26
Mouse colon adenocarcinoma 26 cell line is a chemically induced, undifferentiated carcinoma.10) Although many highly metastatic lines were reported, we began to isolate metastatic variants from mouse colon adenocarcinoma 26 because it was used to screen chemotherapeutic agents by the National Cancer Institute of the U.S. NIH. Establishing metastatic variants of this cell line would, therefore, aid in the study of the anti–metastatic activity of drugs as well as their anti–tumor activity. We tried to isolate metastatic variants by repeated in vivo selection and subsequent in vitro cloning.11),12) In this in vivo selection process, we inoculated excised pieces of whole lung into recipient mice because we could not detect any macroscopic metastatic nodules in the lung during the early in vivo passage. We needed 20 to 26 passages in vivo to obtain reproducible, visible metastatic lung nodules.
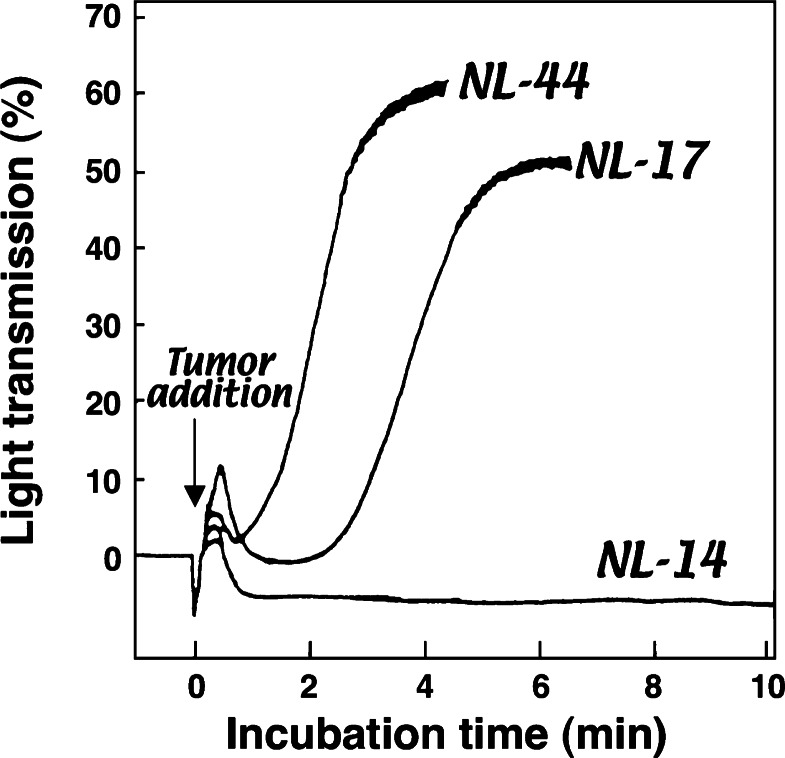
We finally established several clones with different metastatic abilities.11),12) Among these, a highly metastatic clone, NL-17, was found to exhibit high platelet aggregation–inducing activity when incubated with mouse platelet–rich plasma (PRP), while a poorly metastatic clone, NL-14, had a marginal platelet aggregation–inducing capability (Fig. 2).9),13) Therefore, we determined that the ability to induce platelet aggregation was related to the metastatic potential. The NL-44 clone, which possessed high platelet aggregation–inducing activity in vitro, did not form pulmonary metastases, even after platelet clustering. These results suggest that platelet aggregation–inducing capability alone is not enough to cause metastasis. NL-44 cells were thereafter found to proliferate more slowly in vivo than the highly metastatic NL-17 cells.14) In comparison with NL-44 cells, NL-17 cells are stimulated, to a greater extent, for in vitro growth by lung–associated growth factors.14)–17) Thus, the ability of cells to proliferate in the secondary organ may also be important in the formation of metastasis. Because hybridomas between two poorly metastatic clones, one was defective in platelet–aggregating ability but had in vivo growth potential and the other possessed platelet–aggregating ability but was defective in in vivo growth potential, were highly metastatic,18) platelet–aggregating ability and in vivo growth potential are two major determinants for successful experimental lung metastasis of the colon adenocarcinoma 26 cell line.
Fig. 2.
The platelet aggregating capability of highly metastatic NL-17 cells or poorly metastatic NL-14 and NL-44 cells was estimated by incubating the cells with mouse PRP. NL-17 and NL-44, but not NL-14, induced platelet aggregation after a characteristic delay. Because poorly metastatic NL-44 cells possess the platelet aggregation–inducing capability, it becomes clear that platelet aggregating ability alone is not enough for successful hematogenous metastasis formation. The ability to proliferate in the secondary organ may also be important in the formation of metastasis.
Establishment of platelet aggregation–neutralizing monoclonal antibodies
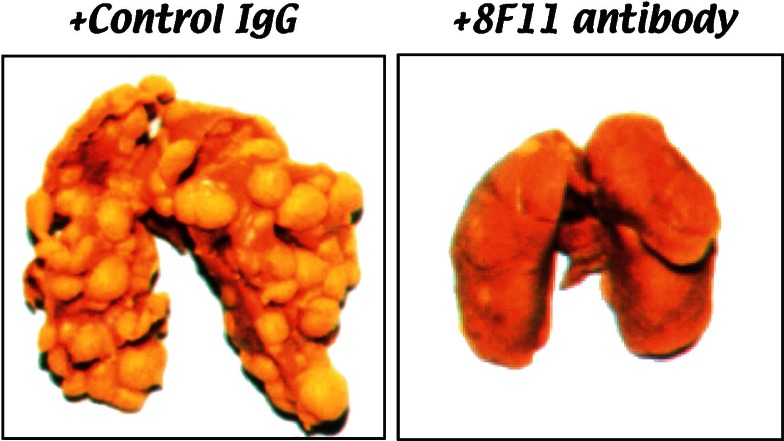
Among the established clones of the mouse colon adenocarcinoma 26 cell line, NL-17 is highly metastatic with high platelet–aggregating ability. We found that NL-17–induced platelet aggregation was dependent upon a trypsin–sensitive protein present on the NL-17 cell membrane.19) Plasma components were not required for aggregation because washed platelets were also aggregated by incubation with NL-17 cells. To elucidate the factor (or factors) that caused platelet aggregation, we immunized rats with membrane fractions of NL-17 cells. Hybridomas were screened by cellular ELISA for monoclonal antibody production that exhibited differential binding to NL-17 and NL-14 cells, which showed high and low platelet–aggregating abilities, respectively (Fig. 2). We successively selected two monoclonal antibodies produced from two, independent hybridomas that showed higher reactivity to NL-17 cells than to NL-14 cells. These monoclonal antibodies were designated 8F11 and 20A11.19) Both 8F11 and 20A11 recognized on the NL-17 cells a membrane sialoglycoprotein with a molecular weight of 44,000 (mouse Aggrus, formerly called gp44).19) It is important to note that 8F11 and 20A11 had the ability to neutralize NL-17–mediated platelet aggregation in vitro.19) The mouse B16 melanoma cell line, which was widely used as a metastasis model,4) could also induce platelet aggregation.20) When the 8F11 was reacted on highly metastatic variants of B16 melanoma cells, it recognized the Mr 41,000 glycoprotein (formerly gp41) as an antigen.20) Moreover, the 8F11 inhibited platelet aggregation induced by the melanoma cells in vitro under conditions that prevent thrombin activity.20) The 8F11 monoclonal antibody exhibited the inhibitory activity of lung colonization of NL-17 cells in vivo (Fig. 3).21) These data suggest that 8F11 antibody–reactive antigen, Aggrus, is a platelet aggregation–inducing factor expressed on metastatic mouse tumor cell lines.
Fig. 3.
NL-17 cells were harvested and resuspended in HBSS supplemented with 1% BALB/c serum. The mice were given intravenous injections of 0.2ml (2.5 × 104 cells) of the tumor suspension via the lateral tail vein. Control rat IgG (500 μg/mouse) or 8F11 (500 μg/mouse) were given intravenously 10 min before the tumor inoculation. Lung metastasis was examined on day 14 after the tumor inoculation.
Molecular identification of Aggrus as a platelet aggregation–inducing factor
Using 8F11 affinity–column chromatography, we purified the antigen and found that purified Aggrus/gp44 itself could induce platelet aggregation with no need for plasma components.22) We tried to identify the protein by conventional peptide sequencing. Unfortunately, we could not obtain the peptide sequence from the purified Aggrus because of the abundant carbohydrate chains.22)
We screened Aggrus–expressing mouse cell lines using immunoblot analysis with 8F11 monoclonal antibody and found that Aggrus was expressed in mouse osteoblastic MC3T3-E1, lymph node stromal and bone–derived endothelial cells.23),24) Several reports have suggested that podoplanin (also known as OTS-8/T1alpha) was expressed in MC3T3-E1 cells, lymphoid tissues, and endothelium.25)–27) We thus assumed that podoplanin could be identical to a then unidentified platelet aggregation–inducing factor, Aggrus. Four lines of evidence support this assumption.23) First, the 8F11 antibody recognized CHO cells (originally Aggrusnegative cells) that had been transfected with mouse podoplanin/OTS-8/T1alpha cDNA; second, the immunoprecipitated mouse podoplanin/OTS-8/T1alpha protein was recognized by 8F11 antibody; third, siRNA directed to mouse podoplanin/OTS-8/T1alpha decreased the 8F11 antibody–reactive mouse Aggrus expression in NL-17 and B16 melanoma cells; and fourth, expression of mouse podoplanin/OTS-8/T1alpha on CHO cell surface induced platelet aggregation with no requirement for plasma components.
Ectopic expression of Aggrus on CHO cells also promoted pulmonary metastasis in vivo.23) The Aggrus-expressing CHO cells formed blood vesseldependent pulmonary metastasis in vivo, while they could not form lymph-node metastasis even in spontaneous metastasis model (data not shown). Because a few metastatic foci could be found when parent CHO cells were intravenously injected into nude mice, CHO cells might originally have the ability to proliferate in lung. Thus, Aggrus expression might enhance their chance to embolize in the microvasculature of the lung, leading to the increase in the number of metastatic foci. Because of the absence of platelets in lymphatic fluid, Aggrus overexpression may not be able to generate tumor emboli in lymphatic vessels and, hence, is not associated with lymph-node metastasis.
The identified mouse aggrus gene was first cloned as an early–response gene (OTS-8) that was induced by phorbol ester in MC3T3-E1 cells.25) Aggrus is a type-I transmembrane sialomucin–like glycoprotein that consists of an extracellular domain, a single transmembrane portion, and a short cytoplasmic tail with putative sites for protein kinase C and cAMP phosphorylation. Its 162 aa protein is extensively O-glycosylated, which results in a doubling of the molecular weight up to about 40,000.28) Sequence analysis indicates that Aggrus does not share common domains with other protein families whose sequences predict their functions. Thus, the physiological role of Aggrus has long been unidentified. The widespread localization of Aggrus led to a multitude of synonyms for its expression forms of human, mouse, rat and dog origin: human gp36,29) mouse Aggrus,23) OTS-8,25) PA2.26 antigen,30) RANDAM-2,31) rat RTI40,32) T1alpha,33) E11 antigen,34) podoplanin35) and dog gp40.28) The aggrus/podoplanin/T1alpha null mice have already been generated by a targeted dysfunction of the gene. The mice, at birth, had respiratory failure, accompanied by immature lymphatic vessel formation.36),37) It is conceivable that Aggrus plays an important role in regulating peripheral lung cell proliferation and lymphatic vascular development.
Identification of the PLatelet AGgregation–stimulating (PLAG) domain in Aggrus
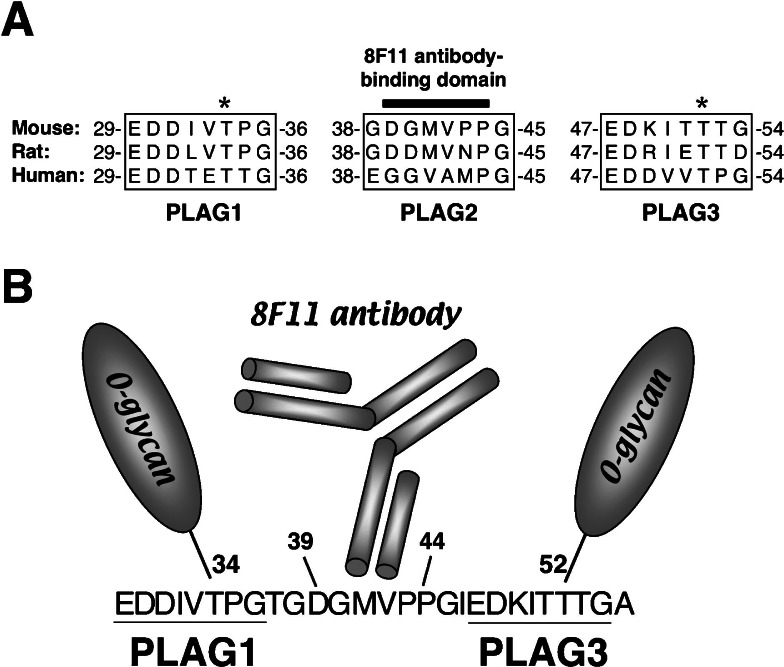
Aggrus–induced mouse platelet aggregation, as mentioned, was perfectly suppressed by pretreatment of the cells with 8F11 monoclonal antibody (Fig. 3).19),20) Thus, 8F11 may recognize the specific epitope of mouse Aggrus that interacts with platelets. The 39-DGMVPP-44 segment was identified as the minimum residue for recombinant mouse Aggrus protein recognition by 8F11 (Fig. 4A).23) The 8F11 antibody recognition domain, however, was not associated with direct binding to platelets because the peptide coding 8F11–recognition domain could not induce platelet aggregation by itself, and mutation of the 8F11 antibody recognition domain did not affect the platelet–aggregating activity of Aggrus. Detailed analysis around the 8F11 recognition domain revealed that Aggrus contained three tandem repeats of the segment EDXXVTPG (in which X may be any amino acid) in the extracellular domain (Fig. 4A).38) The 8F11 recognition domain is located within the second segment.
Fig. 4.
(A) Alignment of the Aggrus homologue amino acid sequence around the PLAG1-3 domains. The Aggrus neutralizing antibody, 8F11, recognizes the DGMVPP segment in the PLAG2 domain. The asterisks indicate the O-glycosylation sites. The O-glycans attached to Aggrus are essential for exhibiting its platelet–aggregating ability. (B) Schematic model of the 8F11 antibody–mediated neutralization of Aggrus–induced platelet aggregation. The 8F11 antibody may neutralize mouse Aggrus–induced platelet aggregation by conformationally interfering with the O-glycans attached on threonine residues in the PLAG1 and PLAG3 domains (threonine-34 and threonine-52 residues).
A striking feature of Aggrus proteins is their extraordinarily high content of serine and threonine residues that might be O-glycosylated. In dog Aggrus/gp40, 14 serine and threonine residues, in total, were identified by Edman degradation to be modified by O-glycosylation.28) We have previously reported that sialylated carbohydrate chains of Aggrus/gp44 were involved in their platelet aggregation–inducing capabilities.22) Because threonine followed by proline is likely to be O-glycosylated, we assumed that the 8F11 might neutralize the platelet–aggregating capability of mouse Aggrus by conformationally interfering with the carbohydrate chains near the 8F11–binding domain (Fig. 4B). We then generated several mouse aggrus point mutants in which putative O-glycosylated threonine residues around the 8F11–binding domain were converted to alanine residues. Mutation of these threonine residues did not affect 8F11 binding nor cell surface expression. However, conversion of threonine residues in the first or third segment eliminated the platelet aggregation–inducing capability and metastasis–promoting ability.39) We, therefore, termed the segments as PLAG (PLatelet AGgregation–stimulating) domains.23) In addition, these PLAG domains are highly conserved among Aggrus homologues from human, mouse, rat, dog, and hamster (Fig. 4A).38) These results indicate that the PLAG domain (especially the O-glycosylated threonine residue) is critical for platelet aggregation and metastasis formation.
Lectin blotting and analyses of glycopeptides produced by Edman degradation following mass spectrometry revealed that the disialyl-core l (NeuAc alpha2-3Gal beta l–3(NeuAc alpha2-6)Gal-NAc alpha l-O-Thr) structure was primarily attached to a glycosylation site at the threonine residues in PLAG domain.40) Using a series of glycosylation–deficient Chinese hamster ovary (CHO) cell mutants—N-glycan–deficient Lec1, CMP–sialic acid transporter–deficient Lec2, and UDP–galactose transporter–deficient Lec8—we found that Aggrus expressed on Lec1 cells could induce platelet aggregation but not Aggrus on Lec2 and Lec8 cells.41) We thus confirmed that sialic acid attached to O-glycans at threonine residues in PLAG domain is essential for exhibiting the platelet–aggregating ability of Aggrus.42),43)
Counterparts and associated proteins of Aggrus
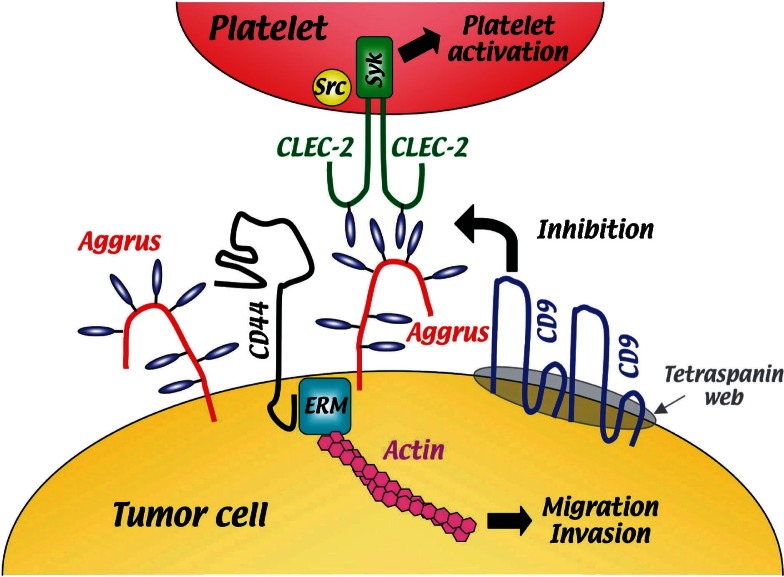
Suzuki-Inoue et al. recently reported that C–type lectin–like receptor 2 (CLEC-2) expressed on platelets was a counterpart of Aggrus (Fig. 5).42) When CLEC-2 binds to Aggrus expressed on tumor cells, it generates activation signals, including Src, Syk and PLCgamma2 in platelets, and eventually induces platelets to degranulate.42) CLEC-2 was originally identified as a counter–receptor of snake venom rhodocytin (also called aggretin).44) Like Aggrus–mediated platelet aggregation (Fig. 2), CLEC-2 activation (by rhodocytin or anti–CLEC-2 antibody) induced platelet aggregation after a characteristic delay.44) Furthermore, the association between Aggrus and CLEC-2 was dependent on sialic acid on O-glycans of Aggrus. Thus, CLEC-2 might be an important counterpart of Aggrus when Aggrus functions as a platelet aggregation–inducing factor.
Fig. 5.
Schematic representation of Aggrus–mediated platelet aggregation. The sialic acid attached to O-glycans (indicated by blue ovals) of Aggrus (red) interacts with CLEC-2 (green) on platelets, induces CLEC-2 activation, and induces platelets to degranulate. The Aggrus function is attenuated by interaction with the tetraspanin family member CD9 (blue). Aggrus also regulates migration, invasion and spontaneous metastasis of tumor cells by interacting with CD44 (black) and ERM (blue square).
Ectopic expression of Aggrus induced adhesion of endothelial cells to fibronectin and type I collagen.36) Moreover, Aggrus overexpression promotes migration, invasion and spontaneous metastasis of the tumor cells.39) The cytoplasmic tail of Aggrus/podoplanin was reported to bind to the ERM (ezrin, radixin and moesin) family protein.45) In addition, Aggrus/OTS-8 was reported to form a complex with CD44.46) Because CD44 and ERM proteins were widely known to regulate cell adhesion and cell motility, Aggrus might regulate cell adhesion and cell motility by regulating CD44 and ERM proteins (Fig. 5).
We recently discovered that a tetraspanin family member, CD9, formed a complex with Aggrus on the cell surface.47) Four transmembrane–domain proteins of the tetraspanin family regulate cell migration, fusion and signaling events by functioning as organizers of a multimolecular membrane complex called the tetraspanin web or tetraspanin–enriched microdomains. The CD9 protein is a member of the tetraspanin family and has been identified as a suppressor of cancer spread. Reduced CD9 expression in various cancers is correlated with the presence of distant metastasis and a poorer prognosis. We found that ectopic expression of CD9 suppressed Aggrus–induced platelet aggregation. We found that CD9 formed a complex with Aggrus, via transmembrane domains 1 and 2 (TM1 and 2) and localized in the tetraspanin web. Because the CD9 mutant lacking TM1 and 2 could not suppress Aggrus–induced platelet aggregation, we concluded that CD9 neutralized Aggrus function by inducing Aggrus localization in the tetraspanin web.47) Other proteins in the tetraspanin web might conformationally interfere with the platelet–aggregating activity of Aggrus (Fig. 5).
Aggrus expression in normal and malignant tissues
In normal human tissues, Aggrus expression can be detected in osteoblasts and osteocytes,25),34) in type I alveolar cells,32),33) in peripheral lymphoid tissues,26) in kidney podocytes, skeletal muscle, placenta, heart (Fig. 6)48) and in epidermal keratinocytes and fibroblasts.30),49) Most important, Aggrus is expressed on lymphatic but not on blood vessel endothelium. Thus, it is widely used as a specific marker for lymphatic endothelial cells and lymphangiogenesis.49) The D2-40 monoclonal antibody is frequently used as the specific antibody to detect lymphatic endothelial cells.50) Schacht et al.51) recently confirmed that the D2-40 antibody recognizes Aggrus/podoplanin by transfecting human aggrus into aggrus–negative rat myoblasts and by using siRNA to knock it out in human lymphatic endothelial cells. Therefore, Aggrus might be a useful marker for lymphatic lineage cells. In addition to its expression in normal cells, recent reports have clarified Aggrus expression in squamous cell carcinoma of the lung, the oral cavity, the cervix, the esophaguand the skin, in Kaposi’s sarcoma, in mesothelioma, in testicular germ cell tumors and in a number of tumors of the central nervous system.48)–56) Therefore, its use as a marker in histopathology is not limited to lymphatic endothelium and lymphangiogenesis.
Fig. 6.
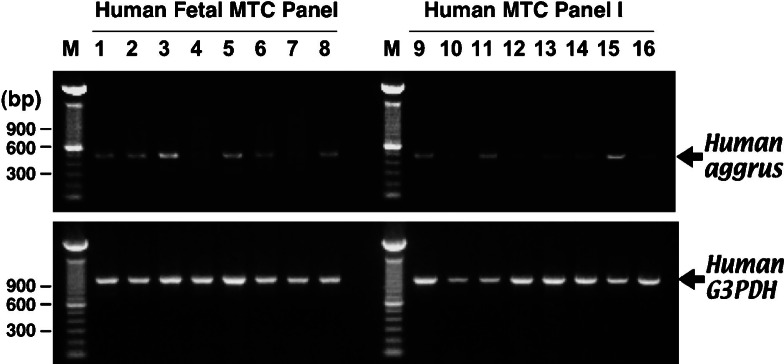
Comparison of tissue expression patterns of aggrus mRNA in human fetal (Lanes 1–8) and adult tissues (Lanes 9–16). Each of the indicated MTC Panel cDNAs (Clontech) was used as a PCR template with the aggrus (top) and G3PDH primers (bottom). After 32 (for aggrus) or 27 (for G3PDH) amplification cycles, samples were electrophoresed onto 1% agarose gels. Lane 1, heart. Lane 2, brain. Lane 3, lung. Lane 4, liver. Lane 5, skeletal muscle. Lane 6, kidney. Lane 7, spleen. Lane 8, thymus. Lane 9, heart. Lane 10, brain. Lane 11, lung. Lane 12, liver. Lane 13, skeletal muscle. Lane 14, kidney. Lane 15, placenta. Lane 16, pancreas. Lanes M, 100-bp DNA ladder marker.
Normal lymphatic endothelial cells and lymphatic stromal cells expressed Aggrus on their surface and exhibited the platelet-aggregating ability in vitro.41) No functional difference between Aggrus expressed on normal cells and that on tumor cells could be found. Normal cells expressing Aggrus on their cell surface, however, may not induce platelet aggregation in vivo because they are unable to interact with platelets under physiological conditions. Thus, platelet aggregation-inducing ability of Aggrus may only be observed under pathological conditions.
Conclusion
There are several mechanisms involved in tumor–induced platelet aggregation, and these can vary among different tumor cells. For example, these cells can activate platelets by tumor cell–induced thrombin generation through a coagulation pathway, releasing ADP, thromboxane A2 (TXA2), and MMP-2.48) We identified a transmembrane sialomucin–like glycoprotein, Aggrus, as a platelet–aggregating factor expressed on tumor cells.23) Aggrus expression promoted hematogenous metastasis without affecting tumor growth, and it diminished survival of mice. Our studies provide clear evidence for a causative role of platelet aggregation in cancer metastasis. Morimoto et al. recently reported that Necl-5/poliovirus receptor is expressed on the tumor cell membrane and was associated with tumor–induced platelet aggregation and metastasis formation. 57) Based on these studies, we suggest that small molecule inhibitors or antibodies against the platelet–aggregating factors could be a therapeutic strategy for inhibiting tumor metastasis and for enhancing cancer patient survival. Further, Aggrus may have other functional implications in vascular pathogenesis.
Acknowledgments
We thank to Drs. Yukinari Kato, Youya Nakazawa, Mika Kato Kaneko, Akiko Kunita, Masahiko Watanabe, Takao Yamori, and Yoshikazu Sugimoto for their great contribution to the research. This study was supported in part by special grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to T.T. and N.F.), a grant from Takeda Science Foundation, Japan (to N.F.), and a grant from the Cell Science Research Foundation, Japan (to N.F.).
Abbreviations:
- CLEC-2
C–type lectin–like receptor 2
- ERM
ezrin, radixin and moesin
- PRP
platelet rich plasma
- TM
transmembrane
Profile
Takashi Tsuruo was born in 1943. He graduated from the University of Tokyo, Faculty of Pharmaceutical Sciences in 1967 and earned a Ph.D. degree in 1972 at the University of Tokyo. He did postdoctoral studies (1974–1977) at St. Louis University and UCLA and worked in the field of DNA and RNA virus replication. In 1977, Dr. Tsuruo joined the Cancer Chemotherapy Center, Japanese Foundation for Cancer Research as a research staff scientist. He studied the mechanism of tumor metastasis and the biological role of cellular calcium in relation to the function of tubulin. He visited Dr. I. J. Fidler’s laboratory at NCI-Frederick for 3 months in 1980 under the Yamagiwa-Yoshida grant from UICC. He began research on tumor cell–induced platelet aggregation, as it had been reported to facilitate hematogenous metastasis by increasing the arrest of tumor cell emboli in the microcirculation. Much of this research is reviewed in this article. Dr. Tsuruo’s work led him to identify Aggrus as a platelet–aggregating factor expressed on a number of human cancers. Aggrus could be an ideal target for development of a drug that would block metastasis. In addition to his metastasis research, Dr. Tsuruo discovered verapamil to be a multidrug resistance (MDR) reversing agent. This was a landmark finding in the field of cancer chemotherapy as verapamil, a calcium channel blocker, actually overcame vinca alkaloid–resistance in animal experiments. He conducted pharmacological and molecular biological studies on MDR mechanisms, and as a result of these studies, P-glycoprotein was first recognized as an ABC (ATP–binding cassette) transporter family protein. Recently, Dr. Tsuruo turned his attention to the study of apoptosis, as many antitumor drugs induce apoptosis in tumor cells. Apoptosis is strongly associated with drug sensitivity, and apoptosis resistance is related to drug resistance.

In addition to his scientific contributions to the field of basic cancer chemotherapy, tumor metastasis and drug resistance, Dr. Tsuruo has made noteworthy contributions to cancer research in Japan. He was a Principal Coordinator for “General Promotion for Cancer Research in Japan,” during 1999–2004. Dr. Tsuruo has been Editor-in-Chief of Cancer Science, the official journal of the Japanese Cancer Association (JCA), since 2003. In 2007, he served as president of the 66th Annual Meeting of the JCA. In 1996, Dr. Tsuruo began “the International Symposium on Cancer Chemotherapy”, and a year later, he founded “the Japanese Association for Molecular Target Therapy of Cancer”. For his many achievements and contributions, Dr. Tsuruo received the Scientific Award from Princess Takamatsu Cancer Foundation in 1991, the Japanese Pharmaceutical Society Award in 1997, and the “Tomizo Yoshida Prize” of the JCA in 2004. He received the Medal with Purple Ribbon as a distinguished scientist from the Japanese Government on November 2, 2005.
Dr. Tsuruo was appointed Chief, Division of Experimental Chemotherapy at the Cancer Chemotherapy Center of the Japanese Foundation for Cancer Research in 1986. He held this position from 1986 to 2006. In 1989, he was promoted to Professor at the University of Tokyo, Institute of Molecular and Cellular Biosciences (formerly the Institute of Applied Microbiology). Between 1999 and 2003, he was Director, Institute of the Molecular and Cellular Biosciences at the University of Tokyo. In 2006, he returned to the Japanese Foundation for Cancer Research and now he is Director of the Cancer Chemotherapy Center. In addition, Dr. Tsuruo is a member of the Science Council of Japan since 2003 and is Professor Emeritus of the University of Tokyo since 2006.
References
- 1).Klinger, M. H. and Jelkmann, W. (2002) Role of blood platelets in infection and inflammation. J. Interferon Cytokine Res. 22, 913–922 [DOI] [PubMed] [Google Scholar]
- 2).Oleksowicz, L. and Dutcher, J. P. (1995) Adhesive receptors expressed by tumor cells and platelets: novel targets for therapeutic anti–metastatic strategies. Med. Oncol. 12, 95–102 [DOI] [PubMed] [Google Scholar]
- 3).Honn, K. V., Tang, D. G. and Crissman, J. D. (1992) Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 11, 325–351 [DOI] [PubMed] [Google Scholar]
- 4).Fidler, I. J. (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 [DOI] [PubMed] [Google Scholar]
- 5).Crissman, J. D., Hatfield, J. S., Menter, D. G., Sloane, B. and Honn, K. V. (1988) Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res. 48, 4065–4072 [PubMed] [Google Scholar]
- 6).Bradley, C. J., Dauer, R. J., Thurlow, P. J. and Connellan, J. M. (1997) Characterization of platelet aggregation induced by the human carcinosarcoma Colo 526: role of platelet activation, tumor cell cytoskeleton and tumor cell plasma membrane. Pathology 29, 189–195 [DOI] [PubMed] [Google Scholar]
- 7).Honn, K. V., Onoda, J. M., Diglio, C. A., Carufel, M. M., Taylor, J. D. and Sloane, B. F. (1984) Inhibition of tumor cell–platelet interactions and tumor metastasis by the calcium channel blocker, nimodipine. Clin. Exp. Metastasis 2, 61–72 [DOI] [PubMed] [Google Scholar]
- 8).Honn, K. V., Onoda, J. M., Pampalona, K., Battaglia, M., Neagos, G., Taylor, J. D., Diglio, C. A. and Sloane, B. F. (1985) Inhibition by dihydropyridine class calcium channel blockers of tumor cell–platelet–endothelial cell interactions in vitro and metastasis in vivo. Biochem. Pharmacol. 34, 235–241 [DOI] [PubMed] [Google Scholar]
- 9).Tsuruo, T., Iida, H., Makishima, F., Yamori, T., Kawabata, H., Tsukagoshi, S. and Sakurai, Y. (1985) Inhibition of spontaneous and experimental tumor metastasis by the calcium antagonist verapamil. Cancer Chemother. Pharmacol. 14, 30–33 [DOI] [PubMed] [Google Scholar]
- 10).Corbett, T. H., Griswold, D. P. Jr., Roberts, B. J., Peckham, J. C., Schabel, F. M. Jr. (1975) Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 35, 2434–2439 [PubMed] [Google Scholar]
- 11).Tsuruo, T., Yamori, T., Naganuma, K., Tsukagoshi, S. and Sakurai, Y. (1983) Characterization of metastatic clones derived from a metastatic variant of mouse colon adenocarcinoma 26. Cancer Res. 43, 5437–5442 [PubMed] [Google Scholar]
- 12).Yamori, T., Tsuruo, T., Naganuma, K., Tsukagoshi, S. and Sakurai, Y. (1984) Isolation and characterization of highly and rarely metastatic clones from murine colon adenocarcinoma. Invasion Metastasis 4, 84–97 [PubMed] [Google Scholar]
- 13).Tsuruo, T., Kawabata, H., Iida, H. and Yamori, T. (1986) Tumor–induced platelet aggregation and growth promoting factors as determinants for successful tumor metastasis. Clin. Exp. Metastasis 4, 25–33 [DOI] [PubMed] [Google Scholar]
- 14).Yamori, T., Iida, H., Tsukagoshi, S. and Tsuruo, T. (1988) Growth stimulating activity of lung extract on lung–colonizing colon 26 clones and its partial characterization. Clin. Exp. Metastasis 6, 131–139 [DOI] [PubMed] [Google Scholar]
- 15).Tsuruo, T., Watanabe, M. and Oh-hara, T. (1989) Stimulation of the growth of metastatic clones of mouse colon adenocarcinoma 26 in vitro by platelet–derived growth factor. Jpn. J. Cancer Res. 80, 136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Koenuma, M., Yamori, T. and Tsuruo, T. (1989) Insulin and insulin–like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn. J. Cancer Res. 80, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Yamori, T., Iizuka, Y., Takayama, Y., Nishiya, S., Iwashita, S., Yamazaki, A., Takatori, T. and Tsuruo, T. (1991) Insulin–like growth factor I rapidly induces tyrosine phosphorylation of a Mr 150,000 and a Mr 160,000 protein in highly metastatic mouse colon carcinoma 26 NL-17 cells. Cancer Res. 51, 5859–5865 [PubMed] [Google Scholar]
- 18).Sugimoto, Y., Oh-hara, T., Watanabe, M., Saito, H., Yamori, T. and Tsuruo, T. (1987) Acquisition of metastatic ability in hybridomas between two low metastatic clones of murine colon adenocarcinoma 26 defective in either platelet–aggregating activity or in vivo growth potential. Cancer Res. 47, 4396–4401 [PubMed] [Google Scholar]
- 19).Watanabe, M., Okochi, E., Sugimoto, Y. and Tsuruo, T. (1988) Identification of a platelet–aggregating factor of murine colon adenocarcinoma 26: Mr 44,000 membrane protein as determined by monoclonal antibodies. Cancer Res. 48, 6411–6416 [PubMed] [Google Scholar]
- 20).Watanabe, M., Sugimoto, Y. and Tsuruo, T. (1990) Expression of a Mr 41,000 glycoprotein associated with thrombin–independent platelet aggregation in high metastatic variants of murine B16 melanoma. Cancer Res. 50, 6657–6662 [PubMed] [Google Scholar]
- 21).Sugimoto, Y., Watanabe, M., Oh-hara, T., Sato, S., Isoe, T. and Tsuruo, T. (1991) Suppression of experimental lung colonization of a metastatic variant of murine colon adenocarcinoma 26 by a monoclonal antibody 8F11 inhibiting tumor cell–induced platelet aggregation. Cancer Res. 51, 921–925 [PubMed] [Google Scholar]
- 22).Toyoshima, M., Nakajima, M., Yamori, T. and Tsuruo, T. (1995) Purification and characterization of the platelet–aggregating sialoglycoprotein gp44 expressed by highly metastatic variant cells of mouse colon adenocarcinoma 26. Cancer Res. 55, 767–773 [PubMed] [Google Scholar]
- 23).Kato, Y., Fujita, N., Kunita, A., Sato, S., Kaneko, M., Osawa, M. and Tsuruo, T. (2003) Molecular identification of Aggrus/T1alpha as a platelet aggregation–inducing factor expressed in colorectal tumors. J. Biol. Chem. 278, 51599–51605 [DOI] [PubMed] [Google Scholar]
- 24).Zhang, Y., Fujita, N., Oh-hara, T., Morinaga, Y., Nakagawa, T., Yamada, M. and Tsuruo, T. (1998) Production of interleukin-11 in bone–derived endothelial cells and its role in the formation of osteolytic bone metastasis. Oncogene 16, 693–703 [DOI] [PubMed] [Google Scholar]
- 25).Nose, K., Saito, H. and Kuroki, T. (1990) Isolation of a gene sequence induced later by tumor–promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras–transformed cells. Cell Growth Differ. 1, 511–518 [PubMed] [Google Scholar]
- 26).Farr, A. G., Berry, M. L., Kim, A., Nelson, A. J., Welch, M. P. and Aruffo, A. (1992) Characterization and cloning of a novel glycoprotein expressed by stromal cells in T-dependent areas of peripheral lymphoid tissues. J. Exp. Med. 176, 1477–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Taniguchi, K., Harada, N., Ohizumi, I., Kinoshita, M., Tsutsumi, Y., Nakagawa, S., Kaiho, S. and Mayumi, T. (2000) Molecular cloning and characterization of antigens expressed on rat tumor vascular endothelial cells. Int. J. Cancer 86, 799–805 [DOI] [PubMed] [Google Scholar]
- 28).Zimmer, G., Lottspeich, F., Maisner, A., Klenk, H. D. and Herrler, G. (1997) Molecular characterization of gp40, a mucin–type glycoprotein from the apical plasma membrane of Madin-Darby canine kidney cells (type I). Biochem. J. 326, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Zimmer, G., Oeffner, F., Von Messling, V., Tschernig, T., Gröness, H. J., Klenk, H. D. and Herrler, G. (1999) Cloning and characterization of gp36, a human mucin–type glycoprotein preferentially expressed in vascular endothelium. Biochem. J. 341, 277–284 [PMC free article] [PubMed] [Google Scholar]
- 30).Gandarillas, A., Scholl, F. G., Benito, N., Gamallo, C. and Quintanilla, M. (1997) Induction of PA2.26, a cell–surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol. Carcinog. 20, 10–18 [DOI] [PubMed] [Google Scholar]
- 31).Kotani, M., Osanai, T., Tajima, Y., Kato, H., Imada, M., Kaneda, H., Kubo, H. and Sakuraba, H. (2002) Identification of neuronal cell lineage–specific molecules in the neuronal differentiation of P19 EC cells and mouse central nervous system. J. Neurosci. Res. 67, 595–606 [DOI] [PubMed] [Google Scholar]
- 32).McElroy, M. C., Pittet, J. F., Hashimoto, S., Allen, L., Wiener-Kronish, J. P. and Dobbs, L. G. (1995) A type I cell–specific protein is a biochemical marker of epithelial injury in a rat model of pneumonia. Am. J. Physiol. 268, L181–L186 [DOI] [PubMed] [Google Scholar]
- 33).Rishi, A. K., Joyce-Brady, M., Fisher, J., Dobbs, L. G., Floros, J., VanderSpek, J., Brody, J. S. and Williams, M. C. (1995) Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev. Biol. 167, 294–306 [DOI] [PubMed] [Google Scholar]
- 34).Wetterwald, A., Hoffstetter, W., Cecchini, M. G., Lanske, B., Wagner, C., Fleisch, H. and Atkinson, M. (1996) Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone 18, 125–132 [DOI] [PubMed] [Google Scholar]
- 35).Breiteneder-Geleff, S., Matsui, K., Soleiman, A., Meraner, P., Poczewski, H., Kalt, R., Schaffner, G. and Kerjaschki, D. (1997) Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down–regulated in puromycin nephrosis. Am. J. Pathol. 151, 1141–1152 [PMC free article] [PubMed] [Google Scholar]
- 36).Schacht, V., Ramirez, M. I., Hong, Y. K., Hirakawa, S., Feng, D., Harvey, N., Williams, M., Dvorak, A. M., Dvorak, H. F., Oliver, G.et al. (2003) T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Ramirez, M. I., Millien, G., Hinds, A., Cao, Y., Seldin, D. C. and Williams, M. C. (2003) T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev. Biol. 256, 61–72 [DOI] [PubMed] [Google Scholar]
- 38).Kaneko, M. K., Kato, Y., Kitano, T. and Osawa, M. (2006) Conservation of a platelet activating domain of Aggrus/podoplanin as a platelet aggregation–inducing factor. Gene 378, 52–57 [DOI] [PubMed] [Google Scholar]
- 39).Kunita, A., Kashima, T. G., Morishita, Y., Fukayama, M., Kato, Y., Tsuruo, T. and Fujita, N. (2007) The platelet aggregation–inducing factor aggrus/podoplanin promotes pulmonary metastasis. Am. J. Pathol. 170, 1337–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Kaneko, M. K., Kato, Y., Kameyama, A., Ito, H., Kuno, A., Hirabayashi, J., Kubota, T., Amano, K., Chiba, Y., Hasegawa, Y.et al. (2007) Functional glycosylation of human podoplanin: glycan structure of platelet aggregation–inducing factor. FEBS Lett. 581, 331–336 [DOI] [PubMed] [Google Scholar]
- 41).Kaneko, M., Kato, Y., Kunita, A., Fujita, N., Tsuruo, T. and Osawa, M. (2004) Functional sialylated O-glycan to platelet aggregation on Aggrus (T1alpha/Podoplanin) molecules expressed in Chinese hamster ovary cells. J. Biol. Chem. 279, 38838–38843 [DOI] [PubMed] [Google Scholar]
- 42).Suzuki-Inoue, K., Kato, Y., Inoue, O., Kaneko, M. K., Mishima, K., Yatomi, Y., Yamazaki, Y., Narimatsu, H. and Ozaki, Y. (2007) Involvement of the snake toxin receptor CLEC-2, in podoplanin–mediated platelet activation, by cancer cells. J. Biol. Chem. 282, 25993–26001 [DOI] [PubMed] [Google Scholar]
- 43).Kato, Y., Kaneko, M. K., Kunita, A., Ito, H., Kameyama, A., Ogasawara, S., Matsuura, N., Hasegawa, Y., Suzuki-Inoue, K., Inoue, O., Ozaki, Y. and Narimatsu, H. (2008) Molecular analysis of the pathophysiological binding of the platelet aggregation–inducing factor podoplanin to the C-type lectin–like receptor CLEC-2. Cancer Sci. 99, 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Suzuki-Inoue, K., Fuller, G. L., García, A., Eble, J. A., Pöhlmann, S., Inoue, O., Gartner, T. K., Hughan, S. C., Pearce, A. C., Laing, G. D.et al. (2006) A novel Syk–dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 107, 542–549 [DOI] [PubMed] [Google Scholar]
- 45).Martín-Villar, E., Megías, D., Castel, S., Yurrita, M. M., Vilaró, S. and Quintanilla, M. (2006) Podoplanin binds ERM proteins to activate RhoA and promote epithelial–mesenchymal transition. J. Cell. Sci. 119, 4541–4553 [DOI] [PubMed] [Google Scholar]
- 46).Ohizumi, I., Harada, N., Taniguchi, K., Tsutsumi, Y., Nakagawa, S., Kaiho, S. and Mayumi, T. (2000) Association of CD44 with OTS-8 in tumor vascular endothelial cells. Biochim. Biophys. Acta 1497, 197–203 [DOI] [PubMed] [Google Scholar]
- 47).Nakazawa, Y., Sato, S., Naito, M., Kato, Y., Mishima, K., Arai, H., Tsuruo, T. and Fujita, N.Tetraspanin family member CD9 inhibits Aggrus/podoplanin–induced platelet aggregation and suppresses pulmonary metastasis. Blood (in press). [DOI] [PubMed] [Google Scholar]
- 48).Ordonez, N. G. (2006) Podoplanin: a novel diagnostic immunohistochemical marker. Adv. Anat. Pathol. 13, 83–88 [DOI] [PubMed] [Google Scholar]
- 49).Wicki, A. and Christofori, G. (2007) The potential role of podoplanin in tumour invasion. Br. J. Cancer 96, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Kahn, H. J., Bailey, D. and Marks, A. (2002) Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod. Pathol. 15, 434–440 [DOI] [PubMed] [Google Scholar]
- 51).Schacht, V., Dadras, S. S., Johnson, L. A., Jackson, D. G., Hong, Y. K. and Detmar, M. (2005) Up–regulation of the lymphatic marker podoplanin, a mucin–type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 166, 913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Kato, Y., Kaneko, M., Sata, M., Fujita, N., Tsuruo, T. and Osawa, M. (2005) Enhanced expression of Aggrus (T1alpha/Podoplanin), a platelet aggregation–inducing factor in lung squamous cell carcinoma. Tumour Biol. 26, 195–200 [DOI] [PubMed] [Google Scholar]
- 53).Mishima, K., Kato, Y., Kaneko, M. K., Nakazawa, Y., Kunita, A., Fujita, N., Tsuruo, T., Nishikawa, R., Hirose, T. and Matsutani, M. (2006) Podoplanin expression in primary central nervous system germ cell tumors: a useful histological marker for the diagnosis of germinoma. Acta Neuropathol. 111, 563–568 [DOI] [PubMed] [Google Scholar]
- 54).Mishima, K., Kato, Y., Kaneko, M. K., Nishikawa, R., Hirose, T. and Matsutani, M. (2006) Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 111, 483–488 [DOI] [PubMed] [Google Scholar]
- 55).Kimura, N. and Kimura, I. (2005) Podoplanin as a marker for mesothelioma. Pathol. Int. 55, 83–86 [DOI] [PubMed] [Google Scholar]
- 56).Kato, Y., Sasagawa, I., Kaneko, M., Osawa, M., Fujita, N. and Tsuruo, T. (2004) Aggrus: a diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene 23, 8552–8556 [DOI] [PubMed] [Google Scholar]
- 57).Morimoto, K., Satoh-Yamaguchi, K., Hamaguchi, A., Inoue, Y., Takeuchi, M., Okada, M., Ikeda, W., Takai, Y. and Imai, T. (2008) Interaction of cancer cells with platelets mediated by Necl-5/ poliovirus receptor enhances cancer cell metastasis to the lungs. Oncogene 27, 264–273 [DOI] [PubMed] [Google Scholar]