SUMMARY
Meningoencephalic herniation (MEH) in the middle ear and mastoid is a rare pathological entity with possible life-threatening complications. We treated 24 patients with a trans-mastoid approach, and the bony defect was closed by heterologous materials positioned in a multilayer fashion. The cause of the bony defect were chronic otitis media with cholesteatoma, iatrogenic, spontaneous and post-traumatic. The major presenting symptoms were meningitis, headache, conductive hearing loss, cerebrospinal fluid (CSF leak), neurologic deficit and pneumoencephalus, and stenosis of a canal wall down cavity. During follow-up, no patient developed complications due to surgery or related to the pathology, and imaging showed a stable occlusion of the bony defect. Different surgical treatments have been proposed to repair MEH, and the choice is based on the localization and size of the bony defect, preoperative auditory function and the presence of a coexisting pathology. We propose the use of collagenous membranes and bone substitutes for reconstruction of the floor of the middle fossa.
KEY WORDS: Meningoencephalic herniations, Tegmen defect, Meningitis, CT, MRI, Transmastoid approach
RIASSUNTO
Le ernie meningoencefaliche nell'orecchio medio e nella mastoide rappresentato una rara entità patologica con complicanze anche mortali. Abbiamo operato 24 pazienti con un approccio trans-mastoideo e abbiamo chiuso il difetto osseo utilizzando più strati di materiale eterologo. Nella nostra casistica il difetto osseo era stato causato nel 42% dei casi da un'otite cronica colesteatomatosa, nel 33% da causa iatrogena, nel 17% il difetto era spontaneo e infine nell'8% post-traumatico. I principali sintomi sono stati: meningite, cefalea, ipoacusia trasmissiva, fistola liquorale, deficit neurologici e pneumoencefalo, stenosi di cavità di timpanoplastica aperta. Durante il follow up nessun paziente ha presentato complicanze legate alla chirurgia o correlate alla patologia e le indagini radiologiche hanno evidenziato una stabile chiusura del difetto osseo. Numerose tecniche chirurgiche sono state proposte per la risoluzione di questa patologia e la scelta si basa sulla localizzazione e la grandezza del difetto osseo, la funzione uditiva e la presenza di patologie concomitanti. Riteniamo pertanto che la tecnica da noi proposta, che si basa sull'utilizzo di una membrana di collagene e di un sostituto osseo, possa essere adatta per la riparazione del pavimento della fossa cranica media e per la risoluzione di questa patologia.
Introduction
Meningoencephalic herniation (MEH) in the middle ear and mastoid is a rare pathological entity, and few series are reported in the recent scientific literature 1. On the other hand, reports of spontaneous or idiopathic brain herniations without a prior history of trauma, chronic ear infection or surgery have increased in the last few years due to the introduction of more sensitive diagnostic tools such as computerized tomography (CT) and magnetic resonance imaging (MRI).
Thanks to the introduction of the antibiotics and improved surgical techniques, the possible life-threatening complications of this pathology, such as meningitis, encephalitis and cerebral abscess, have decreased over the years.
The aetiologies of the bony defect, necessary for the development of a brain herniation, include temporal bone trauma, iatrogenic injuries, chronic otitis media with or without cholesteatoma, congenital cranial base defects, neoplasia, irradiation and idiopathic causes 2 3. The dura is structurally strong and capable of supporting the brain over a large bony defect, so that an isolated temporal bone defect is usually insufficient to cause brain herniation. Increased intracranial pressure, possibly due to local oedema in relation to regional cerebritis, may contribute to this herniation 4.
This case series reports outcomes in the treatment of the MEH analyzing their pathogenesis, clinical presentation and surgical treatment: we propose the use of collagenous membranes and bone substitutes for the repair of the bony defect limited to the tegmen mastoideum and tympani through a transmastoid approach.
Material and methods
From January 2004 to December 2010, we treated 24 patients with MEH at the ENT Clinic of the "A. Gemelli" University Hospital of Rome. A trans-mastoid approach was performed for treating all cases. Through a classical retroauricular incision and mastoidectomy, after the treatment of any middle ear pathology, if present, the herniated brain tissue was exposed and pushed up to the bony defect if possible, or was removed using bipolar electrocoagulation. The dehiscence was then repaired from below using heterologous materials to obtain a multilayer support for the brain. We inserted a layer of a collagenous membrane in contact with the herniated tissue, and beneath this a bone substitute to reinforce the graft; the reconstruction was covered with temporalis fascia and sealed with fibrin glue when CSF leak was present. During follow-up, patients underwent CT and/or MRI at 1 and 2 years after surgery.
Results
We treated 24 patients presenting with MEH: 13 were males and 11 were females; mean age was 56 years, ranging between 33 and 82 years. The cause of the bony defect was chronic otitis media with cholesteatoma in 10 patients (42%), iatrogenic in 8 patients (33%; 6 cases of previous intact canal wall and 2 cases of canal wall down), spontaneous in 4 patients (17%) and post-traumatic in 2 patients (8%).
The major presenting symptoms related to the presence of a MEH were meningitis (8 patients, 33%), headache (2 patients; 8%), conductive hearing loss (2 patients; 8%), headache and conductive hearing loss (2 patients; 8%), CSF leak (1 patient; 4%), neurologic deficit and pneumoencephalus (1 patient; 4%), stenosis of a canal wall down cavity (1 patient; 4%) (Fig. 1). Seven patients (29%) complained only of symptoms of chronic otitis media. Among patients with a MEH due to chronic otitis media, 5 patients presented meningitis, with intra-operative diagnosis; in the group of iatrogenic MEH, 3 patients developed meningitis, 2 complained of headache; for 2 patients diagnosis was intra-operative and 1 presented a stenosis of the cavity. All patients with a spontaneous MEH complained of conductive hearing loss and 2 patients also complained of headache; one patient with a prost-traumatic MEH presented CSF leak and the other developed a neurologic deficit and pneumoencephalus.
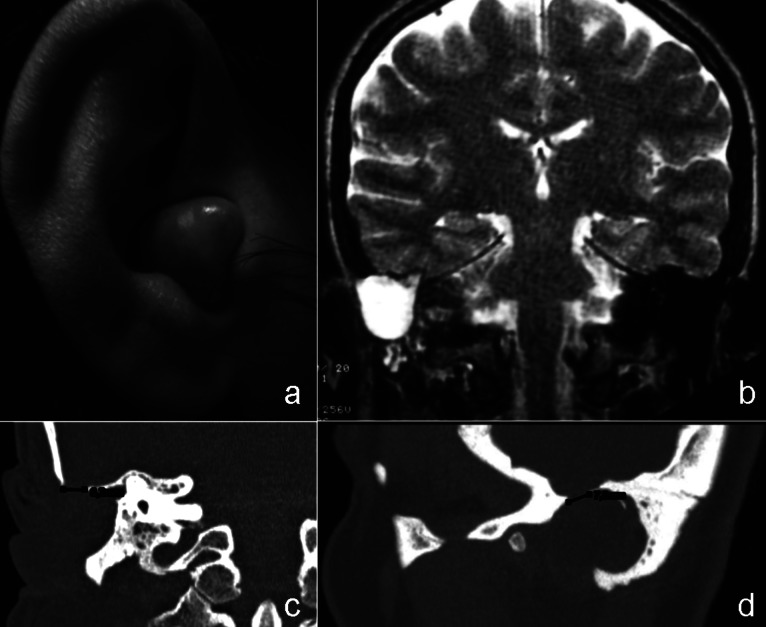
Fig. 1.
Canal wall down cavity occlusion due to a huge meningeal herniation (a) filled by CSF, as showed by the hyperintense signal in T2-weighted MR image (b); the coronal and sagittal CT images show a limited bony dehiscence of 7x6 mm (c-d)
The bony defect was located in the tegmen mastoideum in 13 patients (54%), in the tegmen tympani in 4 cases (17%) and in 7 patients (29%) both tegmen were involved by the dehiscence. All cases of tegmen tympani dehiscence were observed in patients with a spontaneous MEH, and limited to the posterior epitympanum without any ossicular chain involvement. The majority of cases of tegmen mastoideum dehiscence were observed in the iatrogenic group of MEH (n = 8) and among another 5 patients with a cholesteatoma; another 5 patients with COM and 2 with a post-traumatic cause presented a bony defect involving both tegmen.
Follow-up averaged 28 months (range 12-60 months). During follow-up no patients developed complications due to surgery or related to the pathology. CT and MRI showed a stable occlusion of the bony defect (Fig. 2), and only in one case a slight protrusion of brain in the middle ear was observed one year after surgery that remained stable at the following examination.
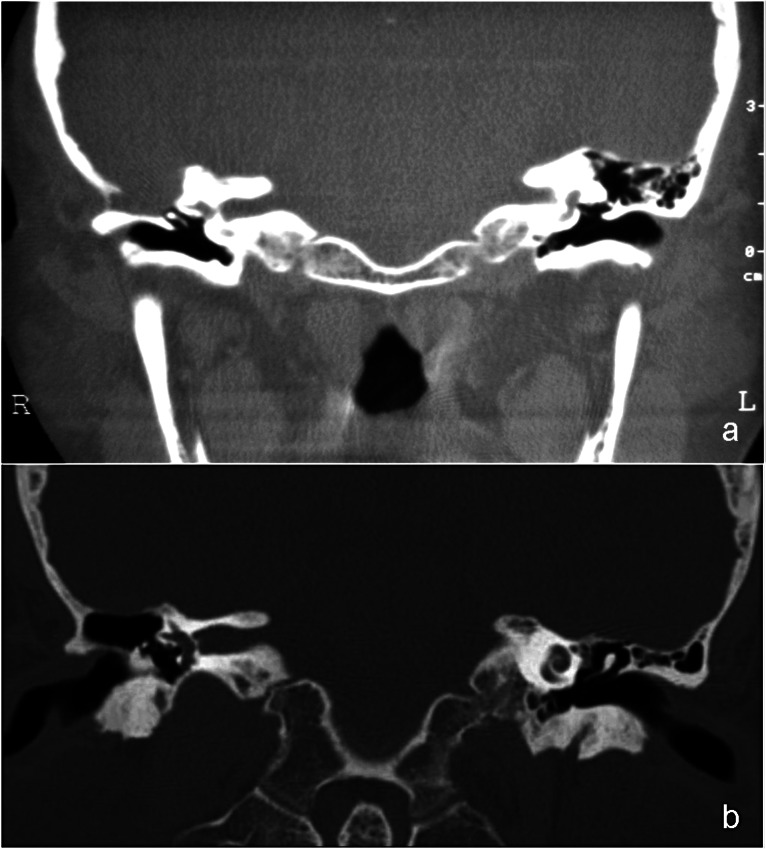
Fig. 2.
Pre-operative CT scan (a) showing a bony dehiscence of the tegmen in a patient with a previous canal wall up tympanoplasty: the cavity appears to be completely filled by a soft tissue mass contiguous with the brain and similar in signal to it. The postoperative image (b) shows a stable occlusion of the bony defect and the cavity is aerated.
Discussion
Herniation of brain tissue in the middle ear is rare and usually occurs following middle ear infection, especially cholesteatoma, or after surgery performed to eradicate the disease. Brain tissue usually protrudes from the middle fossa through a defect either in tegmen tympani or in the mastoid tegmen. Less commonly, the osseous defect is congenital or follows skull fracture, tumour or irradiation. However, cases of spontaneous protrusion of brain tissue in the middle ear are also present in the literature, but their aetiology is unclear. Not all bony defects will lead to a MEH as demonstrated by autopsy findings in which the temporal bone defects were higher than the frequency of MEH 5, this discrepancy reinforces the observation that a tegmental defect is necessary but not sufficient to cause dural/brain herniation without an associated dural deficiency.
Large bony defects, small dehiscences and a thin transparent layer of cortical bone covering the pneumatic cells of the mastoid have been observed close one to another in the same temporal bone 6 7. In addition, the dura of the temporal lobe varies considerably in thickness from one individual to another and even in the same patient, and areas covered by thin and fragile dura may be observed close to areas where the dura is thicker. The combination of a bone defect in the tegmen with a dural defect at the same location may predispose the patient to brain herniation. Such predisposing factors could explain why MEH occurs mainly through the middle cranial fossa, at the level of the tegmen mastoideum or tympani, and rarely through the posterior fossa. Other theories involve variations of intracranial pressure, decades of CSF pulsation, low-grade inflammation, ageing or aberrant arachnoid granulations in the pathogenesis of congenital herniations 3 8 9.
Indeed, histopathological specimens did not show any pathological result that would explain the pathogenesis of the MEH: the herniated tissue was reported to consist of normal but disorganized nervous tissue with numerous well-preserved neurons and synapses near the surface and in the internal part of the herniation that was covered by middle ear mucosa with cuboidal cells 10.
Bony dehiscence and brain tissue herniation can be acquired or spontaneous, and its pathogenesis influences the presenting symptoms. Acquired herniations are most commonly due to chronic otitis media with or without cholesteatoma and, in these cases, may be clinically silent beneath signs and symptoms of chronic middle ear disease, such as hearing loss, chronic otorrhoea, tinnitus and vertigo 4. Bony defects, due to chronic ear surgery or other causes, can result in complications including CSF otorrhoea, pulsatile middle ear or external auditory canal mass, seizures, meningitis, brain abscess, epidural abscess and death 11. Other causes of acquired brain tissue herniation include trauma, neoplasia, irradiation or inflammatory conditions 12. Symptoms of spontaneous brain herniation are generally mild, as serous otitis media, aural fullness, hearing loss and cerebrospinal fluid otorrhoea, although life-threatening complications such as meningitis, brain abscess and temporal lobe seizures are occasionally sentinel events 13 14. In our series, presenting symptoms were mostly related to the presence of brain herniation; excluding 7 patients, who complained only of symptoms of chronic otitis media, the others (79%) complained of symptoms or presented signs related to the presence of the MEH such as meningitis, headache, neurologic deficit and a mass in the middle ear.
The diagnosis of brain herniation is facilitated by the combination of CT and MRI. The former demonstrates a tegmen defect using data reconstructed with a bone algorithm, but has a limited ability to resolve subtle distinctions in density between the brain, cholesteatoma, granulation tissue, and other soft tissue masses inside the middle ear cavity. MRI is the ideal method to differentiate these conditions: herniated meningoencephalic tissue is seen as a non-enhancing contiguous mass that is isointense to brain in all sequences. In contrast, a cholesteatoma appears hyperintense in T2-weighted images, and a cholesterol granuloma appears hyperintense in both T1- and in T2-weighted images. Contrast administration enhances only granulation tissue. Usually, T2-weighted coronal sections are the most useful to identify middle cranial fossa brain herniations. Diffusion-weighted imaging shows a hyperintense signal in cases of cholesteatoma and helps in differential diagnosis 15 (Fig. 3).
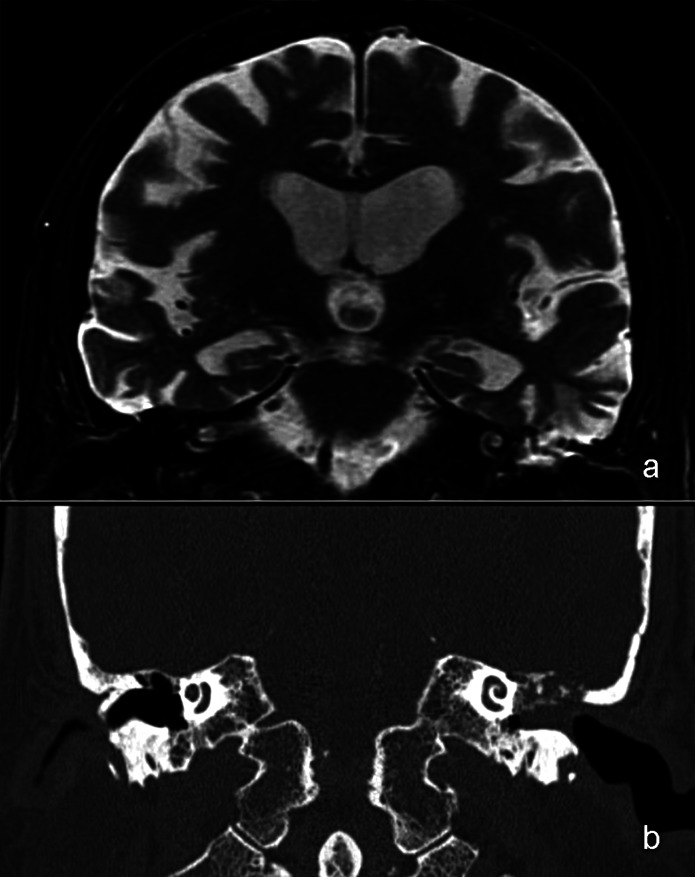
Fig. 3.
Coronal T2-weighted MR image (a) showing a hyperintense signal consistent with a cholesteatoma in the mastoid and a slight protrusion of brain tissue, whereas the coronal CT image (b) shows a bony tegmen defect and indistinguishable soft tissue.
Surgery is the primary modality of treatment, and different techniques and approaches have been described for repair of the bony and dural defect. The surgical approach is based on the aetiology, position and size of the bony defect, presence of chronic infection in the middle ear and/or intraoperative active CSF leakage. These include the middle fossa craniotomy approach, transmastoid approach, combined transmastoid with a minicraniotomy at the level of the temporal fossa and middle ear obliteration with abdominal fat with blind sac closure of the external auditory canal 1. A transmastoid approach was applied uniformly in this series to treat the MEH with a high level of success: this approach is helpful for treating small dehiscences up to 2 cm2, as in our series; obviously, the bony defect cannot reach the anterior epitympanum if the ossicular chain is still in place.
Many techniques for the repair of bony defects of the floor of the middle cranial fossa have been described 3 16-21. The most commonly employed are the fascia-bone-fascia technique 5 and cartilage 1. Kveton and Goravalingappa18 described the use of hydroxyapatite cement via a transmastoid approach, whereas Gubbels et al14 reported the use of the same material but through a middle cranial fossa approach. To our knowledge, the use of a collagenous membrane combined with a bone substitute to repair tegmen defects through a transmastoid approach has not previously been described. The collagenous membrane derived from bovine pericardium was developed for replacing or reinforcing connective tissue structures; it initially provides reliable closure of the tissue defect and serves as a scaffold for the cellular repair mechanism, which will replace this scaffold with the patient's own tissue. The bone substitute has a number of properties that make it a useful material to repair bony defects. Osteoclasts and osteoblasts recognize this matrix as endogenous and begin a remodeling process that lead to the growth of new bone. Moreover, the importance of multilayered over single-layer closure techniques was the preferred method for repair of the defects of the floor of the middle cranial fossa, as clearly demonstrated in literature 19.
Conclusions
Meningoencephalic herniations occur infrequently, but can lead to significant morbidity if not accurately diagnosed and properly treated. Different causes can lead to this condition and, once MEH is suspected, thorough radiologic assessment should be performed based on the combination of CT and MRI. Although multiple approaches have been described for the treatment of encephaloceles of the temporal bone, the most appropriate should be chosen based on the localization and size of the herniated tissue, preoperative auditory function and the presence of active infection or another coexisting pathology. We believe that a transmastoid approach is good for treating small dehiscences up to 2 cm2, which do not reach the anterior epitympanum if the ossicular chain is still in place. The use of collagenous membranes and bone substitutes for reconstruction of the floor of the middle fossa, as described in this study, provides a safe and useful option to the temporal bone surgeon for reconstruction of tegmen defects.
References
- 1.Sanna M, Fois P, Russo A, et al. Management of meningoencephalic herniation of the temporal bone: Personal experience and literature review. Laryngoscope. 2009;119:1579–1585. doi: 10.1002/lary.20510. [DOI] [PubMed] [Google Scholar]
- 2.Valtonen H, Geyer C, Tarlov E, et al. Tegmental defects and cerebrospinal fluid otorrhea. ORL J Otorhinolaryngol Relat Spec. 2001;63:46–52. doi: 10.1159/000055705. [DOI] [PubMed] [Google Scholar]
- 3.Brown NE, Grundfast KM, Jabre A, et al. Diagnosis and management of spontaneous cerebrospinal fluid-middle ear effusion and otorrhea. Laryngoscope. 2004;114:800–805. doi: 10.1097/00005537-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Mosnier I, Fiky LE, Shahidi A, et al. Brain herniation and chronic otitis media: diagnosis and surgical management. Clin Otolaryngol Allied Sci. 2000;25:385–391. doi: 10.1046/j.1365-2273.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 5.Lundy LB, Graham MD, Kartush JM, et al. Temporal bone encephalocele and cerebrospinal fluid leaks. Am J Otol. 1996;17:461–469. [PubMed] [Google Scholar]
- 6.Ahrén C, Thulin C-A. Lethal intracranial complications following inflation in the external auditory canal in treatment of serous otitis media and due to defects in the petrous bone. Acta Otolaryngol. 1965;60:407–421. [Google Scholar]
- 7.Kapur TR, Bangash W. Tegmental and petromastoid defects in the temporal bone. J Laryngol Otol. 1986;100:1129–1132. doi: 10.1017/s0022215100100702. [DOI] [PubMed] [Google Scholar]
- 8.Gacek R, et al. Arachnoid granulation cerebrospinal otorrhea. Ann Otol Rhinol Laryngol. 1990;99:854–862. doi: 10.1177/000348949009901102. [DOI] [PubMed] [Google Scholar]
- 9.Gacek R. Evaluation and management of temporal bone arachnoid granulations. Arch Otolaryngol Head Neck Surg. 1992;118:327–332. doi: 10.1001/archotol.1992.01880030119024. [DOI] [PubMed] [Google Scholar]
- 10.Iurato S, Bux G, Colucci S, et al. Pathology of idiopathic encephaloceles into the middle ear. ORL J Otorhinolaryngol Relat Spec. 2002;64:73–79. doi: 10.1159/000057784. [DOI] [PubMed] [Google Scholar]
- 11.Wootten CT, Kaylie DM, Warren FM, et al. Management of brain herniation and cerebrospinal fluid leak in revision chronic ear surgery. Laryngoscope. 2005;115:1256–1261. doi: 10.1097/01.MLG.0000165455.20118.E3. [DOI] [PubMed] [Google Scholar]
- 12.Ramalingam KK, Ramalingam R, SreenivasaMurthy TM, et al. Management of temporal bone meningo-encephalocoele. J Laryngol Otol. 2008;122:1168–1174. doi: 10.1017/S0022215108001990. [DOI] [PubMed] [Google Scholar]
- 13.Mayeno JK, Korol HW, Nutik SL. Spontaneous meningoencephalic herniation of the temporal bone: case series with recommended treatment. Otolaryngol Head Neck Surg. 2004;130:486–489. doi: 10.1016/j.otohns.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Gubbels SP, Selden NR, Delashaw JB, Jr, et al. Spontaneous middle fossa encephalocele and cerebrospinal fluid leakage: diagnosis and management. Otol Neurotol. 2007;28:1131–1139. doi: 10.1097/MAO.0b013e318157f7b6. [DOI] [PubMed] [Google Scholar]
- 15.Bovo R, Ceruti S, Padovani R, et al. Temporal bone brain herniation. Otol Neurotol. 2006;27:576–577. doi: 10.1097/01.mao.0000233525.51866.b0. [DOI] [PubMed] [Google Scholar]
- 16.Dutt SN, Mirza S, Irving RM. Middle cranial fossa approach for the repair of spontaneous cerebrospinal fluid otorrhoea using autologous bone pate. Clin Otolaryngol Allied Sci. 2001;26:117–123. doi: 10.1046/j.1365-2273.2001.00438.x. [DOI] [PubMed] [Google Scholar]
- 17.Rao AK, Merenda DM, Wetmore SJ. Diagnosis and management of spontaneous cerebrospinal fluid otorrhea. Otol Neurotol. 2005;26:1171–1175. doi: 10.1097/01.mao.0000179526.17285.cc. [DOI] [PubMed] [Google Scholar]
- 18.Kveton JF, Goravalingappa R. Elimination of temporal bone cerebrospinal fluid otorrhea using hydroxyapatite cement. Laryngoscope. 2000;110:1655–1659. doi: 10.1097/00005537-200010000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Savva A, Taylor MJ, Beatty CW. Management of cerebrospinal fluid leaks involving the temporal bone: report on 92 patients. Laryngoscope. 2003;113:50–56. doi: 10.1097/00005537-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Sdano MT, Pensak ML. Temporal bone encephaloceles. Curr Opin Otolaryngol Head Neck Surg. 2005;13:287–289. doi: 10.1097/01.moo.0000179247.51476.f5. [DOI] [PubMed] [Google Scholar]
- 21.Adkins WY, Osguthorpe JD. Mini-craniotomy for management of CSF otorrhea from tegmen defects. Laryngoscope. 1983;93:1038–1040. doi: 10.1288/00005537-198308000-00012. [DOI] [PubMed] [Google Scholar]