SUMMARY
The objective of this study was to analyze the safety and results of intra-operative SAN (spinal accessary nerve) monitoring during selective neck dissection, with emphasis on shoulder syndrome. Twenty-five consecutive patients with head and neck cancer were studied. Selective neck dissection was performed by a single clinical fellow under the supervision of the department chief using an intra-operative SAN monitor. Electrophysiological data were recorded after initial identification of the SAN and continued until just before closure. Electromyographic evaluation was carried out to assess SAN function one month postoperatively. Shoulder disability was also evaluated at this time using a questionnaire for shoulder syndrome (shrug, flexion, abduction, winging, and pain). No patients had postoperative shoulder syndrome involving shrug, flexion, abduction, or winging. Twenty-two of the 25 (88%) patients had shoulder pain, but the average pain score was low (2.3 ± 1.3). No patients had neck recurrence during at least 1 year of follow up. By using nerve monitoring during selective neck dissection, no patient developed significant "shoulder syndrome", with the exception of slight pain.
KEY WORDS: Head and neck, Selective neck dissection, Axonal injury, Spinal accessory nerve, Electrophysiology
RIASSUNTO
L'obiettivo del nostro studio è stato di analizzare la sicurezza ed il risultato del monitoraggio intra-operatorio del nervo accessorio spinale (NAS) durante lo svuotamento selettivo del collo. Sono stati studiati venticinque pazienti con tumore testa-collo. L'intervento chirurgico è stato eseguito da un medico specializzando sotto la supervisione del capo dipartimento con l'utilizzo del monitoraggio intra-operatorio del nervo accessorio spinale. I dati elettrofisiologici sono stati registrati a partire dall'identificazione del nervo spinale accessorio, fino a poco prima della chiusura. Un mese dopo l'intervento è stata effettuata una valutazione elettromiografica per valutare la funzione del nervo spinale accessorio. Allo stesso tempo la disabilità di spalla è stata valutata anche attraverso un questionario specifico per la sindrome della spalla. Nessun paziente ha presentato la suddetta sindorme al termine dello svuotamento. Ventidue dei 25 (88%) pazienti hanno avuto dolore alla spalla, ma il punteggio medio del dolore è stato basso (2,3 ± 1,3). Nessun paziente ha avuto una recidiva a livello del collo per almeno 1 anno di follow-up. Concludendo usando il monitoraggio del nervo durante l'intero intervento, non si è verificata una significativa insorgenza della sindrome della spalla, con l'eccezione della sensazione di dolore.
Introduction
One of the important prognostic factors in head and neck cancer is control of cancer spread to regional lymph nodes during neck dissection. Radical neck dissection (RND) removes all levels of cervical lymph nodes, together with the spinal accessory nerve (SAN), sternocleidomastoid muscle (SCM), and internal jugular vein as defined by the American Academy for Otolaryngology-Head and Neck Surgery (AAO-HNS) classification 1. Due to the various symptoms that appear after RND, including pain over the neck and shoulders, difficulty in shoulder elevation, and scapular winging, patient quality of life is greatly affected 2. In order to minimize postoperative functional impediments, modified RND (MRND) or selective neck dissection (SND) has been preferred in recent years. SND of levels II-IV is effective in N0 laryngeal squamous cell carcinoma 3, and sentinel lymph node biopsy can be considered a useful tool to personalize the surgical approach in N0 carcinomas 4 5. Thus, it is important that head and neck surgeons are trained to perform the functional neck dissection without injury to preserved tissues, especially the SAN, while ensuring patient survival. However, operations that preserve partial or complete functionality require greater surgical skill.
There are several reports 6-8 describing abnormalities in nerve conduction of the SAN and in needle electromyography (EMG) of the trapezius muscle despite SAN preservation during MRND. Clearly, it is important to minimize damage to the SAN, yet there are only few studies 9 10 that have evaluated the feasibility of monitoring the SAN during neck dissection. However, investigators did not use a nerve integrity monitoring system; rather, a 12-channel axon system was used which required the presence of an additional technician during surgery. In one study, it was demonstrated that in the patients without an electrophysiologic threshold increase, 89% did not develop shoulder functional deficits 10. It would appear that preservation of the SAN, the main nerve innervating the trapezius, is key to minimize shoulder dysfunction after neck dissection. Moreover, only a few studies 11 12 have evaluated electrophysiological changes and damage to the trapezius muscle in patients after SND. We therefore examine the outcomes of fellow-performed cases in terms of shoulder syndrome and rate of neck recurrence. This is the first study to determine the effects of intra-operative SAN monitoring with a nerve integrity monitoring system during SND while training head and neck surgical fellows.
Materials and methods
Between October 2007 and July 2008, 25 consecutive patients (all male; mean age, 55 ± 7.7 years; range, 42-69 years) receiving SND or MRND performed by a clinical fellow under the supervision of the department chief (Mu- Kuan Chen, Department of Otorhinolaryngology, Head and Neck Surgery, Changhua Christian Hospital, Changhua, Taiwan) were studied. None of the patients had concomitant rheumatological, metabolic disorders, or previous trauma, and patients who had previous neck biopsy or dissection were excluded. In Taiwan, to become a head and neck surgeon, students complete a 2-year internship, followed by 5.5 years of residency training. Thus, senior residents are defined as clinical fellows. A clinical fellow case is defined as a procedure performed from "skin to skin" by the trainee with the department chief acting as the first assistant or supervising, while another two junior residents directly assist the clinical fellow. This means that the clinical fellow performs all technical aspects of the operation and also acts as supervisor.
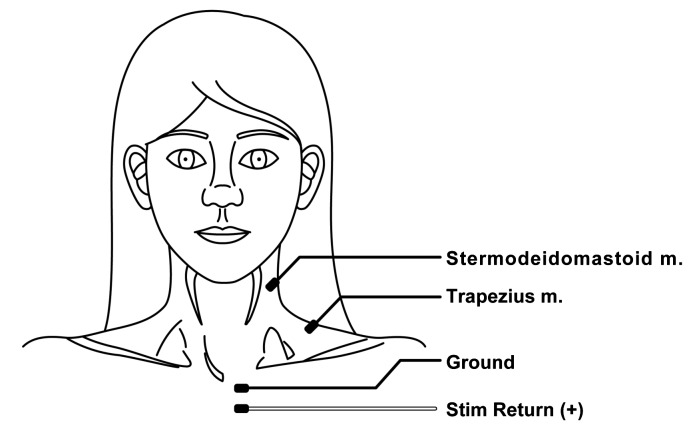
Direct electrical stimulation of neural and non-neural tissue was provided intraoperatively during neck dissection by using a Medtronic-ENT nerve integrity monitoring system (NIM-2.0, Medtronic, USA). Compared with conventional 12-channel axon systems that require the presence of an additional technician during the surgery, the Medtronic-ENT nerve integrity monitoring system can be easily used by the surgeon. The nerve integrity monitoring system is easy to set up and is not time-consuming as it takes only about 5 min. At the beginning of each operation, a blue electrode was inserted into the sternocleidomastoid muscle and a red electrode into the trapezius muscle. Additionally, a ground electrode (green) and a stimulatory electrode (white) were required to complete the electrode setup (Fig. 1). It was important that the patient was not paralyzed during surgery. Electrophysiologic data was recorded from the time of initial identification of the SAN throughout the entire operation, and was terminated immediately before closure. An improved signal-to-noise ratio increases the sensitivity of the NIMResponse 2.0 to lower-level EMG activity. The surgeon can hear and identify small EMG signals, and thus minimize nerve manipulation during surgery.
Fig. 1.
Spinal accessory cranial nerve XI monitoring, left operative side continuous discharges in all areas of the muscle.
Clinical correlation measurements and parameters of "shoulder syndrome" were evaluated at 1 month postoperatively. Functional shoulder status was evaluated using a questionnaire focused on 5 shoulder functions: shrug, flexion, abduction, winging and pain. Shoulder shrugs evaluated the strength of the upper trapezius and was described using a scale from 0 to 5 scale defined as: 0, no active muscle contraction; 1, trace contraction; 2, unable to raise the scapula against gravity; 3, a muscle contraction only sufficient to raise the scapula against gravity; 4, the ability to raise the scapula against partial resistance by the examiner; and 5, the ability to raise the scapula against substantial resistance by the examiner. Shoulder active range of motion, including flexion and abduction, were measured in a seated position. In this study, the active range of motion measures was simplified into outcomes of > 90° and < 90°. The presence of scapular winging was examined with inspections at resting and active scapular positions. Ratings were defined as: 0, no significant displacement; 1, minimal displacement; 2, moderate displacement; and 3, severe displacement. For shoulder pain, patients were asked to specifically describe the pain in the affected shoulder along the superior border of the scapula. The evaluation of shoulder pain used scores of 0 to 10, with 0 no pain and 10 worst pain.
Electromyographic evaluation and denervation potentials (fibrillation and positive sharp waves) at rest were investigated in all patients to assess SAN function at 1 month postoperatively. Electromyography was carried out by a clinical neurophysiologist. Fibrillation potentials and positive sharp wave evaluation scores were created to quantitatively evaluate the severity of SAN injury; the evaluation score included 5 grades, with 0 normal, 1 persistent single runs > 1 sec in two areas, 2 moderate runs > 1 sec in three or more areas, 3 many discharges in most muscle regions and 4 continuous discharges in all areas of the muscle.
Results
The 25 subjects ranged in age from 42 to 69 years. According to TNM staging criteria, 9 cases (36%) were stage I, 4 (16%) were stage II, 7 (28%) were stage III and 5 (20%) were stage IV (Table I). Nine patients had metastatic lymph nodes (2 level I, 7 level II and 1 level III), one of which had multiple ipsilateral lymph nodes (N2b). No patients had shoulder syndromes involving shrug (all were score 5), flexion (all > 90°), abduction (all > 90°), or winging (all were score 0) upon completion of the dissection. Twenty-two of 25 (88%) patients had shoulder pain. The average pain score was 2.3 ± 1.3. Pain scores in 4 subjects (16%) were scored as 0 (normal), 3 (12%) had a score of 1, 11 (44%) had a score of 2, 6 (24%) had a score of 3 and 1 (4%) had a score of 6 points (most severe). The median time period between operation and needle EMG was 35 days. With needle EMG, fibrillation potentials at rest were observed in 10 (40%) cases and positive sharp waves in 16 (64%) cases. Statistical significance was obtained between shoulder pain score and EMG fibrillation or positive sharp wave score (p < 0.005). No patient had recurrence of neck disease during at least 1 year of follow up.
Table I.
TNM stage of the 25 patients.
| N0 | N1 | N2a | N2b | |
|---|---|---|---|---|
| T1 | 9 | 2 | 0 | 0 |
| T2 | 4 | 2 | 0 | 0 |
| T3 | 1 | 2 | 0 | 0 |
| T4 | 2 | 2 | 0 | 1 |
Discussion
There are no published reports on the training of clinical fellows to perform SND with intra-operative spinal accessory nerve monitoring under staff supervision. Some studies have, however, compared the incidence of shoulder symptoms among RND, MRND and SND treatment groups 13-15, with an incidence that was lowest following SND (29-39%), intermediate following MRND (36-77%) and highest following RND (60-100%). Therefore, it was suggested that SND could reduce postoperative shoulder damage to a greater extent than MRND 15. In the present study using the Medtronic-ENT nerve integrity monitoring system during surgery, although 22 of 25 (88%) patients had shoulder pain, the average pain score was only 2.2 ± 1.3. This score may be due to early assessment at 1 month after major surgery, with some of the low pain scores due to postoperative surgical pain. Shoulder syndrome includes pain in the affected shoulder along the superior border of the scapula, shoulder shrug weakness, limitations in active range of motion of the shoulder and scapular winging at rest. No patients in the current cohort experienced shoulder syndrome involving shrug, flexion, abduction or winging following neck dissection. Thus, to prevent shoulder syndrome following neck dissection, use of the Medtronic-ENT nerve integrity monitoring system during surgery may be an effective tool.
By comparing different types of SND, supraomohyoid neck dissection (removal of level I, II and III lymph nodes) had the lowest incidence of shoulder symptoms 16. Patients who underwent SND involving the removal of levels II to IV also had fewer shoulder symptoms. Cappiello et al. 11 concluded that removal of level V lymph nodes increased the incidence of movement disorders of the shoulder. In our series, 13 of the 25 (52.0%) patients received level I to V neck dissection, and using the Medtronic-ENT nerve integrity monitoring system shoulder symptoms were acceptable, despite the fact that the interventions were performed by a training clinical fellow under supervision.
Even if the SAN is spared in SND, some degree of shoulder syndrome may occur due to traction or trauma to the SAN during the operation. Our results suggest that for the training of head and neck fellows in selective neck dissection, the use of intra-operative spinal accessory nerve monitoring results in good functional preservation for at least 1 year of follow up.
Midwinter et al. 19 shows a peripheral nerve monitor and stimulator is useful in identifying and preserving the accessory nerve during nerve-sparing neck dissection and biopsy of a neck mass in the region of the accessory nerve in a series of 10 patients. Remmler et al. 17 showed that the trapezius muscle EMG finding of fibrillation potentials and positive sharp waves at rest were observed in more than half of patients for up to 3 months after MRND surgery. Several other studies 6-8 also reported similar results regarding fibrillation potentials and positive sharp waves at rest following MRND. In the present study, fibrillation potentials at rest were observed in 10 (40%) cases and positive sharp waves in 16 (64%) cases. The possible reasons for EMG findings of fibrillation potentials and positive sharp waves include traction of the nerve, unintentional resection of nerves, nerve injury by electric scalpels, haematoma formation, drain aspiration and infection.
In order to widen the surgical field during neck dissection, damage to the SAN caused by prolonged surgical retraction has been noted by several authors 6 7 16 17. In these reports, neck dissection was impossible to perform without any damage to the SAN. This conclusion emphasizes the importance of taking every measure to avoid damage to the SAN during surgery. With advancements in technology, our data support the use of the SAN monitor during the entire functional neck dissection, especially when performed by a training fellow or a young attending staff member.
Neck recurrence rates in appropriately selected patients are not higher following SAN-preserving neck dissections. As mentioned above, patients undergoing SANsparing neck dissection have less disability than patients undergoing radical neck dissection 18. In the present study, no patients had neck recurrence during at least 1 year of follow up. However, evaluation of the monitoring technique in a larger patient series with longer follow-up is warranted to establish a more conclusive result.
A major limitation of this study is that there was no control group. However, using nerve integrity monitoring system, according previous experience in our centre and compared with other studies, patients will gain some benefit from SAN monitoring; thus, it seems unethical to include a control group to receive the SND without nerve integrity monitoring. Another limitation of the present study is the small number of cases. However, compared with the published studies 6 7 16 17, our preliminary result is encouraging and shoulder syndrome was minimized, with likely improvement in the patients' quality of life.
Conclusions
In conclusion, preservation of the SAN, the main nerve innervating the trapezius, is key to minimize shoulder dysfunction after neck dissection. However, even after SND in which the SAN was preserved, some denervation of the upper trapezius muscle can result from axonal injury of the SAN because of traction of the nerve during surgery. The results of the present pilot study suggest that by using intraoperative spinal accessory nerve monitoring in training the clinical fellow to perform functional neck dissection under supervision, SAN axonal injury and severity of shoulder syndrome are mild without sacrificing patient outcomes.
Part of this study was presented at the First Congress of Asian Society of Head & Neck Oncology (ASHNO) 18- 19 September 2009, Taipei, Taiwan.
References
- 1.Ferlito A, Robbins KT, Silver CE, et al. Classification of neck dissections: an evolving system. Auris Nasus Larynx. 2009;36:127–134. doi: 10.1016/j.anl.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Shone GR, Yardley MP. An audit into the incidence of handicap after unilateral radical neck dissection. J Laryngol Otol. 1991;105:760–762. doi: 10.1017/s0022215100117232. [DOI] [PubMed] [Google Scholar]
- 3.Deganello A, Gitti G, Meccariello G, et al. Effectiveness and pitfalls of elective neck dissection in N0 laryngeal cancer. Acta Otorhinolaryngol Ital. 2011;2011:216–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Manola M, Aversa C, Moscillo L, et al. Status of level IIb lymph nodes of the neck in squamous cell carcinoma of the oral tongue in patients who underwent modified radical neck dissection and lymph node sentinel biopsy. Acta Otorhinolaryngol Ital. 2011;31:130–134. [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio JK, Santini S, Politi D, et al. Sentinel lymph node biopsy in squamous cell carcinoma of the head and neck: 10 years of experience. Acta Otorhinolaryngol Ital. 2012;32:18–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Koybasioglu A, Tokcaer AB, Uslu S, et al. Accessory nerve function after modified radical and lateral neck dissections. Laryngoscope. 2000;110:73–77. doi: 10.1097/00005537-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Patten C, Hillel AD. The 11th nerve syndrome. Accessory nerve palsy or adhesive capsulitis? Arch Otolaryngol Head Neck Surg. 1993;119:215–220. doi: 10.1001/archotol.1993.01880140105016. [DOI] [PubMed] [Google Scholar]
- 8.Sobol S, Jensen C, Sawyer W, 2nd, et al. Objective comparison of physical dysfunction after neck dissection. Am J Surg. 1985;150:503–509. doi: 10.1016/0002-9610(85)90164-3. [DOI] [PubMed] [Google Scholar]
- 9.Witt R, Gillis G, Pratt R, Jr, et al. Spinal accessory nerve monitoring with clinical outcome measures. ENT J. 2006;85:540–544. [PubMed] [Google Scholar]
- 10.Witt RL, Rejto L. Spinal accessory nerve monitoring in selective and modified neck dissection. Laryngoscope. 2007;117:776–780. doi: 10.1097/MLG.0b013e3180341a0c. [DOI] [PubMed] [Google Scholar]
- 11.Cappiello J, Piazza C, Giudice M, et al. Shoulder disability after different selective neck dissections (levels II–IV versus levels II– V): a comparative study. Laryngoscope. 2005;115:259–263. doi: 10.1097/01.mlg.0000154729.31281.da. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji T, Tanuma A, Onitsuka T, et al. Electromyographic findings after different selective neck dissections. Laryngoscope. 2007;117:319–322. doi: 10.1097/01.mlg.0000249781.20989.5c. [DOI] [PubMed] [Google Scholar]
- 13.Leipzig B, Suen JY, English JL, et al. Functional evaluation of the spinal accessory nerve after neck dissection. Am J Surg. 1983;146:526–530. doi: 10.1016/0002-9610(83)90246-5. [DOI] [PubMed] [Google Scholar]
- 14.Pinsolle V, Michelet V, Majoufre Cet, et al. Spinal accessory nerve and lymphatic neck dissection. Rev Stomatol Chir Maxillofac. 1997;98:138–142. [PubMed] [Google Scholar]
- 15.Cheng PT, Hao SP, Lin YH, et al. Objective comparison of shoulder dysfunction after three neck dissection techniques. Ann Otol Rhinol Laryngol. 2000;109:761–766. doi: 10.1177/000348940010900811. [DOI] [PubMed] [Google Scholar]
- 16.Wilgen CP, Dijkstra PU, Laan BF, et al. Shoulder complaints after nerve sparing neck dissections. Int J Oral Maxillofac Surg. 2004;33:253–257. doi: 10.1006/ijom.2003.0507. [DOI] [PubMed] [Google Scholar]
- 17.Remmler D, Byers R, Scheetz J, et al. A prospective study of shoulder disability resulting from radical and modified neck dissections. Head Neck Surg. 1986;8:280–286. doi: 10.1002/hed.2890080408. [DOI] [PubMed] [Google Scholar]
- 18.Short SO, Kaplan JN, Laramore GE, et al. Shoulder pain and function after neck dissection with or without preservation of the spinal accessory nerve. Am J Surg. 1984;148:478–482. doi: 10.1016/0002-9610(84)90373-8. [DOI] [PubMed] [Google Scholar]
- 19.Midwinter K, Willatt D. Accessory nerve monitoring and stimulation during neck surgery. J Laryngol Otol. 2002;116:272–274. doi: 10.1258/0022215021910735. [DOI] [PubMed] [Google Scholar]