Abstract
Podocytes are essential to the structure and function of the glomerular filtration barrier; however, they also exhibit increased expression of MHC class II molecules under inflammatory conditions, and they remove Ig and immune complexes from the glomerular basement membrane (GBM). This finding suggests that podocytes may act as antigen-presenting cells, taking up and processing antigens to initiate specific T cell responses, similar to professional hematopoietic cells such as dendritic cells or macrophages. Here, MHC–antigen complexes expressed exclusively on podocytes of transgenic mice were sufficient to activate CD8+ T cells in vivo. In addition, deleting MHC class II exclusively on podocytes prevented the induction of experimental anti-GBM nephritis. Podocytes ingested soluble and particulate antigens, activated CD4+ T cells, and crosspresented exogenous antigen on MHC class I molecules to CD8+ T cells. In conclusion, podocytes participate in the antigen-specific activation of adaptive immune responses, providing a potential target for immunotherapies of inflammatory kidney diseases and transplant rejection.
Adaptive immune responses are initiated by the stimulation of naive T lymphocytes by antigen-presenting cells (APCs). Briefly, dendritic cells (DCs) and macrophages are present throughout the body; on antigen capture, these cells enter local lymph nodes or the spleen and present the antigens to naive T cells. Whether antigen presentation results in immunity or tolerance depends on additional factors, such as danger signals, the type of APCs, and the local microenvironment.1 T cells recognize antigenic peptides bound to the MHC on the surface of APCs that are presented by three different mechanisms. (I) Intracellularly synthesized antigens, mainly derived from defective ribosomal products of intracellular pathogens or self-proteins, are presented by MHC class I molecules and may activate CD8+ cytotoxic T cells.2 (II) Peptide fragments from extracellular antigens, which are endocytosed and then degraded by proteases, are presented on MHC class II molecules by professional APCs to CD4+ helper T cells. (III) Endocytosed antigens can also be presented to CD8+ lymphocytes through a process called crosspresentation. Depending on the endocytotic process and the activation of APCs, alternative intracellular sorting of the antigen cargo may result in the loading and surface presentation of MHC class I peptide complexes.3,4 Professional APCs such as DCs are, thereby, capable of activating CD8+ cells to provide immunity against viruses that do not infect APCs or nonhematopoietic tumors. Other than the ability for antigen presentation, the activation of naïve T cells by antigen presentation implies additional qualities. First, the APCs have to exhibit high phagocytic capacity and the mechanism to channel the antigen into the according pathway. Thus, antigen presentation requires cell–cell contact; the APCs must have the ability to sense danger signals, like microbiobal components or innate proinflammatory secretions, and after activation, the APCs have to attract the T cells by chemokines. Finally, after T cell receptor ligation, they ensure T cell activation by the expression of several costimulatory receptors.
The main function of the kidney glomerulus is to filter blood plasma into primary urine for subsequent handling by the renal tubular system. Small molecules, such as water, sugars, electrolytes, and small proteins, pass through, whereas the barrier retains high-molecular weight proteins and cells in circulation. The glomerular filtration barrier consists of a specialized fenestrated endothelium, the glomerular basement membrane, and filtration slits, which are generated by intricately interdigitated foot processes from terminally differentiated visceral epithelial cells known as podocytes.5 Several studies defined the critical importance of podocytes for kidney function, and it is now widely accepted that damage to the podocytes is a key event that initiates progression to many glomerular diseases.6
Under inflammatory conditions, podocytes display enhanced expression of MHC class II molecules, which was first described for pauci-immune necrotizing crescentic GN.7 B7-1, a costimulatory molecule that is essential for complete activation of T cells, is likewise upregulated during local renal inflammation (e.g., lipopolysaccharide-induced nephritic syndrome).8 Because podocytes are in continuous contact with blood plasma and hence, foreign antigens present in the blood and because podocytes remove Ig and immune complexes from the glomerular basement membrane through immune globulin receptor type N,9 we suspected that podocytes could act as professional APCs like macrophages. This finding was underlined by the fact that podocytes and macrophages may share lineage commitment (e.g., reflected by their expression of the transcription factor MafB10) as well as other sets of genes.
Results
Podocytes Phagocytose-Labeled Latex Beads and Soluble Ovalbumin
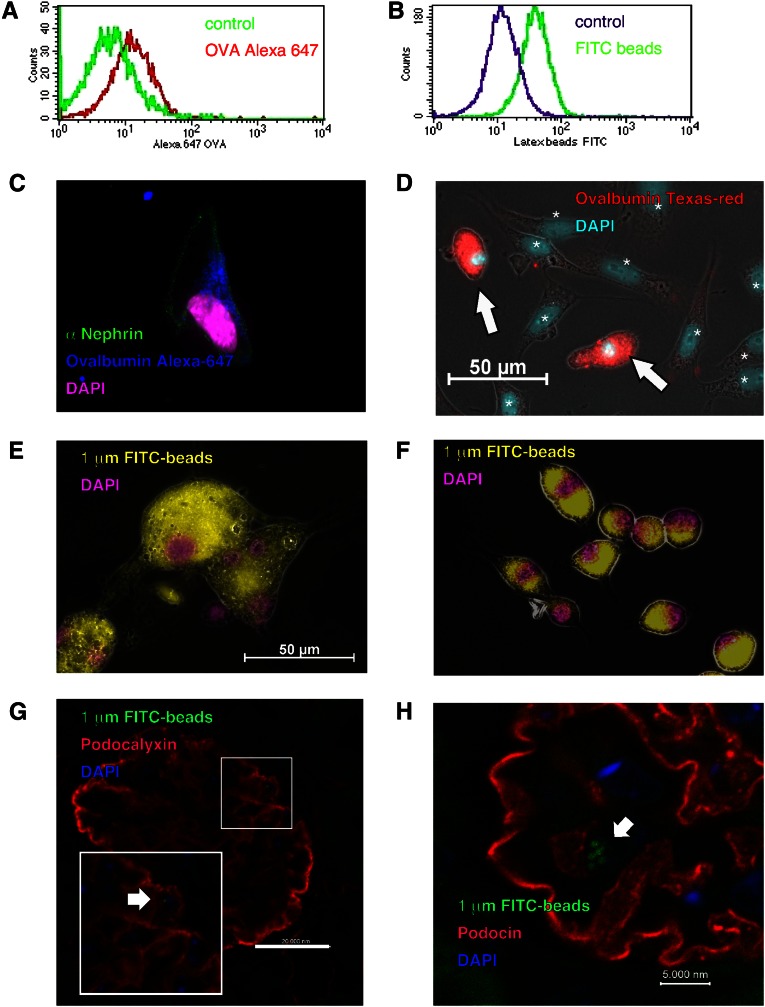
To examine the antigen presentation capacity of podocytes, we used isolated primary podocytes isolated from mouse glomerulus and a conditionally immortalized murine podocyte cell line (PCL) developed and kindly provided by Karlhans Endlich (University of Greifswald, Germany).11 Presentation of antigenic peptides in MHC class II complexes or MHC class I molecules by crosspresentation requires the uptake of extracellular antigens by phagocytosis, pinocytosis, or receptor-mediated mechanisms. We found that podocytes could ingest both labeled latex beads and soluble fluorescence-labeled ovalbumin by phagocytosis. This incorporation was analyzed by FACS or visualized by microscopy (Figure 1, A–F). After intravenous injection, the uptake of labeled beads could also be shown to occur in vivo (Figure 1, G and H). Supplemental Figure 1 shows that PCL cells expressed MHC classes I and II molecules in densities quantitatively comparable with peritoneal exudate macrophages (PEMs). Furthermore, PCL cells express costimulatory molecules, such as CD80 and intercellular adhesion molecule, as well as the podocyte-specific molecule podocalyxin. The analysis of previously published microarray data revealed that the PCL cells as well as sorted primary murine podocytes express all of the genes necessary for MHC classes I and II functions and expression, like the transcription factors Rfxap, Rfx5, Rfxant, and NF-y. In addition, primary podocytes are positive for several other macrophage markers like emr1, sfpi1, MafB, Mpeg1, and Runx1 (Supplemental Figure 2).
Figure 1.
Podocytes ingest both labeled latex beads and soluble ovalbumin. Antigen uptake by conditionally immortalized murine PCLs was analyzed by FACS and is shown by a clear shift in the respective histograms. (A and B) The uptake of particles or soluble protein by different primary cells was visualized by microscopy. (C and E) Uptake rates of isolated primary podocytes, (D) isolated primary podocytes together with mesangium cells, and (F) BMMs were compared. The cells were incubated with (A and C) Alexa647, (D) Texas red-labeled ovalbumin, or (B, E, and F) yellow-green–labeled latex beads. We found that podocytes could ingest both labeled latex beads and soluble fluorescence-labeled ovalbumin. Labeled ovalbumin was incorporated by podocytes (white arrows in D). In contrast, mesangial cells, marked by asterisks and distinguished by the bigger nucleus in D, did not. Furthermore, (E) primary podocytes phagocytosed 1.0-µm beads to the same extent as (F) BMMs. Control staining was performed as shown in Supplemental Figure 5. The phagocytosis in vivo was shown by injecting 1.0-µm latex beads intravenously. After 24 hours, the mice were euthanized and analyzed histologically. The uptake of fluorescent particles into podocin- or podocalyxin-positive cells is shown in G and H.
Podocytes Activate Naive OT-II Cells
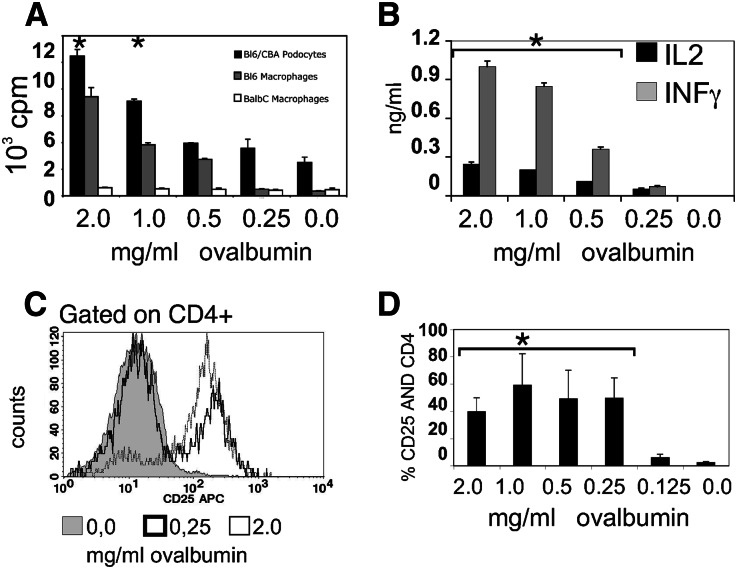
We next addressed the question of whether proteins taken up by podocytes were processed as peptide–MHC complexes for presentation to T cells. PCL cells loaded with ovalbumin induced proliferation of ovalbumin-specific CD4+ T cells in a dose-dependent manner (Figure 2A). As expected, MHC-disparate bone marrow-derived macrophages (BMMs) from BALB/c mice did not, whereas BMM from C57BL/6 mice activated the OT-II cells. OT-II T cells also upregulated the activation marker CD25. A representative histogram is shown in Figure 2C, and a summary of three experiments is shown in Figure 2D. In addition to undergoing activation and proliferation, the CD4+ T cells secreted the Th1 cytokines IL-2 and IFN-γ (Figure 2B).
Figure 2.
Podocytes activate CD4+ T cells by MHC II presentation. BMMs or PCLs were cultivated for 1 day in the presence or absence of ovalbumin. The cells were intensely washed, and 5×105 OT-II cells, purified by magnetic cell sorting, were added at a ratio of 1:1. (B) Supernatants were collected after 48 hours and analyzed for IL-2 and IFN-γ expression by ELISA. Proliferation was measured by 3H uptake, and the CD25 upregulation was analyzed after 48 hours. (A) PCL cells loaded with ovalbumin induced proliferation of ovalbumin-specific MHC class II-restricted CD4+ T cells from OT-II mice in a dose-dependent manner comparable with C57BL/6 BMMs, whereas BALB/c BMMs did not. *Significant differences to medium alone or podocytes without ovalbumin (P<0.05, t test). C shows a representative FACS staining, and D shows quantification of three experiments determining surface expression of the T cell activation marker CD25.
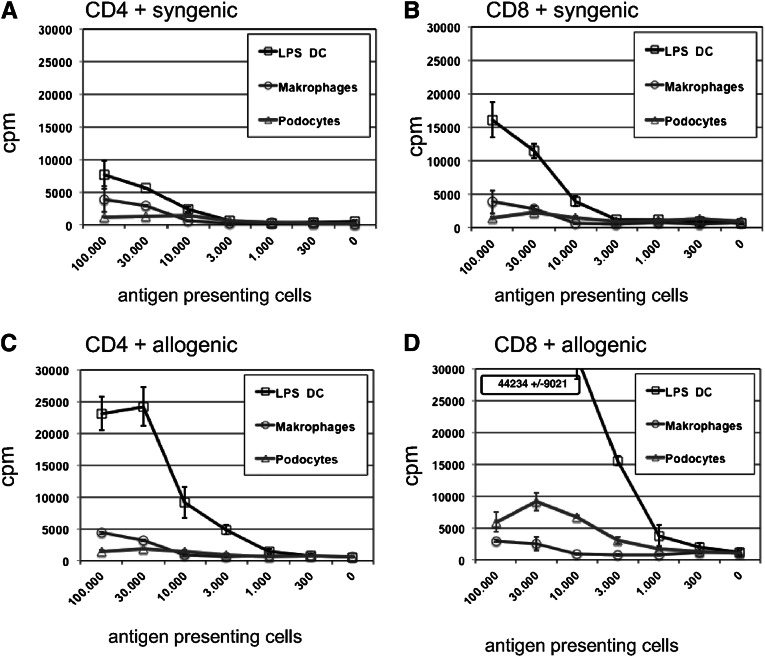
We next asked whether podocytes could also activate CD8+ T cells. In the mixed lymphocyte reactions performed, podocytes were also able to activate allogeneic CD8+ T cells. In comparison, LPS-activated DCs were the best activators of allogenic CD4+ and CD8+ T cells, whereas macrophages were inefficient in our experiments (Figure 3). Also, the observed activation of T cell by DCs in the syngeneic setting may reflect presentation of xenogeneic protein antigens contained in FCS as observed in previous studies. Interestingly, podocytes mainly activated allogeneic CD8+ T cells, whereas their capacity to activate CD4+ T cells was markedly lower (Figure 3, C and D). This strong allogeneic activation was also seen in experiments with unsorted spleen cells from OT-II mice. Because the PCL cells were generated from CBA (H2k) × C57BL/10 (H2b) mice, we were able to analyze the activation of alloreactive cells and ovalbumin-reactive T cells in a mixture of unsorted spleen cells from OT-II transgenic C57BL/6 (H2b) mice simultaneously in one experimental setting (Supplemental Figure 3). In the presence of ovalbumin (Supplemental Figure 3, A and D), a very strong allotypic reaction of the Vα2-negative nontransgenic cells, together with the ovalbumin-specific activation of the transgenic Vα2-positive OT-II T cells, was detected as CD62L down- and CD69 upregulation on the T cells. In contrast, in the absence of ovalbumin, we detected only an allospecific reaction (Supplemental Figure 3, B and E).
Figure 3.
Podocytes activate CD8+ T cells by MHC I presentation. LPS-activated bone marrow DCs, BMMs, or PCLs were seeded in 96-well plates in the indicated numbers; 50,000 CD4+ or CD8+ T cells, purified by magnetic cell sorting, were added at a starting ratio of 1:2 down to 1:486. In the allogeneic setting (C and D), we used CBA/C57BL/6 heterocygote APCs and BALB/c T cells, whereas in the syngeneic setting (A and B), CBA/C57BL/6 heterocygote T cells were used. After 48 hours, the proliferation was measured by 3H uptake. Shown is one representative experiment of three performed. In all experiments, podocytes were more potent activating naïve CD8+ T cells than macrophages, and bone marrow DCs were superior to podocytes and additionally able to activate allogeneic CD4+ T cells.
Crosspresentation by Podocytes
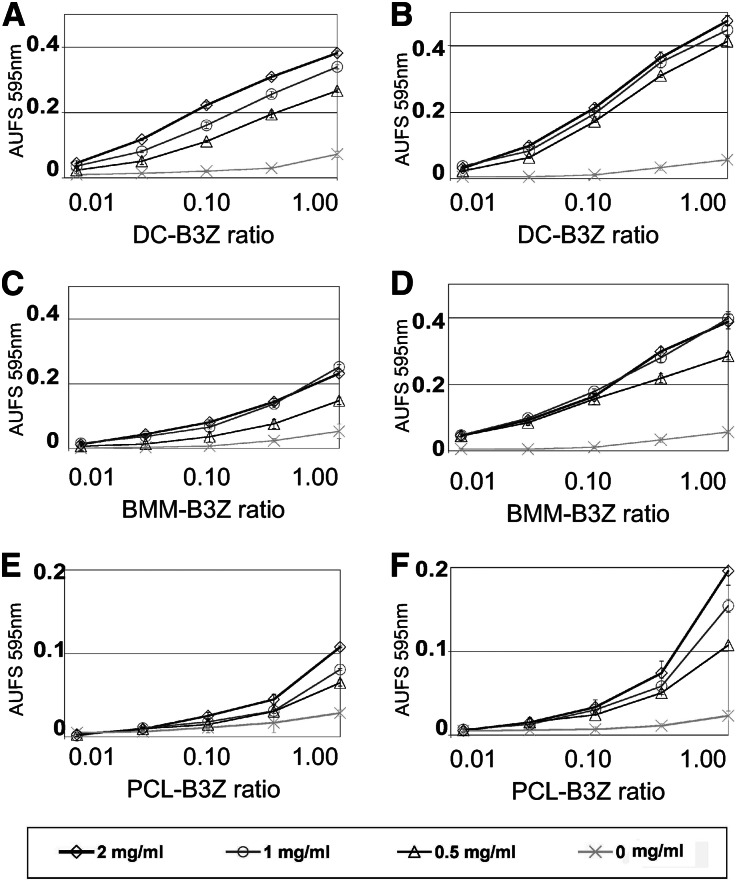
We next wanted to know if podocytes are able to take up exogenous antigen and crosspresent it to CD8+ T cells. For the purpose of comparison, DCs and BMMs were equally loaded for 1 or 2 days with different concentrations of ovalbumin and cocultivated with a mouse T cell hybridoma line, which sensitively recognizes H2Kb loaded with the ovalbumin peptid. LPS-stimulated DCs were very potent activators of antigen-specific CD8+ T cells (Figure 4A). Antigen crosspresentation by BMMs was less potent than for DCs (Figure 4, C and D). We also observed antigen crosspresentation by PCL cells (Figure 4E). The PCL cell capacity was lower than the capacity observed for DCs and BMMs, but it was significantly enhanced by the addition of TNF-α (Figure 4F). Toll-like receptors (TLRs) are essential in DCs for sensing microbial molecules and consequently, inducing maturation. We found that PCL cells expressed high levels of mRNA for almost all TLRs (Supplemental Figure 4). Furthermore, we found that six mostly T cell-attracting chemokines were expressed by PCL cells (Supplemental Figure 4). Interestingly, the subunits of the immunoproteasome (Lmp-2 and Lmp-7) and two proteins essential for efficient crosspresentation (nox-2 and pg91) are also expressed by PCL cells.
Figure 4.
Podocytes activate T cells by crosspresentation. Crosspresentation and activation of the ovalbumin-specific H2-Kb–restricted T cell hybridoma B3Z. DCs, BMMs, or PCLs were incubated with different concentrations of ovalbumin for 18 or 44 hours. After antigen uptake, serial dilutions of the cells were cocultured in 96-well plates with 50,000 B3Z T cells (APC/T cell ratios from 1:1 to 0.01:1). After 16 hours, the T cell activation was analyzed as described. (A and B) LPS-activated DCs incubated with ovalbumin for 18 or 44 hours in the presence of 0.1 µg/ml LPS. (C and D) BMMs incubated with ovalbumin for 18 or 44 hours. (E) Unstimulated PCLs incubated with ovalbumin for 44 hours. (F) PCLs stimulated with TNF-α (20 ng/ml) and incubated with ovalbumin for 44 hours. Individual values present the mean of three individual experiments ± SD.
Freshly Isolated Murine Podocytes Have Features Similar to PCL Cells
To complete the in vitro experiments, we repeated selected experiments with freshly isolated murine podocytes. We isolated glomeruli from murine kidneys by sieve filtration.12 The outgrowing cells were analyzed by immunostaining (Supplemental Figure 5). Thus, our data (Supplemental Figure 6) show that primary podocytes also possess the capacity to activate T cells by MHC classes I and II presentation as well as crosspresentation.
Podocyte/T Cell Contact In Vivo
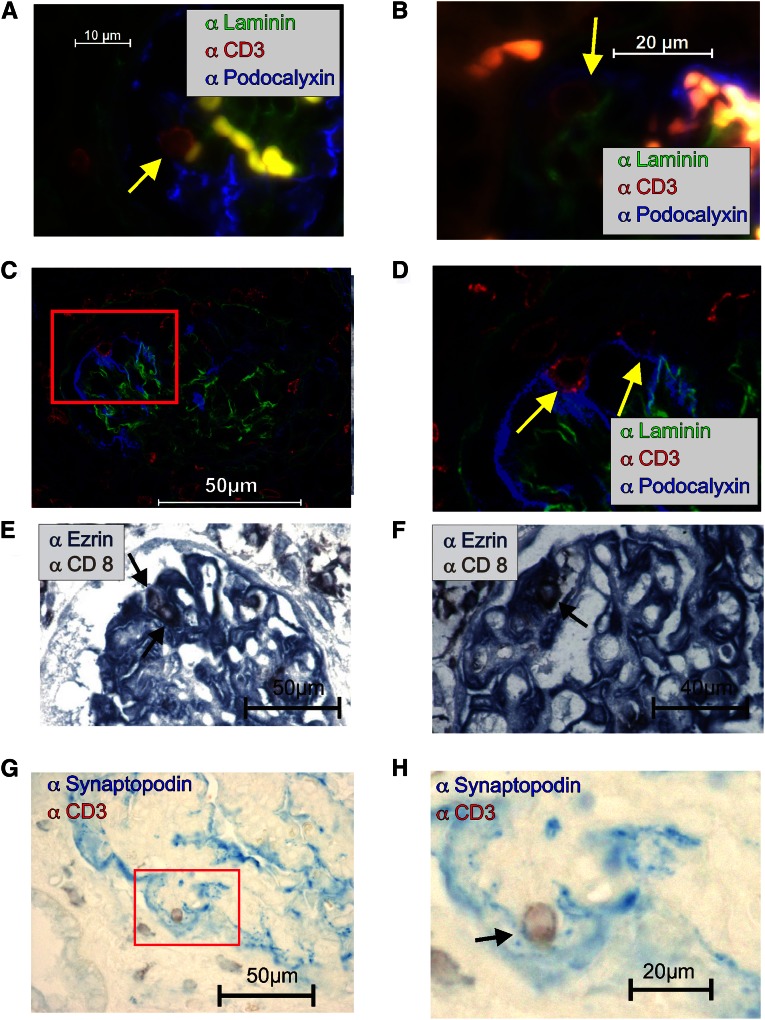
One essential requirement for antigen presentation is close contact between the T cell and the APC. In healthy glomeruli, podocytes are separated from T cells by the glomerular basement membrane (GBM). However, we detected colocalization of T cells in close contact with podocytes in inflamed kidneys. In a glomerulus section from an NZB mouse with lupus nephritis (Figure 5, A and B) and a C57BL/6 mouse 21 days after induction of anti-GBM nephritis (Figure 5, C and D), we observed CD3+ cells surrounded by the podocalyxin-positive foot processes of podocytes (Figure 5, A–D). This contact was laminin-free, indicating a hole in the glomerular membrane. The observation was confirmed by confocal microscopy (Supplemental Movies 1–3). We analyzed several rat models of inflammatory glomerular disease, including the passive Heymann nephritis model (not shown), the 5/6 nephrectomy model (Figure 5E), and a renal transplantation model (Figure 5F). In all models, CD8+ T cells were surrounded by ezrin-positive podocytes and found outside the blood vessel. In tissue sections of human kidneys from a patient with lupus GN (International Society of Nephrology/Renal Pathology Society [ISN/RPS] IV), we also detected close contacts between synaptopodin-stained podocytes and CD3+ T cells (Figure 5, G and H).
Figure 5.
T cell–podocyte contacts are detected in vivo. (A–D) Immunofluorescence showing triple staining for GBM positive for laminin (green), podocalyxin-positive podocytes (blue), and CD3+ T cells (red) in (A and B) the glomerulus of an NZB/W mouse with lupus nephritis or (C and D) a C57BL/6 mouse 21 days after induction of anti-GBM nephritis. (A, B, and D) The close contact (without laminin) between two CD3+ T cells and podocytes is marked with a yellow arrow. Immunohistological double staining of ezrin (blue) as a podocyte marker and CD8 (brown) as a T cell marker in rat models of renal disease. Close contact between CD8+ T cells and podocytes is marked with an arrow in (E) a 5/6 nephrectomy model (a nephron loss model) and (F) the allogeneic Fischer–Lewis renal transplantation model with signs of rejection. Immunohistological double staining of synaptopodin (blue; podocyte marker) and CD3 (brown; T cell marker) in human lupus GN (ISN/RPS IV). (G and H) Close contact between a CD3+ T cell and the foot processes of a podocyte (blue) is visible.
Activation of CD8 OT-I T Cells by Podocytes In Vivo
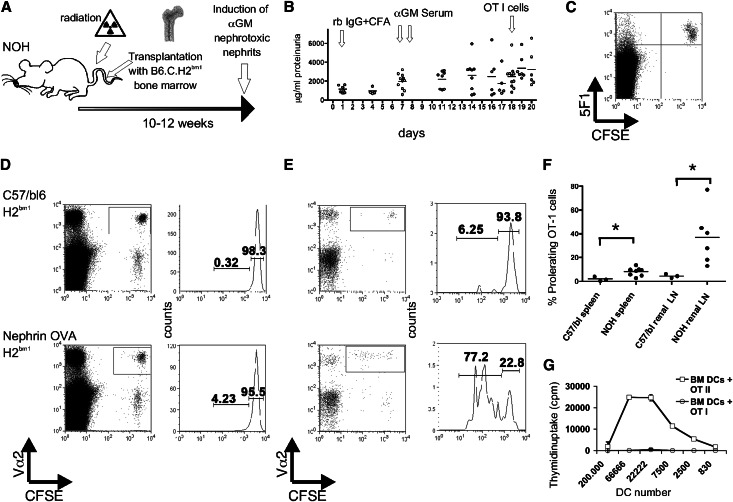
We show that podocytes can act as APCs. To validate that this phenomenon also occurs in vivo, an appropriate model was established. Mice expressing ovalbumin exclusively in their podocytes13 were irradiated, and the hematopoietic system was reconstituted with bone marrow from B6.C.H2bm1 mice. In these mice, a mutation in the binding groove of the MHC class I molecule prevents the binding of the ovalbumin peptide. After bone marrow reconstitution, only nonhematopoietic cells of the chimeric mice are able to present the ovalbumin peptide, and within the nonhematopoietic cells, only podocytes express the antigen. To facilitate podocyte/T cell contacts, we induced αGBM nephritis in the recipient animals. The experimental setup is illustrated in Figure 6A. The development of proteinuria is shown in Figure 6B. Extensive FACS analyses were performed to exclude radiation-resistant hematopoietic cells in the recipient mice. The exemplified FACS blot of spleen cells in Figure 6C indicates the success of the bone marrow transplantation. The antibody 5F1 recognizes the H2kb and not the mutated H2kbm1. Only the transferred Carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-I cells are 5F1-positive. A representative experiment of two mice is shown in Figure 6, D and E. OT-I T cell proliferation was only detected in the nephrin Ova-transgenic mice. The difference in the frequency of proliferating OT-I cells in the spleen was significant (1.9%+−1.11% versus 8.2%+−1.09%, P=0.02). In the renal lymph nodes, the difference in the percentages of proliferating OT-I cells in transgenic or control recipient mice was even more pronounced (3.6%+−1.3% versus 37.0%+−9.5%, P=0.05). The summary is shown in Figure 6F. As a control, bone marrow DCs from the bone marrow chimeric recipient mice were generated and incubated with 0.5 mg/ml ovalbumin and LPS (0.1 µg/ml) for 1 day, and OT-I and -II cells were added in different ratios. As expected, bone marrow DCs from bone marrow chimeric mice did not activate OT-I cells (Figure 6G). In contrast, the bone marrow DCs were able to activate CD4+ transgenic OT-II T cells (Figure 6G), because MHC class II presentation is not altered in these mice. The inability of the hematopoietic cells (like DCs) to induce OT-I T cell proliferation in these animals supports the conclusion that the podocyte–T cell interaction is responsible for the observed antigen-specific T cell activation in vivo.
Figure 6.
Podocytes activate naive T cells in vivo. (A) The schematic drawing illustrates the experimental setup. Transgenic mice expressing ovalbumin exclusively in the podocytes were radiated, and the hematopoietic system was reconstituted with bone marrow from B6.C.H2bm1 mice. These transferred hematopoietic cells are unable to present the ovalbumin peptide SIINFEKL. After complete reconstitution, anti-glomerular basement membrane (αGM) nephritis was induced by preimmunization with rabbit IgG in complete Freund's adjuvant (rb IgG + CFA) followed by two injections of a rabbit serum (αGM) reacting against murine glomerular basal membrane. The time course is shown in B. After 18 days, with the onset of proteinuria, CFSE-labeled OT-I cells were injected intravenously to monitor ovalbumin presentation by the proliferation of the transferred transgenic T cells. (C) Analysis of spleen cells indicating the success of the bone marrow transplantation. Only transferred CFSE-labeled OT-I cells are 5F1-positive, because this antibody does not recognize the H2bm1 antigen. A representative experiment displaying cells of two mice is shown in D for spleen and E for renal lymph nodes. The gates were set on the transgene T cell receptor Vα2. Please note that a T cell proliferation was only detectable in nephrin-OVA transgenic mice, whereas cells of C57BL/6 mice remained negative. The summary of several experiments is shown in F. *P<0.05. Bone marrow DCs from the same mice were generated and incubated with 0.5 mg/ml ovalbumin and incubated with OT-I or OT-II cells. (G) The proliferation was measured by 3H thymidin uptake. Bone marrow DCs from bone marrow chimeric mice did not activate OT-I cells but activated CD4+ transgenic OT-II T cells, showing that MHC class II presentation was not altered in the animals and that bone marrow reconstitutions were complete.
MHC II Expression on Podocytes Is Essential to Induce a Severe Anti-GBM Nephritis
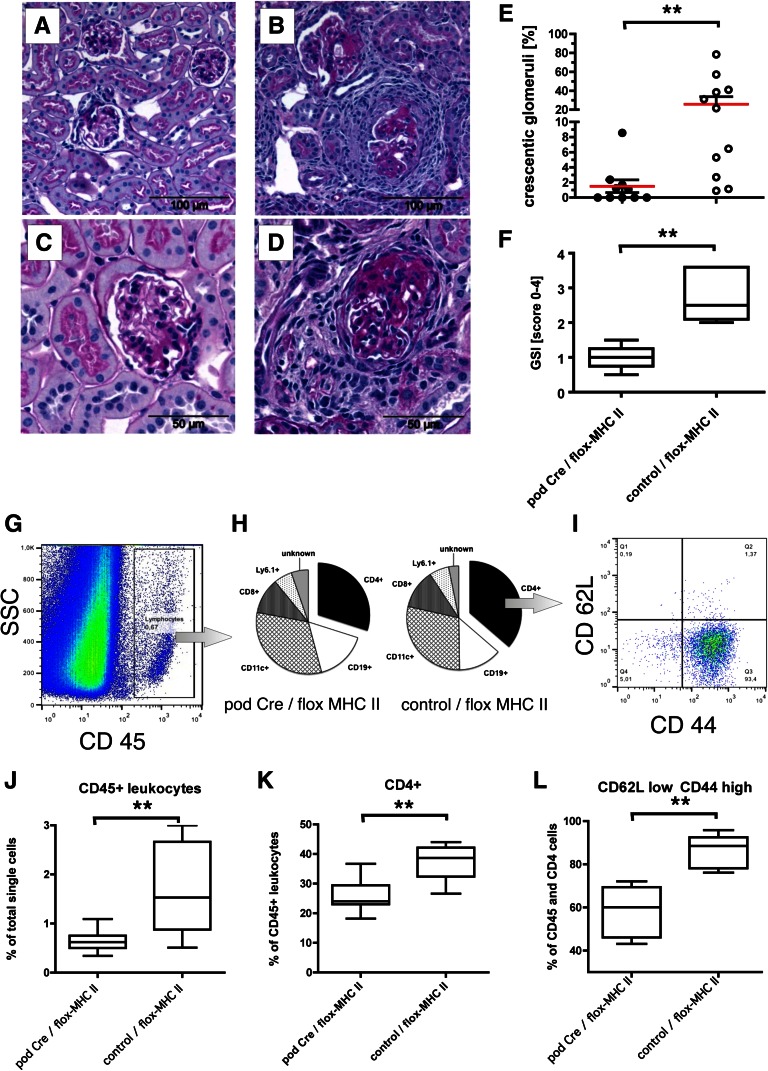
To circumstantiate these results, a second in vivo model was established. Homozygote mice with a loxP-flanked exon 1 of the MHC class II β-1 locus14 were bred with a transgenic mouse line expressing Cre recombinase exclusively in podocytes.15 The F3 offspring from this breeding have a loss of MHC class II only in the podocytes, whereas all other MHC class II-restricted Ag presentation is unchanged (Supplemental Figure 9). To study the role of podocyte MHC class II expression in the pathogenesis of GN, we induced an anti-GBM disease in these mice. Most of the control animals developed a focal and segmental nephrosclerosis and glomerular crescent formation, whereas the animals without MHC class II in the podocytes developed only a very moderate disease (Figure 7, A–F). In addition, in the kidneys from nephritic mice lacking MHC class II on podocytes, we found decreased numbers of CD45+ and CD45/CD4 double-positive leukocytes compared with kidneys from mice with podocytic MHC class II expression (Figure 7, H, J, and K). Furthermore, CD4 activation, as assessed by the appearance of a population, expressing the effector memory phenotype (CD62Llow/CD44high) was significantly stronger in control animals (80.17%±5.0% versus 54.9%±3.4% of all intra-renal CD4 cells) compared with animals lacking MHC class II on podocytes (Figure 7, I and L). These experiments strongly suggest that T cell activation by podocytes occurs in vivo and plays an important role in CD4-driven immune response.
Figure 7.
MHC II expressed on podocytes is essential for the induction of severe anti-GBM nephritis. Transgenic mice expressing the Cre recombinase exclusively in podocytes were crossed in a mouse line expressing a floxed MHC class II gene. In the F3 offspring and control littermates, an anti-GBM nephritis was induced as described in Figure 6. The kidneys were analyzed by histology and FACS. Exemplified periodic acid–Schiff staining is shown from mice without MHC class II (A and C) on podocytes and (B and D) from control animals. The quantification of three independent experiments is shown in E (percent crescentic glomeruli) and F (glomerulosclerosis index). The FACS gating strategy is shown in G–I. (J–L) The quantifications are shown as box plots (box-and-whisker diagrams graphically depicting the lowest number observed, lower quartile, median, upper quartile, and largest observation). **P<0.001. (G and J) First, the relative frequencies of CD45+ leukocytes in the kidneys were calculated; (H and I) subsequently, the main subpopulations of the leukocytes were analyzed, and within the CD45/CD4 double-positive populations, the frequencies of effector memory cells (CD44 high/CD62L low) were determined. Please note that most control animals displayed serve glomerulosclerosis, pronounced and frequent crescent formation, and infiltration of CD4+ effector memory cells. This result was not seen in mice lacking MHC class II expression selectively on podocytes.
Discussion
For decades, podocytes have been considered to be a major passive antigenic target in many different glomerular diseases in man, such as membranous glomerulopathy, minimal change disease, and FSGS as well as many other glomerulonephritides. Our study is the first to show that podocytes in vitro and in vivo are able to act as activating specific T cell responses by all three of the modes usually used by professional hematopoetic APCs. Based on our findings, we propose a new and unexpectedly active role for podocytes in the adaptive immune responses, which may have broad importance for initiation and progression of immunologic kidney disease as well as alloimmune responses after renal transplantation.
Recent studies showed that podocytes can acquire macrophage-like functions such as expression of CD80 on LPS stimulation16 as well as upregulation/activation of TLRs leading to the local release of chemokines.17 Using immortalized as well as primary murine podocytes, we found expression of MHC classes I and II molecules and costimulatory molecules in amounts quantitatively comparable with macrophages. In addition, primary podocytes are positive for several other macrophage markers (Supplemental Figure 7). This finding is in agreement with a published gene array analysis9 that showed the expression of several macrophage transcripts (CD68, F4/80, CD206, DEC205, and the transcription factor PU.1) in podocytes (Supplemental Figure 2). Transcriptome analysis of laser-captured glomeruli from patients with lupus nephritis revealed that myeloid linage transcripts were expressed in the biopsy samples.18 In crescentic GN, podocytes transform into crescent-forming cells, which are double-positive for podocalyxin and CD68, showing several characteristics of macrophages, such as hydrogen peroxide and cytokine production. This finding was first described for rat glomeruli19 and subsequently, idiopathic collapsing glomerulopathy in humans.20 Uptake of latex beads was, so far, thought to be an identifying exclusive feature of professional macrophages. We showed a high phagocytic activity in cultured podocytes and in vivo as well. We also showed that not just macrophages but also podocytes express many TLRs being used to recognize danger signals and specifically, modulated on TNF-α stimulation, leading to the release of T cell-attractive chemokines. A microarray study analysing the transcriptional profile of podocyte cell line from Peter Mundel21 using challenge with TNFa also shows the upregulation of these chemokines.22 There are increasing data supporting a role of TLRs in inflammatory kidney diseases.17,23,24 Endocytosed antigens usually enter the MHC class II pathway to permit CD4+ T cell activation. Only professional APCs are able to also pass extracellular material in the MHC class I pathway, allowing the generation of effector CD8+ cells, a process denominated crosspresentation. So far, crosspresentation capacity has been shown for macrophages and DCs and only a few other hematopoietic (e.g., B cells25 and neutrophils26) and nonhematopoietic (liver sinusoidal endothelial cells27) cell types. In contrast to liver sinusoidal endothelial cells, which induce a liver-specific T cell tolerization,28 we found that activated podocytes are potent activators of CD8+ T cells. Next, we showed that this finding could also happen in vivo. Double staining for podocytes together with T cells showed that, in many inflammatory kidney disease models in rats, man, and mice, T cells can be observed next to podocytes. This result could also been shown by FACS analysis of cell doublets from kidney (Supplemental Figure 8).
Finally, using two in vivo model systems, we proved that, in a situation of anti-GBM nephritis, that the MHC class I antigen presentation by the podocytes is sufficient to activate CD8+ OT-I cells in the murine kidney and furthermore, that MHC class II antigen presentation by podocytes is necessary to induce the CD4+ T cell-driven glomerular disease. The fact that intrinsic renal cells expressing MHC class II are required for the development of immune-mediated renal injury was shown before,29 and our results show that these intrinsic renal cells are the podocytes.
Although we performed very extensive FACS control staining, the persistence of a very small population of host DCs in the kidney, acting as a potential confounding factor for ovalbumin presentation to OT-I cells in the bone marrow chimeras, cannot be excluded. Because the newly generated podocyte-specific MHC class II-deficient mice were virtually resistant to the induction of anti-GBM nephritis, MHC II presentation by podocytes plays a decisive role in this T cell-driven disease. Thus, we propose a new kidney-intrinsic T cell activation pathway through the antigen-presenting properties of podocytes. Our data suggest an important direct role of podocytes in glomerular-mediated immune reaction leading to glomerular diseases and during rejection of kidney grafts, where T cell-mediated immune mechanisms are mandatory for renal tissue damage.13,30,31 However, the relative contribution of renal DCs presenting podocyte antigens or alternatively, podocytes themselves functioning as APCs for the initiation and perpetuation of a T cell immune response remains an important question to be solved by future studies. We consider it very likely that antigen presentation by DCs and podocytes is not mutually exclusive. It has been shown previously in elegant studies that renal DCs present glomerular autoantigens to T cells after ingestion and migration to the draining renal lymph nodes.13,32 A putative transfer of MHC II molecules from podocytes to the DCs or the migration of podocytes to the lymph nodes cannot be completely excluded. However, because activated DCs are extremely potent MHC class II expressors, the additive cargo of some podocyte MHC molecules by trochocytosis or exosome transfer would most probably play only a minor role. Under conditions where the deterioration of the glomerular basal membrane facilitates the podocyte T cell contact in vivo, podocytes might contribute to the tissue-intrinsic activation of T cells recruited by podocyte-derived chemokines. This glomerular preconditioning may be operative early on whenever kidney damage is caused by drugs, toxins, ischemia, or infections, even before DC migration from the renal tissue to the draining lymph nodes has occurred. Whether this newly described kidney intrinsic pathway of T cell activation is specific for podocytes needs to be evaluated in future studies, because within the kidney, different nonhemopoetic cells with potential antigen-presenting capacity have been described, such as renal proximal tubular33 as well as activated vascular endothelial cells.34
In summary, our studies suggest that podocytes should be regarded not only as victims but also culprits of immune-mediated glomerular diseases and potentially, kidney transplant rejection, being able to directly activate T cells as newly defined kidney APCs.
Concise Methods
Full methods are in Supplemental Material.
The murine PCL,11 developed from the H-Kb-tsA58 transgenic mouse strain,35 was kindly provided by Karl-Hans Endlich (University of Greifswald, Greifswald, Germany). The PCLs were maintained and differentiated as described.11
To isolate primary murine podocytes, glomeruli from the kidney cortexes of approximately 30–40 mice were purified by the sieving method based on the original work of Krakower and Greenspon.12,36 The primary cellular outgrowth was harvested and analyzed by immunostaining.
The preparation and culture of bone marrow cells from C57BL/6 and BALB/c mice to generate DCs and macrophages, respectively, have been previously described.37 Granulocyte macrophage colony-stimulating factor and macrophage colony-stimulating factor (PeproTech/Tebu) were used at 200 U/ml.
PEMs were elicited by intraperitoneal injection of thioglycollate (Sigma, Steinheim, Germany). PEMs were harvested 4 days postintraperitoneal injection by peritoneal lavage with 10 ml sterile PBS and enriched by adhesion. The purity of the resulting cell population was analyzed by FACS.
Immunostaining
Primary cells grown in chamber slides and frozen on paraffin-embedded sections of kidney biopsies were used and stained with the indicated antibodies. The slides were embedded with 4',6-diamidino-2-phenylindole–containing mounting medium and evaluated with a fluorescence microscope (Zeiss Axiovert 220M with AxionCam MRm and ApoTome together with the software AxioVision 4.7.1; Oberkochen, Germany) or confocal microscopy (Leica SP5 together with the software LAS 2.3.0; Mannheim, Germany).
To visualize podocyte–T cell contacts, renal biopsies were taken from rats 12 weeks after 5/6 nephrectomy, 5 days after induction of passive Heymann nephritis, 11 days after puromycin injection, day 6 of the anti-Thy1 model, and 11 days after renal transplantation of a Fischer rat kidney into a Lewis rat recipient. In addition, renal biopsies from 24-week-old NZB/W mice with proven lupus nephritis and human kidney biopsies with proven immune complex GN were analyzed. Biopsies from pauci-immune crescentic GN and normal renal tissues served as controls.
Flow Cytometry (FACS)
To block Fc receptors, ex vivo-prepared or -cultured cells were incubated with Fc block (BD Biosciences) or 5 µl normal mouse serum for 10 minutes on ice. Subsequently, cells were stained with 1:100 dilutions of the monoclonal antibodies for 30 minutes on ice. The antibodies used in immunohistochemistry were also used for FACS. All FACS analyses were done on an FACSCalibur (BD Biosciences) equipped with Cell Quest Pro 4.1 and FlowJo 9.3.1 software.
OT-II Cell Stimulation Assays
For stimulation assays, defined populations of bone marrow macrophages, podocytes, DCs, or PEMs were cultured for 1 day in the presence or absence of ovalbumin. The cells were washed intensively at least five times with PBS, and 5×105-purified CD4+ cells from OT-II mice38 were added at a ratio of 1:1. Because of the limited numbers of primary podocytes, the effector/target cell ration E/T ratio was diminished in these assays to 0.2:1. CD4+ OT-II cells were purified from spleens by magnetic cell sorting. The purity of the resulting cell population was analyzed by FACS as described above, and it was >95%. After 24 hours of coculture, an aliquot was used to measure the upregulation of CD69 by FACS. After 48 hours, the supernatants of cultures were collected and analyzed for the presence of IL-2 and IFN-γ by ELISA using the DuoSet ELISA development system according to the manufacturer’s instructions (R&D Systems). Additionally, proliferation was measured by 3H uptake; downregulation of CD62L and upregulation of CD25 were analyzed by FACS.
Crosspresentation and B3Z Activation
To analyze the MHC class I-specific T cell activation, the SL-H2-Kb–specific, murine CD8+ T cell B3Z hybridoma was used.39,40 These cells were kindly provided by Nilabh Shastri at the University of California at Berkeley (Berkeley, CA). The antigen-specific activation was monitored by a colorimetric assay. APCs were incubated with indicated concentrations of ovalbumin, and serial dilutions of these cells were then cocultured with B3Z cells. To exclude nonspecific reactions, serial dilutions of TNF-α (PeproTech/Tebu), LPS, or ovalbumin alone were tested in parallel. Ionomycin served as a positive control.
OT-I Cell Activation In Vivo
The podocin ovalbumin (NOH) or nontransgene littermates were radiated two times with 3 Gy. Immediately, 30 mio fresh isolated bone marrow cells from a B6.C.H2bm1 mouse (Jackson Laboratories) were injected in the tail vain. After reconstitution, the nephrotoxic nephritis was induced by a standard protocol. First, the mice were immunized subcutaneously with 50 µg rabbit IgG together with 50 µl Freund's complete adjuvant. After 6 and 7 days, a rabbit serum reacting with the glomerular basal membrane was injected intravenously. With the beginning signs of proteinuria (on average, 10 days postinduction), 107 CFSE-labeled OT-I cells were transferred intravenously. To display the in vivo T cell activation, the spleen and the renal lymph node were removed, and cells were analyzed by FACS as described above. Additionally, the bone marrow was used to generate bone marrow DCs. After differentiation, these cells were loaded with ovalbumin, and serial dilutions were used in the proliferation experiments. After 2 days coculture with either OT-I or -II cells, the thymid uptake was measured.
Anti-GBM GN in Mice with MHC Class II-Deficient Podocytes
C57/BL6 mice with a podocyte-specific MHC class II knockout were generated by breeding mice with a loxP-flanked exon 1 of the MHC class II β-1 locus14 and another C57BL/6 transgenic mouse line expressing the Cre recombinase exclusively in podocytes.15 The genotype was determined by PCR. All experimental mice are homozygote for the floxed MHC II gene. One group was double transgenic (causing no MHC II expression on podocytes) or control single transgenic littermates (no Cre expression results in a unchanged MHC II expression). In these mice, we induced an anti-GBM disease and analyzed the disease progression by FACS or histologic evaluation.
Disclosures
None.
Acknowledgments
We thank Christina Saemann, Leonie Littmann, Miriam Reutelshöfer, and Romy Böhme for technical support. We are especially grateful to Joachim Gläsner for helpful discussions and critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grants GE 671/12-2, SFB 643, B1, and SFB 423, Z2 and the Interdisciplinary Center for Clinical Research (IZKF, A45) at the University Hospital Erlangen–Nuremberg.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012020133/-/DCSupplemental.
References
- 1.Vyas JM, Van der Veen AG, Ploegh HL: The known unknowns of antigen processing and presentation. Nat Rev Immunol 8: 607–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldwich A, Hahn SS, Schreiber S, Meier S, Kämpgen E, Wagner R, Lutz MB, Schubert U: Targeting HIV-1 Gag into the defective ribosomal product pathway enhances MHC class I antigen presentation and CD8+ T cell activation. J Immunol 180: 372–382, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C: Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316: 612–616, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Burgdorf S, Schölz C, Kautz A, Tampé R, Kurts C: Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol 9: 558–566, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Michaud JL, Kennedy CR: The podocyte in health and disease: Insights from the mouse. Clin Sci (Lond) 112: 325–335, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Coers W, Brouwer E, Vos JT, Chand A, Huitema S, Heeringa P, Kallenberg CG, Weening JJ: Podocyte expression of MHC class I and II and intercellular adhesion molecule-1 (ICAM-1) in experimental pauci-immune crescentic glomerulonephritis. Clin Exp Immunol 98: 279–286, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S: MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol 26: 5715–5727, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiwek D, Endlich N, Holzman L, Holthöfer H, Kriz W, Endlich K: Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int 66: 91–101, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Krakower CA, Greenspon SA: Factors leading to variation in concentration of nephrotoxic antigen(s) of glomerular basement membrane. AMA Arch Pathol 58: 401–432, 1954 [PubMed] [Google Scholar]
- 13.Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Gröne HJ, Kurts C: Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimoda M, Mmanywa F, Joshi SK, Li T, Miyake K, Pihkala J, Abbas JA, Koni PA: Conditional ablation of MHC-II suggests an indirect role for MHC-II in regulatory CD4 T cell maintenance. J Immunol 176: 6503–6511, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Reiser J, Mundel P: Danger signaling by glomerular podocytes defines a novel function of inducible B7-1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol 15: 2246–2248, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, Hauser P, Pippin JW, Shankland SJ, Smith KD, Stoelcker B, Liu G, Gröne HJ, Krämer BK, Alpers CE: TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19: 704–713, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson KS, Huang JF, Zhu J, D’Agati V, Liu X, Miller N, Erlander MG, Jackson MR, Winchester RJ: Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J Clin Invest 113: 1722–1733, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orikasa M, Iwanaga T, Takahashi-Iwanaga H, Kozima K, Shimizu F: Macrophagic cells outgrowth from normal rat glomerular culture: Possible metaplastic change from podocytes. Lab Invest 75: 719–733, 1996 [PubMed] [Google Scholar]
- 20.Bariéty J, Nochy D, Mandet C, Jacquot C, Glotz D, Meyrier A: Podocytes undergo phenotypic changes and express macrophagic-associated markers in idiopathic collapsing glomerulopathy. Kidney Int 53: 918–925, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Shankland SJ, Pippin JW, Reiser J, Mundel P: Podocytes in culture: Past, present, and future. Kidney Int 72: 26–36, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Bruggeman LA, Drawz PE, Kahoud N, Lin K, Barisoni L, Nelson PJ: TNFR2 interposes the proliferative and NF-κB-mediated inflammatory response by podocytes to TNF-α. Lab Invest 91: 413–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith KD: Toll-like receptors in kidney disease. Curr Opin Nephrol Hypertens 18: 189–196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krüger B, Banas MC, Walberer A, Böger CA, Farkas S, Hoffmann U, Fischereder M, Banas B, Krämer BK: A comprehensive genotype-phenotype interaction of different Toll-like receptor variations in a renal transplant cohort. Clin Sci (Lond) 119: 535–544, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Ke Y, Kapp JA: Exogenous antigens gain access to the major histocompatibility complex class I processing pathway in B cells by receptor-mediated uptake. J Exp Med 184: 1179–1184, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter NS, Harding CV: Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol 167: 2538–2546, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA: Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med 6: 1348–1354, 2000 [DOI] [PubMed] [Google Scholar]
- 28.von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, Geerts A, Kolanus W, Knolle P, Diehl L: Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 49: 1664–1672, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Li S, Kurts C, Köntgen F, Holdsworth SR, Tipping PG: Major histocompatibility complex class II expression by intrinsic renal cells is required for crescentic glomerulonephritis. J Exp Med 188: 597–602, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao L, Haas M, Pippin J, Wang Y, Miwa T, Chang A, Minto AW, Petkova M, Qiao G, Song WC, Alpers CE, Zhang J, Shankland SJ, Quigg RJ: Focal and segmental glomerulosclerosis induced in mice lacking decay-accelerating factor in T cells. J Clin Invest 119: 1264–1274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer TN, Schwesinger C, Wahlefeld J, Dehde S, Kerjaschki D, Becker JU, Stahl RA, Thaiss F: A new mouse model of immune-mediated podocyte injury. Kidney Int 72: 841–852, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int 68: 1096–1108, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kreisel D, Krasinskas AM, Krupnick AS, Gelman AE, Balsara KR, Popma SH, Riha M, Rosengard AM, Turka LA, Rosengard BR: Vascular endothelium does not activate CD4+ direct allorecognition in graft rejection. J Immunol 173: 3027–3034, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kreisel D, Krupnick AS, Balsara KR, Riha M, Gelman AE, Popma SH, Szeto WY, Turka LA, Rosengard BR: Mouse vascular endothelium activates CD8+ T lymphocytes in a B7-dependent fashion. J Immunol 169: 6154–6161, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D: Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A 88: 5096–5100, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burlington H, Cronkite EP: Characteristics of cell cultures derived from renal glomeruli. Proc Soc Exp Biol Med 142: 143–149, 1973 [DOI] [PubMed] [Google Scholar]
- 37.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G: An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Barnden MJ, Allison J, Heath WR, Carbone FR: Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 76: 34–40, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Karttunen J, Shastri N: Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci U S A 88: 3972–3976, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson S, Shastri N: LacZ inducible, antigen/MHC-specific T cell hybrids. Int Immunol 6: 369–376, 1994 [DOI] [PubMed] [Google Scholar]