Abstract
Central distribution of body fat is associated with a higher risk of renal disease, but whether it is the distribution pattern or the overall excess weight that underlies this association is not well understood. Here, we studied the association between waist-to-hip ratio (WHR), which reflects central adiposity, and renal hemodynamics in 315 healthy persons with a mean body mass index (BMI) of 24.9 kg/m2 and a mean 125I-iothalamate GFR of 109 ml/min per 1.73 m2. In multivariate analyses, WHR was associated with lower GFR, lower effective renal plasma flow, and higher filtration fraction, even after adjustment for sex, age, mean arterial pressure, and BMI. Multivariate models produced similar results regardless of whether the hemodynamic measures were indexed to body surface area. Thus, these results suggest that central body fat distribution, independent of BMI, is associated with an unfavorable pattern of renal hemodynamic measures that could underlie the increased renal risk reported in observational studies.
Central body fat distribution is associated with increased long-term renal risk, as shown in several recent studies.1–3 This increased risk is often attributed to associated conditions, such as weight excess, hypertension, dyslipidemia, and diabetes.4–10 However, after adjustment for these conditions, central body fat distribution, estimated from waist-to-hip ratio (WHR), remains an independent determinant of increased long-term renal risk.1–3 The mechanisms underlying this increased renal risk in association with a central body fat distribution are not well established. Small studies suggest that central body fat distribution is associated with an unfavorable renal hemodynamic profile;11,12 however, these studies did not control for concomitant presence of overall weight excess as commonly reflected by body mass index (BMI), a well established determinant of an unfavorable renal hemodynamic profile in itself.5,13 We therefore investigated whether body fat distribution is associated with renal hemodynamics, independent of BMI, in a cohort of 315 normotensive persons with normal fasting glucose levels.
Results
Participant characteristics are shown in Table 1. All persons were healthy and, by default, had normal renal function, BP, and plasma glucose levels. Mean WHR ± SD was 0.87±0.09.
Table 1.
Participant characteristics (n=315)
| Variable | Data |
|---|---|
| Men, n (%) | 167 (53) |
| Age (yr) | 39±15 |
| WHR | 0.87±0.09 |
| Waist circumference (cm) | 86±11 |
| Hip circumference (cm) | 99±9 |
| Weight (kg) | 78±14 |
| Height (cm) | 176±10 |
| BMI (kg/m2) | 24.9±3.9 |
| BSA (m2) | 1.94±0.20 |
| Overweight, n (%) | 109 (35) |
| Obesity, n (%) | 31 (10) |
| Fasting plasma glucose (mmol/L) | 4.7±0.5 |
| Systolic BP (mmHg) | 123±12 |
| Diastolic BP (mmHg) | 75±8 |
| MAP (mmHg) | 89±9 |
| GFR/BSA (ml/min per 1.73 m2) | 109±15 |
| ERPF/BSA (ml/min per 1.73 m2) | 415±92 |
| GFR (ml/min) | 122±20 |
| ERPF (ml/min) | 464±112 |
| FF (%) | 27±5 |
| Albuminuria (mg/d) (n=198) | 5 (3–8) |
| Urinary sodium excretion (mmol/d) (n=192) | 205±79 |
Overweight and obesity are defined as BMI 25–29.9 and ≥30 kg/m2, respectively. Unless otherwise noted, data are shown as mean ± SD or median (interquartile range).
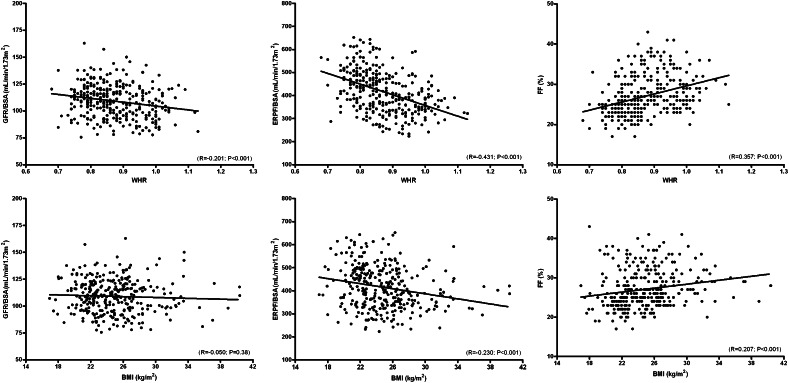
The univariate associations of WHR and BMI, respectively, with body surface area (BSA)–indexed renal hemodynamic measures are shown in Figure 1. WHR was inversely associated with GFR/BSA (R=−0.201; P<0.001) and effective renal plasma flow (ERPF)/BSA (R=−0.431; P<0.001) and positively with filtration fraction (FF) (R=0.357; P<0.001). For BMI, the R values were 0.230 for ERPF/BSA (P<0.001) and 0.207 for FF (P<0.001), whereas no significant association was found with GFR/BSA. Multivariate analyses are shown in Table 2. WHR (upper panel) was associated with GFR/BSA (β=−4.01 [95% confidence interval (CI), −6.17 to −1.85]; P<0.001), ERPF/BSA (β=−46.6 [95% CI, −58.0 to −35.1]; P<0.001), and FF (β=2.05 [95% CI, 1.41 to 2.70]; P<0.001), independent of sex, age, mean arterial pressure (MAP), and BMI. BMI (lower panel) was associated with ERPF/BSA (β=−16.2 [95% CI, −26.3 to −6.0]; P<0.002) and FF (β=0.78 [95% CI, 0.22 to 1.33]; P<0.001), independent of sex, age, and MAP, but the associations were lost after inclusion of WHR into the model. No association between BMI and GFR/BSA was present.
Figure 1.
Univariate associations between WHR and BMI with renal hemodynamics measures. Scatter plots showing the univariate associations between WHR (upper panel) and BMI (lower panel), respectively, with BSA-indexed GFR and ERPF and with FF.
Table 2.
Multivariate data for WHR and BMI with renal hemodynamic measures, indexed for BSA
| Variable | GFR/BSA | ERPF/BSA | FF | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| WHR | ||||||
| Model 1 | −2.95 (−4.67 to −1.23) | 0.001 | −38.7 (−48.2 to −29.2) | <0.001 | 1.72 (1.19 to 2.24) | <0.001 |
| Model 2 | −3.48 (−5.26 to −1.70) | <0.001 | −46.0 (−55.5 to −36.5) | <0.001 | 2.05 (1.52 to 2.57) | <0.001 |
| Model 3 | −3.39 (−5.18 to −1.61) | <0.001 | −45.7 (−55.2 to −36.2) | <0.001 | 2.05 (1.51 to 2.58) | <0.001 |
| Model 4 | −3.13 (−4.99 to −1.28) | 0.001 | −43.0 (−52.8 to −33.2) | <0.001 | 1.93 (1.38 to 2.48) | <0.001 |
| Model 5 | −4.01 (−6.17 to −1.85) | <0.001 | −46.6 (−58.0 to −35.1) | <0.001 | 2.05 (1.41 to 2.70) | <0.001 |
| BMI | ||||||
| Model 1 | −0.78 (−2.52 to 0.96) | 0.38 | −20.8 (−31.0 to −10.7) | <0.001 | 0.98 (0.43 to 1.52) | <0.001 |
| Model 2 | −0.70 (−2.45 to 1.04) | 0.43 | −19.8 (−29.9 to −9.7) | <0.001 | 0.93 (0.39 to 1.48) | 0.001 |
| Model 3 | −0.69 (−2.43 to 1.05) | 0.44 | −19.8 (−29.8 to −9.7) | <0.001 | 0.93 (0.39 to 1.48) | 0.001 |
| Model 4 | −0.34 (−2.12 to 1.44) | 0.71 | −16.2 (−26.3 to −6.0) | 0.002 | 0.78 (0.22 to 1.33) | 0.006 |
| Model 5 | 1.61 (−0.42 to 3.64) | 0.12 | 6.5 (−4.3 to 17.3) | 0.24 | −0.22 (−0.83 to 0.38) | 0.47 |
In models of WHR, sex, age, MAP, and BMI are entered as independent values; BSA-indexed GFR and ERPF and FF are entered as dependent variables. In models of BMI, sex, age, MAP, and WHR are entered as independent values. Regression coefficients and confidence intervals are reported as standardized values. Model 1: crude; model 2: model 1 plus adjustment for sex; model 3: model 2 plus adjustment for age; model 4: model 3 plus adjustment for MAP; model 5: model 4 plus adjustment for BMI in model of WHR or adjustment for WHR in model of BMI.
For the crude renal hemodynamic measures, univariate analysis confirmed the association of WHR with ERPF (R=−0.210; P<0.001) and with FF (R=0.357; P<0.001). The association of WHR with GFR was borderline significant (R=0.107; P=0.06). BMI was associated with GFR (R=0.254; P<0.001) and with FF (R=0.207; P<0.001). Multivariate analyses are shown in Table 3. WHR (upper panel) was independently associated with GFR (β=−4.53 [95% CI, −6.90 to −2.16]; P<0.001), ERPF (β=−52.7 [95% CI, −65.2 to −40.1]; P<0.001) and FF (β=2.03 [95% CI, 1.39 to 2.67]; P<0.001), after adjustment for BSA, sex, age, MAP, and BMI. BMI (lower panel) was independently associated with GFR (β=3.92 [95% CI, 1.11 to 6.72]; P=0.006), after adjustment for BSA, sex, age, MAP, and WHR. The association of BMI with ERPF (β=−22.0 [95% CI, −36.9 to −7.2]; P=0.004) and FF (β=−1.46 [95% CI, 0.74 to 2.18]; P<0.001) was independent of BSA, sex, age, and MAP; however, this association was eliminated after inclusion of WHR in the model. Similar results were found with analysis for height-indexed GFR and ERPF (data not shown).
Table 3.
Multivariate data for WHR and BMI with renal hemodynamic measures, unindexed
| Variable | GFR | ERPF | FF | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| WHR | ||||||
| Model 1 | 2.21 (−0.09 to 4.51) | 0.06 | −22.8 (−35.3 to −10.4) | <0.001 | 1.72 (1.19 to 2.24) | <0.001 |
| Model 2 | −3.41 (−5.53 to −1.29) | 0.002 | −53.5 (−64.9 to −42.1) | <0.001 | 2.29 (1.72 to 2.85) | <0.001 |
| Model 3 | −3.46 (−5.57 to −1.35) | 0.001 | −54.0 (−65.0 to −42.9) | <0.001 | 2.30 (1.74 to 2.86) | <0.001 |
| Model 4 | −3.34 (−5.45 to −1.23) | 0.002 | −53.6 (−64.7 to −42.4) | <0.001 | 2.30 (1.74 to 2.87) | <0.001 |
| Model 5 | −3.11 (−5.28 to −0.94) | 0.005 | −51.0 (−62.4 to −39.7) | <0.001 | 2.19 (1.61 to 2.76) | <0.001 |
| Model 6 | −4.53 (−6.90 to −2.16) | <0.001 | −52.7 (−65.2 to −40.1) | <0.001 | 2.03 (1.39 to 2.67) | <0.001 |
| BMI | ||||||
| Model 1 | 5.06 (2.82 to 7.29) | <0.001 | −1.2 (−13.9 to 11.4) | 0.85 | 0.98 (0.43 to 1.52) | <0.001 |
| Model 2 | −0.44 (−2.64 to 1.76) | 0.69 | −30.4 (−43.0 to −17.7) | <0.001 | 1.45 (0.84 to 2.06) | <0.001 |
| Model 3 | 1.30 (−1.29 to 3.88) | 0.32 | −25.4 (−40.4 to −10.4) | 0.001 | 1.59 (0.87 to 2.32) | <0.001 |
| Model 4 | 1.35 (−1.23 to 3.93) | 0.30 | −25.1 (−40.1 to −10.1) | 0.001 | 1.59 (0.86 to 2.31) | <0.001 |
| Model 5 | 1.63 (−0.96 to 4.23) | 0.22 | −22.0 (−36.9 to −7.2) | 0.004 | 1.46 (0.74 to 2.18) | <0.001 |
| Model 6 | 3.92 (1.11 to 6.72) | 0.006 | 4.5 (−10.4 to 19.4) | 0.55 | 0.44 (−0.32 to 1.19) | 0.26 |
In models of WHR, BSA, sex, age, MAP, and BMI are entered as independent values; unindexed GFR and ERPF, and FF are entered as dependent variables. In models of BMI, BSA, sex, age, MAP, and WHR are entered as independent values. Regression coefficients and confidence intervals are reported as standardized values. Model 1: crude; model 2: model 1 plus adjustment for BSA; model 3: model 2 plus adjustment for sex; model 4: model 3 plus adjustment for age; model 5: model 4 plus adjustment for MAP; model 6: model 5 plus adjustment for BMI in model of WHR or adjustment for WHR in model of BMI.
No significant interaction was present between WHR and sex on GFR, ERPF, or FF, suggesting that sex was not a major modifier of the independent relationship between WHR and renal hemodynamics. Albuminuria was available and above the detection limit in 185 persons; in this subpopulation, no association was seen between WHR and albuminuria excretion (R=−0.076; P=0.289). Urinary sodium excretion was available in 192 persons and was positively associated with both unindexed GFR (R=0.248; P=0.001) and ERPF (R=0.233; P=0.001), but not with FF. These associations with urinary sodium excretion disappeared when renal hemodynamics were indexed for BSA or height.
To test whether recruitment source (i.e., prospective kidney donors versus healthy volunteers in several renal hemodynamic studies) affected the relationship between WHR and renal hemodynamics, linear regression analysis was repeated with recruitment source included in the model. This did not materially change the results (data not shown), indicating lack of effect of the recruitment source.
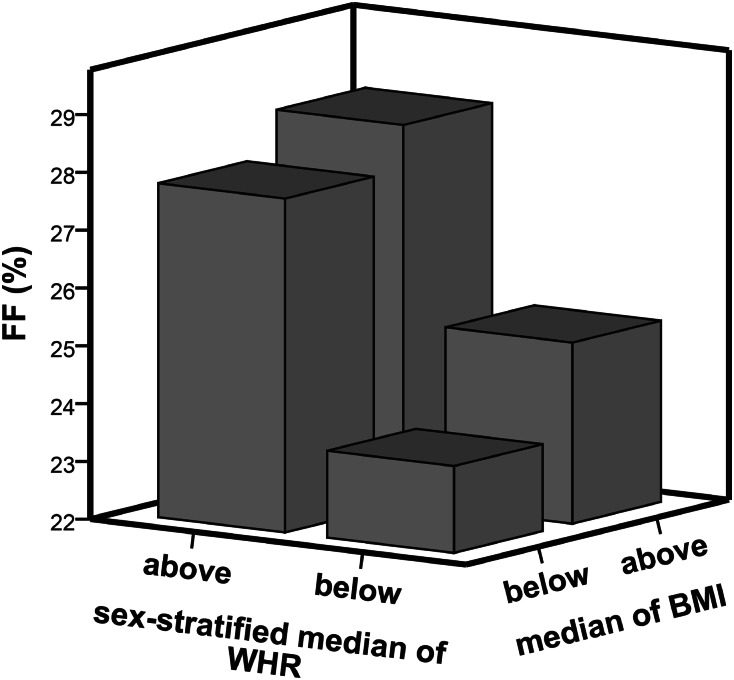
The combined effects of WHR and BMI on FF are illustrated in Figure 2, which shows mean FF by sex-stratified median of WHR (0.86) and median of BMI (24.6 kg/m2), respectively. It clearly illustrates that a WHR above the median was associated with a substantially and significantly higher FF in persons with lower BMI and in those with higher BMI (P<0.001 for both). No significant interaction between WHR and BMI on FF was detected.
Figure 2.
Mean FF per median WHR and BMI. Three-dimensional bar graph showing the combined effects of WHR and BMI on FF, with mean FF (y-axis) per sex-stratified median of WHR (x-axis) and median BMI (z-axis). Median WHR was 0.86 and median BMI was 24.6 kg/m2.
Discussion
We found that higher WHR was associated with an unfavorable renal hemodynamic profile, with lower GFR and ERPF, and with higher FF, independent of BMI, in a population of nonhypertensive, nondiabetic healthy persons. These data are in line with epidemiologic studies showing that central body fat distribution is an independent risk factor for renal damage and suggest that renal hemodynamic factors could be involved in the increased renal risk.
For our analysis, we used renal data normalized for BSA, as well as the crude renal data. BSA normalization is warranted to account for differences in body size between individuals, but it can lead to systematic error in analyses of associations with participant characteristics that relate to body dimensions, such as weight excess or, in this case, body fat distribution.14–17 To account for such bias, height normalization has been recommended,14 as well as normalization to extracellular volume.18,19 However, there is no validated gold standard. Unfortunately, data on extracellular volume were available in only a relatively small subset of persons in this study. Therefore, we presented BSA-normalized data, as well as crude data. In both analyses, higher WHR was associated with lower GFR and ERPF in multivariate analysis, supporting the robustness of the association, which was further confirmed in the height-indexed data. Moreover, higher WHR was also associated with higher FF. The latter is calculated as GFR/ERPF and hence is insensitive to assumptions underlying normalizations. Taken together, this finding supports the validity of the associations of renal hemodynamics with WHR detected here.
The associations of GFR, ERPF, and FF with WHR were independent of BMI, as demonstrated by the multivariate analysis. The independence was not due to lack of effect of BMI on renal hemodynamics because BMI was associated with ERPF and FF as well, with lower ERPF and higher FF in persons with higher BMI. The association of BMI with renal hemodynamics is in line with our prior findings in healthy persons5,20 and in transplant recipients,21 along with data in the literature on obese and morbidly obese persons.22–25 Our data also agree with prior studies on the association of body fat distribution with renal hemodynamics in obese and hypertensive persons, respectively. In obese persons with central body fat distribution, Scaglioni and colleagues found a lower nonindexed ERPF and a somewhat higher FF compared with obese persons who have peripheral body fat distribution.11 Reid and colleagues found an inverse association between WHR and renal blood flow in hypertensive patients with and without microalbuminuria.12 Hence, both studies found a univariate association between WHR and renal hemodynamics. However, to our knowledge, we are the first to report that the effect of WHR on renal hemodynamics is independent of BMI. Furthermore, we demonstrated that this association is present in a population of healthy persons.
Increasing evidence links body fat distribution with well established renal risk factors, as well as long-term renal damage. Several studies reported that WHR was associated with albuminuria26–28 and BP,29,30 both irrespective of BMI. In the current study, however, we have not found a robust association between WHR and albuminuria, possibly because of the very low albuminuria excretion rates, which reflect the strict inclusion criteria of this healthy population. As to long-term data, when the distribution of body fat is considered, central distribution was a stronger independent predictor of renal damage than general adiposity estimated by BMI (i.e., persons with central fat distribution, whether lean, overweight, or obese, showed a greater risk of decreased filtration rate).1 In an even larger cohort study in 13,000 individuals with long-term follow-up, WHR was independently associated with incident CKD and a composite endpoint of all-cause mortality and incident CKD.2 Finally, in an elderly population, central body fat distribution was more accurate in predicting CKD than BMI.3
Several mechanisms may play a role in the deleterious effects of central fat accumulation on the kidney, such as oxidative stress and inflammation by upregulation of proinflammatory adipokines and cytokines.31,32 Furthermore, central fat is associated with insulin resistance,33 dyslipidemia,34–36 metabolic syndrome,37 and type 2 diabetes,38–40 most likely in mutual interaction. An association with the nervous system has also been proposed.41 Our study does not allow dissection of different mechanisms underlying the deleterious effect of central body fat distribution on the kidney; however, it can add to current literature that abnormalities in renal hemodynamics occur in absence of hypertension and impaired glucose tolerance and diabetes.
Our data demonstrate an independent association of higher WHR with lower GFR and ERPF, and with higher FF. This renal hemodynamic profile has similarities with renal hemodynamics in sodium-sensitive hypertensive patients42,43 and with renal hemodynamic profile in overweight and morbid obesity,5,13,44 whereas in the latter condition GFR tends to be elevated, usually in association with comorbid diabetes.23 On the basis of extensive animal data, including micropuncture data from Brenner’s group, an elevated FF is assumed to reflect elevated glomerular pressure, which contributes to long-term renal risk by glomerular capillary hypertension.45 The validity of the glomerular hypertension hypothesis in humans is supported by intervention data in CKD, demonstrating predictive power of FF reduction for long-term renal prognosis46,47 and by observational data in renal transplant recipients.21 The combination of lower ERPF and higher FF indicates a higher postglomerular efferent arteriolar tone that can affect renal sodium handling by altering peritubular Starling forces, hampering sodium excretion, and hence contributing to sodium-sensitive hypertension.42 Of note, a high sodium intake, in turn, can be a determinant of this unfavorable renal hemodynamic profile, as shown in hypertension42 and otherwise healthy overweight persons.20 In this study we also found an association between sodium status and both GFR and ERPF; however, this association disappeared after normalization for BSA or height. Intervention data in obese subjects showed that the renal hemodynamic profile was at least partly normalized by bariatric surgery, supporting its hemodynamic nature, and the potential reversibility.22 The latter is also supported by data on the renal effects of renin-angiotensin-aldosterone system blockade in relation to BMI.48 Whether this also applies to the renal hemodynamic changes in relation to body fat distribution remains to be explored in further studies.
This study has several limitations. First, because of its cross-sectional design, this study cannot assess causality. Second, WHR provides only an indirect measurement of central body fat distribution, and it would be interesting to assess intra-abdominal fat or liver fat more directly and more specifically (e.g., by means of computed tomography and dual-energy x-ray absorptiometry of the abdomen or by means of magnetic resonance imaging and spectroscopy of the liver, respectively).49,50 Yet, despite the indirect nature as a marker of abdominal fat, WHR is an accepted measure to asses central body fat and is commonly used in epidemiologic studies showing the association with long-term renal outcome. Third, it would also be interesting to have indirect markers of intra-abdominal fat available. Liver enzymes are increasingly appreciated as indirect measures of hepatic fat accumulation (i.e., nonalcoholic steatosis hepatis), which in turn is strongly associated with intra-abdominal fat accumulation and central body fat distribtution.51–53 It would have been interesting if we could have investigated whether liver enzymes, in particular alanine aminotransferase and γ-glutamyl transpeptidase, are associated with renal hemodynamic measures, independent of BMI, in a similar fashion as WHR. This is a relevant subject for future studies. Furthermore, no detailed data on metabolic status (other than the exclusion of diabetes) were available. Fourth, because the association between renal hemodynamics and BSA changes with increasing adiposity, this could possibly bias correlations between renal hemodynamics and WHR. However, in that respect, it is noteworthy that we found essentially similar results when studying the association between WHR and height-indexed and unindexed renal hemodynamics for GFR and ERPF and, by default, for FF, supporting the robustness of our data against assumptions on body composition.
In conclusion, a higher WHR was associated with lower GFR and ERPF, and with higher FF, in healthy persons; these are considered an unfavorable renal hemodynamic profile. Of note, this association was independent of BMI. These data suggest the possibility that an altered renal hemodynamic profile is involved in the long-term renal risk associated with body fat distribution, as seen in epidemiologic studies.
Concise Methods
Study Population
The total population consisted of 408 healthy persons in whom renal hemodynamic and anthropometric measurements were performed. For the current study we excluded persons who used antihypertensive medication to avoid possible interaction with the renin-angiotensin-aldosterone system and thereby on renal hemodynamics (n=54) and persons with an impaired fasting glucose (defined as fasting plasma glucose ≥5.6 mmol/L according to American Diabetes Association guidelines;54 n=39), leaving 315 healthy persons for the current analysis. The participants were either healthy persons screened and subsequently found eligible for kidney donation (n=208; 51% men; mean age ± SD, 47±12 years) or healthy volunteers previously studied in three different experimental protocols (n=107; 56% men; mean age ± SD, 24±5 years), of whom two protocols have been previously published together.55 Inclusion criteria for the three experimental protocols were similar and entailed normal BP (defined as systolic and diastolic BP <140 and <90 mmHg, respectively), normal renal function, and normoalbuminuria. For all persons, medical histories were without significant disease and physical examination did not reveal any abnormalities. No persons had diabetes mellitus, and persons did not use nonsteroidal anti-inflammatory drugs frequently. Data on use of oral contraceptives were not available. Healthy persons were studied during a standardized liberal sodium intake (200 mmol sodium per day), and prospective kidney donors were assessed during unrestricted sodium intake. The study is in accordance with the Declaration of Helsinki and was conducted according to the guideline for good clinical practice.
Renal Hemodynamic and Anthropometric Measurements
GFR and ERPF were measured by constant infusion of radiolabeled tracers, 125I-iothalamate, and 131I-hippurate, respectively, when the persons were in a quiet room and in a semi-supine position. After a blank blood sample was drawn, a priming solution containing 0.04 ml of the infusion solution per kg body weight (0.04 MBq of 125I-iothalamate and 0.03 MBq of 131I-hippurate) plus an extra bolus of 0.06 MBq of 125I-iothalamate was given at 08:00 hours, followed by constant infusion at 12 ml/h. To attain stable plasma concentrations of both tracers, a 2-hour stabilization period followed, after which baseline measurements started at 10:00 hours. The clearances were calculated as (U×V)/P and (I×V)/P, respectively. U×V represents the urinary excretion of the tracer, I×V represents the infusion rate of the tracer, and P represents the tracer value in plasma at the end of each clearance period. This method corrects for incomplete bladder emptying and dead space, by multiplying the urinary clearance of 125I-iothalamate with the ratio of the plasma and urinary clearance of 131I-hippurate.56,57 To comply with common practice in the literature, GFR and ERPF were indexed for BSA as calculated using the DuBois-DuBois formula.58 In addition, we indexed GFR and ERPF for height. The filtration fraction (FF) was calculated as the ratio of GFR and ERPF and expressed as percentage. This renal hemodynamic parameter was not indexed for BSA. BP was measured during renal function measurements by noninvasive automatic BP assessment (Dinamap) and expressed as MAP (diastolic pressure plus one third of pulse pressure). Albuminuria and sodium excretion were measured in 24-hour urine collections. BMI as a measure of overall obesity was calculated by dividing body weight by height squared (kg/m2). Waist and hip circumference were measured on bare skin, at the natural indentation between the 10th rib and iliac crest and at the region of the trochanter major, respectively. Waist circumference was measured after an overnight fast and at the end of normal expiration to avoid influence of stomach content and respiration phase on measurements. Values of waist and hip circumference were expressed in whole centimeters, and WHR was calculated as waist circumference divided by hip circumference.
Statistical Analyses
Data are given as mean with SD when normally distributed or as median with interquartile range (IQR). Numbers and percentages were used to summarize categorical variables. A Pearson correlation test was performed to explore the univariate associations between WHR and BMI, respectively, with renal hemodynamics. Multivariate linear regression analysis was used to investigate the cross-sectional relations between WHR and BMI, respectively, with renal hemodynamics, with adjustment for sex, age and MAP. In line with general population studies using creatinine clearance or eGFR,59 the relation of age with GFR was nonlinear and was better described with addition of a quadratic term of age in the regression models. To test whether effects of WHR on renal hemodynamics were independent of BMI, the latter was forced into the model that tested the effects of WHR. To test whether effects of BMI were independent of WHR, the latter was forced into the model that tested the effects of BMI.
Primary analyses were performed with ERPF and GFR indexed for BSA to comply with common practice in the literature. However, in analyses of associations with measures of body dimensions, such as BMI and WHR, this can induce bias.14–17 Therefore, we additionally performed the same analyses with the crude values of ERPF and GFR, and finally, for height-indexed ERPF and GFR. Because the latter results were similar to those for the crude values, these data are not shown. We included BSA in the unindexed models to confirm results found with the BSA-indexed models. To check for collinearity between WHR and BMI, and for BSA with WHR or BMI, variance inflation factor diagnostics were used. Although WHR and BMI were correlated (R=0.496; P≤0.001) and BSA was correlated with WHR and BMI (R=0.447 and R=0.488, respectively; both P≤0.001), no co-linearity was present. All regression coefficients are reported as standardized regression coefficients to facilitate comparison between variables and models. We investigated potential interactions between WHR, BMI, and age by entering age, the investigated characteristic, and their product term in multivariate linear regression analyses. Finally, to ensure that the relationship between WHR and renal hemodynamics was not biased by recruitment source (i.e., prospective kidney donor or healthy volunteer in renal hemodynamic studies), we repeated linear regression analysis with adding recruitment source in the model. Data were analyzed using SPSS, version 18.0 (SPSS Inc., Chicago, IL), and GraphPad Prism, version 5 (GraphPad Software Inc., San Diego, CA). A two-sided P value <0.05 was considered to represent a statistically significant difference.
Disclosures
None.
Acknowledgments
We thank Dirkina Hesseling-Swaving, Roelie Karsten-Bartelds, and Marian Vroom-Dallinga for performing renal hemodynamic and anthropometric measurements.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE, PREVEND Study Group : A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41: 733–741, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, Levey AS, Weiner DE: Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis 52: 29–38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chou CY, Lin CH, Lin CC, Huang CC, Liu CS, Lai SW: Association between waist-to-hip ratio and chronic kidney disease in the elderly. Intern Med J 38: 402–406, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O: Obesity and risk for chronic renal failure. J Am Soc Nephrol 17: 1695–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G: Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int 65: 259–265, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J: Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ: Risk of end-stage renal disease in diabetes mellitus: A prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA 278: 2069–2074, 1997 [PubMed] [Google Scholar]
- 8.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J: The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140: 167–174, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Kurella M, Lo JC, Chertow GM: Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16: 2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Scaglione R, Ganguzza A, Corrao S, Parrinello G, Merlino G, Dichiara MA, Arnone S, D’Aubert MD, Licata G: Central obesity and hypertension: pathophysiologic role of renal haemodynamics and function. Int J Obes Relat Metab Disord 19: 403–409, 1995 [PubMed] [Google Scholar]
- 12.Reid M, Bennett F, Wilks R, Forrester T: Microalbuminuria, renal function and waist:hip ratio in black hypertensive Jamaicans. J Hum Hypertens 12: 221–227, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Kwakernaak AJ, Tent H, Rook M, Krikken JA, Navis GJ: Renal hemodynamics in overweight and obesity: Pathogenetic factors and targets for intervention. Expert Rev Endocrinol Metab 2: 539–552, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Schmieder RE, Beil AH, Weihprecht H, Messerli FH: How should renal hemodynamic data be indexed in obesity? J Am Soc Nephrol 5: 1709–1713, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Delanaye P, Radermecker RP, Rorive M, Depas G, Krzesinski JM: Indexing glomerular filtration rate for body surface area in obese patients is misleading: Concept and example. Nephrol Dial Transplant 20: 2024–2028, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Hihara E: Human body surface area: A theoretical approach. Eur J Appl Physiol 91: 425–428, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Verbraecken J, Van de Heyning P, De Backer W, Van Gaal L: Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 55: 515–524, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Visser FW, Muntinga JH, Dierckx RA, Navis G: Feasibility and impact of the measurement of extracellular fluid volume simultaneous with GFR by 125I-iothalamate. Clin J Am Soc Nephrol 3: 1308–1315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visser FW, Krikken JA, Muntinga JH, Dierckx RA, Navis GJ: Rise in extracellular fluid volume during high sodium depends on BMI in healthy men. Obesity (Silver Spring) 17: 1684–1688, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Krikken JA, Lely AT, Bakker SJ, Navis G: The effect of a shift in sodium intake on renal hemodynamics is determined by body mass index in healthy young men. Kidney Int 71: 260–265, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bosma RJ, Kwakernaak AJ, van der Heide JJ, de Jong PE, Navis GJ: Body mass index and glomerular hyperfiltration in renal transplant recipients: Cross-sectional analysis and long-term impact. Am J Transplant 7: 645–652, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y: The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U: Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol 278: F817–F822, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Reisin E, Messerli FG, Ventura HO, Frohlich ED: Renal haemodynamic studies in obesity hypertension. J Hypertens 5: 397–400, 1987 [PubMed] [Google Scholar]
- 25.Ribstein J, du Cailar G, Mimran A: Combined renal effects of overweight and hypertension. Hypertension 26: 610–615, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Liese AD, Hense HW, Döring A, Stieber J, Keil U: Microalbuminuria, central adiposity and hypertension in the non-diabetic urban population of the MONICA Augsburg survey 1994/95. J Hum Hypertens 15: 799–804, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Chandie Shaw PK, Berger SP, Mallat M, Frölich M, Dekker FW, Rabelink TJ: Central obesity is an independent risk factor for albuminuria in nondiabetic South Asian subjects. Diabetes Care 30: 1840–1844, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Mulyadi L, Stevens C, Munro S, Lingard J, Bermingham M: Body fat distribution and total body fat as risk factors for microalbuminuria in the obese. Ann Nutr Metab 45: 67–71, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, Welborn TA, AusDiab Steering Committee : Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med 254: 555–563, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Feldstein CA, Akopian M, Olivieri AO, Kramer AP, Nasi M, Garrido D: A comparison of body mass index and waist-to-hip ratio as indicators of hypertension risk in an urban Argentine population: a hospital-based study. Nutr Metab Cardiovasc Dis 15: 310–315, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Wajchenberg BL: Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr Rev 21: 697–738, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Hunley TE, Ma LJ, Kon V: Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens 19: 227–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyko EJ, de Courten M, Zimmet PZ, Chitson P, Tuomilehto J, Alberti KG: Features of the metabolic syndrome predict higher risk of diabetes and impaired glucose tolerance: A prospective study in Mauritius. Diabetes Care 23: 1242–1248, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Balkau B, Sapinho D, Petrella A, Mhamdi L, Cailleau M, Arondel D, Charles MA, D.E.S.I.R. Study Group : Prescreening tools for diabetes and obesity-associated dyslipidaemia: Comparing BMI, waist and waist hip ratio. The D.E.S.I.R. Study. Eur J Clin Nutr 60: 295–304, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barzi F, Woodward M, Czernichow S, Lee CM, Kang JH, Janus E, Lear S, Patel A, Caterson I, Patel J, Lam TH, Suriyawongpaisal P, Huxley R: The discrimination of dyslipidaemia using anthropometric measures in ethnically diverse populations of the Asia-Pacific Region: the Obesity in Asia Collaboration. Obes Rev 11: 127–136, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Huxley R, James WP, Barzi F, Patel JV, Lear SA, Suriyawongpaisal P, Janus E, Caterson I, Zimmet P, Prabhakaran D, Reddy S, Woodward M, Obesity in Asia Collaboration : Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev 9[Suppl 1]: 53–61, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB: Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 165: 777–783, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, Azambuja MI, Tracy RP, Heiss G: Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet 353: 1649–1652, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Harding AH, Day NE, Khaw KT, Bingham S, Luben R, Welsh A, Wareham NJ: Dietary fat and the risk of clinical type 2 diabetes: The European prospective investigation of Cancer-Norfolk study. Am J Epidemiol 159: 73–82, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Ohlson LO, Larsson B, Svärdsudd K, Welin L, Eriksson H, Wilhelmsen L, Björntorp P, Tibblin G: The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 34: 1055–1058, 1985 [DOI] [PubMed] [Google Scholar]
- 41.Hall JE: The kidney, hypertension, and obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Campese VM, Parise M, Karubian F, Bigazzi R: Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 18: 805–812, 1991 [DOI] [PubMed] [Google Scholar]
- 43.van Paassen P, de Zeeuw D, Navis G, de Jong PE: Does the renin-angiotensin system determine the renal and systemic hemodynamic response to sodium in patients with essential hypertension? Hypertension 27: 202–208, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Krikken JA, Bakker SJ, Navis GJ: Role of renal haemodynamics in the renal risks of overweight. Nephrol Dial Transplant 24: 1708–1711, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Brenner BM: Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 23: 647–655, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Apperloo AJ, de Zeeuw D, de Jong PE: Short-term antiproteinuric response to antihypertensive treatment predicts long-term GFR decline in patients with non-diabetic renal disease. Kidney Int Suppl 45: S174–S178, 1994 [PubMed] [Google Scholar]
- 47.Hansen HP, Rossing P, Tarnow L, Nielsen FS, Jensen BR, Parving HH: Increased glomerular filtration rate after withdrawal of long-term antihypertensive treatment in diabetic nephropathy. Kidney Int 47: 1726–1731, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Price DA, Lansang MC, Osei SY, Fisher ND, Laffel LM, Hollenberg NK: Type 2 diabetes, obesity, and the renal response to blocking the renin system with irbesartan. Diabet Med 19: 858–861, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL: Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 20: 1109–1114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF: Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol 16: 1560–1566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tahan V, Canbakan B, Balci H, Dane F, Akin H, Can G, Hatemi I, Olgac V, Sonsuz A, Ozbay G, Yurdakul I, Senturk H: Serum gamma-glutamyltranspeptidase distinguishes non-alcoholic fatty liver disease at high risk. Hepatogastroenterology 55: 1433–1438, 2008 [PubMed] [Google Scholar]
- 52.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ: Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev 22: 437–443, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Kotronen A, Yki-Järvinen H: Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 28: 27–38, 2008 [DOI] [PubMed] [Google Scholar]
- 54.American Diabetes Association : Diagnosis and classification of diabetes mellitus. Diabetes Care 31[Suppl 1]: S55–S60, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Krikken JA, Dallinga-Thie GM, Navis G, Dullaart RP: Renin-angiotensin-aldosterone responsiveness to low sodium and blood pressure reactivity to angiotensin-II are unrelated to cholesteryl ester transfer protein mass in healthy subjects. Expert Opin Ther Targets 12: 1321–1328, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Donker AJ, van der Hem GK, Sluiter WJ, Beekhuis H: A radioisotope method for simultaneous determination of the glomerular filtration rate and the effective renal plasma flow. Neth J Med 20: 97–103, 1977 [PubMed] [Google Scholar]
- 57.Apperloo AJ, de Zeeuw D, Donker AJ, de Jong PE: Precision of glomerular filtration rate determinations for long-term slope calculations is improved by simultaneous infusion of 125I-iothalamate and 131I-hippuran. J Am Soc Nephrol 7: 567–572, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–311, discussion 312–313, 1989 [PubMed] [Google Scholar]
- 59.Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D: C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 63: 654–661, 2003 [DOI] [PubMed] [Google Scholar]