Abstract
Regulatory T cells (Tregs) exert their immunosuppressive activity through several immunoregulatory mechanisms, including the production of anti-inflammatory cytokines such as IL-10. Although several studies suggest a role for Tregs in modulating crescentic GN, the underlying mechanisms are not well understood. Here, using IL-10 reporter mice, we detected IL-10–producing Foxp3+ T cells in the kidney, blood, and secondary lymphoid tissue in a mouse model of crescentic GN. Specific inactivation of Il10 in Foxp3+ Tregs eliminated the ability of these cells to suppress renal and systemic production of IFNγ and IL-17; these IL-10–deficient Tregs lost their capacity to attenuate renal tissue injury. These data highlight the suppressive functions of Tregs in crescentic GN and suggest the importance of Treg-derived IL-10 in ameliorating disease severity and in modulating both the Th1 and most notably Th17 immune response.
The discovery of CD4+CD25+Foxp3+ regulatory T cells (Tregs) in the 1990s and their indispensable role in (self) tolerance and autoimmunity marked the beginning of a new era in immunology.1 Since then, different suppressive mechanisms mediated by various Treg cell subsets were identified,2,3 particularly in well studied models of autoimmune diseases such as Crohn's disease,4 multiple sclerosis,5 or rheumatoid arthritis.6,7 Until now, only limited numbers of studies have assessed the function of regulatory T cells in crescentic GN. Adoptive cell transfer experiments in mice showed the beneficial role of exogenous wild-type (wt) CD4+CD25+ Tregs in attenuation of crescentic GN,8 whereas CCR6- and CCR7-deficient CD4+CD25+ Tregs failed to protect mice against GN.9,10 Recently, our own published data revealed the importance of endogenous Foxp3+ Tregs in suppressing the Th1 immune response and consequently ameliorating the disease severity in the T cell–dependent GN model of nephrotoxic nephritis (NTN).11 Concurrently, Ooi and coworkers confirmed the relevance of endogenous Foxp3+ Tregs in an accelerated model of experimental crescentic GN.12
However, the mechanisms of Treg cell-mediated suppression in crescentic GN are still unclear. One important player might be the anti-inflammatory cytokine IL-10, which is known to be released by Tregs in order to suppress immune responses and therefore might protect against autoimmunity.13 Indeed, endogenous IL-10 regulates the Th1 immune response in an accelerated model of experimental crescentic GN, as kidney damage is aggravated in IL-10–deficient mice.14 However, the source of protective IL-10 still needs to be clarified. Because IL-10 detection and tracking in vivo is difficult, most findings are based on studies with IL-10−/− mice.
Therefore, to study the cell-specific function of IL-10, we used a double-knockin reporter mouse model (Foxp3-IRES-mRFP (FIR) x IL-10 ires gfp-enhanced reporter [tiger]), which enables detection of the well-defined and simultaneous expression of IL-10 (green fluorescent protein [GFP]) and Foxp3 (monomeric red fluorescent protein [mRFP]). Indeed, we detected a distinct population of renal mRFP+(Foxp3+) Tregs expressing GFP (IL-10) upon induction of NTN. Thus, to investigate the role of Treg cell-derived IL-10 in NTN, we first adoptively transferred CD4+CD25+ Tregs from wt or IL-10−/− mice into wt mice subsequently challenged with nephrotoxic sheep serum. Adoptively transferred wt Tregs attenuated the course of NTN, whereas IL-10−/− Tregs did not. Furthermore, to analyze the role of endogenous IL-10 produced by Tregs, we generated Foxp3YFP-Cre x Il10flox/flox mice, in which IL-10 is selectively inactivated in Foxp3+ Tregs.15 Indeed, lack of Treg-derived IL-10 resulted in an aggravated course of NTN. In summary, we demonstrated a crucial role of Treg cell-derived IL-10 in regulating the Th1 and most notably the Th17 immune response in NTN. Hence, this study contributes to the understanding of the suppressive mechanisms of Tregs in crescentic GN and will have biologic implications for designing therapeutic approaches.
Results
Increased Mortality Rate and Disease Severity in IL-10–deficient Mice
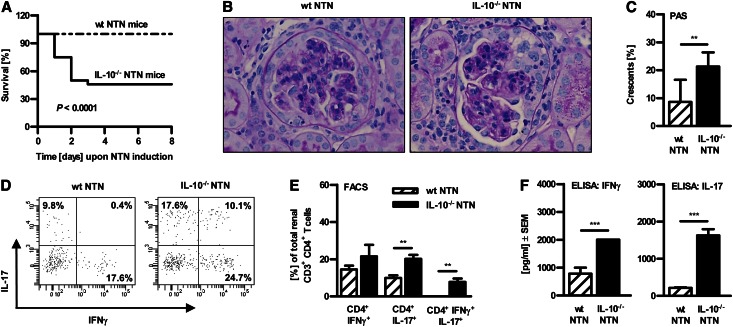
To investigate the role of IL-10 in the nonaccelerated model of crescentic GN, we induced NTN in C57BL/6 IL-10–deficient mice and wt mice. IL-10–deficient mice died within 72 hours upon NTN induction, whereas all wt mice survived (Figure 1A). Hence, we performed further experiments with a lower dose of the nephrotoxic serum (500 µl per mouse). Indeed, half of the IL-10−/− mice survived. Histologic analyses revealed more severe glomerular and interstitial damage in surviving nephritic IL-10−/− mice compared with nephritic wt mice, as shown by representative pictures of periodic acid-Schiff (PAS)–stained kidney sections and quantification of glomerular crescent formation (Figure 1, B and C). These data correlate with an increased Th1 and Th17 immune response in the kidney of nephritic IL-10–deficient mice compared with nephritic wt mice, as measured by flow cytometry analysis (Figure 1, D and E), as well as in spleen quantified by ELISA (Figure 1F).
Figure 1.
Protective role of IL-10 in the murine model of NTN. (A) Survival was monitored in wt versus IL-10–deficient mice upon NTN induction (Kaplan-Meier survival analysis; P = 0.0001, log-rank test; n≥24). (B) Representative photographs of PAS-stained kidney sections and (C) quantification of glomerular crescent formation from nephritic IL-10−/− and/or wt mice (400× magnification; **P<0.01). (D and E) Renal single cell suspensions from wt and IL-10−/− NTN mice were prepared for flow cytometry analysis. Cells were gated on CD3+CD4+ T cells and intracellular cytokine production of IFNγ and IL-17 was measured. Representative dot plots and quantification are depicted (**P<0.01; n>4). Numbers represent events in quadrants in percentage of all gated events. (F) Cytokine secretion of IFNγ and IL-17 by sheep IgG-treated splenocytes from nephritic wt and IL-10−/− mice was measured by ELISA (***P<0.0001). Results were obtained 7 days after NTN induction. Error bars represent standard error of the mean (SEM).
Upregulated Renal and Systemic IL-10 Production by Tregs of Nephritic Mice
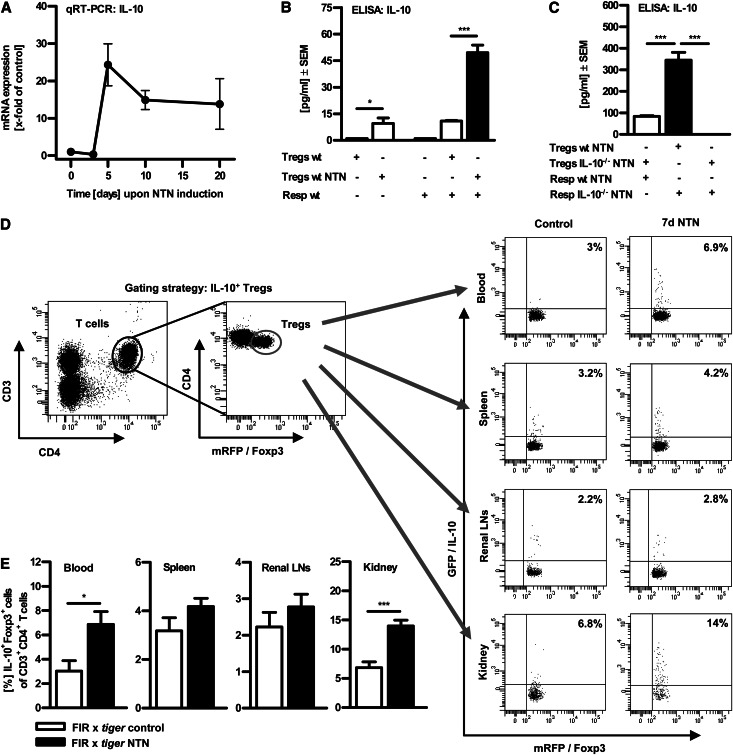
To evaluate the relevance of IL-10 for regulation of crescentic GN, we quantified the IL-10 expression level in renal tissue upon induction of NTN in a time course experiment. Of note, IL-10 mRNA expression was strongly upregulated in the autologous phase from day 5 onward (Figure 2A). This correlates with the time kinetics of Treg numbers in the kidney, as previously shown in our NTN model.11 Furthermore, we compared the immunosuppressive capacity of splenic CD4+CD25+ Tregs isolated from nephritic mice and their counterparts from healthy control mice and performed in vitro coculture experiments with naive CD4+CD25- responder T cells (= Resp wt). Purified CD4+CD25+ Tregs were also Foxp3+ (>95%, data not shown). ELISA analysis of the supernatants indicated that Tregs from nephritic mice exhibited a more pronounced regulatory phenotype because these Tregs released significantly more IL-10 upon single cultivation and even induced a five-fold IL-10 release upon cocultivation with naive CD4+CD25- responder T cells in contrast to Tregs from healthy controls (Figure 2B). Moreover, cocultivation of responder T cells from nephritic IL-10−/− mice with Tregs from nephritic wt mice revealed a significantly higher IL-10 production compared with cocultures of responder T cells from wt NTN mice with Tregs from IL-10−/− NTN mice. These results identify CD4+CD25+Foxp3+ Tregs as the main source of IL-10 in coculture with responder T cells (Figure 2C).
Figure 2.
IL-10 production is upregulated by Tregs upon NTN induction. (A) Quantitative real-time PCR analysis of renal IL-10 mRNA expression in the time course of NTN. (B) 1×105 splenic CD4+CD25− responder T cells (Resp wt) were cultured without or with 1×105 splenic CD4+CD25+ Tregs from non-nephritic wt controls (Tregs wt) or nephritic wt mice (Tregs wt NTN) and stimulated with plate-bound anti-CD3 mAb for 72 hours. (C) Under the same conditions, responder T cells isolated from wt or IL-10−/− NTN mice were cocultured with Tregs from wt or IL-10−/− NTN mice. Secretion of the cytokine IL-10 was assessed in supernatants by ELISA (*P<0.05, ***P<0.0001). (D) Single cell suspensions of blood, spleens, renal lymph nodes, and kidneys from non-nephritic and nephritic IL-10 reporter (FIR x tiger) mice were prepared and analyzed by flow cytometry at day 7 upon NTN induction. Gating strategy is depicted, as exemplified in blood. Cells were further analyzed for mRFP (Foxp3) and GFP (IL-10) expression. Representative dot plots are depicted. Numbers represent events in quadrants in percentage of all gated events. (E) Quantification of IL-10–producing CD4+Foxp3+ Tregs. LN, lymph node. (*P<0.05, ***P<0.0001). Error bars represent standard error of the mean (SEM).
To validate these results in the target organ, namely the kidney, and to detect IL-10–producing cell populations in vivo, we used double-knockin reporter mice, which allow detection of IL-10/GFP as well as Foxp3/mRFP (FIR x tiger mice).16 Indeed, we measured a distinct population of GFP+(IL-10+) and mRFP+(Foxp3+) double-positive cells in the murine kidney 7 days upon induction of NTN via flow cytometry (Figure 2, D and E). The frequency of renal IL-10+Foxp3+ Tregs significantly increased from 6.8% ± 1% in non-nephritic FIR x tiger mice (n=10) to about 14% ± 1% in nephritic animals (n=14). Similarly, a significant increase of IL-10+Foxp3+ Tregs upon NTN induction was detectable in blood (3% ± 0.9% versus 6.9% ± 1%), whereas frequencies were only slightly increased in spleen (3.2% ± 0.5% versus 4.2% ± 0.3%) and renal draining lymph nodes (2.2% ± 0.4% versus 2.8% ± 0.3%). Accordingly, adoptive transfer experiments with Tregs from IL-10−/− mice revealed that these cells failed to suppress nephrotoxic nephritis (Supplemental Figure 1).
Detection of Renal IL-10–producing Helper T Cells, Dendritic Cells, Macrophages and B Cells in Nephritic Mice
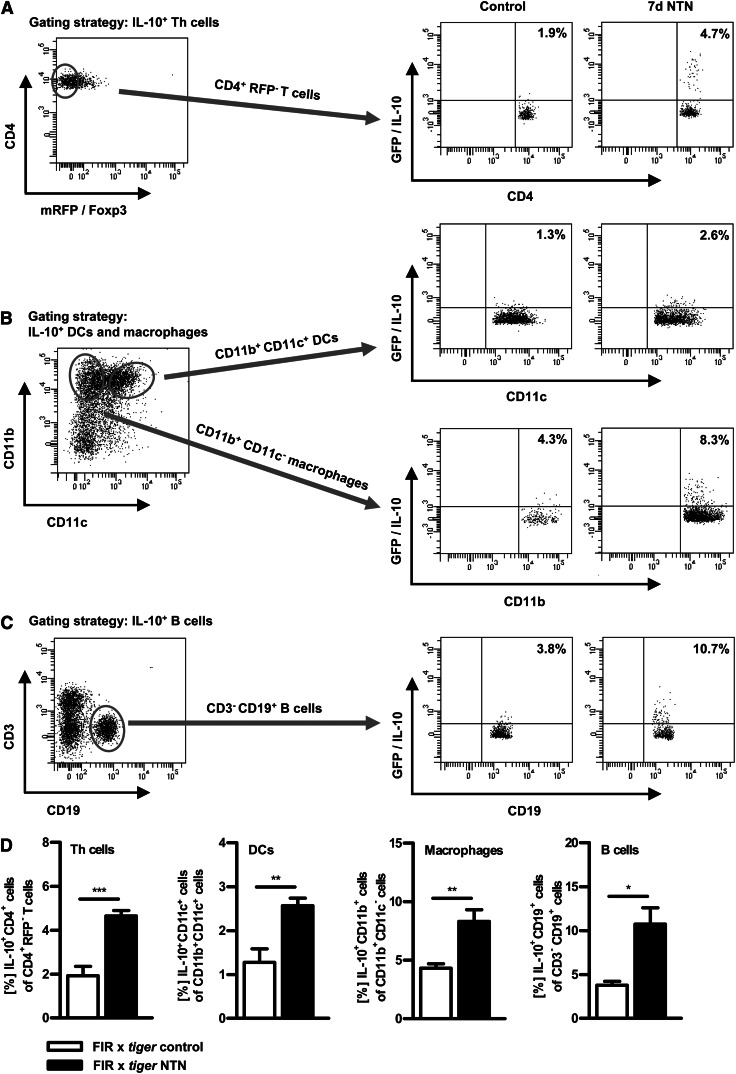
Through use of IL-10 reporter (FIR x tiger) mice, the renal IL-10 production of helper T (Th) cells (non-Tregs), dendritic cells (DCs), macrophages, and B cells was analyzed via flow cytometry 7 days upon induction of NTN (Figure 3). The gating strategy is depicted. We detected a significantly higher proportion of renal IL-10+CD4+(Foxp3-) Th cells (Figure 3A), as well as IL-10–producing CD11b+CD11c+ DCs (Figure 3B), CD11b+CD11c- macrophages (Figure 3B), and CD19+ B cells (Figure 3C), in nephritic IL-10 reporter (FIR x tiger) mice compared with healthy controls. This clearly shows that in addition to Tregs, other cell populations are also capable of producing IL-10 in the inflamed kidney. Frequencies of the renal IL-10 producer were quantified (Figure 3D).
Figure 3.
IL-10-producing T cells, B cells, DCs and macrophages infiltrate the inflamed kidney. Renal single cell suspensions from non-nephritic and nephritic IL-10 reporter (FIR x tiger) mice were prepared and analyzed by flow cytometry 7 days after NTN induction. Cells were gated on the appropriate cell surface markers for (A) Th cells (CD4+RFP−), (B) DCs (CD11b+CD11c+), (B) macrophages (CD11b+CD11c−), and (C) B cells (CD3−CD19+) and further analyzed for GFP (IL-10) expression. Representative dot plots are depicted. Numbers represent events in quadrants in percentage of all gated events. (D) Quantification of renal IL-10–producing cells (*P<0.05, **P<0.01, ***P<0.0001). Error bars represent standard error of the mean (SEM).
Cell-specific IL-10 Deletion in Foxp3YFP-Cre x Il10flox/flox Mice
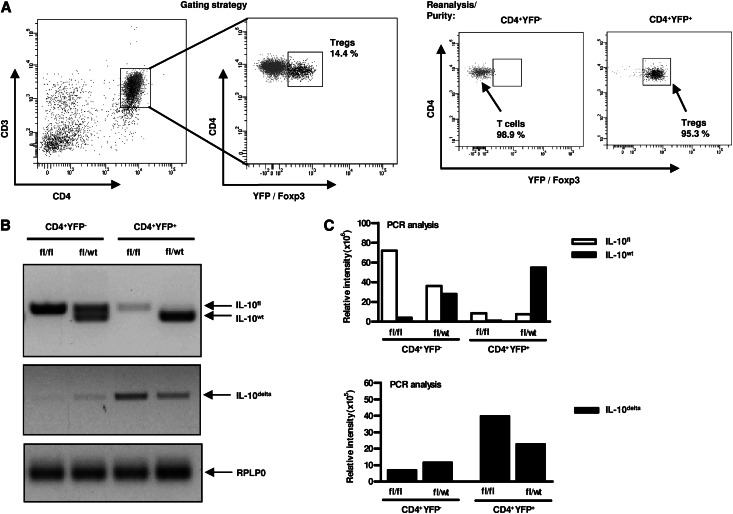
In the next step, we analyzed the specific role of endogenous IL-10–producing Tregs by the usage of Foxp3YFP-Cre x Il10flox/flox mice. To generate a selective IL-10 gene inactivation in Foxp3+ Tregs, we bred Il10flox/flox mice harboring loxP sites with Foxp3YFP-Cre mice. Splenic YFP-Cre+(Foxp3+) CD4+ T cells and YFP-Cre-(Foxp3-) CD4+ T cells from Foxp3YFP-Cre x Il10flox/wt or Foxp3YFP-Cre x Il10flox/flox mice were FACS-sorted to a high cell purity in order to quantify the wt (Il10wt), the floxed undeleted (Il10flox) and the recombined deleted (Il10delta) Il10 gene product. The gating strategy is depicted in Figure 4A. Enriched splenic CD4+ T cells were stained with anti–CD3-APC and anti–CD4-PE antibodies. CD3+CD4+ T cells were further analyzed for YFP (= Foxp3) expression. Analysis of sorted cells indicated a purity of 95.3% (YFP+) and 98.1% (YFP-), respectively. Genomic DNA was isolated from FACS-sorted YFP-Cre+(Foxp3+) CD4+ T cells and YFP-Cre-(Foxp3-) CD4+ T cells. PCR analysis showed an efficient deletion of IL-10 in YFP-Cre+CD4+ T cells (= Foxp3+ Tregs) isolated from male Foxp3YFP-Cre x Il10flox/flox mice, whereas the Il10delta gene product was absent in YFP-Cre-CD4+ T cells (i.e., nonregulatory T cells). Moreover, we analyzed heterozygous Foxp3YFP-Cre x Il10flox/wt mice: YFP-Cre-CD4+ T cells displayed an intact Il10wt and Il10flox allele, whereas YFP-Cre+CD4+ Tregs exhibited an intact Il10wt and Il10delta allele (Figure 4B). In Figure 4C, the relative fluorescence intensities of the three PCR products (IL-10flox, IL-10wt, and IL-10delta) were quantified. The Il10flox allele was deleted to about 90% in CD4+YFP+ cells, whereas it was still detectable and intact in CD4+YFP- cells, thereby demonstrating the successful Treg cell-specific deletion of IL-10.
Figure 4.
IL-10 is specifically inactivated in Tregs in Foxp3YFP-Cre x Il10flox/flox mice. Genomic DNA was isolated from FACS-sorted YFP-Cre−CD4+ and YFP-Cre+CD4+ splenocytes (2×105 cells) of non-nephritic Foxp3YFP-Cre x Il10flox/wt (heterozygous) mice and Foxp3YFP-Cre x Il10flox/flox mice (hemizygous). (A) FACS-sorting strategy for CD4+YFP− cells and CD4+YFP+ cells is depicted. Cells were gated on CD3+CD4+ T cells and measured for YFP (=Foxp3) expression. Analysis of sorted cells indicated a purity of 95.3% (YFP+) and 98.1% (YFP−), respectively. (B) PCR analysis for the presence of wt (IL-10wt) and undeleted (IL-10flox) or deleted (IL-10delta) Il10 alleles were performed and are represented by photographs of agarose gel electrophoresis. RPLP0 (60S acidic ribosomal protein P0) was used as a loading control. (C) Quantification of the relative fluorescence intensity of the PCR products. Error bars represent standard error of the mean (SEM).
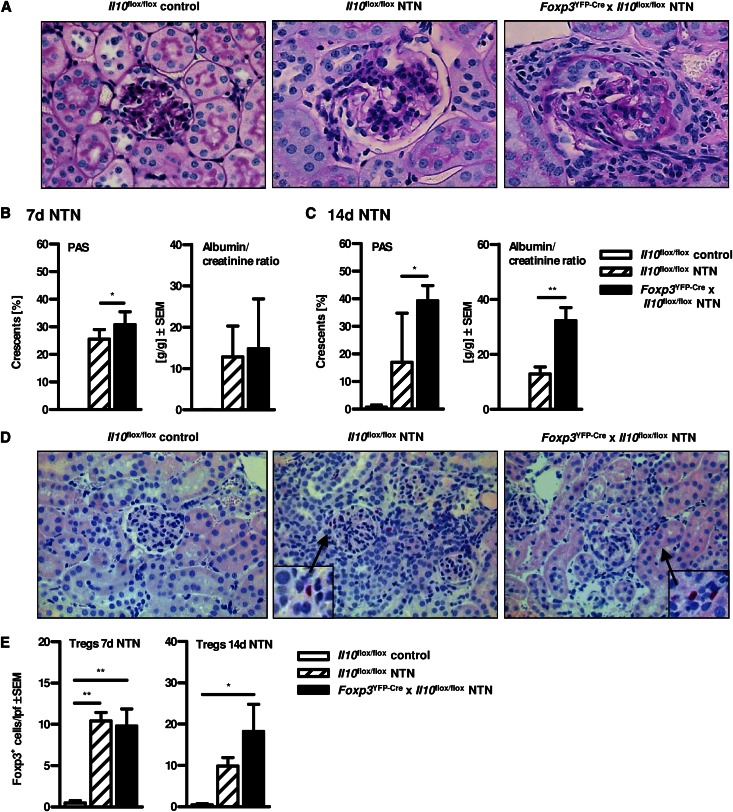
Aggravated NTN in Mice Lacking IL-10–producing Tregs
We induced NTN in Foxp3YFP-Cre x Il10flox/flox and Il10flox/flox mice. To quantify glomerular and tubular tissue damage, PAS-stained kidney sections were evaluated as previously described.9 The frequency of glomerular crescent formation on day 7 of NTN was significantly increased in Foxp3YFP-Cre x Il10flox/flox mice, which selectively lack IL-10–producing Tregs, in contrast to IL-10–competent Il10flox/flox mice (Figure 5, A and B). This effect was more pronounced 14 days upon induction of NTN (Figure 5C). Noteworthy, both untreated Foxp3YFP-Cre x Il10flox/flox (data not shown) and Il10flox/flox mice (Figure 5A) did not show any signs of tissue pathology in the kidney per se, and nephritic Foxp3YFP-Cre mice and Il10flox/flox mice exhibited a similar degree of kidney damage compared with nephritic wt mice, excluding significant pathologic effects induced by the transgene (Figure 5A and data not shown). Moreover, mice selectively lacking IL-10–producing Foxp3+ Tregs exhibited significantly elevated levels of albuminuria in comparison with IL-10–competent mice 14 days upon induction of NTN (Figure 5C). Again, this clinical measure was less prominent on day 7 (Figure 5B). Levels of BUN showed no significant difference between nephritic Il10flox/flox and Foxp3YFP-Cre x Il10flox/flox mice (data not shown). Nevertheless, Foxp3YFP-Cre x Il10flox/flox mice exhibited an aggravated course of NTN.
Figure 5.
Aggravated nephrotoxic nephritis in mice lacking IL-10–producing Tregs. (A) Representative photographs of PAS-stained kidney sections (400× magnification). (B and C) Quantification of glomerular crescents of non-nephritic Il10flox/flox control mice and nephritic Il10flox/flox and Foxp3YFP-Cre x Il10flox/flox mice and quantification of albumin-to-creatinine ratio was performed (*P<0.05, **P<0.01). (D) Kidney sections of nephritic Il10flox/flox and Foxp3YFP-Cre x Il10flox/flox mice were stained for the Treg-specific transcription factor Foxp3 and (E) numbers of Foxp3+ cells per low-power field (100× magnification) were determined (*P<0.05, **P<0.01). All experiments were performed at days 7 and 14 after induction of NTN. Error bars represent standard error of the mean (SEM).
To exclude an influence on Foxp3 expression in Foxp3YFP-Cre x Il10flox/flox mice due to the transgene, we quantified the number of Foxp3+ cells in Foxp3YFP-Cre x Il10flox/flox and Il10flox/flox mice by histologic staining of Foxp3. Indeed, the numbers of Tregs were similar in both Foxp3YFP-Cre x Il10flox/flox and Il10flox/flox mice verifying the intact expression of Foxp3 in Tregs lacking IL-10 (Figure 5, D and E). Hence, renal Treg frequency is unaffected. Therefore, aggravated kidney damage in Foxp3YFP-Cre x Il10flox/flox mice resulted from qualitative rather than from quantitative differences in the Treg compartment.
Increased Renal IFNγ and IL-17 Response and Augmented Systemic Immune Response in Mice with Selective Deletion of Treg-derived IL-10
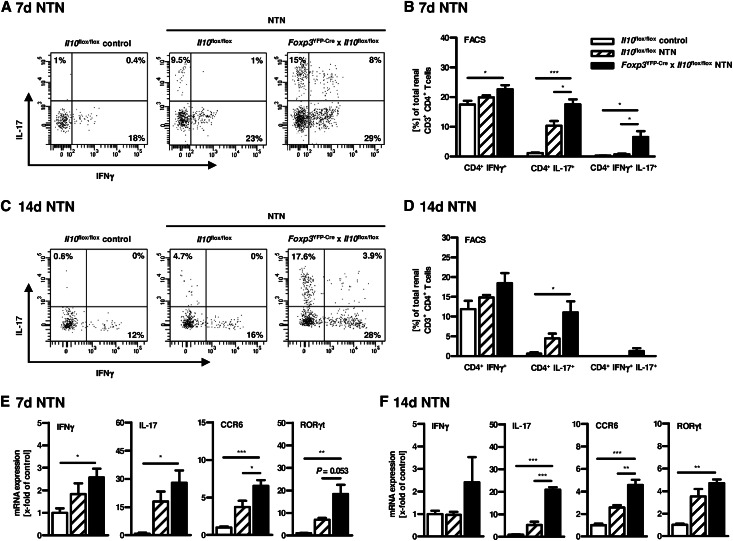
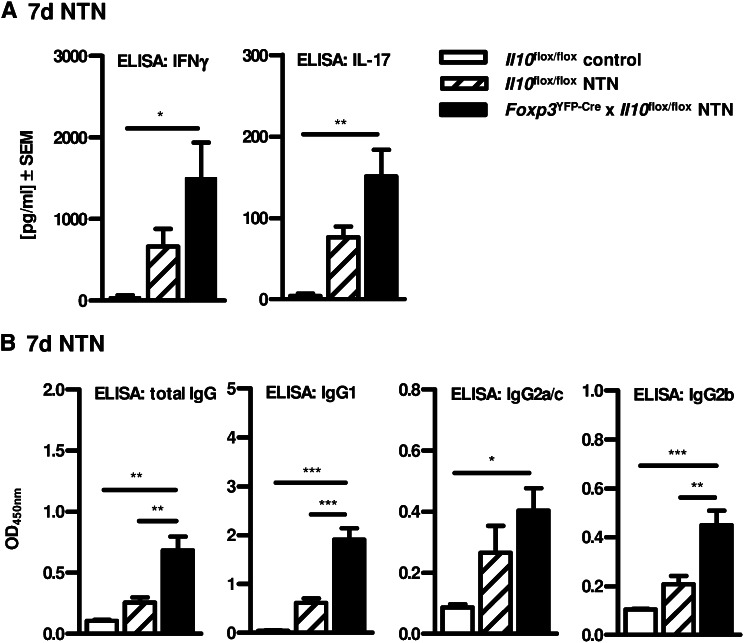
To evaluate the renal T helper cell response in Foxp3YFP-Cre x Il10flox/flox mice 7 and 14 days upon NTN induction, we performed intracellular cytokine staining in renal CD4+ T cells after in vitro stimulation with PMA/ionomycin. Indeed, Foxp3YFP-Cre x Il10flox/flox mice showed an increased renal Th1 and Th17 immune response in contrast to Il10flox/flox mice, as measured by flow cytometry (Figure 6, A–D). Of note, besides the slightly increased frequency of renal IFNγ+CD4+ T cells, the number of renal IL-17+ and IL-17+IFNγ+ double-positive CD4+ T cells was strikingly elevated in Foxp3YFP-Cre x Il10flox/flox mice. In line with this, IFNγ and especially IL-17 mRNA expression levels were strongly increased in kidney tissue isolated from nephritic mice lacking IL-10–producing Tregs (Figure 6, E and F). The increased Th17 immune response in Foxp3YFP-Cre x Il10flox/flox mice was associated with expression of the Th17-specific transcription factor RORγt and the Th17-relevant chemokine receptor CCR6 (Figure 6, E and F). To investigate the role of Treg-derived IL-10 regarding the systemic immune response in NTN, splenocytes were isolated from Foxp3YFP-Cre x Il10flox/flox and Il10flox/flox mice 7 or 14 days after induction of NTN and stimulated in vitro with sheep IgG, the nephritogenic antigen. Consistent with the results obtained in the inflamed kidney, ELISA analysis of splenocyte supernatants indicated that lack of Treg-derived IL-10 resulted in an elevated systemic IFNγ and IL-17 secretion in nephritic mice compared with NTN mice with IL-10–competent Tregs (Figure 7A and Supplemental Figure 2A). Furthermore, we determined the IgG antibody response directed against the nephritogenic antigen in serum samples by ELISA for sheep IgG-specific mouse IgG subclasses. Specific deletion of IL-10 in Tregs resulted in significantly increased levels of total IgG and the isotypes of IgG1, IgG2a/c, and IgG2b, which was most evident for IgG1 and IgG2b (Figure 7B and Supplemental Figure 2B), correlating with an increased Th17 immune response as previously shown.17 For demonstration that disease induction by the nephrotoxic serum was not influenced by the transgene, we performed semi-quantitative analysis of glomerular sheep/mouse IgG and complement factor C3 deposition, which showed no differences between nephritic Il10flox/flox mice and Foxp3YFP-Cre x Il10flox/flox mice (Supplemental Figure 3, 14 days upon NTN induction).
Figure 6.
Increased renal Th1 and Th17 immune response in nephritic mice lacking IL-10–producing Tregs. Renal single cell suspensions from non-nephritic Il10flox/flox control mice and nephritic Il10flox/flox mice and nephritic Foxp3YFP-Cre x Il10flox/flox mice were stimulated in vitro with PMA/ionomycin 7 days (A and B) or 14 days (C and D) after NTN induction. Intracellular cytokine production of IFNγ and IL-17 was analyzed by flow cytometry. Representative dot plots are depicted. Cells are gated on CD4+ T cells, and numbers represent events in quadrants in percentage of all gated events. The frequencies of IFNγ–/IL-17–producing renal CD4+ T cells were quantified (*P<0.05, ***P<0.0001). (E and F) Total RNA was extracted from kidneys. Subsequently, quantitative real-time RT-PCR was performed for IFNγ, IL-17, RORγt, and CCR6 expression 7 days or 14 days after NTN induction (*P<0.05, **P<0.01, ***P<0.0001). The expression levels are indicated as x-fold of non-nephritic Il10flox/flox control mice. Error bars represent standard error of the mean (SEM).
Figure 7.
Enhanced systemic Th1 and Th17 immune response in nephritic mice lacking IL-10–producing Tregs 7 days after NTN induction. (A) Cytokine secretion of IFNγ and IL-17 in supernatants of cultured and sheep IgG-treated splenocytes from non-nephritic Il10flox/flox control mice and nephritic Il10flox/flox mice and Foxp3YFP-Cre x Il10flox/flox mice was measured by ELISA (*P<0.05, **P<0.01). (B) Circulating titers of mouse antisheep total IgG and isotypes of IgG1, IgG2a/c, and IgG2b were measured in sera by ELISA (*P<0.05, **P<0.01, ***P<0.0001). Serum was diluted as indicated. All analyses were performed 7 or 14 days after NTN induction. Error bars represent standard error of the mean (SEM).
Discussion
Regulatory T cells exhibit a wide repertoire with respect to their immunosuppressive mechanisms.13,18 Under steady-state conditions, Tregs are thought to suppress T cells by deprivation of activation signals and consumption of IL-2, whereas under inflammatory conditions Tregs are activated or induced and obtain further suppressive mechanisms.18 These induced Tregs (iTregs) might create an immune-regulatory environment mediated by IL-10 and/or TGF-β, which results in antigen-specific as well as bystander immunosuppression.
Here, we describe a protective role of IL-10–producing Tregs in a murine model of crescentic GN. Very recently, we and others demonstrated that systemic depletion of endogenous Foxp3+ Tregs resulted in exacerbation of glomerular crescent formation and mouse antisheep IgG antibody production as well as in enhanced systemic and renal IFNγ production.11,12 However, up to now little has been known about the suppressive mechanisms mediated by Tregs and their behavior in renal inflammation. Recently, Eller et al. showed that Treg-derived IL-9 mediated the recruitment of anti-inflammatory mast cells into kidney-draining lymph nodes in NTN.19 Furthermore, Tregs were shown to protect against renal ischemia-reperfusion injury through IL-10–mediated suppression.20 Total IL-10–deficient mice showed an aggravated phenotype in accelerated and nonaccelerated NTN (Kitching et al.14 and our own observations) and renal IL-10 expression is strongly upregulated in wt mice upon NTN induction, indicating the protective function of endogenous IL-10 during NTN. Although innate IL-10 might be more critical for survival (mice died in the early phase of NTN), we investigated the role of Treg-derived IL-10 because its protective function in the inflamed kidney as well as therapeutic approaches are largely unknown and detection via flow cytometry is difficult. Indeed, IL-10–producing Tregs were clearly detectable in the kidney as well as in blood and secondary lymphoid organs in IL-10 reporter (FIR x tiger) mice. Frequencies of IL-10+ Tregs were significantly increased in kidney and blood upon NTN induction. During intestinal inflammation, it is well known that IL-10 released by Tregs plays an essential role,21–23 which was confirmed by the use of Il10flox/flox x Foxp3YFP-Cre mice carrying endogenous Treg cell-specific deficiency of IL-10.15
To investigate the role of endogenously Treg-derived IL-10 release during GN, we also generated Foxp3YFP-Cre x Il10flox/flox mice and, indeed, observed aggravated kidney damage upon induction of NTN. This clearly identifies the release of Treg-derived IL-10 as an important mechanism of regulating the proinflammatory Th1/Th17 response during NTN. It has to be emphasized that our experiments were performed at the age of 8–10 weeks in order to exclude any influence due to spontaneous colitis development in aged Il10flox/flox x Foxp3YFP-Cre mice.15 Furthermore, we confirmed the importance of Treg-derived IL-10 by demonstrating that adoptively transferred IL-10–deficient Tregs did not exert any suppressive and thereby protective function in nephritic mice (Supplemental Figure 1).
A recent study demonstrated that IL-10 is required to maintain Foxp3 expression because Tregs lacking the IL-10 receptor lost their Foxp3 expression.24 However, there are controversial results since Chaudhry and coworkers did not detect any differences in Foxp3 expression by IL-10R–deficient Foxp3+ Tregs.25 Moreover, it is postulated that IL-10 released by Tregs acts in an autocrine manner in order to self-regulate and expand Tregs themselves.18,23,24,26 Therefore, to exclude any influence of IL-10 deficiency with respect to Foxp3 stability, we determined the number of Foxp3+ Tregs in the inflamed kidney by immunohistochemistry. Of note, the numbers of renal Tregs were similar both in nephritic Foxp3YFP-Cre x Il10flox/flox and Il10flox/flox mice, verifying the intact expression of Foxp3 in Tregs lacking IL-10. Thus, aggravated kidney damage in Foxp3YFP-Cre x Il10flox/flox mice resulted from qualitative rather than quantitative differences in the Treg compartment.
These qualitative differences particularly affected the Th17 response. Both transfer of IL-10–deficient Tregs as well as use of Foxp3YFP-Cre x Il10flox/flox mice resulted in more pronounced IL-17 expression in the inflamed kidney and IL-17 release by splenocytes. This finding suggests a selective suppression pattern of IL-10–producing Tregs with respect to the Th17 immune response. We have recently demonstrated that Treg-pool depletion neither increased the local Th17 response in the kidney nor induced the systemic IL-17 production. However, the Th1 immune response was predominantly affected in these nephritic DEREG (DEpletion of REGulatory T cells) mice.11 The importance of IL-10 signaling in Tregs with respect to selective control of the Th17-mediated inflammatory process has already been investigated in the intestine.25–27 Chaudhry and colleagues demonstrated that ablation of the IL-10 receptor in Treg cells resulted in dysregulation of the Th17 response, followed by the spontaneous development of severe colitis.25 Additionally, Huber and coworkers showed that IL-17A–producing CD4+ T cells express the IL-10 receptor. Subsequently, specific blockade of the IL-10 signaling in T cells resulted in an increased frequency of IL17A+ and IL-17A+IFNγ+ CD4+ T cells during intestinal inflammation.27 These observations are in line with our results in the murine model of GN because lack of Treg-derived IL-10 led to higher numbers of both renal IL-17+ and IL-17+IFNγ+ CD4+ T cells during NTN.
The differentially regulated Th17 immune response in nephritic mice with either systemic Treg depletion in our recent study11 or with selective lack of IL-10–producing Tregs in the present study might be due to the plasticity of RORγt+ Th17 cells and Foxp3+ Tregs. Indeed, several studies in humans and mice have identified cell subsets, which coexpress the Treg master transcription factor Foxp3 and the Th17 cell-related transcription factor RORγt.28,29 Therefore, RORγt+Foxp3+ double-positive cells, which might differentiate into Th17 cells under inflammatory conditions, could have been depleted in DEREG mice upon injection of diphtheria toxin in our recent study. In line with this, we were able to detect a distinct renal Foxp3+IL-17+ T cell population in nephritic mice via flow cytometry (data not shown). This might explain why we observed an upregulation of the Th1 rather than the Th17 immune response in DEREG mice.11 In contrast, Foxp3YFP-Cre x Il10flox/flox mice lack only IL-10–producing Tregs, whereas the frequency of Foxp3+ cells is still unaltered. Hence, RORγt+Foxp3+ double-positive cells are still present in these mice and might potentially differentiate into RORγt+CCR6+ Th17 cells under pathogenic conditions. In general, CD4+ T cell plasticity/stability has a greater effect on the course of inflammatory diseases than previously thought. Further experiments are clearly needed to address this point in more detail, such as by using recently established IL-17 fate reporter mice.30
Furthermore, we reemphasize that Foxp3YFP-Cre x Il10flox/flox mice as well as recipient mice in transfer experiments still harbor a large proportion of IL-10+ immune-regulatory cells other than Tregs, as shown by own (Figure 3, A–D) and other observations. Thus, innate (e.g., DCs, macrophages) as well as adaptive immune cells (B cells and CD4+ Th cells [non-Tregs]) might influence the course of inflammatory reactions by producing IL-10.31–35 Further studies using the FIR x tiger mice as well as the Cre/loxP system are intended to study the potential role of these cells in more detail.
In summary, our study provides the first evidence for an important role of endogenous Treg-derived IL-10 in the immune regulation of crescentic GN. Of note, the Th1 and Th17 immune response during NTN is differentially regulated by distinct subsets of Tregs. IL-10–producing Tregs mainly influence the regulation of IL-17–releasing CD4+ effector T cells, whereas systemic depletion of Foxp3+ cells resulted in a strongly increased pathogenic Th1 cell response. These data might be helpful for the development of Treg-based treatment strategies in the future.
CONCISE METHODS
Animals
IL-10–deficient gene knockout mice (IL-10−/− mice)36 were originally obtained from Janvier (Le Genest-St-Isle, France) and bred in the animal facilities of the University Medical Center Hamburg-Eppendorf. Il10flox/flox mice were kindly provided by Axel Roers (Institute of Immunology, TU Dresden, Germany)37 and Foxp3YFP-Cre mice by Alexander Y. Rudensky (Howard Hughes Medical Institute and Immunology Program, Sloan Kettering Institute, New York, USA). Foxp3YFP-Cre mice and Il10flox/flox mice were crossed to generate Foxp3YFP-Cre x Il10flox/flox mice (C57BL/6 background) as previously described.15 Double-knockin Foxp3-IRES-mRFP (FIR) x tiger mice16 and sex-/age-matched (8–10 weeks old) C57BL/6 wt controls were bred in the animal facilities of the University Medical Center Hamburg-Eppendorf. Animals received humane care according to guidelines of the National Institutes of Health in Germany. Experiments were approved by the institutional review board, Behörde für Soziales, Familie, Gesundheit und Verbraucherschutz (Hamburg, Germany; approval code G09/122).
Animal Treatment and Functional Studies
NTN was induced in mice by intraperitoneal injection of 500–650 µl of nephrotoxic sheep serum per mouse. Controls received an equal amount of nonspecific sheep IgG. Blood samples for assessment of systemic antibody response were obtained at the time of sacrifice. For urine sample collection, mice were housed in metabolic cages for 6 hours. Urinary creatinine was measured by standard laboratory methods. Albuminuria was determined by standard ELISA analysis (mice-albumin kit; Bethyl Laboratories). In adoptive transfer experiments, splenic CD4+CD25+ Tregs (1×106) isolated from naive IL-10−/− or wt mice were injected intravenously into wt mice 1 day before NTN induction.
PCR Analysis and Agarose Gel Electrophoresis
Genomic DNA from splenocytes (2×105 cells) was isolated using an AllPrep DNA/RNA Micro Kit (Qiagen, Hilden, Germany), and 20 ng of template DNA was used for further PCR analysis. The standard PCR protocol was performed regarding primer pairs and their characteristics. For detecting of IL-10flox or IL-10wt alleles and the IL-10 allele lacking excised floxed fragment (IL-10delta), primers were used as described elsewhere.15 The 60S acidic ribosomal protein P0 (RPLP0; primer pair 5′-TGCCACACTCCATCATCAAT-3′ and 5′-CGAAGAGACCGAATCCCATA-3′) was used as loading control to ensure loading of equal amounts of template. DNA fragments were separated and analyzed by agarose gel electrophoresis. The relative fluorescence intensity of the PCR products was quantified using Image Lab software (Bio-Rad, Munich, Germany).
Real-time Qualitative RT-PCR Analysis
Total RNA was isolated with a Total RNA Isolation Kit (Macherey-Nagel, Düren, Germany) and reverse-transcribed, followed by qualitative RT-PCR using Biorad CFX96 real-time system and ABsolute QPCR SYBR mix (Thermo Fischer). Primer pairs were used as described previously.9,11 Relative mRNA levels were calculated after normalization to 18S rRNA using CFX96 Manager software.
Morphologic Examinations
Light microscopy and immunohistochemistry were performed by routine procedures.11 Briefly, crescent formation was assessed in 30 glomeruli per mouse in a blinded fashion in PAS-stained paraffin sections. Paraffin-embedded sections (2 µm) were also stained with an antibody directed against Foxp3 (FJK-16s, eBioscience, San Diego, CA), sheep or mouse IgG (Jackson ImmunoResearch Laboratories Europe Ltd., Newmarket, United Kingdom), or complement factor C3 (Cappel Laboratories, Organon Teknika, West Chester, PA). For quantification of Foxp3+ Tregs, at least seven low-power fields (100× magnification) were counted. Glomerular deposition of sheep IgG, mouse IgG, and C3 was scored from 0 to 3 in 30 glomeruli per mouse as previously described.38
Antigen-specific Humoral Immune Response
Mouse antisheep IgG antibody titers were measured by ELISA, as previously shown11 using sera collected at the time of sacrifice. Bound mouse IgG was detected using peroxidase-conjugated goat antimouse IgG (Biozol, Eching, Germany), and immunoglobulin isotypes were detected using peroxidase-conjugated rabbit antimouse IgG1, IgG2a/c, and IgG2b antibodies (Zymed-Invitrogen, Karlsruhe, Germany).
Leukocyte Isolation from Various Tissues
Previously described methods for leukocyte isolation from murine kidneys were used.9,39 In brief, kidneys were finely minced and digested for 40 minutes at 37°C with 0.4 mg/ml collagenase D (Roche, Mannheim, Germany) and 0.01 mg/ml DNAse I in RPMI 1640 medium supplemented with 10% heat-inactivated FCS (Invitrogen). Cell suspensions were filtered through 70- and 40-µm nylon meshes and washed with HBSS without Ca2+ and Mg2+ (Life Technologies GmbH, Darmstadt, Germany). Single-cell suspensions of spleens and renal lymph nodes were prepared according to standard laboratory procedures.11 Viability of the cells was assessed by trypan blue staining prior to flow cytometry.
Flow Cytometry
Leukocytes were stained using a standard protocol. For T cell differentiation, isolated cells were stained with anti-CD3 (APC, eBioscience, San Diego, CA), anti-CD4 (APC-AlexaFluor750), and anti-CD45 (PerCP; both Becton Dickinson, Heidelberg, Germany) upon a blocking step. Staining of intracellular IFNγ, IL-17, and Foxp3 was performed as described previously.11,40 In case of FIR x tiger mice, intracellular Foxp3 and IL-10 expression were determined via mRFP or GFP expression, respectively. Staining of CD11b, CD11c, and CD19 was performed as described previously.41 Data were recorded using BD LSRII Flow Cytometry system and BD FACSDiva software. Moreover, splenocytes from hemizygous Foxp3YFP-Cre x Il10flox/flox mice or heterozygous Foxp3YFP-Cre x Il10flox/wt mice were sorted using an FACS Aria III cell sorter.
Isolation and Culture of Splenic CD4+CD25+ Tregs and Responder T Cells
Spleens were excised from C57BL/6 wt or IL-10−/− mice 7 days after induction of NTN, and from healthy controls and passed through 100-µm nylon meshes. Sorting procedures were carried out by magnetic-activated cell sorting according to the manufacturers’ instructions (MACS CD4+ T-Cell-Isolation Kit; Miltenyi Biotec, Germany). Briefly, CD4+ T cells were enriched using a biotinylated antibody cocktail depleting all other blood cell types and antibiotin microbeads. CD4+CD25+ T cells were isolated by positive selection using PE-labeled anti-CD25 mAb and anti-PE microbeads. Purity and intracellular Foxp3 expression was controlled by flow cytometry. We cultured 1×105 wt responder T cells (CD4+CD25-) isolated from healthy mice alone or with 1×105 CD4+CD25+ Tregs from nephritic wt or healthy controls for 72 hours in 96-well plates precoated with anti-CD3 mAb (5 µg/ml; clone 145–2C11, BD Biosciences). Under the same conditions, responder T cells isolated from nephritic wt or IL-10−/− mice were cocultured with Tregs from nephritic wt or IL-10−/− mice. IL-10 concentrations were measured in supernatants by ELISA.
Statistical Analyses
Results are expressed as mean ± SEM. Differences between individual experimental groups were compared by t test. In case of multiple comparisons, one-way ANOVA with post analysis by Tukey-Kramer test was used. Experiments that did not yield enough independent data for statistical analysis because of the experimental setup were repeated at least three times.
Disclosure
None.
Acknowledgments
We thank Elena Tasika, Anett Peters, Sabrina Bennstein, and Mareike Holz for the perfect technical assistance. Moreover, we thank the members of the FACS Core Facility of the University Medical Center Hamburg-Eppendorf for cell sorting.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Klinische Forschergruppe 228: PA 754/7-2 to UP and TI169/9-2 to GT).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070684/-/DCSupplemental.
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155: 1151–1164, 1995 [PubMed] [Google Scholar]
- 2.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T: Regulatory T cells: How do they suppress immune responses? Int Immunol 21: 1105–1111, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Vignali DA, Collison LW, Workman CJ: How regulatory T cells work. Nat Rev Immunol 8: 523–532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paust S, Cantor H: Regulatory T cells and autoimmune disease. Immunol Rev 204: 195–207, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Costantino CM, Baecher-Allan C, Hafler DA: Multiple sclerosis and regulatory T cells. J Clin Immunol 28: 697–706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notley CA, Ehrenstein MR: The yin and yang of regulatory T cells and inflammation in RA. Nat Rev Rheumatol 6: 572–577, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Bettini M, Vignali DA: Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol 21: 612–618, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf D, Hochegger K, Wolf AM, Rumpold HF, Gastl G, Tilg H, Mayer G, Gunsilius E, Rosenkranz AR: CD4+CD25+ regulatory T cells inhibit experimental anti-glomerular basement membrane glomerulonephritis in mice. J Am Soc Nephrol 16: 1360–1370, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Turner JE, Paust HJ, Steinmetz OM, Peters A, Riedel JH, Erhardt A, Wegscheid C, Velden J, Fehr S, Mittrücker HW, Tiegs G, Stahl RA, Panzer U: CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol 21: 974–985, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eller K, Weber T, Pruenster M, Wolf AM, Mayer G, Rosenkranz AR, Rot A: CCR7 deficiency exacerbates injury in acute nephritis due to aberrant localization of regulatory T cells. J Am Soc Nephrol 21: 42–52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paust HJ, Ostmann A, Erhardt A, Turner JE, Velden J, Mittrücker HW, Sparwasser T, Panzer U, Tiegs G: Regulatory T cells control the Th1 immune response in murine crescentic glomerulonephritis. Kidney Int 80: 154–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ooi JD, Snelgrove SL, Engel DR, Hochheiser K, Ludwig-Portugall I, Nozaki Y, O’Sullivan KM, Hickey MJ, Holdsworth SR, Kurts C, Kitching AR: Endogenous foxp3(+) T-regulatory cells suppress anti-glomerular basement membrane nephritis. Kidney Int 79: 977–986, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM: Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30: 636–645, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Kitching AR, Tipping PG, Timoshanko JR, Holdsworth SR: Endogenous interleukin-10 regulates Th1 responses that induce crescentic glomerulonephritis. Kidney Int 57: 518–525, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY: Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28: 546–558, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galán JE, Harhaj E, Flavell RA: Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25: 941–952, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mitsdoerffer M, Lee Y, Jäger A, Kim HJ, Korn T, Kolls JK, Cantor H, Bettelli E, Kuchroo VK: Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A 107: 14292–14297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi T, Wing JB, Sakaguchi S: Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol 23: 424–430, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie AN, Maurer M, Rosenkranz AR, Wolf AM: IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune suppression. J Immunol 186: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F: An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190: 995–1004, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F: Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol 177: 5852–5860, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annacker O, Asseman C, Read S, Powrie F: Interleukin-10 in the regulation of T cell-induced colitis. J Autoimmun 20: 277–279, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M: Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10: 1178–1184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W, Rudensky AY: Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34: 566–578, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravano DM, Vignali DA: The battle against immunopathology: Infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci 69: 1997–2008, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber S, Gagliani N, Esplugues E, O’Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA: Th17 cells express interleukin-10 receptor and are controlled by Foxp3⁻ and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34: 554–565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ: Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 106: 4793–4798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G: In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med 205: 1381–1393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B: Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12: 255–263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter NA, Rosser EC, Mauri C: Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther 14: R32, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez W, Mold C, Kataranovski M, Hutt JA, Marnell LL, Verbeek JS, Du Clos TW: C-reactive protein-mediated suppression of nephrotoxic nephritis: role of macrophages, complement, and Fcgamma receptors. J Immunol 178: 530–538, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wilson HM, Stewart KN, Brown PA, Anegon I, Chettibi S, Rees AJ, Kluth DC: Bone-marrow-derived macrophages genetically modified to produce IL-10 reduce injury in experimental glomerulonephritis. Mol Ther 6: 710–717, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Tadagavadi RK, Reeves WB: Endogenous IL-10 attenuates cisplatin nephrotoxicity: Role of dendritic cells. J Immunol 185: 4904–4911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cope A, Le Friec G, Cardone J, Kemper C: The Th1 life cycle: Molecular control of IFN-γ to IL-10 switching. Trends Immunol 32: 278–286, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W: Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Müller W, Roers A: Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol 36: 3248–3255, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Paust HJ, Turner JE, Riedel JH, Disteldorf E, Peters A, Schmidt T, Krebs C, Velden J, Mittrücker HW, Steinmetz OM, Stahl RA, Panzer U: Chemokines play a critical role in the cross-regulation of Th1 and Th17 immune responses in murine crescentic glomerulonephritis. Kidney Int 82: 72–83, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Turner JE, Paust HJ, Steinmetz OM, Peters A, Meyer-Schwesinger C, Heymann F, Helmchen U, Fehr S, Horuk R, Wenzel U, Kurts C, Mittrücker HW, Stahl RA, Panzer U: CCR5 deficiency aggravates crescentic glomerulonephritis in mice. J Immunol 181: 6546–6556, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Paust HJ, Turner JE, Steinmetz OM, Peters A, Heymann F, Hölscher C, Wolf G, Kurts C, Mittrücker HW, Stahl RA, Panzer U: The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol 20: 969–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riedel JH, Paust HJ, Turner JE, Tittel AP, Krebs C, Disteldorf E, Wegscheid C, Tiegs G, Velden J, Mittrücker HW, Garbi N, Stahl RA, Steinmetz OM, Kurts C, Panzer U: Immature renal dendritic cells recruit regulatory CXCR6(+) invariant natural killer T cells to attenuate crescentic GN. J Am Soc Nephrol 23: 1987–2000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]