Abstract
Mutations in inverted formin 2 INF2 are a common cause of familial FSGS. INF2 interacts with diaphanous-related formins (mDia) and antagonizes mDia-mediated actin polymerization in response to active Rho signaling, suggesting that dysregulation of these pathways may mediate the development of INF2-related FSGS. However, the precise mechanisms by which INF2 regulates actin-dependent podocyte behavior remain largely unknown. Here, we investigated the possible role of INF2 in both lamellipodia-associated actin dynamics and actin-dependent slit diaphragm (SD) protein trafficking by manipulating the expression of INF2 and the activity of Rho/mDia signaling in cultured podocytes. Activation of mDia in the absence of INF2 led to defective formation of lamellipodia and abnormal SD trafficking. Effects of mutations disrupting the INF2-mDia interaction suggested the specificity of the mDia-antagonizing effect of INF2 in maintaining the lamellipodium. Furthermore, we found that SD trafficking requires INF2 interaction with lipid raft components. In summary, INF2 regulates lamellipodial actin dynamics and the trafficking of slit diaphragm proteins by opposing Rho/mDia-mediated actin polymerization. Thus, in podocytes, INF2 appears to be an important modulator of actin-dependent behaviors that are under the control of Rho/mDia signaling.
Podocytes are terminally differentiated epithelial cells with interdigitating processes that wrap around and support the capillaries of the kidney’s glomeruli. The terminal portions of these actin-rich extensions, known as foot processes (FPs), are bridged by cell-cell junctions called slit diaphragms (SD), a complex of proteins anchored at adjacent FPs.1,2 FPs are polarized cytoplasmic processes with an apical-basal junction demarcated by the SD complex.3 The SD protein complex participates in regulating the morphology and filter function of podocytes through crosstalk with actin remodeling pathways.4,5
Ultrastructural studies have shown that the FPs contain a central actin bundle surrounded by a cortical actin network. This cortical actin is essential for maintaining the morphology and function of podocytes.6 Through direct connections with the plasma membrane of FPs, cortical actin serves as a scaffold for SD proteins and their communication with actin filaments through signaling pathways and actin-binding proteins.6,7 Actin reorganization and SD protein translocation accompany the foot process effacement and loss of the filtration barrier seen in proteinuric diseases.8
Members of the Rho family of small GTPases play a central role in controlling the actin architecture of cells.9 Perturbations in the activity of several of the Rho family GTPases can disrupt the morphology of podocytes and lead to proteinuria. Constitutive activation of RhoA in podocytes in mice causes proteinuric kidney disease and FSGS, with FP effacement, prominent intracellular stress fibers, and altered distribution of SD proteins nephrin and podocin.10,11 HIV nephropathy and nephrotoxicity studies have also confirmed that factors altering Rho activity lead to FP effacement and SD dysfunction.12,13 Conversely, treatments that antagonize overactivated Rho may be able to restore proper actin dynamics, FP morphology, and the targeting of SD proteins,10,13 although expression of a dominant negative RhoA in podocytes also leads to glomerular disease.11 These and other studies point to the significance of fine-tuning RhoGTPase activity for preserving the actin-dependent phenotype of podocytes.14 Nevertheless, the precise understanding of the regulation of Rho-mediated actin dynamics required for maintaining the podocyte phenotype remains rudimentary.
Members of the family of the diaphanous subfamily of mammalian formins (or mDia) are major downstream effectors of Rho signaling.15 The mDias contain an N-terminal GTPase-binding domain (GBD) that overlaps a diaphanous inhibitory domain (DID), two regions affecting actin dynamics, the formin homology 1 and 2 (FH1 and FH2) domains, and a C-terminal diaphanous autoregulatory domain (DAD). mDia’s actin polymerizing activity is normally silenced by an intra-molecular DID/DAD interaction (termed autoinhibition). GTP-bound RhoA binding to the GBD disrupts the DID/DAD interaction, allowing mDia-mediated actin nucleation and elongation through the FH1 and FH2 domains.16,17
Previously, we identified a direct interaction between mDia and inverted formin 2 (INF2) using a yeast two-hybrid screen.18 INF2, like other formin family members, contains an N-terminal DID, a C-terminal DAD, and FH domains.19,20 Mutations in the INF2-DID cause FSGS that typically develops in adolescence or adulthood, often leading to overt kidney failure.21–23 Some patients with INF2 mutations display both FSGS and the demyelinating condition Charcot-Marie-Tooth disease.24 In vitro actin polymerization assay and serum responsive factor assays demonstrate that the binding of INF2-DID to mDia-DAD interferes with mDia-mediated actin assembly and gene transcription in response to actin polymerization.18 We therefore hypothesized that (1) INF2 preserves podocyte actin dynamics by negatively regulating Rho/mDia signaling through the INF2-DID/mDia-DAD interaction and (2) INF2 mutations that interfere with this interaction may impair its Rho/mDia-antagonizing property in maintaining normal actin dynamics. In this study, we explored the cellular significance of INF2’s mDia-antagonizing effect in preserving the actin cytoskeleton architecture of cultured podocytes, especially in response to Rho activation. We examined the role of INF2 in regulating cortical actin dynamics, lamellipodia extension, and peripheral membrane trafficking of SD proteins.
Results
INF2 Maintains Podocyte Cortical Actin on Lamellipodial Membrane by Opposing Rho/mDia Activity
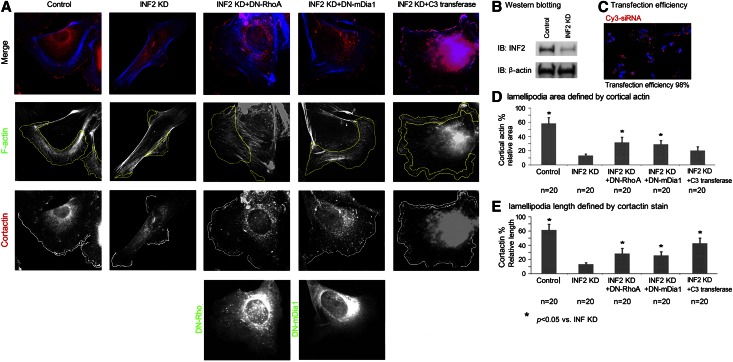
Previously we showed that INF2 antagonizes the effect of Rho/mDia on actin polymerization. FSGS-causing INF2 mutations lead to an ultrastructural phenotype characterized by abnormally thick actin filament bundles.21 We used small interfering RNA (siRNA) to knock down the endogenous expression of INF2 in cultured podocytes As shown in Figure 1, knockdown of INF2 to approximately 30% of normal, as indicated by immunoblotting (with an siRNA transfection efficiency of >98%), led to a loss of the cortical actin network (as labeled by cortactin) with more prominent actin stress fibers and parallel actin bundles compared with control cells. These INF2-deficient cells also exhibited elongated morphology. We quantified the fraction of cells containing lamellipodia by measuring the relative area of cortical actin meshwork and the relative length of plasma membrane enriched with cortactin25 (Figure 1), a protein that is highly associated with the branched lamellipodial actin network. We observed a statistically significant decrease in the relative size of the lamellipodia in cells with INF2 depletion.
Figure 1.
INF2 maintains podocyte cortical actin by opposing Rho/mDia activity. (A) Podocytes (undifferentiated) were respectively transfected with universal negative control siRNA duplex (control), INF2-targeting siRNA (INF2 KD), INF2 KD+DN-RhoA, INF2 KD+DN-mDia1, or INF2 KD, followed by treatment with cell permeable C3 transferase (Cytoskeleton Inc.; Rho inhibitor I, 1.0 µg/ml for 2 hours). Cortactin, DN RhoA (DN mDia1), and actin filaments (F-actin) were co-illustrated by immunofluorescent stain (Cortactin in red, DN-RhoA/DN mDia1 in green, and F-actin in blue). (B) INF2 knockdown by siRNA was confirmed by Western blotting. (C) High transfection efficiency of siRNA as reflected by Cy3-labeled siRNA duplex. The transfection efficiency was calculated as the number of Cy3-positive cells/100 cells with positive stain of DAPI. Both the relative area of cortical actin mesh (D) and the relative length of cortactin-positive membrane (E) were calculated for 20 randomly selected cells in each group; data were expressed as mean ± standard deviation. The difference among groups was statistically analyzed using one-way ANOVA and least significant difference test. *P<0.05 versus cells with INF2 KD only.
In an earlier study, we showed that INF2-DID interacts with and antagonizes members of the mDia formin subfamily, major downstream effectors of Rho. Therefore, we examined whether the effect of INF2 depletion on cortical actin retraction might result from loss of inhibition of Rho-mediated mDia activity. We coexpressed dominant negative forms of either RhoA (DN-RhoA, encoded by pEGFP (Enhanced Green Fluorescent Protein)-RhoA T19N mutant11) or mDia1 (DN-mDia1, encoded by pEGFP-mDia1-ΔN3 (Hind III)26) in cells with INF2 knockdown and found that DN-RhoA or DN-mDia1 could partially restore the normal cortical actin meshwork. We found that treatment with a potent Rho inhibitor, permeable C3 transferase, could also antagonize the retraction of lamellipodia induced by INF2 knockdown (Figure 1). Therefore, we propose that the altered actin architecture in INF2-deficient cells is mediated, at least in part, by loss of inhibition of Rho-mediated actin polymerization and filament elongation in the absence of sufficient INF2 to antagonize the action of mDia.
INF2 Counteracts mDia in Maintaining Cortical Actin Dynamics Independent of Rho
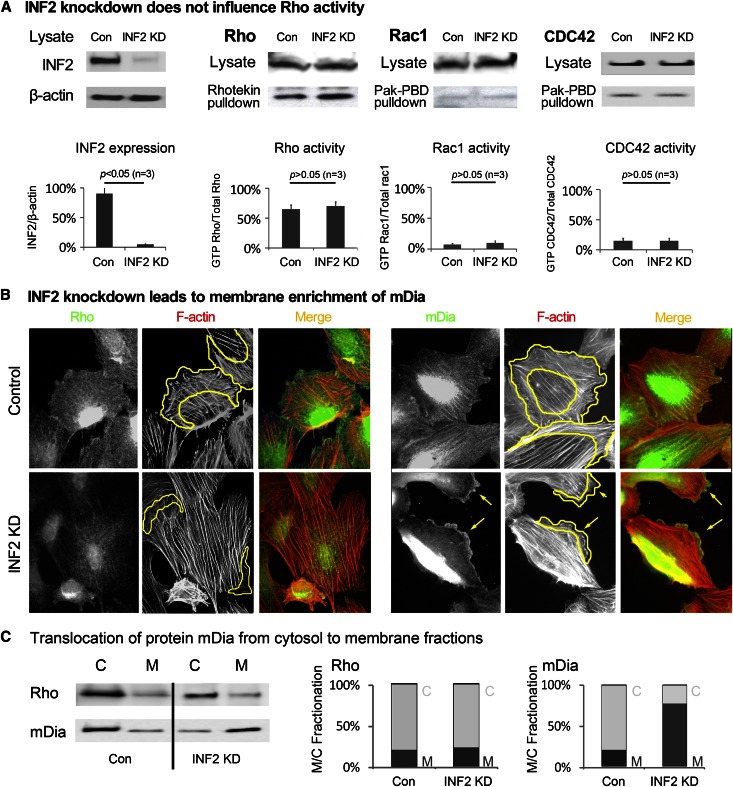
The dynamic balance between stress fiber formation and cortical actin is thought to be coordinated by Rho/Rac/CDC42 GTPase activity.27,28 To exclude the possibility that direct changes in Rho/Rac/Cdc42 GTPase activity after INF2 depletion might underlie the altered actin dynamics, we compared Rho/Rac/CDC42 activity in cells with and without INF2 knockdown by pulldown assays. We observed no changes in Rho, Rac, or CDC42 activity (Figure 2A). This suggests that INF2 depletion induced actin reorganization independent of direct activation of Rho but occurs through disinhibition of mDia. By immunofluorescence staining, we observed peripheral membrane enrichment of mDia, but not RhoA, in INF2 knockdown cells. We also observed a shift in mDia, but not Rho, from cytosolic to membrane fractions of cell lysates upon INF2 knockdown (Figure 2C). In INF2 knockdown cells, the size of the actin cortex decreased in concert with plasma membrane enrichment of mDia and a more fusiform cell morphology with prominent parallel actin bundles (Figure 2B). Thus, INF2 depletion disinhibits mDia activity downstream of RhoA.
Figure 2.
INF2 counteracts mDia in maintaining cortical actin dynamics independent of Rho. (A) Rho/Rac/CDC42 activity in podocytes (undifferentiated) with or without INF2 KD was measured by pulldown assay. Expression of INF2 in cells was illustrated by immunoblotting and normalized to the protein level of β-actin. Cell lysates were incubated with Rhotekin or Pak-PBD–conjugated agarose, and the precipitates were subjected to immunoblotting using anti-Rho for Rhotekin pulldown or anti-Rac/CDC42 for Pak-PBD pulldown. The fraction of active (GTP bound, pulldown) Rho/Rac/CDC42 was compared as a percentage of the total Rho/rac/CDC42 level. (B) Podocytes with or without INF2 KD were costained with anti-Rho/anti-mDia and phalloidin. The changes in peripheral membrane recruitment of Rho/mDia (green) in association with actin filaments (red) were compared. Cortical actin area is circled by yellow lines. Local aggregation of mDia and retraction of lamellipodia in cells with INF2 depletion was highlighted by arrows. (C) Rho and mDia in the cytosolic and membrane fractions of cells were detected by Western blotting, and the membrane/cytosolic (M/C) fractions of Rho and mDia were compared in cells with or without INF2 knockdown. HSP 70 and Na+/K+ ATPase were used as markers for cytosolic and membrane fractions, respectively.
INF2 Preserves Lamellipodial Trafficking of SD Proteins by Opposing Rho/mDia Activity
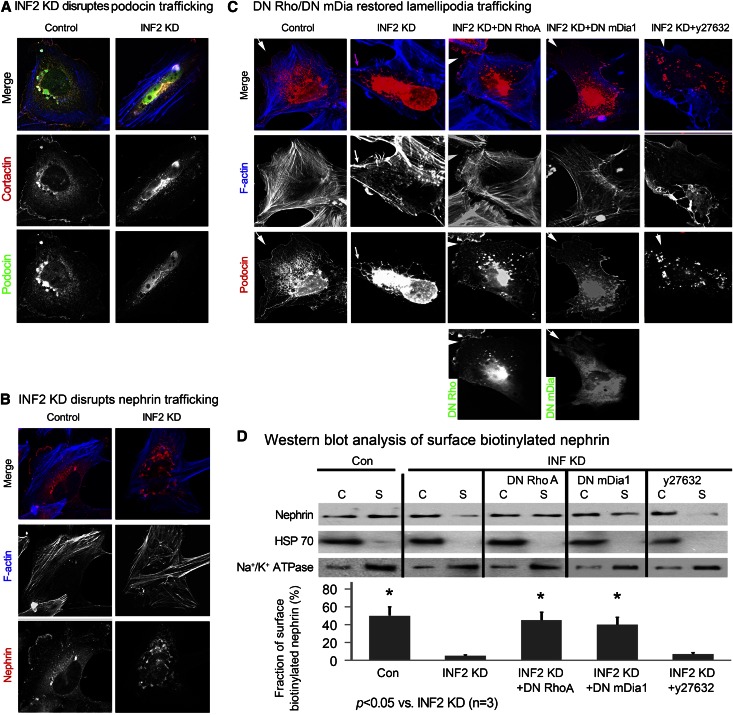
The cortical actin that is maintained through the active polymerization and depolymerization dynamics of actin provides a platform for cellular events, such as vesicle trafficking and membrane protein turnover.29,30 Loss of cortical actin impairs the trafficking of endosomes and newly synthesized membrane receptor proteins.31 We found that absence of INF2 leads to a decrease in cortical actin and lamellipodia formation. We therefore wished to also determine the role of INF2 in coordinating cortical actin remodeling and membrane trafficking of SD proteins. As shown in Figure 3A, podocin resides along the lamellipodial membrane distal to the cortical mesh, together with areas of cortactin enrichment. However, with INF2 knockdown, the plasma membrane distribution of podocin was strongly reduced. We also compared the distribution of SD protein nephrin in podocytes with and without INF2 knockdown. Nephrin is normally enriched at the plasma membrane adjacent to the cortical actin meshwork in control cells, whereas INF2 knockdown cells showed a decreased cortical actin mesh and a defect in the membrane trafficking nephrin, which appeared to be trapped in vesicular structures (Figure 3B).
Figure 3.
INF2 preserves peripheral membrane trafficking of SD proteins by opposing Rho/mDia activity. Cultured podocytes (undifferentiated) were transfected with INF2-targeting siRNA duplex (INF2 KD) or universal negative control RNA duplex (control). Forty-eight hours later, the cells were cotransfected with plasmids encoding HA-podocin or nephrin. (A) HA (newly synthesized podocin, green) was costained with cortactin (red), and the lamellipodial distribution of podocin was compared in cells with or without INF2 KD. (B) The distribution of newly synthesized nephrin (red) and the association with actin filaments (blue) were compared in cells with or without INF2 KD. (C and D) Cells with INF2 KD were cotransfected with DN RhoA or DN mDia1 or were treated with y27632, a Rock inhibitor. (C) The distribution of podocin and the association with F-actin are illustrated by immunofluorescent stain and phalloidin stain. (D) Surface biotinylation was used to measure the surface membrane trafficking of nephrin. The cytosolic (C) and surface (S) nephrin was measured by Western blotting, and the membrane trafficking of nephrin was quantified as the fraction of biotinylated nephrin (S/S+C)%. HSP 70 and Na+/K+ ATPase were used as controls for unbiotinylated and surface-biotinylated proteins, respectively.
In transgenic mouse experiments, Wang et al. found that active RhoA-induced proteinuria and FSGS were associated with reorganization of the podocyte actin cytoskeleton, FP retraction, and redistribution of SD proteins from a linear to a discontinuous pattern.11 Therefore, to determine whether altered membrane trafficking of SD proteins after INF2 knockdown was also mediated by Rho/mDia signaling, we studied the distribution of podocin and nephrin in the presence of DN-RhoA or DN-mDia1. As shown in Figure 3C, in INF2 knockdown cells, coexpression of DN-RhoA or DN-mDia1 restored the cortical actin meshwork as well as membrane trafficking of podocin. Surface biotinylation experiments confirmed the ability of DN-RhoA or DN-mDia1 to restore the plasma membrane localization of nephrin lost by INF2 knockdown (Figure 3D). To exclude the involvement of Rock, an alternative downstream effecter of Rho in regulating SD protein trafficking, we inhibited Rock using Y27632. This treatment did not restore the membrane trafficking of podocin or nephrin, supporting the assertion that the function of INF2 in maintaining the membrane trafficking of SD proteins is mediated by counterbalancing the activity of the otherwise Rho-activated mDia.
INF2 Directs Lipid Raft–Mediated Lamellipodial Trafficking of SD Proteins
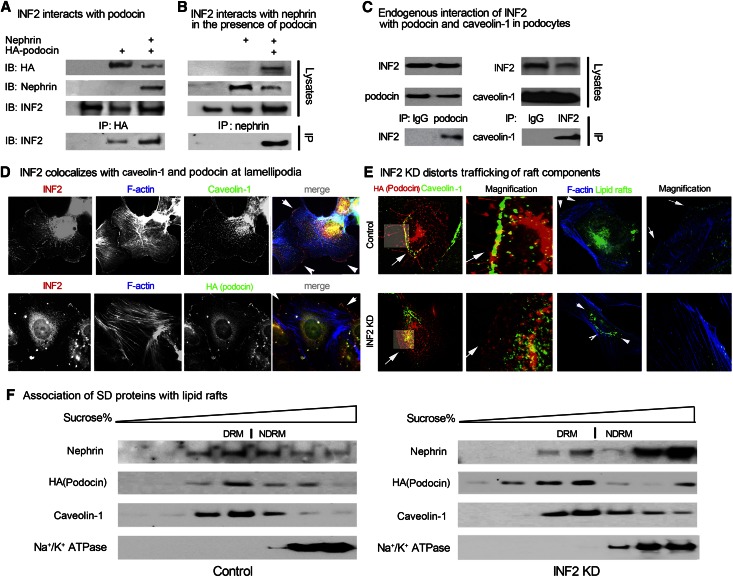
The trafficking of the nephrin-podocin protein complex to the SD membrane is a lipid raft–mediated process.4 Podocin, a prohibitin homology domain containing protein with affinity for lipid rafts, helps recruit nephrin to the membrane and serves as a scaffolding protein for SD complex organization.32–34 To understand the role of INF2 in maintaining nephrin-podocin complex trafficking, we investigated the interaction of INF2 with podocin and nephrin, as well as the lipid raft scaffolding protein caveolin-1.35 As shown in Figure 4, A and B, in HEK 293T cells that overexpressed specific SD proteins, endogenous INF2 was co-immunoprecipitated with overexpressed hemagglutinin (HA)-podocin, but was immunoprecipitated by nephrin only in the presence of HA-podocin This finding indicates that podocin mediates an association of INF2 with nephrin. The interactions of endogenous INF2 with endogenous lipid raft components podocin and caveolin-1 were verified by co-immunoprecipitation in differentiated podocytes (Figure 4C). We observed that INF2 co-localizes with both podocin and caveolin-1 along the lamellipodial edge adjacent to the cortical actin meshwork (Figure 4D). With INF2 knockdown, caveolin-1 and podocin, as well as lipid raft components labeled by cholera B toxin, are no longer detected at the plasma membrane edge but rather relocalize to intracellular vesicular structures (Figure 4E). Although INF2 knockdown did not prevent association of the SD proteins nephrin and podocin with detergent resistant membranes, we did observe a qualitative increase in the fraction of nephrin in nondetergent resistant membranes (Figure 4F). Taken together, these results suggest that INF2 plays a necessary role not only in the trafficking of lipid rafts to the surface but also the recuitment of nephrin to the rafts.
Figure 4.
INF2 is essential for lipid raft–mediated trafficking of SD proteins. (A and B) HEK293T cells were transfected with plasmids encoding HA-podocin, nephrin, both, or neither as indicated. Twenty-four hours later, the cell lysates were subjected to immunoprecipitation with anti-HA (A) or ant-inephrin (B) antibody, and the pulldown products were subjected to immunoblotting by using anti-INF2. (C) The endogenous interaction of INF2 with podocin and caveolin-1 was detected in podocytes (differentiated at 37°C for 2 weeks on collagen I–coated plates) by co-immunoprecipitation; control IgG was used as a comparison. (D) The association of INF2 with caveolin-1 or HA-podocin was studied by immunostain, and F-actin was stained by phalloidin. (E) The distribution of HA-podocin, caveolin-1, and rafts labeled by cholera B toxin in podocytes with or without INF2 KD was illustrated by immunostain. (F) The association of overexpressed SD proteins with lipid rafts in podocytes with or without INF2 knockdown was shown by immunoblotting of nephrin, podocin in lipid rafts (DRM, caveolin-1 rich), and nonrafts (NDRM, Na+/K+ ATPase rich) fractions isolated by sucrose gradient centrifuge.
INF2 Preserves Lamellipodial Trafficking in Response to Rho/mDia Activation
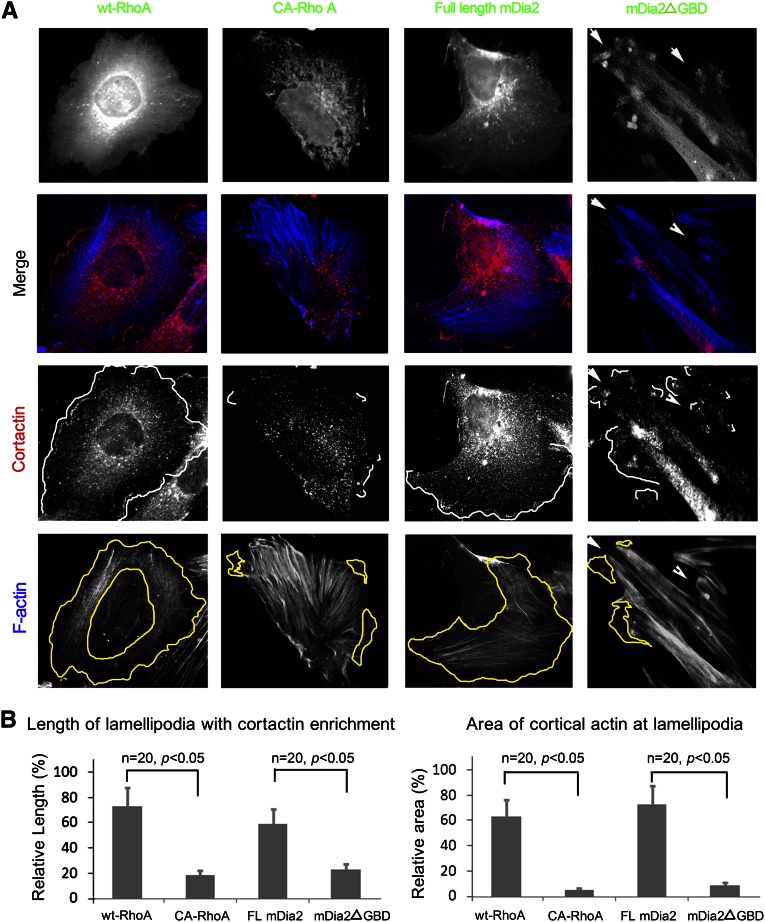
As shown in Figure 5, when we activated Rho by expressing constitutive active mutant of Rho (CA-RhoA), we found prominent stress fibers (panel A) with loss of cortical actin meshwork and lamellipodia (panel B, quantification) compared with control cells expressing wt-RhoA, which were characterized by a well developed cortical actin. In cells expressing active mDia2ΔGBD (constitutively active mDia2 that lacks the Rho GBD and is therefore incapable of autoinhibiting its actin polymerization activity), we found a diminished actin meshwork at the retracted lamellipodia and aggregation of active mDia2 near the cell membrane (panel A, arrows). By contrast, cells expressing full-length mDia2 (autoinhibited mDia2 as a control) showed a well developed cortical actin network. Compared with the control cells with a fine distribution of podocin along the lamellipodia, the podocytes with Rho (CA-RhoA) or mDia (mDia2ΔGBD) activation showed a failure in membrane trafficking of nephrin (Figure 6A) and podocin (Figure 6C). When INF2 was coexpressed with CA-RhoA or mDia2ΔGBD, we observed restoration of lamellipodial morphology and membrane trafficking of nephrin (Figure 6A) and podocin (Figure 6C, arrows). We quantified the membrane trafficking of nephrin by measuring the fraction of surface biotinylated protein. We observed a statistically significant decrease in the membrane trafficking of nephrin in cells with active RhoA or mDia2ΔGBD (Figure 6B; P<0.05 CA-RhoA versus wt-RhoA; P<0.05 mDia2ΔGBD versus full-length mDia2), both of which could be restored partially by coexpression of wild-type (wt) INF2 (P<0.05 CA-RhoA+wt-INF2 versus CA-RhoA; P<0.05 mDia2ΔGBD+wt-INF2 versus mDia2ΔGBD). Furthermore, we found that the coexpression of INF2 variant 3, a truncated transcription variant of only INF2-DID without direct actin-binding activity, was sufficient to rescue the disrupted membrane trafficking of nephrin and podocin. This result highlights the importance of INF2's mDia-antagonizing effects, as opposed to the intrinsic actin polymerization/depolymerization activity of INF2, in maintaining the lamellipodial biology of podocytes.
Figure 5.
Active Rho/mDia signaling inhibits cortical actin dynamics at lamellipodia. Podocytes were transfected with plasmids encoding different forms of RhoA (wt or consistent active mutant Q63L) or mDia2 (full-length mDia2 or mDia2ΔGBD). (A) Actin conformation in cells with different activity of RhoA or mDia2 was illustrated by phalloidin stain. Lamellipodia were illustrated by immunostain of cortactin. (B) Lamellipodial changes were quantified and statistically analyzed for 20 randomly selected cells in each group by measurement of cortical area or ruffle length with cortactin enrichment, as in Figure 1.
Figure 6.
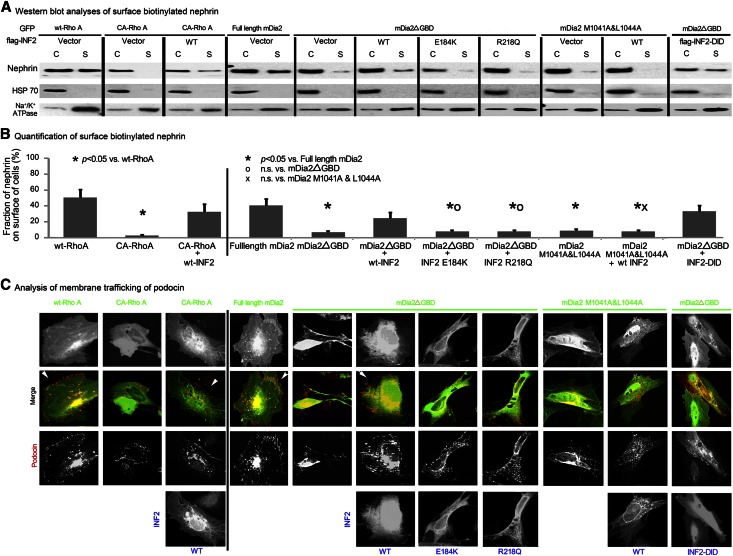
Mutations disassociating INF2 from mDia impair INF2’s ability to rescue lamellipodial trafficking in response to Rho/mDia activation. Podocytes were transfected with plasmids encoding different forms of Rho (wt or CA-RhoA, GFP fusion), with or without cotransfection of WT-INF2 or were cotransfected with plasmids encoding different forms of INF2 (wt full length, E184K full length, or R218Q full-length, WT-INF2-DID) and different forms of mDia2 (full-length mDia2 as an autoinhibited form, mDia2ΔGBD and mDia2 1041A&1044A mutant as two forms of disinhibited or constitutively active mDia2). (A) The cytosol (C) and the surface biotinylated nephrin (S) in cells with different treatments was measured by Western blotting. (B) Surface biotinylation was used to measure the surface membrane trafficking of nephrin. HSP 70 and Na+/K+ ATPase were used as controls for unbiotinylated and surface-biotinylated proteins, respectively. The membrane trafficking of nephrin was quantified as the fraction of biotinylated nephrin (S/S+C)% in three independent experiments of each group; data were expressed as mean ± standard deviation. The difference among groups was analyzed by using one-way ANOVA and least significant difference test. (C) Podocin trafficking in cells with different treatments was illustrated by immunostain.
Mutations Disassociating INF2 from mDia Impair INF2’s Rescuing Effects on Lamellipodial Trafficking
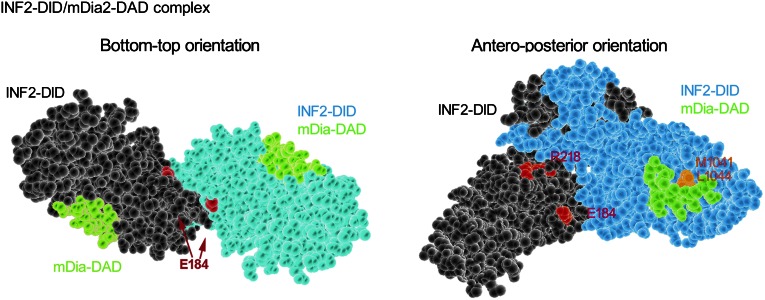
We used the Swiss-model protein structure homology-modeling server (http://swissmodel.expasy.org/)36 to model the INF2-DID homodimer/mDia-DAD interaction based on the crystal structure of mDia1-DID/DAD complex (MMDB: 38033, PDB: 2BAP; Figure 7). Previously, we found that mutations changing amino acid residues E184 and R218 cause human FSGS.21 These residues localize at the interface of INF2-DID homodimer and might stabilize the spatial conformation of the dimer and facilitate binding of INF2 to mDia-DAD. Both E184K and R218Q disrupt the INF2/mDia association as shown by both co-immunoprecipitation and yeast two-hybrid studies.18 In addition, M1041 and L1044 in mDia are key residues that localize to the DID/DAD binding interface and mediate the direct interaction of DID and DAD. Mutations engineered to alter these DAD residues also inhibit INF2-DID/mDia-DAD binding.18 To determine whether maintenance of cortical actin dynamics and trafficking of SD proteins depend on the mDia-DAD/INF2-DID interaction, we introduced mutations into INF2 (E184K, R218Q, human disease-causing mutations) or mDia (M1041A, L1044A). We sought to determine whether these mutations influenced the ability of INF2 to rescue the altered SD protein trafficking caused by active mDia.
Figure 7.
Molecular model of INF2-DID dimer in complex with mDia-DAD. Molecular model of INF2-DID dimer in complex with mDia-DAD was established by using Swiss-model homology server based on the mDia1 DID/DAD crystal structure (MMDB# 38033, PDB# 2BAP). FSGS-causing mutations in INF2 are highlighted in pink. mDia residues that mediate direct INF2-DID/mDia-DAD interaction are highlighted in orange.
In cells expressing full-length mDia2, SD proteins nephrin and podocin (Figure 6, A and C) were targeted to the lamellipodial membrane. In cells expressing active mDia2 mutants mDia2ΔGBD or M1041A and L1044A, nephrin was reserved in cytosolic fraction of cells (Figure 6A, by Western blotting analysis of surface biotinylation of nephrin) and podocin was confined to intracellular vesicular structures (Figure 6C). The disrupted trafficking of the proteins by mDia2ΔGBD was restored by the coexpression of WT-INF2. However, the E184K and R218Q INF2 mutants did not restore the membrane trafficking of nephrin (Figure 6B, fraction of nephrinon surface of cells: P>0.05 mDia2ΔGBD+E184K-INF2 versus mDia2ΔGBD; P>0.05 mDia2ΔGBD+R218Q-INF2 versus mDia2ΔGBD) and podocin (Figure 6C). In addition, wt-INF2 did not rescue the altered trafficking induced by mutant mDia2 M1041A and L1044A (P>0.05 mDia2 M1041A &L1044A+wt-INF2 versus mDia2 M1041A &L1044A). Thus, the specific interaction between INF2-DID and mDia-DAD is essential for mediating the anti-Rho/mDia property of INF2 in preserving cortical actin dynamics and the trafficking of SD proteins in podocytes.
Abnormal Distribution of SD Proteins in Patients with FSGS Carrying R218Q Mutation
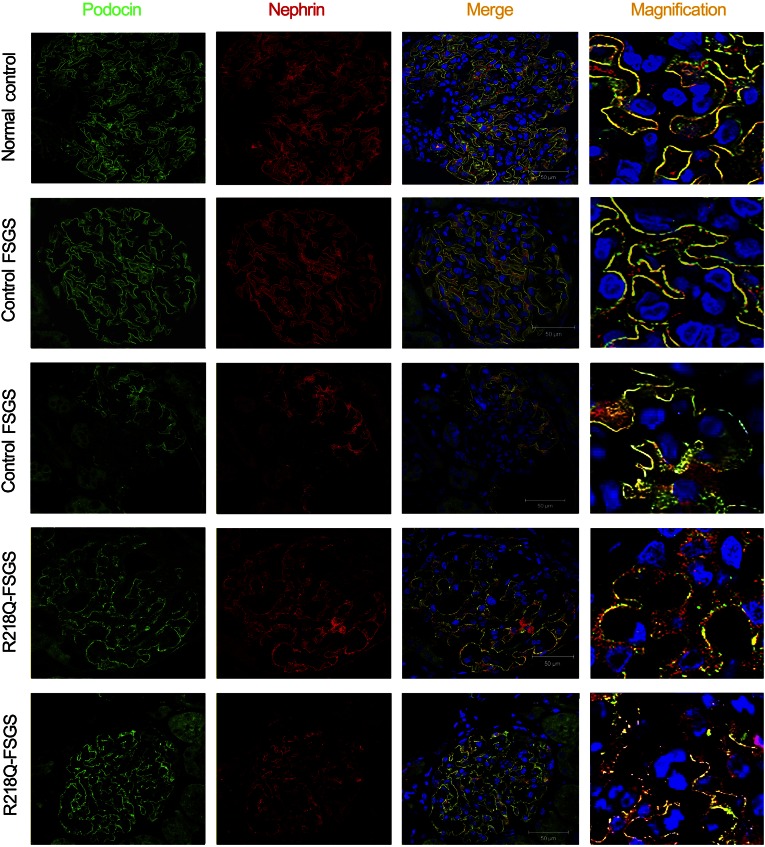
To determine whether disease-causing mutations of INF2 lead to failure in the trafficking of SD proteins in patients with FSGS who have these mutations, we immunostained a kidney biopsy section from a patient with FSGS who had an R218Q mutation for podocin and nephrin (proband of pedigree FG-JN21). We previously demonstrated that this mutation prevents the interaction of INF2 with mDia.18 As shown in Figure 8, in both a nonproteinuric person (normal control) and in a kidney biopsy specimen from a patient with FSGS that was secondary to hypertension and obesity, both podocin (in green) and nephrin (in red) assume a linear or continuous distribution along the capillary of the glomerulus with a high degree of colocalization. However, in the glomerulus from the patient with FSGS who carried the INF2 R218Q mutation (R218Q-FSGS), both the proteins show a granular or discontinuous pattern of distribution.
Figure 8.
Abnormal distribution of SD proteins in patients with FSGS who carry R218Q mutation. Immunostaining of podocin (green) and nephrin (red) in renal biopsy sections from a nonproteinuric person (normal control), a secondary FSGS (control FSGS), and an individual with FSGS caused by an INF2 R218Q mutation (R218Q-FSGS).
Discussion
Point mutations in the INF2-DID can cause isolated FSGS or FSGS together with Charcot-Marie-Tooth disease.24 INF2 antagonizes Rho-activated actin assembly by binding and inactivating mDia formin proteins.18,21 Ultrastructural analysis of an INF2-mutant kidney biopsy specimen showed abnormal aggregation of actin filaments,21 a common consequence of Rho signaling activation in cultured cells. These findings led us to hypothesize that disease-causing mutations of INF2 could lead to unregulated activation of Rho-actin dynamics in podocytes, possibly through unbalanced actin polymerizing activity of mDia, a target for INF2.
In exploring INF2’s role in regulating actin-dependent features of podocytes in culture, we noticed changes in ruffle morphology. In podocytes depleted of INF2 using siRNA, we observed loss of lamellipodia and prominent parallel actin bundles. The actin reorganization induced by INF2 depletion could be reversed by DN-Rho, Rho inhibitor, or DN-mDia, suggesting that INF2 antagonizes Rho/mDia signaling. We found no evidence of a direct effect of INF2 knockdown on Rho/Rac/CDC42 activity. We did note an increase in the aggregation of mDia at the retracted lamellipodia. Thus, we speculate that INF2 knockdown may lead to actin reorganization caused by uncontrolled mDia activity. Lamellipodia are areas of active actin polymerization and depolymerization turnover and vesicle trafficking. mDia, the major Rho effecter mediating actin elongation and stress fiber formation, localizes to lamellipodia. In these studies, we found that selective Rho/mDia activation in podocytes using a constitutively active Rho or mDia leads to more pronounced stress fibers and loss of cortical actin at lamellipodial edges. These changes were reversed by expression of INF2.
In parallel with changes in lamellipodial actin dynamics, we observed altered trafficking of SD complex proteins nephrin and podocin. In cultured podocytes, we observe a fine distribution of SD proteins along the lamellipodial membrane. However, in INF2-depleted podocytes demonstrating a retracted lamellipodia, SD proteins lose their membrane distribution and are trapped in cytoplasmic vesicles. Trafficking of SD protein complex is a lipid raft–dependent process. The SD is a lipid raft–enriched membrane in which the critical SD protein nephrin is recruited.33,37 Mutations disrupting the raft localization of podocin or the nephrin/podocin interaction disassociate nephrin from the raft-mediated trafficking pathway, disrupt its membrane localization, and cause congenital proteinuria.33,38 We found that INF2 interacts with podocin and caveolin-1, proteins both involved in lipid raft–mediated trafficking; this finding suggests a direct role for INF2 in lipid raft–mediated SD protein trafficking. In addition to podocin and caveolin-1, recent studies have also identified INF2’s association with raft proteins MAL and MAL2, which mediate membrane trafficking of functional molecules in Schwann cells,24 T lymphocytes,39 Madin-Darby canine kidney cells, and HepG2 hepatocytes.39 Here we found that the association of nephrin with INF2 depended on the presence of podocin, indicating the involvement of INF2 in podocin-mediated trafficking of nephrin. We also found that the depletion of INF2 decreased the recruitment of nephrin to lipid rafts and the mobility of the rafts during the surface targeting of SD proteins. Therefore, by connecting actin dynamics and lipid raft–based transporters, INF2 appears to serve as an essential protein in driving the trafficking of SD proteins in podocytes.
The altered distribution of SD proteins upon INF2 knockdown is consistent with recent findings in transgenic mice expressing constitutively active RhoA. These mice develop podocyte FP effacement, proteinuria, and FSGS.10,11 In podocytes of these animals, SD protein distribution was granular and discontinuous, in contrast to the linear distribution normally seen. Wallar and colleagues have shown that controlled Rho/mDia activity is critical for the actin assembly and disassembly dynamics that drive membrane remodeling and directional endosome transport. Excess active mDia disrupts directional myosin-driven vesicle transport.40 Here we found that the impaired membrane trafficking of SD proteins caused by INF2 knockdown could be restored by dominant negative Rho or mDia, indicating that the failure in trafficking is mediated by uncontrolled Rho/mDia signaling after INF2 loss. This trafficking failure was not rescued by Y27632, an inhibitor for Rock, another downstream effecter of Rho, suggesting the effect of INF2 deletion is specific to mDia. We introduced mutations that destroy the INF2-DID/mDia-DAD interaction into both the INF2 and mDia sequence to confirm the selective targeting of active Rho/mDia signaling by INF2 in preserving the lamellipodial trafficking. We found that the trafficking defect caused by active mDia could not be rescued by the disease-causing INF2 mutations E184K or R218Q, nor could wt INF2 rescue the effects of mDia-harboring mutations (M1041A and L1044A) that prevent the INF2-mDia interaction. Together, these results confirm a role for the interaction of the INF2 with mDia in maintaining normal podocyte function. In parallel, we found that SD protein trafficking disrupted by active mDia could also be rescued by INF2-DID, a domain that targets mDia-DAD without direct actin binding or processing functions. This result indicates that the role of INF2 in mediating lamellipodial trafficking is largely due to the mDia-antagonizing effect of INF2, instead of the direct activity of the protein on actin filaments.
In summary, on the basis of our studies, we believe that INF2 mutations lead to uncontrolled Rho signaling and unbalanced actin polymerization. We have demonstrated an essential role of INF2 as a modulator of mDia activity downstream of Rho. INF2 helps maintain lamellipodial actin dynamics and coordinate the trafficking of SD proteins, especially in response to Rho activation. Abnormal activation of Rho signaling has been identified in multiple forms of kidney injury, including diabetic nephropathy,41 acute ischemia, ischemia-reperfusion,42 and hypertensive kidney disease.43 In patients with INF2 mutations, uncontrolled mDia-mediated actin dynamics may predispose to podocyte injury. In the absence of normal INF2 activity, the changes in actin regulation in response to Rho activation may alter podocyte cytoskeletal function, impair the trafficking of SD proteins, and disrupt glomerular filtration function.
Concise Methods
Antibodies
Rabbit anti-INF2 is a polyclonal antibody raised against amino acids 994–1273 of mouse INF2. Rabbit anti-mDia1/2 is a polyclonal antibody that recognizes both mDia1 and mDia2.18 Mouse-anti HA and peroxidase conjugate antibodies are from Cell Signaling Technology (Beverly, MA); guinea pig–derived antinephrin is a product of Progenbiotechnik (Germany); rabbit antipodocin is from Sigma-Aldrich Corp., mouse-anti Rho, mouse-anti CDC42, mouse-anti Rac1, and mouse-anti cortactin are from EMD Millipore (Billerica, MA); and mouse-anti-Na+/K+ ATPase α1 and rabbit-anti caveolin-1 are from Santa Cruz Biotechnology, Inc. Mouse anti-HSP70 antibody (Heat Shock Protein 70) was obtained from Stressgen Bioreagents. Alexa Fluor labeled secondary antibodies and phalloidin are products of Molecular Probes.
Plasmids
pEGFP-mDia2 and pEGFP-mDia2 ΔGBD were generated by H.N.H.’s laboratory (Department of Biochemistry, Geisel School of Medicine, Dartmouth University). pCMV6-AN-DDK-INF2 plasmid and pCMV6-AN-Myc-DDK-INF2 Variant3 (containing DID only) plasmid was obtained from OriGene Technologies (Rockville, MD). E184K, S186P, and R218Q mutations were introduced into INF2, and M1041A&L1044A mutations were introduced into mDia using a QuikChange site direct mutagenesis kit (Stratagene). Human podocin was cloned into pcDNA3.1 by PCR to generated HA-tagged podocin. Human nephrin was cloned into pcDNA3.1 by PCR (nontagged). pEGFP-mDia1ΔN3 (HindIII) was a generous gift from Dr. Shuh Narumiya, Department of Pharmacology, Kyoto University, Japan.44
RNA Interference
Human podocytes were transfected with INF2-targeting Dicer-Substrate siRNA duplexes (TriFECTa kit from IDT) using Hyperfect transfection reagent (Qiagen) at a final concentration of 10 nM. DS Scrambled-Neg, a randomized RNA duplex sequence (not present in human genome sequence) served as a negative control. The transfection efficiency was evaluated by Cy3 fluorescent-labeled transfection control duplex. The cells were processed for Western blotting, immunofluorescent stain, or immunoprecipitation 72 hours after the transfection.
Cell Culture
The conditionally immortalized human podocyte cell line developed by transfection with the temperature-sensitive SV40-T gene has been previously described.45 The podocytes were maintained in RPMI1640 with 10% FBS, Insulin-transferrin-selenium (Gibco), and 50 IU/ml penicillin-streptomycin at 33°C (permissive condition). Podocytes maintained at permissive condition were used for transfection experiments. To measure the endogenous interaction of INF2 with podocin and caveolin-1, podocytes were seeded on collagen I–coated plates and differentiated at 37°C for 2 weeks (nonpermissive condition). For the co-immunoprecipitation experiment, HEK 293T cells were maintained in DMEM with 10% FBS at 37°C. Experiments were performed with subconfluent cultures of podocytes to allow for the accurate analysis of lamellapodial structure. We cannot rule out the possibility that increased cell-cell contact might have additional effects on nephrin and podocin trafficking, either independent or dependent on INF2 function.
Lamellipodia Quantification
Immunofluorescent staining was performed to illustrate the expression and distribution of cortactin and actin architecture in podocytes with different treatments, as described elsewhere.25 Following the standard quantification method described previously,25 images were captured with a Cool-SNAPCF camera attached to a Nikon (Tokyo, Japan) Eclipse 80i microscope. Podocytes immunostained for cortactin and F-actin were used for the quantification of lamellipodia. Background fluorescence was subtracted from the image, and individual cells were then digitally outlined with Image J software (National Institutes of Health) to measure total cell fluorescence for cortactin or phalloidin staining. All areas outside the cell were cleared to best visualize the leading edges of lamellipodia. The fluorescence intensity within an entire cell was summed. Lamellipodia was defined as an actin-rich fringe with fluorescence intensity gradually declining with the distance from the edge in phalloidin-stained cells. Cell image intensity thresholds were set automatically by ImageJ with an isodata algorithm. The threshold pixels in lamellipodia were selected by drawing regions of interest, and the fluorescence intensities within the selected lamellipodia regions were summed. The fraction of a cell containing lamellipodia was expressed as a percentage of total cell area. The rim of cortactin staining at the leading edge was then digitally outlined and its length was expressed as a percentage of the total cell fluorescence. The summed length of lamellipodia was expressed as a percentage of total cell circumferences. Quantification was performed using a blind experimental procedure by an uninformed observer on unidentified samples.
Immunofluorescence Staining
Expression of nephrin and podocin were studied by indirect immunofluorescence in renal biopsy sections from an affected member of family FG-JN with autosomal dominant FSGS caused by an INF2 R218Q mutation,21 as well as a patient diagnosed with secondary FSGS (no INF2 mutation), and a portion of normal kidney tissue (contained within a nephrectomy specimen done for renal carcinoma) as a nonproteinuric control. Formalin-fixed, paraffin-embedded biopsy sections were de-waxed and rehydrated gradually through graded alcohols, followed by antigen retrieval through 3 minutes of autoclaving in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). The sections were then blocked in 10% FCS and sequentially incubated successively with rabbit antipodocin (dilution 1:100) and guinea pig anti-nephrin (dilution 1:100) antibodies, followed by Alexa Fluor 488-labeled goat anti-rabbit IgG (dilution 1:100) and Alexa Fluor 592-labeled goat anti-guinea pig IgG (dilution 1:100). After mounting, the sections were observed under a confocal microscope.
Quantification of Cytosol to Membrane Transfer of RhoA and mDia
Podocytes were transfected with INF2-targeting siRNA or control RNA duplex for 72 hours.46 After starvation in medium containing 0.5% FBS overnight, the cells were scraped and homogenized in cold homogenization buffer (10 mM Tris-HCl, pH 7.5; 1 mM EGTA; 1 mM MgCl2) supplemented with complete protease inhibitor cocktail (Roche LTD). Cells were disrupted by passing through a 22-gauge needle, and lysates were clarified by centrifugation at 4300 g for 10 minutes. Supernatants were subsequently centrifuged at 50,000 g and 4°C for 60 minutes to produce cytosolic and membrane fractions in a Beckman Optima TLX ultracentrifuge with TLA45 rotor (Beckman Coulter, Inc.). The membrane fraction was washed three times with the hypotonic buffer and dissolved in 1% SDS in buffer A (50 mM Tris-HCl, pH 7.5; 140 mM NaCl; 10% glycerol; 1% Triton X-100) supplemented with protease inhibitor cocktail. Protein concentrations were determined by using Bradford reagent. Equal amounts of protein were loaded on a 4%–20% SDS gel and separated by electrophoresis. Rho and mDia protein in the cytosolic and membrane fractions of cells were measured by Western blotting (mouse anti-Rho A, B&C, rabbit anti-mDia 1/2). HSP 70 and Na+/K+ ATPase were used as markers for cytosolic and membrane fractions, respectively.47,48 Protein band intensities were measured using ImageJ software. The fractionation of cytosolic to membrane-associated Rho and mDia was expressed as the percentage of total protein.
Lipid Raft Fractionation
Undifferentiated podocytes were transfected with INF2-targeting siRNA or control RNA duplex for 48 hours, followed by cotransfection of plasmids encoding nephrin and HA-podocin. Twenty-four hours later, lipid rafts were isolated from the cells by discontinuous sucrose density gradient ultracentrifugation, as described elsewhere.49 Cells were washed in ice-cold PBS and lysed in a modified buffer: 1% Triton X-100 in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (25 mM MES, pH 6.5, 150 mM NaCl, 2 mM EDTA complemented with protease inhibitors) on ice. Lysates were homogenized and mixed with an equal volume of 85% (wt/vol) sucrose in MES buffer, and then overlaid with 5.5 ml of 36% sucrose and 2.5 ml of 5% sucrose. The gradients were subjected to ultracentrifugation at 250,000 g at 4°C for 18 hours with a 90-Ti rotor in an Beckman L8M ultracentrifuge (Beckman Coulter, Inc.). Fractions of 1 ml were collected from the top of the gradients. The detergent resistant microdomain (lipid rafts) and the nondetergent resistant microdomain (nonraft microdomain) were labeled by enrichment of caveolin-1 and Na+/K+ ATPase.50
Surface Biotinylation
The surface trafficking of nephrin was measured by using the Pierce Cell Surface Protein Isolation Kit. After different treatments, podocytes grown on six-well plates were surface-biotinylated on ice using Sulfo-NHS-SS-biotin after different treatments, followed by lysis in modified radioimmunoprecipitation buffer. Lysates were incubated with streptavidin beads at 4°C and spun down to collect the supernatants as the cytosolic fraction. After washes, the streptavidin beads were eluted in equal volume of 1XSDS loading buffer as the surface fraction. The level of nephrin in cytoplasm and surface fractions of cells were analyzed by Western blotting. HSP 70 and Na+/K+ ATPase were used as controls for unbiotinylated and surface-biotinylated proteins, respectively.47,48
Others
Rho activation assay and co-immunoprecipitation were performed as described elsewhere.18
Disclosures
None.
Acknowledgments
We thank Drs. Moin Saleem and Jochen Reiser for the generous gift of immortalized human podocytes.
This work was supported by Nature Science Foundation of China grant 81000282/H0502, Research Fund for Young Teachers at Shanghai University grant JDY08061 (to H.S.), and grants from the U.S. National Institutes of Health: DK088826 (to M.P. and H.H.) and DK080947 (to J.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Pätäri-Sampo A, Ihalmo P, Holthöfer H: Molecular basis of the glomerular filtration: Nephrin and the emerging protein complex at the podocyte slit diaphragm. Ann Med 38: 483–492, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Michaud JL, Kennedy CR: The podocyte in health and disease: Insights from the mouse. Clin Sci (Lond) 112: 325–335, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Asanuma K, Mundel P: The role of podocytes in glomerular pathobiology. Clin Exp Nephrol 7: 255–259, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Ichimura K, Kurihara H, Sakai T: Actin filament organization of foot processes in vertebrate glomerular podocytes. Cell Tissue Res 329: 541–557, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Moeller MJ, Holzman LB: Imaging podocyte dynamics. Nephron, Exp Nephrol 103: e69–e74, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Mathieson PW: Podocyte actin in health, disease and treatment. Nephrol Dial Transplant 25: 1772–1773, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Heasman SJ, Ridley AJ: Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690–701, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T: Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, Ruiz P, Fields TA, Spurney RF: Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int 81: 1075–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE: HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem 283: 8173–8182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata S, Nagase M, Fujita T: Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol 17: 754–764, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop AL, Hall A: Rho GTPases and their effector proteins. Biochem J 348: 241–255, 2000 [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Higgs HN: The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol 13: 1335–1340, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Wallar BJ, Stropich BN, Schoenherr JA, Holman HA, Kitchen SM, Alberts AS: The basic region of the diaphanous-autoregulatory domain (DAD) is required for autoregulatory interactions with the diaphanous-related formin inhibitory domain. J Biol Chem 281: 4300–4307, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Sun H, Schlondorff JS, Brown EJ, Higgs HN, Pollak MR: Rho activation of mDia formins is modulated by an interaction with inverted formin 2 (INF2). Proc Natl Acad Sci U S A 108: 2933–2938, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chhabra ES, Higgs HN: INF2 Is a WASP homology 2 motif-containing formin that severs actin filaments and accelerates both polymerization and depolymerization. J Biol Chem 281: 26754–26767, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Chhabra ES, Ramabhadran V, Gerber SA, Higgs HN: INF2 is an endoplasmic reticulum-associated formin protein. J Cell Sci 122: 1430–1440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gbadegesin RA, Lavin PJ, Hall G, Bartkowiak B, Homstad A, Jiang R, Wu G, Byrd A, Lynn K, Wolfish N, Ottati C, Stevens P, Howell D, Conlon P, Winn MP: Inverted formin 2 mutations with variable expression in patients with sporadic and hereditary focal and segmental glomerulosclerosis. Kidney Int 81: 94–99, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer O, Benoit G, Gribouval O, Nevo F, Tête MJ, Dantal J, Gilbert-Dussardier B, Touchard G, Karras A, Presne C, Grunfeld JP, Legendre C, Joly D, Rieu P, Mohsin N, Hannedouche T, Moal V, Gubler MC, Broutin I, Mollet G, Antignac C: Mutations in INF2 are a major cause of autosomal dominant focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 239–245, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer O, Nevo F, Plaisier E, Funalot B, Gribouval O, Benoit G, Cong EH, Arrondel C, Tête MJ, Montjean R, Richard L, Karras A, Pouteil-Noble C, Balafrej L, Bonnardeaux A, Canaud G, Charasse C, Dantal J, Deschenes G, Deteix P, Dubourg O, Petiot P, Pouthier D, Leguern E, Guiochon-Mantel A, Broutin I, Gubler MC, Saunier S, Ronco P, Vallat JM, Alonso MA, Antignac C, Mollet G: INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N Engl J Med 365: 2377–2388, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y: Gbetagamma signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther 333: 393–403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S: Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol 161: 381–391, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobes CD, Hall A: Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81: 53–62, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Ridley AJ: Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16: 522–529, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Pollard TD, Borisy GG: Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Saarikangas J, Zhao H, Lappalainen P: Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev 90: 259–289, 2010 [DOI] [PubMed] [Google Scholar]
- 31.McCarthy AM, Spisak KO, Brozinick JT, Elmendorf JS: Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am J Physiol Cell Physiol 291: C860–C868, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, Saleem MA, Walz G, Benzing T: Molecular basis of the functional podocin-nephrin complex: Mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum Mol Genet 12: 3397–3405, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shono A, Tsukaguchi H, Yaoita E, Nameta M, Kurihara H, Qin XS, Yamamoto T, Doi T: Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephrol 18: 2525–2533, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Sörensson J, Fierlbeck W, Heider T, Schwarz K, Park DS, Mundel P, Lisanti M, Ballermann BJ: Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol 13: 2639–2647, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Schwede T, Kopp J, Guex N, Peitsch MC: SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res 31: 3381–3385, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrakka J, Tryggvason K: Nephrin—a unique structural and signaling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Otaki Y, Miyauchi N, Higa M, Takada A, Kuroda T, Gejyo F, Shimizu F, Kawachi H: Dissociation of NEPH1 from nephrin is involved in development of a rat model of focal segmental glomerulosclerosis. Am J Physiol Renal Physiol 295: F1376–F1387, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Madrid R, Aranda JF, Rodríguez-Fraticelli AE, Ventimiglia L, Andrés-Delgado L, Shehata M, Fanayan S, Shahheydari H, Gómez S, Jiménez A, Martín-Belmonte F, Byrne JA, Alonso MA: The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev Cell 18: 814–827, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Wallar BJ, Deward AD, Resau JH, Alberts AS: RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res 313: 560–571, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, McKenzie R, Krepinsky JC: RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes 57: 1683–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Shin HK, Salomone S, Ayata C: Targeting cerebrovascular Rho-kinase in stroke. Expert Opin Ther Targets 12: 1547–1564, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Rupérez M, Sánchez-López E, Blanco-Colio LM, Esteban V, Rodríguez-Vita J, Plaza JJ, Egido J, Ruiz-Ortega M: The Rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int Suppl S39–S45, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Tsuji T, Ishizaki T, Okamoto M, Higashida C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai H, Narumiya S: ROCK and mDia1 antagonize in Rho-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol 157: 819–830, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto S, Ishizaki T, Okawa K, Watanabe S, Arakawa T, Watanabe N, Narumiya S: Liprin-α controls stress fiber formation by binding to mDia and regulating its membrane localization. J Cell Sci 125: 108–120, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, Huang DC, Adams JM: Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci U S A 105: 18081–18087, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logar CM, Brinkkoetter PT, Krofft RD, Pippin JW, Shankland SJ: Darbepoetin alfa protects podocytes from apoptosis in vitro and in vivo. Kidney Int 72: 489–498, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Wang M, Tan L, Wang C, Ma J, Li N, Li Y, Xu G, Li J: Docosahexaenoic acid changes lipid composition and interleukin-2 receptor signaling in membrane rafts. J Lipid Res 46: 1904–1913, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Waheed AA, Jones TLZ. Hsp90 interactions and acylation target the G protein Gα12 but not Gα13 to lipid rafts. J Biol Chem 277: 32409–32412, 2002 [DOI] [PubMed] [Google Scholar]