Abstract
The effects of high-flux dialysis and ultrapure dialysate on survival of hemodialysis patients are incompletely understood. We conducted a randomized controlled trial to investigate the effects of both membrane permeability and dialysate purity on cardiovascular outcomes. We randomly assigned 704 patients on three times per week hemodialysis to either high- or low-flux dialyzers and either ultrapure or standard dialysate using a two-by-two factorial design. The primary outcome was a composite of fatal and nonfatal cardiovascular events during a minimum 3 years follow-up. We did not detect statistically significant differences in the primary outcome between high- and low-flux (HR=0.73, 95% CI=0.49 to 1.08, P=0.12) and between ultrapure and standard dialysate (HR=0.90, 95% CI=0.61 to 1.32, P=0.60). Posthoc analyses suggested that cardiovascular event-free survival was significantly better in the high-flux group compared with the low-flux group for the subgroup with arteriovenous fistulas, which constituted 82% of the study population (adjusted HR=0.61, 95% CI=0.38 to 0.97, P=0.03). Furthermore, high-flux dialysis associated with a lower risk for cardiovascular events among diabetic subjects (adjusted HR=0.49, 95% CI=0.25 to 0.94, P=0.03), and ultrapure dialysate associated with a lower risk for cardiovascular events among subjects with more than 3 years of dialysis (adjusted HR=0.55, 95% CI=0.31 to 0.97, P=0.04). In conclusion, this trial did not detect a difference in cardiovascular event-free survival between flux and dialysate groups. Posthoc analyses suggest that high-flux hemodialysis may benefit patients with an arteriovenous fistula and patients with diabetes and that ultrapure dialysate may benefit patients with longer dialysis vintage.
Cardiovascular mortality is substantially higher in patients undergoing maintenance hemodialysis (HD) compared with the nonuremic population.1 Several risk factors unique to uremia are implicated in the high rates of cardiovascular disease (CVD) and mortality in these patients (for example, accumulation of medium-sized or large middle molecules and predominance of a chronic inflammatory state).2 Consequently, use of high-flux synthetic membranes, providing enhanced removal of higher-molecular weight uremic toxins coupled with better biocompatibility, together with ultrapure dialysis fluid might be expected to improve patient outcomes.
Because of proven clinical benefits and a possible survival advantage reported by several observational studies,3–5 high-flux HD is increasingly the most common dialysis treatment modality.6 Although the Hemodialysis (HEMO) Study was not able to confirm superiority of high- over low-flux HD,7 a survival benefit was reported in long-term HD patients in a posthoc analysis of the study, where high-flux dialyzers showed reduced cardiovascular mortality.8–11 In the multicenter European Membrane Permeability Outcome (MPO) Study, subgroup analyses showed better survival rates with high-flux HD in patients with hypoalbuminemia and patients with diabetes.12
Ultrapure dialysis fluid has also been associated with less inflammation, resulting in clinically relevant improvements, including amelioration of erythropoietin response,13 better nutritional status,14,15 and reduction in the incidence of β2-microglobulin amyloidosis.16 Although preliminary reports suggested lower cardiovascular morbidity with the use of ultrapure dialysis fluid,17 no randomized and controlled trial has been performed addressing hard clinical outcomes.
This randomized controlled trial was designed to determine whether membrane permeability and dialysate purity affect the incidence of fatal and nonfatal cardiovascular events and overall survival in prevalent maintenance HD patients.
Results
Baseline Characteristics
Subjects’ characteristics at baseline did not significantly differ between the dialyzer groups as well as between the dialysate groups (Table 1). In the overall population, prevalences of diabetes mellitus and CVD disease history were each 22.7%. The majority of the enrolled patients (81.8%) had native arteriovenous fistula (AVF) as vascular access. BP was adequately controlled in 79.9% of the subjects (systolic BP≤140 and diastolic BP≤90 mmHg), 13.9% had hyperphosphatemia (>5.5 mg/dl), and 53.6% had serum albumin levels below 4 g/dl.
Table 1.
Baseline characteristics in the treatment arms
| Characteristics | All Patients (n=704) | High-Flux HD (n=352) | Low-Flux HD (n=352) | Ultrapure Dialysate (n=352) | Standard Dialysate (n=352) |
|---|---|---|---|---|---|
| Demographical and clinical parameters | |||||
| Age (yr) | 58.6±14.2 | 58.5±13.8 | 58.7±14.5 | 59.1±14.3 | 58.2±14.1 |
| Sex (women; %) | 46 | 45 | 47 | 44 | 48 |
| Diabetes (%) | 22.7 | 22.7 | 22.7 | 21.9 | 23.6 |
| CVD history (%) | 22.7 | 23.3 | 22.1 | 22.3 | 23.2 |
| Time on HD (mo) | 42.8 (21.4–73.1) | 43.6 (22.5–73.0) | 42.1 (20.1–74.2) | 43.3 (22.1–72.0) | 42.2 (20.0–75.9) |
| Body mass index (kg/m2) | 24.1±4.2 | 24.2±4.2 | 24.0±4.2 | 24.0±4.1 | 24.2±4.3 |
| AVF (%) | 81.8 | 82.4 | 81.3 | 82.7 | 81.0 |
| Permanent catheter (%) | 12.8 | 10.9 | 14.7 | 12.7 | 12.9 |
| Systolic BP (mmHg) | 125±16 | 126±16 | 125±16 | 125±16 | 125±16 |
| Diastolic BP (mmHg) | 75±9 | 75±9 | 75±9 | 75±8 | 75±9 |
| IDWG (L) | 2.30±0.93 | 2.33±0.92 | 2.27±0.95 | 2.24±0.87 | 2.36±0.99 |
| IDWG (% body wt) | 3.59±1.47 | 3.66±1.54 | 3.52±1.41 | 3.53±1.41 | 3.65±1.53 |
| Laboratory data | |||||
| Urea reduction rate (%) | 75.5±6.8 | 75.6±6.6 | 75.4±7.0 | 75.6±6.6 | 75.5±6.9 |
| eKt/V | 1.41±0.29 | 1.41±0.27 | 1.40±0.31 | 1.40±0.26 | 1.41±0.32 |
| Creatinine (mg/dl) | 8.4±2.0 | 8.5±2.1 | 8.4±1.9 | 8.4±2.0 | 8.4±2.0 |
| Calcium (mg/dl) | 9.3±0.8 | 9.3±0.8 | 9.2±0.7 | 9.3±0.8 | 9.2±0.8 |
| Phosphate (mg/dl) | 4.2±1.2 | 4.3±1.2 | 4.2±1.1 | 4.2±1.1 | 4.3±1.2 |
| Ca-P product (mg2/dl2) | 39.4±11.5 | 39.9±11.8 | 38.8±11.2 | 39.1±11.3 | 39.7±11.7 |
| Parathormone (pg/ml) | 83.3 (34.1–178.2) | 85.6 (31.9–189.8) | 78.8 (34.8–164.9) | 78.0 (30.6–184.4) | 91.5 (34.9–172.3) |
| Albumin (g/dl) | 3.91±0.32 | 3.92±0.32 | 3.90±0.33 | 3.90±0.31 | 3.91±0.34 |
| Total cholesterol (mg/dl) | 183±46 | 182±45 | 184±47 | 181±44 | 185±48 |
| Triglyceride (mg/dl) | 142 (100–200) | 138 (90–196) | 146 (107–204) | 135 (96–196) | 147 (102–205) |
| Bicarbonate (mEq/L) | 23.8±3.5 | 23.8±3.5 | 23.8±3.5 | 23.9±3.5 | 23.7±3.5 |
| Hemoglobin (g/dl) | 11.2±1.5 | 11.2±1.4 | 11.1±1.5 | 11.2±1.5 | 11.1±1.4 |
| HDL cholesterol (mg/dl) | 39±11 | 39±11 | 39±11 | 39±11 | 39±11 |
| LDL cholesterol (mg/dl) | 110±36 | 110±34 | 110±37 | 110±34 | 111±37 |
| CRP (mg/dl) | 0.70 (0.27–1.59) | 0.65 (0.28–1.66) | 0.71(0.25–1.52) | 0.73 (0.28–1.64) | 0.67 (0.25–1.57) |
| Transferrin (mg/dl) | 169±39 | 170±38 | 168±40 | 167±39 | 171±39 |
| β-2 Microglobulin (mg/L) | 30.4±11.5 | 30.2±12.0 | 30.6±10.9 | 30.2±11.8 | 30.5±11.2 |
| Medication data | |||||
| Vitamin D usage (%) | 10.5 | 13 | 9 | 13 | 9 |
| Vitamin D dose (mcg/wk) | 0.24±0.82 | 0.27±0.82 | 0.21±0.81 | 0.28±0.90 | 0.19±0.72 |
| Phosphate binder dose (elementary Ca; mg/d) | 552±724 | 550±748 | 553±699 | 526±761 | 578±684 |
| Erythropoietin usage (%) | 40 | 41 | 40 | 40 | 41 |
| Erythropoietin dose (IU/wk) | 2162±3134 | 2120±3049 | 2204±3221 | 2196±3198 | 2129±3074 |
| Antihypertensive drug (%) | 21 | 20 | 22 | 22 | 20 |
P>0.05 between the treatment groups at the time of randomization, and all results were given as mean ± SD, except time on HD, CRP, parathormone, and triglyceride levels, which were not normally distributed and given as median (interquartile range) values. IDWG, interdialytic weight gain.
During follow-up, duration of dialysis sessions and blood flow rates did not significantly differ between the randomization groups (Table 2). Mean urea reduction ratio and eKt/V values were similar.
Table 2.
Mean clinical, laboratory, and medication characteristics of the treatment arms in the studied period
| Parameters | High-Flux HD (n=352) | Low-Flux HD (n=352) | P Value | Ultrapure Dialysate (n=352) | Standard Dialysate (n=352) | P Value |
|---|---|---|---|---|---|---|
| Clinical parameters | ||||||
| Body mass index (kg/m2) | 23.9±4.1 | 23.7±4.4 | 0.52 | 23.7±4.1 | 24.0±4.4 | 0.36 |
| Systolic BP (mmHg) | 123±13 | 123±12 | 0.72 | 123±13 | 123±12 | 0.85 |
| Diastolic BP (mmHg) | 75±7 | 75±6 | 0.49 | 75±6 | 75±6 | 0.83 |
| IDWG (L) | 2.09±0.71 | 2.11±0.70 | 0.68 | 2.08±0.69 | 2.12±0.72 | 0.43 |
| IDWG (% body wt) | 3.25±1.06 | 3.29±1.06 | 0.66 | 3.26±1.07 | 3.28±1.05 | 0.86 |
| Duration of dialysis session (min) | 236±4 | 236±5 | 0.82 | 235±5 | 236±5 | 0.79 |
| Blood flow rate (ml/min) | 360±32 | 359±30 | 0.83 | 358±31 | 361±31 | 0.45 |
| Biochemical parameters | ||||||
| Urea reduction rate (%) | 75±5 | 76±4 | 0.41 | 75±5 | 75±5 | 0.70 |
| eKt/V | 1.47±0.21 | 1.47±0.21 | 0.85 | 1.48±0.22 | 1.47±0.21 | 0.50 |
| Creatinine (mg/dl) | 8.23±1.79 | 8.25±1.73 | 0.85 | 8.25±1.68 | 8.24±1.83 | 0.92 |
| Phosphate (mg/dl) | 4.8±0.9 | 4.8±1.0 | 0.60 | 4.8±1.0 | 4.8±0.9 | 0.78 |
| Albumin (g/dl) | 4.0±0.2 | 4.0±0.2 | 0.29 | 4.0±0.2 | 4.0±0.2 | 0.83 |
| Transferrin (mg/dl)) | 172±39 | 168±38 | 0.23 | 168±36 | 171±41 | 0.37 |
| Total cholesterol (mg/dl) | 168±36 | 172±40 | 0.15 | 169±38 | 171±38 | 0.46 |
| Triglyceride (mg/dl) | 141 (104––202) | 158 (119–209) | 0.01 | 144 (110–199) | 156 (111–213) | 0.36 |
| Bicarbonate (mEq/L) | 22.0±1.6 | 21.9±1.7 | 0.77 | 22.0±1.6 | 21.9±1.6 | 0.83 |
| Hemoglobin (g/dl) | 11.2±1.0 | 11.0±1.0 | 0.02 | 11.1±1.0 | 11.0±1.0 | 0.23 |
| Ferritin (ng/ml) | 600 (430–737) | 601 (467–782) | 0.94 | 587 (441–744) | 625 (466–765) | 0.05 |
| Transferrin saturation (%) | 25.9±9.7 | 26.3±10.2 | 0.57 | 26.2±10.4 | 26.0±9.5 | 0.76 |
| HDL cholesterol (mg/dl) | 39±10 | 38±9 | 0.19 | 38±10 | 38±9 | 0.57 |
| LDL cholesterol (mg/dl) | 95±27 | 97±30 | 0.47 | 96±29 | 96±28 | 0.71 |
| CRP (mg/dl) | 0.91 (0.58–1.86) | 1.12 (0.57–2.15) | 0.03 | 1.07 (0.61–2.06) | 1.00 (0.51–1.95) | 0.51 |
| β-2 Microglobulina (mg/L) | 25.3±9.9 | 32.9±13.1 | <0.001 | 29.0±11.4 | 29.2±13.0 | 0.82 |
| Δβ-2 Microglobulin (mg/L) | −5.0±13.6 | 2.7±14.3 | <0.001 | −1.1±13.6 | −1.1±15.4 | 0.96 |
| Medication data | ||||||
| Vitamin D (mcg/wk) | 0.39±0.54 | 0.36±0.54 | 0.49 | 0.38±0.52 | 0.36±0.56 | 0.73 |
| Ca-based P binder (elementary Ca; mg/d) | 545±450 | 586±503 | 0.25 | 559±483 | 572±472 | 0.71 |
| Erythropoietin (U/wk) | 2087 (537–3523) | 2066 (438–3649) | 0.97 | 1914 (331–3348) | 2285 (571–3928) | 0.04 |
| Erythropoietin resistance index (U/wk per kg per g/dl) | 2.89 (0.59–5.31) | 2.92 (0.53–5.58) | 0.94 | 2.62 (0.46–5.04) | 3.27 (0.67–5.67) | 0.04 |
| Iron (mg/wk) | 17.2±20.1 | 18.1±19.3 | 0.56 | 17.6±20.4 | 17.6±19.07 | 0.97 |
All results were given as mean ± SD, except triglyceride, ferritin, CRP, erythropoietin dose, and resistance index, which were not normally distributed and given as median (interquartile range) values. IDWG, interdialytic weight gain.
β-2 Microglobulin level at the end of the study
Primary Outcome
Mean follow-up was 35.2±16.3 months (1–50 months). During the study, 186 subjects prematurely terminated the study: 46 subjects received a kidney transplant, and 140 subjects transferred to nonparticipating centers (Table 3). Baseline characteristics of the patients from treatment arms who left the study were similar. Mean follow-up time of those patients was 22.5±13.3 months when they left (range=1–45 months). There were 172 (24.4%) deaths, 92 from cardiovascular causes.
Table 3.
Outcomes of the treatment arms in the studied period
| Outcomes | High-Flux HD (n=352) | Low-Flux HD (n=352) | P Value | Ultrapure Dialysate (n=352) | Standard Dialysate (n=352) | P Value |
|---|---|---|---|---|---|---|
| Kidney transplantation (n, %) | 24 (6.8) | 22 (6.3) | 0.43 | 17 (4.8) | 29 (8.2) | 0.10 |
| Transfer to nonparticipating centers (n, %) | 78 (22.2) | 62 (17.6) | 0.20 | 69 (19.6) | 71 (20.2) | 0.85 |
| Death (n, %) | 75 (21.3) | 97 (27.6) | 0.05 | 87 (24.7) | 85 (24.1) | 0.93 |
| Cardiovascular death (n, %) | 39 (11.1) | 53 (15.1) | 0.11 | 47 (13.4) | 45 (12.8) | 0.82 |
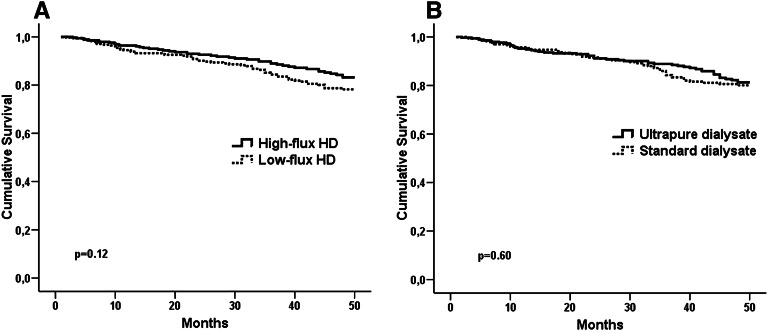
Fatal and nonfatal cardiovascular event rate was 5.03 per 100 patient years (n=104) in the entire study population. Event-free survival rates with respect to primary outcome were 83.2% in the high-flux group and 78.2% in the low-flux group at the end of 48-month follow-up (log-rank=2.41, P=0.12) (Figure 1A). Primary outcome was 27% less in the high-flux group (hazard ratio[HR]=0.73, 95% confidence interval [95% CI]=0.49 to 1.08) compared with the low-flux group, but this difference did not reach statistical significance (P=0.12). Event-free survival rates with respect to primary outcome were 81.3% in the ultrapure dialysate group and 80.1% in the standard dialysate group (P=0.60) (Figure 1B).
Figure 1.
Comparison of fatal and nonfatal cardiovascular event-free survival in treatment arms. (A) high-flux/low-flux dialyzers and (B) ultrapure/standard dialysate.
The risk of primary outcome was not significantly different (HR=0.90, 95% CI=0.61 to 1.32, P=0.60) between dialysate groups.
In adjusted Cox regression analysis, high-flux HD was associated with a nonsignificant 24% relative risk reduction in primary outcome (HR=0.76, 95% CI=0.50 to 1.14, P=0.19). Age (HR=1.04, 95% CI=1.02 to 1.06, P<0.001), presence of diabetes (HR=1.64, 95% CI=1.06 to 2.52, P=0.02), CVD history (HR=1.77, 95% CI=1.15 to 2.70, P=0.008), systolic BP (HR=1.04, 95% CI=1.02 to 1.06, P<0.001), serum albumin (HR=0.22, 95% CI=0.10 to 0.48, P<0.001), and hemoglobin levels (HR=0.72, 95% CI=0.57 to 0.91, P=0.007) were independent predictors for primary outcome (model chi-squared=93.5, P<0.001).
The subjects who prematurely terminated the study were younger and less likely to have diabetes, CVD history, and higher serum albumin levels at baseline compared with the subjects who remained in the study (data not shown). Additional analysis using the study group excluding patients who prematurely terminated the study yielded very similar results to the primary analysis in adjusted models (HR=0.75, 95% CI=0.51 to 1.11, P=0.16 for high-flux HD group and HR=0.88, 95% CI=0.60 to 1.29, P=0.52 for ultrapure dialysate group).
Secondary Outcomes
Overall survival rates were 74.1% in the high-flux group and 67.9% in the low-flux group (log-rank=2.76, P=0.09). Overall mortality rate was 23% lower (HR= 0.77, 95% CI=0.57 to 1.04, P=0.09) in the high-flux group compared with the low-flux group.
Overall survival rate was equal in the dialysate arms (70.9% in the ultrapure dialysate group and 70.8% in the standard dialysate group, P=0.82).
Overall mortality rate was 8.32 per 100 patient years in the whole study population. In adjusted Cox regression analysis, age, presence of diabetes, permanent catheter use, and C-reactive protein (CRP) were shown to be independent predictors for overall mortality (model chi-squared=96.6, P<0.001).
Mean values of biochemical parameters during follow-up are listed in the Table 2. Predialysis serum β2-microglobulin level significantly decreased in the high-flux group compared with the low-flux group (Δβ2-microglobulin=−5.0±13.6 mg/L in the high-flux group versus 2.7±14.3 mg/L in the low-flux group, P<0.001). There was no difference between the dialysate groups regarding serum β2-microglobulin levels.
Time-averaged CRP levels were lower in the high-flux group compared with the low-flux group (Table 2). During follow-up, mean hemoglobin level was significantly higher in the high-flux HD group than the low-flux HD group, despite similar ferritin levels and prescribed erythropoietin dosage.
In the dialysate arms, mean dialysate endotoxin level decreased from 0.16±0.26 EU/ml (median=0.07 [0.03–0.21]) to 0.01±0.01 EU/ml (P<0.001; median=0.00 [0.00–0.02]) in the ultrapure dialysate arm and remained stable in the standard dialysate arm (from 0.14±0.23 to 0.15±0.22 EU/ml, P=0.87; from median=0.07 [0.03–0.16] to 0.08 [0.04–0.17]). However, time-averaged CRP levels were not significantly different between the groups. Prescribed erythropoietin dosage and erythropoietin resistance index were lower in the ultrapure dialysate group compared with the standard dialysate group, despite similar hemoglobin and ferritin levels (Table 2).
Posthoc Analyses
Subjects with AVF
Vascular access was native AVF fistula in 576 of 704 subjects (81.8%) at the baseline and remained as fistula in 98.1% of them during follow-up.
The subgroup of subjects with AVF was younger, more likely to be men, had longer time on HD, had lower frequency of diabetes, had higher serum albumin, and had higher hemoglobin compared with those subjects with permanent catheters at the baseline (data not shown). Mean blood flow rate was higher in patients with AVF compared with patients without (361±31 versus 350±31 ml/min, respectively, P=0.01).
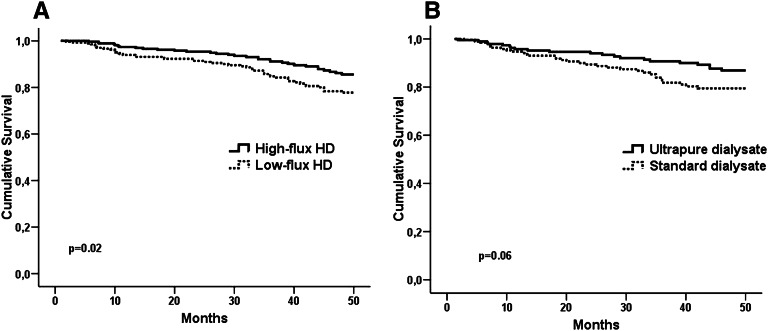
Baseline characteristics were not significantly different between the dialyzer arms in the subgroup of subjects with AVF. Composite cardiovascular event-free survival was significantly higher in the high-flux compared with the low-flux group (85.6% versus 77.8%, P=0.02) in subjects with AVF at the end of 48 months of follow-up (Figure 2A). In Cox regression analysis adjusted for age, sex, diabetes, CVD history, and time on dialysis, primary outcome risk was 39% lower in the high- compared with low-flux group (HR=0.61, 95% CI=0.38 to 0.97, P=0.03). Mean blood flow rate was similar between the high- and low-flux HD groups (362±32 versus 362±30ml/min, P=0.58); there was no interaction between blood flow and the flux arms (P=0.42).
Figure 2.
Cardiovascular event-free survival in subgroups analysis. (A) patients with AVF and (B) HD duration longer than 3 years.
Overall survival was also better in the high-flux group (P=0.04). Of the study population, 75.8% had been treated with high-flux dialysis at the time of randomization (76.1% and 74.6% in the high- and low-flux groups, respectively). The best overall survival was seen in the subjects who were on high-flux dialysis before the randomization and remained on high-flux dialysis during the study period (77.8% in high flux/high flux, 77.0% in low flux/high flux, 71.9% in high flux/low flux, and 63.3% in low flux/low flux, P=0.03).
Composite of cardiovascular event-free survival was similar in the dialysate arms (82.7% in the ultrapure dialysate and 80.6% in the standard dialysate group, P=0.45). Overall survival was also not significantly different between the dialysate arms (P=0.57).
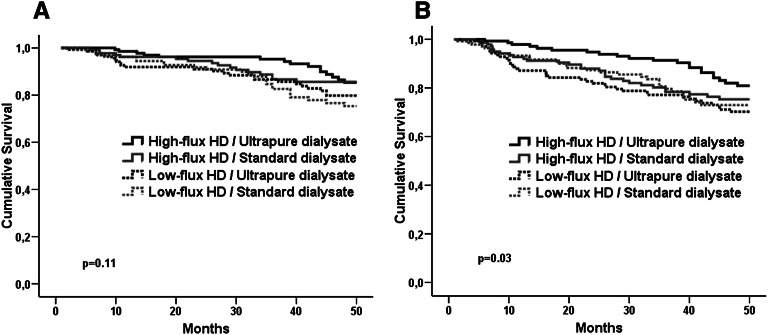
Among subjects with AVF, composite of cardiovascular event-free survival rates was slightly different between the combination groups of flux and dialysate arms (P=0.11). In Cox regression analysis, the patients in the high-flux dialyzer/ultrapure dialysate group had better cardiovascular event-free survival compared with the patients in the low-flux dialyzer/standard dialysate group (HR=0.50, 95% CI=0.26 to 0.95, P=0.03). Patients treated with a combination of high-flux and ultrapure dialysate also had the highest overall survival rate (P=0.03) (Figure 3).
Figure 3.
Cardiovascular event-free survival and overall survival. (A) event-free survival and (B) overall survival among the combination of treatment arms.
In subjects with permanent catheter, neither primary outcome nor overall mortality was different between flux and dialysate groups (data not shown). Subjects with permanent catheters had slightly higher CRP levels during follow-up (1.39 mg/dl [0.75–2.21] versus 0.97 mg/dl [0.54–1.88], P=0.001) and lower overall survival compared with those subjects with AVF (53.5% versus 74.9%, P<0.001).
Diabetic Subjects
In diabetic subjects (n=160), high-flux dialysis was associated with better cardiovascular event-free survival compared with low-flux dialysis (73.9% versus 58.0%, respectively, P=0.05). Also, overall survival (60.5% versus 41.6%, P=0.02) was better in the high-flux group compared with the low-flux group. In adjusted Cox regression analysis, the relative risk for primary outcome was 0.49 (95% CI=0.25 to 0.94, P=0.03) in the high-flux group. There were no differences between the dialysate arms in terms of cardiovascular event-free survival and overall survival.
Subjects with HD Duration Longer Than 3 Years at Baseline
In this subgroup of 399 subjects, composite of fatal and nonfatal cardiovascular event-free survival was slightly higher in the ultrapure dialysate arm than the standard dialysate arm (86.9% versus 79.5%, P=0.06); relative risk for primary outcome was 0.59 (95% CI=0.34 to 1.03, P=0.06) in the ultrapure dialysate arm (Figure 2B). In adjusted Cox regression analysis, the risk for primary outcome was significantly lower in the ultrapure dialysate arm compared with the standard dialysate arm (HR=0.55, 95% CI=0.31 to 0.97, P=0.04).
In this subgroup, there were no differences regarding composite cardiovascular event-free survival and overall survival between the high- and low-flux groups.
Discussion
Many observational studies have suggested that the use of high-flux dialyzers improves survival.3–5 However, primary analyses of the two randomized clinical trials (RCTs) failed to show improvement in overall survival with the use of high-flux dialyzers.7–12 A nonstatistically significant 8% mortality risk reduction with high-flux membrane was reported in the HEMO Study.7 The study has been criticized because of several confounding factors, such as dialyzer reuse, predominance of African Americans, and presence of a high CVD history (80.1%). The MPO Study was started in incident patients with albumin levels below 4 g/dl, but later, it was expanded to include patients with albumin levels over 4 g/dl12; the survival advantage with high-flux dialyzers (24%) was of borderline significance (P=0.09). The current trial is the third RCT addressing the topic of membrane flux and the first RCT investigating the impact of dialysate microbial purity. The primary outcome was the composite of fatal and nonfatal cardiovascular events, and the secondary outcome was overall survival.
In regard to the primary end point, the patient population randomized to high-flux HD showed a risk reduction of 27% compared with the population on low-flux HD, but this difference failed to reach statistical significance. Likewise, the 10% risk reduction with ultrapure dialysate was not statistically significant. In fact, it has to be noted that the statistical power was lower than hypothesized during the design of the study, and therefore, a type II error cannot be excluded; it is attributable to the much lower event rate during the study than estimated: an event-free survival of 78.2% was observed in the low-flux HD group compared with the anticipated 72.9%.
Subgroup analyses of the HEMO Study revealed that high-flux dialysis is associated with better overall survival in patients with HD duration longer than 3.7 years.7 High-flux use was associated with decreased cardiac mortality and the composite outcome of first cardiac hospitalization or cardiovascular death in secondary analyses.10 Subgroup analyses of the MPO Study showed survival benefit with high-flux membrane in patients with serum albumin below 4.0 g/dl and patients with diabetes.12
The current study showed, for the first time, better outcomes with high-flux membranes in the subgroup of patients dialyzed with AVF. Although it was not a prespecified subgroup analysis, patients with AVF constitute the majority of the population (81.8%). Prevalence of patients with AVF was 33.2% in the HEMO Study and 80.1% in the MPO Study. Neither the HEMO Study nor the MPO Study reported the impact of high-flux membrane on survival in patients with AVF who have survival advantage over the patients with permanent catheter.18 Catheter-related complications or patient factors associated with having a catheter could negatively influence patient outcome, which was also shown in the current study. Thus, the impact of high-flux membranes on cardiovascular event-free survival and overall survival was shown in this subgroup that was free of confounding factors associated with catheter use. Not surprisingly, achieved blood flow rate was higher in patients with AVFs compared with patients with catheters. It can be speculated that higher blood flow rate is a prerequisite to improve outcomes with use of high-flux dialyzers.
As a result of a strict volume control strategy applied over the long-term in the study clinics, our patient population has a quite low prevalence of hypertension (20.1%) and hypervolemia, which strongly affect cardiovascular outcomes and possibly interact with assessment of any therapeutic interventions (in the HEMO Study, prevalence of hypertension and congestive heart failure were 96% and 39.7%, respectively). Elimination of these well known cardiovascular risk factors might facilitate demonstration of the beneficial effect of high-flux use in this study, namely a survival advantage in a large proportion of HD patients (patients with AVFs). Additionally, exclusion of patients with substantial residual function may have contributed to a more prominent benefit from high-flux dialysis in the current study, which is in accordance with the favorable outcome in patients with longer dialysis duration shown in the HEMO Study, suggesting that high-flux membrane use may have higher importance in patients without significant residual renal function.
In the secondary analysis restricted to diabetic patients, high-flux HD was found to have beneficial effects on both the primary and secondary outcomes. This result is in line with the 38% risk reduction with high flux reported in the MPO Study12 and the 59% increase in mortality with low-flux use in the 4D Study.5 Clearance of circulating advanced glycosylated end products and oxidized LDLs with high-flux HD may be responsible for this benefit.19,20 This consistent finding in three separate studies strongly supports the use of high-flux dialyzers in diabetic patients.
The use of ultrapure dialysis fluid is associated with lower levels of circulating proinflammatory cytokines and acute-phase proteins compared with standard dialysate meeting current quality recommendations. Most of these reports have shown that ultrapure dialysate use might lead to better outcomes.14–17 However, no study has investigated the effect on survival in a randomized, controlled manner. This study showed no difference between the ultrapure and standard dialysate groups in the primary analysis. However, it should be noted that mean level of endotoxin was 0.15±0.22 EU/ml in the standard dialysate group, which is much lower than recommended levels for endotoxin by many national authorities until quite recently.21 Therefore, it is possible that relatively good quality of standard dialysate in the current study might blunt the demonstration of a beneficial effect of ultrapure dialysate.
Despite lower endotoxin levels in the ultrapure group, CRP levels were similar in the dialysate groups during follow-up. IL-6 has been shown to be a more sensitive marker for inflammation than CRP22; therefore, it cannot be excluded that less source of inflammation is present in the ultrapure dialysate group. Indeed, lower exposure to endotoxin by dialysate may be the reason for the lower erythropoietin requirement and erythropoietin resistance index, despite similar hemoglobin levels in the ultrapure dialysate group. Moreover, in the posthoc analysis, beneficial effects of ultrapure dialysate on cardiovascular event-free survival were evident in patients on dialysis for more than 3 years. This more prominent beneficial effect of ultrapure dialysate on outcome in patients on HD for long term suggests a long-standing effect of inflammation.
In the subgroup of the patients with AVFs, the highest overall survival was observed in patients treated with the combination of high-flux dialyzer and ultrapure dialysate. This result suggests a synergystic effect of these two interventions. In the HEMO Study and the MPO Study, data on dialysate purity were not reported. It seems that ultrapure dialysate was not used routinely in the HEMO Study, and dialysate endotoxin levels were not available; additionally, recommendations for endotoxins in dialysate were less strict at that time.11 Use of ultrapure dialysate in one half of the high-flux group in our study might contribute to better outcomes with high flux.
Strengths of the current study are absence of dialyzer reuse, use of same synthetic membranes in both flux arms, homogenicity of treatment procedures in the study clinics, and use of the same central laboratory for analyses. However, our study has some important limitations. Although we described better outcomes with high-flux HD and ultrapure dialysate in some subgroups, these were not prespecified subgroup analyses. Also, a substantial proportion of the study population had already been treated with high-flux dialysis at the time of randomization, which may reduce the effect of the intervention in the follow-up because of carryover effects of prior treatment. More importantly, perhaps, is the fact that the study population was relatively healthy (low prevalence of diabetes, hypertension, and CVD history), yielding a quite lower annual mortality rate of 8.32 per 100 patient years. Therefore, the results may not be applicable to all HD populations.
In summary, this study showed no statistically significant benefit in cardiovascular event-free survival with use of high- over low-flux membranes or use of ultrapure over standard dialysate. However, in posthoc analyses, high-flux dialysis was associated with better composite of cardiovascular-event free survival and overall survival in patients with AVF, which constitutes the majority of the population, as well as patients with diabetes, which is in keeping with a previous major trial.12 Ultrapure dialysate has favorable effects on survival in long-term dialysis patients, even compared with dialysate quality lower than the quality widely recommended. These posthoc analyses results should be interpreted cautiously and require additional investigation for confirmation.
Concise Methods
Study Design
The Multiple Interventions Related to Dialysis Procedures in Order to Reduce Cardiovascular Morbidity and Mortality in HD Patients was a prospective and randomized clinical trial (Clinical Trials ID NCT00295191). Maintenance dialysis patients (704) undergoing three times per week bicarbonate HD were randomized to high-flux/low-flux dialyzer and ultrapure/standard quality dialysate arms in a two-by-two factorial design. Primary and secondary outcomes were evaluated during a minimum 3 years of follow-up period. The study was completed in March of 2010. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and compliance with Good Clinical Practice Guidelines; all patients provided written informed consent. The local ethics committee of Ege University approved the study protocol. Physicians assessed all subjects three times per week according to national health authority regulations and clinical practice guidelines. An independent institution (Data Management Service) at Ege University managed the collection and database storage of all trial data.
Patient Selection
Based on inclusion and exclusion criteria, 704 patients were enrolled between December of 2005 and March of 2006 in eight HD centers operated by Fresenius Medical Care (FMC), Turkey. Enrolled patients were then randomized centrally in a 1:1 ratio to high- or low-flux dialyzers and ultrapure or standard dialysate in a two-by-two factorial design during a period of 3 months (Figure 4). Eligibility criteria were age over 18 years, three times per week 12 hours/week maintenance bicarbonate HD for a minimum of 3 months, mean single pool Kt/V above 1.2, and willingness to participate in the study and sign informed consent. Patients were excluded for recruitment if scheduled for living donor renal transplantation, known to have serious life-limiting comorbidities (namely active malignancy, active infection, end stage cardiac, pulmonary, or hepatic disease), or requiring HD more than three times per week because of medical comorbid conditions. In addition, patients with urine volume output more than 250 ml/d, patients lacking intellectual capabilities to understand the study objectives, and patients who were pregnant or lactating were also excluded.
Figure 4.
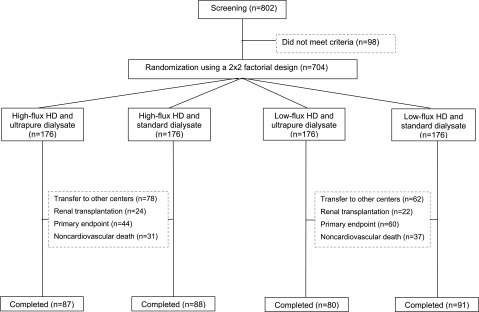
Flow chart of the study.
Treatment Characteristics
All subjects were prescribed three times per week bicarbonate HD aiming for a spKt/V over 1.2. Planned dialysis duration was 240 minutes, with a blood flow rate between 250 and 400 ml/min and a dialysate flow rate of 500 ml/min. High-flux HD was performed using FX60 and FX80 synthetic Helixone dialyzers (FMC, Bad Homburg, Germany), and low-flux HD was performed using F7HPS and F8HPS synthetic dialyzers (FMC, Bad Homburg, Germany). All dialyzers were sterilized by in-line steam sterilization. Dialyzer reuse was not allowed. Before entry into the study, 76.1% of the subjects in the high-flux arm and 74.6% of the subjects in the low-flux arm were being treated with high-flux HD. Ultrapure dialysate was not used at any clinic before the study was launched, which means that all study cases had been treated with standard dialysate before study entry. In the ultrapure dialysate arm, ultrapure dialysate (<0.1 CFU/ml; endotoxin level<0.03 EU/ml) was produced online using a polysulfone-based dialysate filter (Diasafe; FMC, Bad Homburg, Germany) placed next to the dialyzer in Fresenius 4008S dialysis machines. The Diasafe filters were changed every 3 months to ensure that operation time not exceed 1000 hours (in practice, approximately 750 hours of operation were typical). The dialysate was regularly tested for levels of colony-forming units and endotoxins just before change of ultrafilters. Water treatment systems were the same in the study clinics. Water and dialysate quality had to comply with criteria specified by the Association for the Advancement of Medical Instrumentation in both dialysate arms.23 Determination of microbial count was carried out by standard agar tests and expressed as colony-forming units per milliliter dialysate. Endotoxin concentration was quantified with the Limulus amoebocyte lysate assay (Coatest Endotoxin Chromogenix, Mölndal, Sweden) and expressed as Limulus amoebocyte lysate activity (EU/ml).
Study Outcomes
Primary outcome was the composite of fatal and nonfatal cardiovascular events (myocardial infarction, stroke, unstable angina pectoris requiring hospitalization, and revascularization). Diagnosis of myocardial infarction and unstable angina pectoris was based on clinical symptoms and electrocardiography findings as well as markers of cardiac muscle damage, including creatine kinase and creatine kinase-heart specific isoenzyme. Sudden death was considered as cardiovascular in nature unless a noncardiovascular cause could be identified. Definition of stroke was based on neurologic symptoms caused by an ischemic or hemorrhagic event detected by radiologic methods. The main secondary outcome was overall mortality.
Follow-up visits were performed monthly during the study period, and all clinical data of the preceding month were documented. A history of CVD was defined as history of chronic stable angina or unstable angina, prior myocardial infarction, coronary stent or angioplasty, prior coronary bypass grafting, carotid artery disease, transient ischemic attack, ischemic cerebral vascular accident, peripheral artery disease, or peripheral artery angioplasty or stent.
Biochemical parameters were measured monthly using standard automated techniques (Architect C 8000 autoanalyzer and Axsyme third-generation immunoassay system; Abbott, IL) unless otherwise stated. Iron, iron binding capacity, and ferritin were analyzed every 2 months; lipids and high-sensitivity CRP were analyzed every 3 months, and β2-microglobulin was analyzed at baseline and the 18th and 36th months of follow-up (Axsyme third-generation immunoassay system; Abbott, IL). Intact parathormone was measured with an immunoradiometric assay (Roche Elecsys 2010) every 3 months. Blood samples were obtained under fasting conditions immediately before the subjects’ scheduled midweek HD session; blood samples were studied within 2 hours after centrifugation, or sera samples were stored at −70°C until measurement. All analyses were carried out at a central laboratory (DIALAB) registered according to external quality control programs.
Statistical Analyses
Sample size estimation was based on the hypothesis to increase cardiovascular event-free survival with high-flux HD by 15% at the end of the minimum 3-year follow-up if 3-year event-free survival in the control group was 72.9% with annual rate of primary end point of 10% (a bilateral α-risk equal to 5% and power of 90%). The required sample size was a total of 598 study subjects. Expecting a of 15% dropout rate, 704 patients were enrolled and randomly assigned during a period of 3 months.
Data are reported as mean ± SD or median with interquartile range depending on their distribution unless otherwise stated. All repeated measures of each subject during the study period were time-averaged. Between-group comparisons were made with t tests or Wilcoxon rank sum tests as appropriate. Categorical variables were compared with chi-squared tests. Survival analysis was performed using the Kaplan–Meier method testing for statistical significance using the log-rank test. Subjects who were transferred to another treatment modality or other dialysis centers were censored at the time of premature study termination. For independent predictors associated with primary outcome, adjusted forward stepwise Cox regression analyses were performed including those variables statistically significant in the univariate analysis (age, diabetes, CVD history, systolic BP, serum albumin, CRP, and hemoglobin) and those variables that could have an effect on primary outcome (sex and time on HD). Additionally, sensitivity analysis for primary outcome was performed in the study group after excluding subjects who terminated the study prematurely. For independent predictors associated with overall mortality, adjusted Cox regression analysis was performed including variables statistically significant in the univariate analysis.
The impact of high-flux HD and ultrapure dialysate usage on the primary and secondary end points was also examined in unspecified subgroups as posthoc analyses: subjects with AVF, diabetic subjects, and subjects with HD duration longer than 3 years. Statistical significance was defined as P<0.05. All analyzes were performed using SPSS software version 13.0 (SPSS Inc., Chicago, IL).
Disclosures
A.B. and E.O. are members of the scientific advisory board of Fresenius Medical Care, Turkey. Other authors declare no conflict of interest.
Acknowledgments
The study was performed in Fresenius Medical Care hemodialysis clinics in Izmir, Turkey. We thank all doctors and nurses working in the study clinics for their support.
The study was supported by Fresenius Medical Care, Turkey, with an unrestricted grant.
E.O. was the chief investigator, wrote both the study protocol and report, and participated in data collection and interpretation. G.A. and E.O. are the guarantors for the report, had full access to all the data in the study, and take responsibility for the integrity of the data and accuracy of the data analysis. G.A. participated in data verification and analysis. G.A., H.T., M.O., S.D., and A.B. were the coinvestigators and participated in data collection and interpretation. M.S.D., M.C., S.S., H.D., D.B., F.K., E.S.O., S.E., and M.E. participated in data collection. J.G.R. and N.W.L. participated in interpretation of data and revising the manuscript. All authors participated in drafting or revising the manuscript, and all authors reviewed and approved the final version of the report.
Fresenius Medical Care had no role in study design and conduction, data management, collection and analysis, preparation, and submission of the manuscript.
The following investigators also participated in the EGE Study: Ege Nefroloji Dialysis Clinic: Can Boydak, Taskin Colak; Nefron Dialysis Clinic: Dilhan Yucedag, Burcu Ozmalkoc; ANKA Dialysis Clinic: Yuksel Yucedag, Mehmet Sonbahar; Gaziemir Dialysis Clinic: Yesim Peker, Erhan Lale, Pinar Uretmen; Sevgi Dialysis Clinic: Banu Kinay, Erdal Karaca; Nasir Dialysis Clinic: Ali Ilaslan, Mujgan Sifil; Hatay Dialysis Clinic: Ertan Bertan, Erdal Sevinc, Mehmet Tanr\x{0131}sev; Narlidere Dialysis Clinic: Nurdan İsik; Ege University School of Medicine: Suha Süreyya Ozbek, Naim Ceylan, Recep Savas, Selen Bayraktaroglu, Necati Sezgin, Ender Hur, Gulperi Celik, Cenk Demirci, Levent Can, and Meral Kayikcioglu.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.US Renal Data System : Overall hospitalization and mortality. Am J Kidney Dis 42[Suppl 5]: S1–S230, 2003. 14520607 [Google Scholar]
- 2.Bouré T, Vanholder R: Biochemical and clinical evidence for uremic toxicity. Artif Organs 28: 248–253, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Port FK, Wolfe RA, Hulbert-Shearon TE, Daugirdas JT, Agodoa LY, Jones C, Orzol SM, Held PJ: Mortality risk by hemodialyzer reuse practice and dialyzer membrane characteristics: Results from the usrds dialysis morbidity and mortality study. Am J Kidney Dis 37: 276–286, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chauveau P, Nguyen H, Combe C, Chêne G, Azar R, Cano N, Canaud B, Fouque D, Laville M, Leverve X, Roth H, Aparicio M, French Study Group for Nutrition in Dialysis : Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis 45: 565–571, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Krane V, Krieter DH, Olschewski M, Marz W, Mann JF, Ritz E, Wanner C: Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis 49: 267–275, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Blankestijn PJ, Ledebo I, Canaud B: Hemodiafiltration: Clinical evidence and remaining questions. Kidney Int 77: 581–587, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cheung AK, Levin NW, Greene T, Agodoa L, Bailey J, Beck G, Clark W, Levey AS, Leypoldt JK, Ornt DB, Rocco MV, Schulman G, Schwab S, Teehan B, Eknoyan G: Effects of high-flux hemodialysis on clinical outcomes: Results of the HEMO study. J Am Soc Nephrol 14: 3251–3263, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Delmez JA, Yan G, Bailey J, Beck GJ, Beddhu S, Cheung AK, Kaysen GA, Levey AS, Sarnak MJ, Schwab SJ, Hemodialysis (HEMO) Study Group : Cerebrovascular disease in maintenance hemodialysis patients: Results of the HEMO Study. Am J Kidney Dis 47: 131–138, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS, HEMO Study Group : Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Cheung AK, Greene T, Leypoldt JK, Yan G, Allon M, Delmez J, Levey AS, Levin NW, Rocco MV, Schulman G, Eknoyan G, HEMO Study Group : Association between serum 2-microglobulin level and infectious mortality in hemodialysis patients. Clin J Am Soc Nephrol 3: 69–77, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R, Membrane Permeability Outcome (MPO) Study Group : Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitter T, Bergner A, Schiffl H: Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients. Nephrol Dial Transplant 15: 1207–1211, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Schiffl H, Lang SM, Stratakis D, Fischer R: Effects of ultrapure dialysis fluid on nutritional status and inflammatory parameters. Nephrol Dial Transplant 16: 1863–1869, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Rahmati MA, Homel P, Hoenich NA, Levin R, Kaysen GA, Levin NW: The role of improved water quality on inflammatory markers in patients undergoing regular dialysis. Int J Artif Organs 27: 723–727, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Koda Y, Nishi S, Miyazaki S, Haginoshita S, Sakurabayashi T, Suzuki M, Sakai S, Yuasa Y, Hirasawa Y, Nishi T: Switch from conventional to high-flux membrane reduces the risk of carpal tunnel syndrome and mortality of hemodialysis patients. Kidney Int 52: 1096–1101, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Lederer SR, Schiffl H: Ultrapure dialysis fluid lowers the cardiovascular morbidity in patients on maintenance hemodialysis by reducing continuous microinflammation. Nephron 91: 452–455, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ: Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis 47: 469–477, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Weiss MF, Erhard P, Kader-Attia FA, Wu YC, Deoreo PB, Araki A, Glomb MA, Monnier VM: Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int 57: 2571–2585, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Wanner C, Bahner U, Mattern R, Lang D, Passlick-Deetjen J: Effect of dialysis flux and membrane material on dyslipidaemia and inflammation in haemodialysis patients. Nephrol Dial Transplant 19: 2570–2575, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Ward RA: Worldwide water standards for hemodialysis. Hemodial Int 11[S1]: S18–S25, 2007 [Google Scholar]
- 22.Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B: Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Association for the Advancement of Medical Instrumentation : Dialysate for hemodialysis (ANSI/AAMI RD52:2004), Arlington, VA, American National Standard, 2004 [Google Scholar]