Abstract
Anopheles funestus is one of the most proficient malaria vectors in the world, mainly because of its remarkable ability to populate a wide range of ecological settings across Africa. Its formidable environmental plasticity has been primarily associated to high amounts of genetic and inversion polymorphisms. However, very little is known about the morphological changes that this ecological adaptation entails. Here, we report on wing morphometric variations in karyotyped specimens of this species collected throughout a wide range of eco-geographical conditions in Cameroon (Central Africa). Our results revealed strong selection on mosquito wing traits. Variation of wing size was dependent on temperature and elevation (p<0.001), while wing shape did not exhibit a specific environmental pattern. On the other hand, we observed a significant correlation of wing shape variation (p<0.001), but not size (p>0.05), with regard to karyotype. This pattern was maintained across different environmental conditions. In conclusion, our findings cast strong evidence that change in morphometric traits are under natural selection and contribute to local adaptation in Anopheles funestus populations. Furthermore, the robust relation between chromosome polymorphisms and wing shape suggests new evolutionary hypotheses about the effect of chromosomal inversions on phenotypic variation in this malaria vector.
Keywords: Anopheles funestus, morphometric variation, environmental adaptation, chromosomal inversions, Cameroon
1. Introduction
One of the major factors which establish the ability of an insect to become an important vector for human diseases is its “anthropophilic” behavior, e.g. its preference for feeding human blood and/or exploiting man-made resting or breeding habitats. In this sense, medically important insects, such as malaria vector mosquitoes, coevolve with to control pressures and habitat changes due to their close contact with human populations, revealing frequent genotypic and phenotypic variations (Dujardin, 2008). In the field, these variations can be based on either genetic divergence, direct environmental effects, or both. Thus, studies on phenotypic variation can provide relevant insights into the evolution of vector systems and help detect local populations with potentially important characters, which might affect disease transmission (Dujardin, 2008; Pigliucci, 2005). However, despite their importance, little is known about phenotypic variation and morphological plasticity in Anopheles species across the different habitats that they populate. This is an important limitation for studies aimed at exploring ecological adaptation in malaria vectors with direct consequences on disease epidemiology.
Morphometric traits have been employed to analyze adaptive variation in natural populations of animals and plants (Mayr, 1942). Patterns of morphological variation involving size or shape dimensions have been often interpreted with regard to their evolutionary importance (Pigliucci, 2005). In this sense, insect wings have been reported as an excellent model for studying morphological evolution in natural populations. Wing size is directly related to body size (Sokoloff, 1966) and there exists considerable evidence that size and shape are targets of natural selection (Soto et al., 2006). Moreover, they respond to environmental variation in complex ways, suggesting that the reaction norm may be part of an adaptive response (Carreira et al., 2006; Weber, 1990). Consequently, investigations on morphological traits variation necessarily require to involve the simultaneous analysis of genetic and environmental factors, which somehow cause intra-specific variation and interspecific divergence (Mackay, 2004).
Insect chromosomal polymorphism has been frequently associated with environmental adaptation (Coluzzi et al., 1979; Hoffmann et al., 2004; Krimbas and Powell, 1992; Krimbas, 1967). Natural populations of Drosophila and Anopheles species have recurrently shown clinal variation in some paracentric chromosomal inversions along latitudinal or altitudinal gradients (Balanya et al., 2003; Collinge et al., 2006; Hoffmann et al., 2004; Simard et al., 2009). Numerous chromosomal rearrangements have been linked to effects on Drosophila morphometric traits, establishing additional variation on which selection may be acting (Colombo et al., 2004; Colombo et al., 2001; Orengo and Prevosti, 2002; Santos et al., 2004). Hence, the observations of adaptive environmental clines where chromosomal polymorphisms and morphometric traits running in parallel suggest that both might be related and subject to similar evolutionary forces (Orengo and Prevosti, 2002). In Africa, malaria transmission is primordially ensured by three anopheline species, Anopheles gambiae, An. funestus and An. arabiensis, which are widely distributed across sub-Saharan Africa. The ability of those malaria vectors to thrive the wide range of habitats present in Africa has been associated to the richness of chromosomal polymorphisms (Ayala et al., 2010; Coluzzi et al., 2002; Pombi et al., 2008). However, to date, no study has examined how this ecological plasticity has modeled their phenotypic traits, contributing to increasing their local population fitness.
The purpose of this study was to investigate the association of chromosomal polymorphism and environmental conditions in phenotypic variation (wing size and shape) of An. funestus in Cameroon, Central Africa. In this sense, natural populations of adult females An. funestus were sampled across nine distinct eco-geographical zones displaying large variation in environmental conditions. First, we compared patterns of wing size and shape among ecological zones. Second, we related wing traits to environmental variables and we elucidated the contribution of each variable in morphometric variation. Finally, we studied the effect of chromosomal inversion polymorphisms and wing morphology. Our findings revealed significant effect of local environmental conditions on wing morphology. Chromosomal polymorphism was associated to wing shape variation across populations. These outcomes are discussed in a context of environmental adaptations and their impact on malaria epidemiology and vector control strategies.
2. Materials and Methods
2.1 Study sites and mosquito sampling
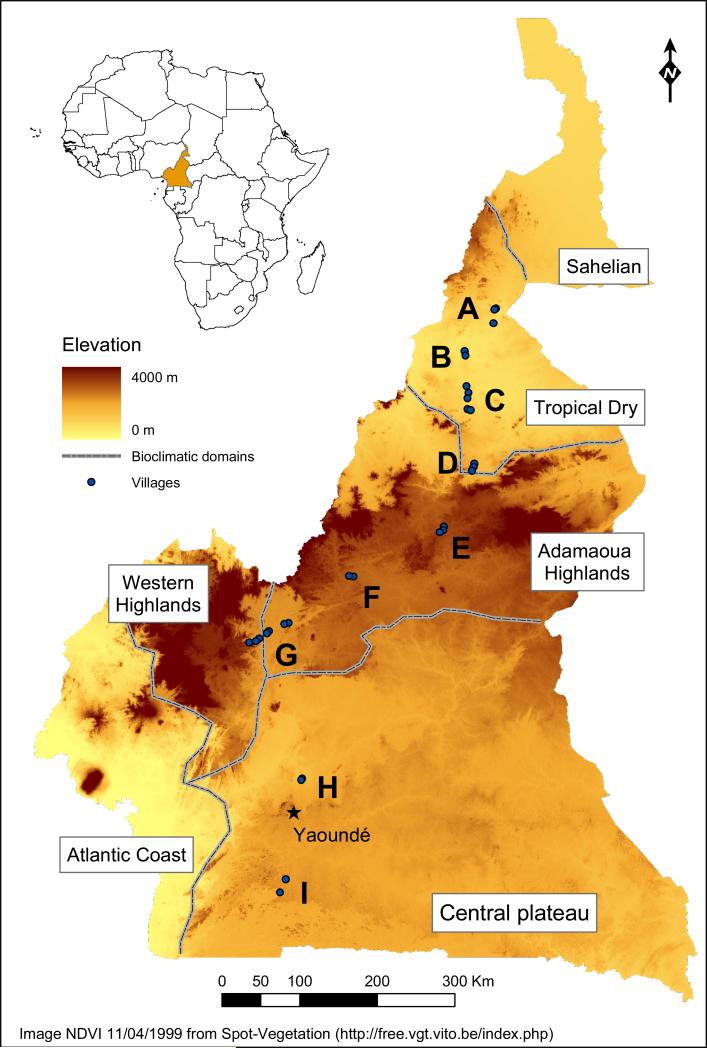
Wing morphometric trait variations in An. funestus were compared between nine locations in five different bioclimatic domains across Cameroon (Figure 1). Latitudinal survey covered most bioclimatic domains present in that country, from the northern arid savannas to the evergreen rainforest in the south (Olivry, 1986). To reduce possible local effects and increase the number of specimens, mosquitoes were collected from 2-7 villages in each zone (average distance between villages 7.83 km). Adult females An. funestus were captured by day-time spraying aerosols of pyrethroid insecticides inside human dwellings (Service, 1993). Anopheline mosquitoes were identified using morphological identification keys (Gillies and de Meillon, 1968). Ovaries from half-gravid An. funestus females were dissected and stored in Carnoy fixative solution (3 parts of 100% ethanol: 1 part glacial acetic acid by volume) for subsequent cytogenetic analysis. Carcasses were stored individually in labeled tubes containing a desiccant and kept at -20°C.
Figure 1.
Topographic Map of Cameroon showing sampling zones (A to I) and villages in each zone (dots). Dotted lines delimit biogeographical domains (Olivry, 1986).
2.2 Mosquito PCR identification and karyotyping
Genomic DNA was extracted from the body of adult mosquito females using the protocol described in Morlais et al. (2004). DNA was then resuspended in sterile water in individual tubes. Morphological identification of An. funestus s.s. (hereafter An. funestus) was confirmed by molecular identification (Cohuet et al., 2003; Koekemoer et al., 2002). Polytene chromosomes obtained from the ovaries of half-gravid females An. funestus, were squashed and stained according to standard protocols (della Torre, 1997). The preparations were examined under a phase-contrast microscope, and paracentric chromosomal inversions were scored according to the An. funestus cytological map (Guelbeogo et al., 2005; Sharakhov et al., 2004).
2.3 Environmental data
A set of seven eco-geographical variables (EGVs) was used to describe the average environmental conditions in each zone (source: LocClim database developed by the Food Agriculture Organization – FAO, http://www.fao.org/sd/2002/EN1203a_en.htm): elevation (in m), rainfall (in mm), temperature (in °C), evapotranspiration (in mm), relative humidity (water vapor pressure in %), mean number of hours of sunlight per day (hours), and wind speed (in m.s-1). Climate data are yearly means, averaged over the past 30 years, obtained from interpolation of field stations data. Computational operations linked to geo-analysis requirements were performed by using the software ArcGIS 8.3 (http://www.esri.com/software/arcgis/index.html).
2.4 Morphometric Analysis
2.4.1 Sample processing
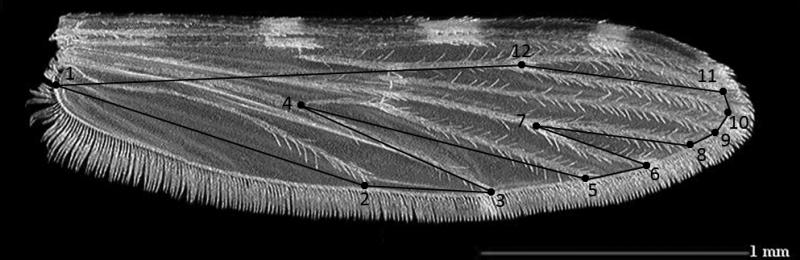
A digital image of each mosquito female wing, left and right (dorsal view) was taken through a binocular microscope (Leica MZ6). Both wings were removed and mounted on microscope slides under cover slips. Morphometric measurements were taken from both wings of each female, except when only one undamaged wing was available. Twelve morphometric measurements, as recommended by Bookstein (1991), were scored from the digital images of each wing (Figure 2) using the free software COOw (http://www.mpl.ird.fr/morphometrics/). All measurements were taken by the same person for more consistency (Bookstein, 1991).
Figure 2.
Selected landmarks on Anopheles funestus mosquito wing.
2.4.2 Repeatability
Random measurement error is common in morphometric analysis, and it can cause serious statistical problems (Arnqvist and Martensson, 1998). To detect this kind of error, we repeated measures of all individuals twice, and we quantified their repeatability by the ratio between the individual variance and the total variance. For this purpose, we used the free software VAR 1.4 (http://www.mpl.ird.fr/morphometrics/).
2.4.3 Size and Shape
For comparing overall wing size between zones, we used the isometric estimator known as “centroid size” (CS) derived from coordinates data: it is defined as the square root of the sum of squared distances between the center of the configuration of landmarks and each individual landmark (Bookstein, 1991). Variation in shape was examined by using geometric morphometrics based on generalized least squares Procrustes superimposition methods (Rohlf, 1990; Rohlf and Slice, 1990). Procrustes methods analyze shape by superimposing configurations of landmarks of two or more individuals to achieve an overall best fit. The first 11 Relative Warps (RW), principal components of shape variables or “Partial warps” (PW), representing > 95 % of the total shape variance, were retained for further analysis. The free software MOG (http://www.mpl.ird.fr/morphometrics/) was used to compute size and shape variation.
2.5 Statistical Analysis
One-way ANOVAs were employed to infer size variation across zones, EGVs and karyotypes. Furthermore, pairwise wilcoxon signed-rank tests were performed to construct a matrix based on differences among zones. To analyze shape variation with regard to zones, EGVs and karyotypes, we performed a MANOVA using the first 11 RWs. Computational statistics were performed by using the free software R (http://cran.r-project.org/).
3. Results
3.1 Macro-environmental survey
A total of 265 Anopheles funestus females were karyotyped and processed for morphometric analysis (Table 1). An average of 29 mosquitoes were collected in each zone, with a range of 18 (Zone I) to 59 specimens (Zone C). Most of the specimens were processed from both wings (66%), and 34% of mosquitoes were analyzed only from one wing. Our results revealed a high level of precision and repeatability in the collection of landmarks, which guaranteed the reliability of the wing size and shape analyses (Supplementary Table 1).
Table 1.
Chromosomal inversion frequencies and wing size details for Anopheles funestus specimens collected in Cameroon
| Zone* | Bioclimatic Domain | Temperature (¼C) | Rainfall (mm) | Elevation (m) | Number of villages | Number of mosquitoes | Inversion Frequency (%) | Wing Size (mm) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 3Ra | 3Rb | 3La | ||||||||
| A | Dry tropical | 27 | 1,000 | 285 | 3 | 27 | 0.0 | 0.0 | 0.0 | 2.66 ± 0.12 |
| B | 2 | 33 | 1.5 | 0.0 | 3.0 | 2.67 ± 0.15 | ||||
| C |
5 | 59 | 63.6 | 68.6 | 66.9 | 2.70 ± 0.16 | ||||
| D |
Cliff | 24 | 1,330 | 610 | 3 | 24 | 87.5 | 97.9 | 95.8 | 2.76 ± 0.15 |
| E | Highlands | 23 | 1,500 | 870 | 3 | 21 | 76.2 | 73.8 | 100.0 | 2.90 ± 0.14 |
| F |
2 | 26 | 71.2 | 80.8 | 100.0 | 2.84 ± 0.12 | ||||
| G |
Highlands | 22 | 1,860 | 780 | 7 | 22 | 63.6 | 72.7 | 100.0 | 2.93 ± 0.12 |
| H | Rainforest | 25 | 1,700 | 570 | 2 | 35 | 100.0 | 100.0 | 100.0 | 2.70 ± 0.13 |
| I | 2 | 18 | 100.0 | 100.0 | 100.0 | 2.63 ± 0.10 | ||||
Zone denomination refers to Figure 1. Elevation: mean elevation (in meters); Rainfall: mean yearly rainfall (in mm); Temperature: mean yearly temperature (in °C).
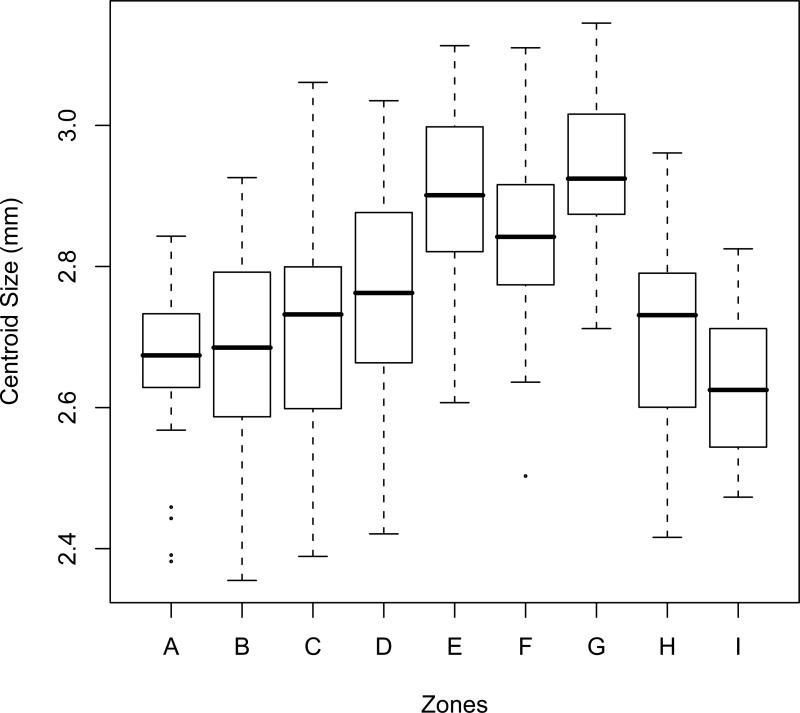
Size differences between ecological zones were highly significant in the general ANOVA (F=14.85, p< 0.001). Pairwise comparisons inferred two groups: mosquitoes collected in highland areas in the central part of the country (Zones E, F and G) were significantly different from those collected in the northern (Zone A, B and C) and southern zones (Zones H and I) (Wilcoxon signed-rank test, p< 0.01). Zone D, located close to the highlands was intermediate (Table 1, Figure 3). Wing sizes in northern savannah areas (A, B and C) were not significantly different from those in the southern rainforest biome (H, I) (Wilcoxon signed-rank test, p>0.05). That reveals the important role of the Adamaoua Highlands in the ecological diversification of this species, as it was already observed in other animals (Bauer et al., 2006). Before proceeding to analysis of wing shape variation, an analysis of the correlation between wing size and shape was carried out (allometry). The correlation coefficient between centroid size (CS) and the 11 RWs was highly significant (F=6.63; p<0.001), which means that changes in wing size will carry with it changes in wing shape. Among the zones, wing shape varied significantly (F=2.24, p<0.001).
Figure 3.
Boxplot representing wing size (CS, centroid size) variation in different sampling zones. Expand: Median, percentile and outliers.
Association between 7 eco-geographical variables (EGVs) and wing morphology were analyzed by ANOVA. The overall effect of EGVs in wing size (CS) variation was highly significant (F= 25.74; r2=0.33, p<0.001). However, only temperature and elevation were highly significantly associated to CS variation (Table 2). In Cameroon, elevation and temperature are highly correlated (Spearman test, p<0.001). Therefore, wing size variation might be explained solely by temperature effect. Rainfall and wind speed revealed marginally significant values (Table 2). Similar procedure was employed to study wing shape variation, using the first 11 RWs. Again, temperature was highly significantly associated to variation in wing shape (MANOVA test F= 9.06, p<0.001). However, the rest of the EGVs were also highly statistically associated to wing shape variation (P<0.005, Table 2). As a result, and in agreement with the analysis of wing shape variation across zones, we were not able to infer a key role of any EGV on wing shape variation (Table 2).
Table 2.
Statistical correlation between eco-geographical variables (EGVs) and wing morphometric traits (size and shape).
| EGV | Wing size1 | Wing shape2 | ||
|---|---|---|---|---|
| F-statistic | pvalue | F-statistic | pvalue | |
| Elevation | 18.21 | <0.001 | 6.352 | <0.001 |
| Evapotranspiration | 2.06 | 0.152 | 7.889 | <0.001 |
| Sunlight exposure | 0.00 | 0.982 | 5.774 | <0.001 |
| Rainfall | 4.71 | 0.031 | 7.779 | <0.001 |
| Wind speed | 5.14 | 0.024 | 6.322 | <0.001 |
| Temperature | 14.16 | <0.001 | 9.060 | <0.001 |
| Water Vapor Pressure | 3.27 | 0.072 | 2.549 | 0.005 |
Wing size was estimated by centroid size (CS) measures. Statistical significance was studied by ANOVA test.
Wing shape was estimated by the first 11 relative warps (RW) which represent >95% of total wing shape variance. Statistical significance was studied by MANOVA test
3.2 Chromosomal polymorphism and wing traits
Mosquito karyotyping was carried out for the independent (non-overlapping) and common inversions on chromosome 3. Two inversions were scored on the right arm (3Ra, 3Rb) and one on the left arm (3La). These inversions have been involved in local environmental adaptation and speciation in different populations of An. funestus throughout Africa (Ayala et al., 2010; Cohuet et al., 2005; Costantini et al., 1999; Guelbeogo et al., 2005). Chromosomal polymorphisms are not randomly distributed in Cameroon (Ayala et al., 2010). Latitudinal variation in inversion frequencies was observed in all three inversion systems (Table 1). The three inversions were absent in the northern dry savannahs (Zones a and B) and gradually increased in frequency southwards until they were fixed in the humid southern rainforest areas (Zones H and I) (Table 1). In Cameroon, clinal latitudinal inversion frequencies have been previously reported in An. funestus (Ayala et al., 2010; Cohuet et al., 2005) and An. gambiae (Simard et al., 2009; Wondji et al., 2005).
When comparing wing traits among karyotype forms (Homozygote standard – Heterozygote – Homozygote inverted), significant differences were observed. Wing size was studied separately for each chromosomal rearrangement and alternative karyotype. Inversion 3Rb and 3La revealed highly significant wing size differences between standard and inverted homozygotes, while inversion 3Ra was marginally significant (Pairwise Wilcoxon rank sum test, 3Ra p=0.043; 3Rb p=0.002; 3La p<0.001 after Bonferroni correction). Because highland specimens (Zones E and F) are bigger than those from other zones in Cameroon, they were removed from the analysis. As result, the distinction of size between standard and inverted homozygotes in all three inversions disappeared (Pairwise Wilcoxon rank sum test, p>0.05). On the other hand, highly significant differences were observed when comparing heterozygotes with both homozygotes for inversions 3Ra and 3Rb (Pairwise Wilcoxon rank sum test, p<0.001, after Bonferroni correction). Most of heterozygotes occur in the Adamaoua Highlands. Hence, this divergence could be due to environmental conditions rather than genetic background. Statistical analysis for 3La heterozygotes was not carried out due to the low number of specimens (n=3).
Similar analyses were performed for wing shape morphometry among chromosomal inversions and karyotypes. Pairwise comparisons revealed significant differences among karyotypes for each inversion. However, main dissimilarities were observed between standard and inverted forms for the three chromosomal rearrangements across Cameroon, even when the Adamaoua highlands’ populations were not included in the analysis (Pairwise Wilcoxon rank sum test p<0.001, after Bonferroni correction). Besides, wing shape of 3Ra and 3Rb heterozygotes remained slightly different from standard homozygotes (Pairwise Wilcoxon rank sum test, 3Ra p=0.03; 3Rb p=0.035 after Bonferroni correction) and were not significantly different from inverted homozygotes (Pairwise Wilcoxon rank sum test p>0.5). The low number of heterozygotes for 3La inversion (n=3) made it impossible to carry out robust statistical analysis with this karyotype.
To reduce potential environmental determinants in wing trait variation, we further focused our analysis on Zone C, where both homozygotes (standard and inverted) for the three inversions systems (3Ra, 3Rb and 3La) occur in sympatry. In this area, the three inversions appear in almost absolute linkage disequilibrium (G-test, p<0.001). We computed the Hardy-Weinberg Equilibrium test using the software GENEPOP v4 (Raymond and Rousset, 1995; Rousset, 2008). All inversions showed strong and significant deficit in heterozygotes (Exact Test, FIS >0.8, p<0.001). Due to low numbers of heterozygotes in the 3 inversions, they were removed from further analyses, and we only performed comparisons between specimen homozygotes for all 3 inversions, e.g. 19 and 34 specimens for standard and inverted homozygotes, respectively. At first glance, the results did not reveal significant levels of wing size variation between homozygotes in Zone C (MANOVA test, F=0.43, p>0.05). However, when we analyzed wing shape, highly significant variation was observed between standard and inverted homozygotes (MANOVA test, F=13.35, p<0.001). To confirm these differences in wing shape between standard and inverted homozygotes, we extended the wing shape variation analysis to the rest of the ecological zones. We compared standard individuals from zones A, B and C to inverted homozygotes collected in zones C, D, E, F, G, H and I. We did not observe any significant wing shape difference within standard (MANOVA test, F= 1.20, p>0.05) and inverted homozygotes (MANOVA test, F= 1.28, p>0.05). However, differences in wing shape between standard and inverted homozygotes was highly significant (MANOVA test, F= 36.04, p<0.001). These results revealed that the pattern of wing shape variation, but not size, depends upon the chromosomal background and karyotype of An. funestus specimens.
4. Discussion
This study is the first of its kind to demonstrate environmental and genetic effects associated with phenotypic variation in an Anopheles species. We showed i) that wing size variation in An. funestus from Cameroon was almost exclusively mediated by temperature effects and independent of the genetic background; and ii) that chromosomal polymorphism is associated with wing shape variation, but not size. The statistical analysis suggested that this latter pattern is stout and maintained across different environmental conditions.
4.1 Wing size variation
Clines in body size have repeatedly been observed in many insects, such as in fruit flies and mosquitoes, and were commonly related to eco-geographical variables such as latitude, temperature or elevation (Capy et al., 1993; Collinge et al., 2006; Weeks et al., 2002) or to biological processes such as larval crowding and/or availability of limited resources during larval development (Aboagye-Antwi and Tripet, 2010; Manoukis et al., 2006). It is not clear why wing size evolves in response to temperature or elevation. It has been suggested that natural selection could act on the ratio between the surface area and the volume of the body, and hence on the rate of heat exchange, a theory known as Bergmann's rule (Mayr, 1963). Although this feature is typical for endothermic animals, it has been also reported for ectothermic animals, like insects (Partridge et al., 1994; vanVoorhies, 1996).
Several hypotheses could explain wing size variation among An. funestus populations. First, larval development conditions affect final adult size in mosquitoes (Aboagye-Antwi and Tripet, 2010; Briegel, 1990a, 1990b; Lyimo et al., 1992). Water temperature during mosquito larval development may affect the amount of food eaten, therefore, the efficiency of assimilation and growth efficiency. In Drosophila, larval development at low temperature reduces growth rate but increases final adult size (French et al., 1998). If we consider a direct relationship between body size and wing size to be common in Diptera (Briegel, 1990a; Briegel et al., 2001; Sokoloff, 1966), it could explain why larger wings were found in the colder regions of the Adamaoua highlands. Second, biotic processes affecting larvae are directly correlated to final adult size in Anopheles (Lyimo et al., 1992; Manoukis et al., 2006). For instance, larval crowding affects adult size negatively. However, (adult) mosquitoes abundance has been negatively related to elevation (Tchuinkam et al., 2010; Zhou et al., 2007). Unfortunately, we do not have any information on larval density of An. funestus in Cameroon but high density is not expected because these mosquitoes typically breed in large bodies of water such as in lakes and semi permanent swamps (Coetzee and Fontenille, 2004). Therefore, we cannot conclude on the implication of biological process in the variation of wing size. A third hypothesis relates wing size and altitudinal environments based on air conditions in high elevations. On a developmental or evolutionary timescale, flying insects in high altitudinal environments may compensate for reduced air density by altering wing length and/or wing area relative to body size (Dillon et al., 2006). Insects with greater wing area relative to body size decrease the induced velocity necessary to sustain flight, therefore minimizing the energetic cost in reduced air density conditions (Dillon et al., 2006). Some morphological data support this hypothesis. Wing length in Drosophila robusta is greater at higher elevation whereas thoracic dimensions remain constant (Stalker and Carson, 1948). Similarly, mountain honeybees have longer wings and a greater wing area but invariant body mass relative to their lowland counterparts (Hepburn et al., 1998). Unfortunately, in our analysis we only measured mosquito's wings and no other body traits. Consequently, we could not verify this hypothesis, something which remains open for future studies. The last hypothesis is related to chromosomal inversion background. Chromosomal inversion polymorphism has been associated with a large number of traits (Hoffmann and Rieseberg, 2008; Hoffmann et al., 2004). In Drosophila, several authors have sought relationships between body (or wing) size and chromosomal inversions karyotype, leading to diverse results (Bitner-Mathe et al., 1995; Fanara et al., 1997; Orengo and Prevosti, 2002; Prevosti, 1967; Santos et al., 2004; Yadav and Singh, 2006). Moreover, in seaweed flies, Day and Gilburn related the male body size with one inversion (Day and Gilburn, 1997). In general, body size is affected only by some inversions, and negatively or positively associated with one of the homozygotes. Moreover, several studies have revealed a positive effect of heterosis (heterozygotes) in body size (Prevosti, 1967; Yadav and Singh, 2003). In our study, both homozygotes (standard and inverted) for the different inversions systems did not show significant wing size variation at local or country scale. Instead, heterozygotes for inversion 3Ra and 3Rb were mostly found in the highlands and they exhibited significantly bigger wings than either homozygote. Our data therefore suggest that chromosomal polymorphisms do not play a significant role in determining wing size, but heterosis does. These results should be tested experimentally in the laboratory under controlled environmental conditions. However, unfortunately, conventional approaches based on formal laboratory crosses are not possible with An. funestus because of scarcity of laboratory colonies
Some studies have correlated mosquito body size with malaria parasite transmission efficiency: larger An. gambiae females were shown to live longer, to bite more often and to utilize the blood meal more efficiently than smaller ones (Aboagye-Antwi and Tripet, 2010; Manoukis et al., 2006; Takken et al., 1998). Thus, variations in body size may have a direct impact on vector capacity and malaria transmission dynamics, because larger mosquitoes will be able to transmit the parasite more efficiently. Accordingly, it might be expected that in the highlands of Cameroon, mosquitoes might able to transmit malaria better than in the lowlands. However, many other local factors are known to affect transmission intensity and vector capacity of mosquito populations (Cohuet et al., 2010).
4.2 Wing shape variation
In morphometrics, the shape of a configuration of landmarks is represented by their relative positions as contained in their coordinates. Since each form can be explained by the change in linear dimensions, it is obvious that size and shape are not independent attributes. In this study we analyzed the relationship between size and shape (called ‘allometry’). Our results showed a significant correlation between both traits. As shown above, environmental changes affected the size, which in turn should produce passive shape changes (allometric effects). However, wing shape did not follow the same environmental pattern than wing size, showing higher environmental diversity across Cameroon and within ecological zones. It was therefore not possible to attribute the variations in shape to allometric effects only.
Although latitudinal and/or temperature clines were described in many animal species in size-related traits, the relationship between shape and environmental conditions is still unclear (Huey et al., 2000; James et al., 1997). Birdsall et al., (2000) concluded that wing shape in D. melanogaster is poorly affected by developmental temperature. However, there is circumstantial evidence suggesting that developmental and evolutionary temperature-related cell size divergence have contrasting effects on wing shape in D. subobscura (Calboli et al., 2003). Although hypothetical functional explanations for subtle wing shape variation are missing for environmental variables, apparently wing shape would affect aerodynamics in insects. Because wing shape largely determines the high energetic costs of flight, it may be expected that inter- or intraspecific differences be partly due to selection. One of the environmental variables considered in this study was wind speed, which could be presumed to have a direct effect on insect flight. However, again, no significant pattern in wing shape variation could be linked to variations in this EGV. One study in dragonflies revealed important modifications in wing shape between migrant and non-migrant populations (Johansson et al., 2009). In mosquitoes, migration is not a well-known phenomenon. Anopheles funestus breeds in semi-permanent or permanent sites whose distribution is patchy, such as dams, agricultural irrigation schemes, and marshes (Coetzee and Fontenille, 2004). Migration therefore, does not appear to be a major biological attribute of An. funestus. However, low levels of neutral genetic differentiation between geographically distant An. funestus populations in Cameroon (Ayala et al., 2010; Cohuet et al., 2005) suggests that intensive gene flow occurs across long distances may be facilitated by some levels of migratory patterns. This area of research clearly deserves further attention, and the evolutionary significance of long distance migration, and the physiological, morphological and behavioral traits that favor it in An. funestus and other major malaria vectors needs to be clearly assessed.
Few studies have paid attention to wing shape variation with regard to chromosomal inversions. Santos et al., (2004) showed significant differences in wing shape depending on chromosomal inversions in D. subobscura. Actually, shape appears as a classical polygenic character (Patterson and Klingenberg, 2007). Many genes with small additive effects on wing shape are dispersed along the Drosophila genome (Weber et al., 2001; Zimmerman et al., 2000). This suggests plentiful chance for gene-inversion linkage disequilibria in inversion-rich species such as D. subobscura. In contrast to what was observed for environmental variables, there was a consistent pattern of wing shape variation among populations of An. funestus carrying the same karyotype across ecological areas. Mosquitoes sharing identical full homozygote state (standard or inverted for the 3 inversions systems on chromosome 3) did not show significant differences in wing shape between localities across Cameroon. This pattern was consistently observed, even when both homozygotes occurred in sympatry (Zone C). Non-random distribution of chromosomal inversions, strong and significant heterozygote deficits, and linkage disequilibrium between inversions within natural populations of An. funestus have been described and interpreted as indicative of environmental adaptation and/or incipient speciation, a situation that is reminiscent of An. gambiae (Cohuet et al., 2005; Coluzzi et al., 2002; Costantini et al., 1999; Guelbeogo et al., 2005; Michel et al., 2005). Recently, one study in six sympatric cryptic species in the neotropical genus Blepharoneura (Tephritidae) suggests a direct link between behavioral traits exhibited by males of different species, the degree of wing shape dimorphism, and the extent of reproductive isolation (Marsteller et al., 2009). Such divergence operating on courtship and mate recognition (e.g., prezygotic reproductive isolation) might also exist in An. funestus and contribute to some extent to the strong deficits in heterozygote we observed. Indeed, significant levels of genetic differentiation have been reported between sympatric populations characterized by different levels of chromosomal polymorphism (i.e., the “Folonzo” and “Kiribina” chromosomal forms described by Costantini et al., (1999) and Guelbeogo et al., (2005)) in Burkina Faso (Michel et al., 2005). Here again, further research is needed to explore mating behavior and mating preferences, and their genetic bases in anophelines mosquitoes.
4.3 Conclusion
In conclusion, our study demonstrated that wing morphology in Anopheles funestus shows significant phenotypic variation in different bioclimatic settings. In particular, An. funestus wing size exhibits significant variation according to temperature and elevation, suggesting this character is potentially targeted by natural selection. Because wing size is correlated to body size in mosquitoes and variations in body size were shown to impact on the overall fitness and vector efficiency, further studies aiming at unraveling the genetic bases of such plasticity are needed. Moreover, wing shape morphology analyses revealed significant variation between as well as within eco-climatic regions of Cameroon, and were shown to correlate with chromosomal polymorphisms. Differences in wing shape related to chromosomal inversions might be involved in mate recognition and therefore impact on the population structure of this vector. This has major consequences for the development and implementation of any vector control strategies, including innovative vector control using genetically altered mosquitoes for population suppression and/or replacement (Boete and Koella, 2002).
Supplementary Material
Supplementary Table 1
Test of repeatability for landmark measurements. All individuals were measured twice. Estimates of landmarks correlation between both measures were obtained by comparing aligned coordinates X and Y (after Procustes analysis) as in (Arnqvist and Martensson, 1998). Size correlation between both measures was analyzed using the centroid size (CS correlation = 0.999). Landmark numbers correspond to positions showed in Figure 1.
Acknowledgements
We thank Jean-Pierre Agbor, Serge Donfack, Collince Kandem, Joachim Etouna of the Laboratoire de Recherche sur le Paludisme at OCEAC, Yaoundé for excellent assistance in the field. We especially thank Gilbert LeGoff for his valuable technical assistance and Sébastien Pion for help in statistical analysis. Financial support was provided by the Institut de Recherche pour le Développement and student fellowship grant from Fundacion CAJA MADRID (Madrid, Spain) to D.A. Field work was supported in part by the National Institutes of Health grant R01-AI063508 to Nora J. Besansky.
References
- Aboagye-Antwi F, Tripet F. Effects of larval growth condition and water availability on desiccation resistance and its physiological basis in adult Anopheles gambiae sensu stricto. Malaria Journal 9. 2010 doi: 10.1186/1475-2875-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G, Martensson T. Measurement error in geometric morphometrics: Empirical strategies to assess and reduce its impact on measures of shape. Acta Zool. Acad. Sci. Hung. 1998;44:73–96. [Google Scholar]
- Ayala D, Fontaine MC, Cohuet A, Fontenille D, Vitalis R, Simard F. Chromosomal inversions, natural selection and adaptation in the malaria vector Anopheles funestus Mol. Biol. Evol. 2010;28:745–758. doi: 10.1093/molbev/msq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanya J, Serra L, Gilchrist GW, Huey RB, Pascual M, Mestres F, Sole E. Evolutionary pace of chromosomal polymorphism in colonizing populations of Drosophila subobscura: an evolutionary time series. Evolution: International Journal of Organic Evolution. 2003;57:1837–1845. doi: 10.1111/j.0014-3820.2003.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Chirio L, Ineich I, LeBreton M. New Species of Cnemaspis (Squamata: Gekkonidae) from Northern Cameroon, a Neglected Biodiversity Hotspot. Journal of Herpetology. 2006;40:510–519. [Google Scholar]
- Birdsall K, Zimmerman E, Teeter K, Gibson G. Genetic variation for the positioning of wing veins in Drosophila melanogaster. Evolution & Development. 2000;2:16–24. doi: 10.1046/j.1525-142x.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- Bitner-Mathe BC, Peixoto AA, Klaczko LB. Morphological variation in a natural population of Drosophila mediopunctata: altitudinal cline, temporal changes and influence of chromosome inversions. Heredity. 1995;75:54–61. doi: 10.1038/hdy.1995.103. [DOI] [PubMed] [Google Scholar]
- Boete C, Koella JC. A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malar J. 2002;1:3. doi: 10.1186/1475-2875-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric Tools For Landmark Data: Geometry and Biology. Cabridge Universyt press; Cambridge: 1991. [Google Scholar]
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera, Culicidae), vectors of malaria. Journal of Medical Entomology. 1990a;27:839–850. doi: 10.1093/jmedent/27.5.839. [DOI] [PubMed] [Google Scholar]
- Briegel H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology. 1990b;36:165–172. [Google Scholar]
- Briegel H, Knusel I, Timmermann SE. Aedes aegypti: size, reserves, survival, and flight potential. Journal of Vector Ecology. 2001;26:21–31. [PubMed] [Google Scholar]
- Calboli FCF, Gilchrist GW, Partridge L. Different cell size and cell number contribution in two newly established and one ancient body size cline of Drosophila subobscura. Evolution. 2003;57:566–573. doi: 10.1111/j.0014-3820.2003.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Capy P, Pla E, David JR. Phenotypic and genetic variability of morphometrical traits in natural populations of Drosophila melanogaster and D. simulans I. Geographic variations. Genet Sel Evol. 1993;25:517–536. [Google Scholar]
- Carreira VP, Soto IM, Hasson E, Fanara JJ. Patterns of variation in wing morphology in the cactophilic Drosophila buzzatii and its sibling D. koepferae. Journal of Evolutionary Biology. 2006;19:1275–1282. doi: 10.1111/j.1420-9101.2005.01078.x. [DOI] [PubMed] [Google Scholar]
- Coetzee M, Fontenille D. Advances in the study of Anopheles funestus, a major vector of malaria in Africa. Insect Biochemistry and Molecular Biology. 2004;34:599–605. doi: 10.1016/j.ibmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cohuet A, Dia I, Simard F, Raymond M, Rousset F, Antonio-Nkondjio C, Awono-Ambene PH, Wondji CS, Fontenille D. Gene flow between chromosomal forms of the malaria vector Anopheles funestus in Cameroon, Central Africa, and its relevance in malaria fighting. Genetics. 2005;169:301–311. doi: 10.1534/genetics.103.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohuet A, Harris C, Robert V, Fontenille D. Evolutionary forces on Anopheles: what makes a malaria vector? Trends in Parasitology. 2010;26:130–136. doi: 10.1016/j.pt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Cohuet A, Simard F, Toto JC, Kengne P, Coetzee M, Fontenille D. Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. American Journal of Tropical Medicine and Hygiene. 2003;69:200–205. [PubMed] [Google Scholar]
- Collinge JE, Hoffmann AA, McKechnie SW. Altitudinal patterns for latitudinally varying traits and polymorphic markers in Drosophila melanogaster from eastern Australia. Journal of Evolutionary Biology. 2006;19:473–482. doi: 10.1111/j.1420-9101.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Pensel S, Isabel RM. Chromosomal polymorphism, morphometric traits and mating success in Leptysma argentina Bruner (Orthoptera). Genetica. 2004;121:25–31. doi: 10.1023/b:gene.0000019924.96257.97. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Pensel SM, Remis MI. Chromosomal polymorphism, morphological traits and male mating success in Leptysma argentina (Orthoptera). Heredity. 2001;87:480–484. doi: 10.1046/j.1365-2540.2001.00932.x. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V. A polytene chromosome analysis of the Anopheles gambiae species complex. Science. 2002;298:1415. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, Dideco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Costantini C, Sagnon N, Ilboudo-Sanogo E, Coluzzi M, Boccolini D. Chromosomal and bionomic heterogeneities suggest incipient speciation in Anopheles funestus from Burkina Faso. Parassitologia. 1999;41:595–611. [PubMed] [Google Scholar]
- Day TH, Gilburn AS. Sexual selection in seaweed flies. Advances in the Study of Behavior. 1997;26:1–57. [Google Scholar]
- della Torre A. Polytene chromosome preparation from Anopheline mosquitoes. In: Crampton JM, Beard CB, Louis C, editors. Molecular Biology of Insect Disease Vectors: a Methods Manual. Chapman & Hall; London: 1997. pp. 329–336. [Google Scholar]
- Dillon ME, Frazier MR, Dudley R. Into thin air: Physiology and evolution of alpine insects. Integrative and Comparative Biology. 2006;46:49–61. doi: 10.1093/icb/icj007. [DOI] [PubMed] [Google Scholar]
- Dujardin JP. Morphometrics applied to medical entomology. Infection, Genetics and Evolution. 2008;8:875–890. doi: 10.1016/j.meegid.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Fanara JJ, Hasson E, Rodriguez C. The effect of polymorphic inversions on body size in two natural populations of Drosophila buzzatii from Argentina. Hereditas. 1997;126:233–237. doi: 10.1111/j.1601-5223.1997.00233.x. [DOI] [PubMed] [Google Scholar]
- French V, Feast M, Partridge L. Body size and cell size in Drosophila: the developmental response to temperature. Journal of Insect Physiology. 1998;44:1081–1089. doi: 10.1016/s0022-1910(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Gillies MT, de Meillon B. The anophelinae of Africa, south of the Sahara. The South African Institute for Medical Research; Johannesburg: 1968. [Google Scholar]
- Guelbeogo WM, Grushko O, Boccolini D, Ouedraogo PA, Besansky NJ, Sagnon NF, Costantini C. Chromosomal evidence of incipient speciation in the Afrotropical malaria mosquito Anopheles funestus. Medical and Veterinary Entomology. 2005;19:458–469. doi: 10.1111/j.1365-2915.2005.00595.x. [DOI] [PubMed] [Google Scholar]
- Hepburn HR, Youthed C, Illgner P, Radloff SE, Brown RE. Production of aerodynamic power in mountain honeybees (Apis mellifera). Naturwissenschaften. 1998;85:389–390. [Google Scholar]
- Hoffmann AA, Rieseberg LH. Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annual Review of Ecology, Evolution, and Systematics. 2008;39:21–42. doi: 10.1146/annurev.ecolsys.39.110707.173532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Sgro CM, Weeks AR. Chromosomal inversion polymorphisms and adaptation. Trends in Ecology & Evolution. 2004;19:482–488. doi: 10.1016/j.tree.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308–309. doi: 10.1126/science.287.5451.308. [DOI] [PubMed] [Google Scholar]
- James AC, Azevedo RBR, Partridge L. Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics. 1997;146:881–890. doi: 10.1093/genetics/146.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson F, Soderquist M, Bokma F. Insect wing shape evolution: independent effects of migratory and mate guarding flight on dragonfly wings. Biol. J. Linnean Soc. 2009;97:362–372. [Google Scholar]
- Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. American Journal of Tropical Medicine and Hygiene. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- Krimbas C, Powell J. Drosophila inversion polymorphism. CRC Press; Boca Raton: 1992. [Google Scholar]
- Krimbas CB. The genetics of Drosophila subobscura populations. 3. Inversion polymorphism and climatic factors. Mol Gen Genet. 1967;99:133–150. doi: 10.1007/BF00426158. [DOI] [PubMed] [Google Scholar]
- Lyimo EO, Takken W, Koella JC. Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomologia Experimentalis Et Applicata. 1992;63:265–271. [Google Scholar]
- Mackay TFC. The genetic architecture of quantitative traits: lessons from Drosophila. Current Opinion in Genetics & Development. 2004;14:253–257. doi: 10.1016/j.gde.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Manoukis NC, Toure MB, Sissoko I, Doumbia S, Traore SF, Diukwasser MA, Taylor CE. Is Vector Body Size the Key to Reduced Malaria Transmission in the Irrigated Region of Niono, Mali? Journal of Medical Entomology. 2006;43:820–827. doi: 10.1603/0022-2585(2006)43[820:ivbstk]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsteller S, Adams DC, Collyer ML, Condon M. Six cryptic species on a single species of host plant: morphometric evidence for possible reproductive character displacement. Ecol. Entomol. 2009;34:66–73. [Google Scholar]
- Mayr E. Systematics and the Origin of Species. Columbia University Press; New York: 1942. [Google Scholar]
- Mayr E. Animal species and evolution. Harvard University Press; Cambridge, Mass: 1963. [Google Scholar]
- Michel AP, Guelbeogo WM, Grushko O, Schemerhorn BJ, Kern M. Molecular differentiation between chromosomally defined incipient species of Anopheles funestus. Insect Molecular Biology. 2005;14:375. doi: 10.1111/j.1365-2583.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- Morlais I, Poncon N, Simard F, Cohuet A, Fontenille D. Intraspecific nucleotide variation in Anopheles gambiae: new insights into the biology of malaria vectors. Am J Trop Med Hyg. 2004;71:795–802. [PubMed] [Google Scholar]
- Olivry JC. Fleuves et Rivières du Cameroun. ORSTOM; Paris, France: 1986. [Google Scholar]
- Orengo DJ, Prevosti A. Relationship between chromosomal polymorphism and wing size in a natural population of Drosophila subobscura. Genetica. 2002;115:311–318. doi: 10.1023/a:1020640112673. [DOI] [PubMed] [Google Scholar]
- Partridge L, Barrie B, Fowler K, French V. Evolution and development of body-size and cell-size in Drosophila melanogaster in response to temperature. Evolution. 1994;48:1269–1276. doi: 10.1111/j.1558-5646.1994.tb05311.x. [DOI] [PubMed] [Google Scholar]
- Patterson JS, Klingenberg CP. Developmental buffering: how many genes? Evolution & Development. 2007;9:525–526. doi: 10.1111/j.1525-142X.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Evolution of phenotypic plasticity: where are we going now? Trends in Ecology & Evolution. 2005;20:481–486. doi: 10.1016/j.tree.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Pombi M, Caputo B, Simard F, Di Deco MA, Coluzzi M, Della Torre A, Costantini C, Besansky NJ, Petrarca V. Chromosomal plasticity and evolutionary potential in the malaria vector Anopheles gambiae sensu stricto: insights from three decades of rare paracentric inversions. BMC Evolutionary Biology. 2008;8:309. doi: 10.1186/1471-2148-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevosti A. Inversion heterozygosity and selection for wing legth in Drosophila subobscura. Genetical Research. 1967;10:81–&. doi: 10.1017/s0016672300010788. [DOI] [PubMed] [Google Scholar]
- Raymond M, Rousset F. GENEPOP, Version 1.2. A population genetics software for exact testsand ecumenicism. Journal of Heredity. 1995;26:248–249. [Google Scholar]
- Rohlf FJ. Morphometrics. Annual Review of Ecology and Systematics. 1990;21:299–316. [Google Scholar]
- Rohlf FJ, Slice DE. Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Zoology. 1990;39:40–59. [Google Scholar]
- Rousset F. GENEPOP’ 007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources. 2008;8:103–106. doi: 10.1111/j.1471-8286.2007.01931.x. [DOI] [PubMed] [Google Scholar]
- Santos M, Iriarte PF, Cespedes W, Balanya J, Fontdevila A, Serra L. Swift laboratory thermal evolution of wing shape (but not size) in Drosophila subobscura and its relationship with chromosomal inversion polymorphism. Journal of Evolutionary Biology. 2004;17:841–855. doi: 10.1111/j.1420-9101.2004.00721.x. [DOI] [PubMed] [Google Scholar]
- Service M. Mosquito ecology field sampling methods. 2nd ed. Elsevier Applied Science; New York: 1993. [Google Scholar]
- Sharakhov I, Braginets O, Grushko O, Cohuet A, Guelbeogo WM, Boccolini D, Weill M, Costantini C, Sagnon N, Fontenille D, Yan G, Besansky NJ. A microsatellite map of the African human malaria vector Anopheles funestus. Journal of Heredity. 2004;95:29–34. doi: 10.1093/jhered/esh011. [DOI] [PubMed] [Google Scholar]
- Simard F, Ayala D, Kamdem G, Pombi M, Etouna J, Ose K, Fotsing J-M, Fontenille D, Besansky N, Costantini C. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecology. 2009;9:17. doi: 10.1186/1472-6785-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff A. Morphological variation in natural and experimental populations of Drosophila pseudoobscura and Drosophila persimilis. Evolution. 1966;20:49. doi: 10.1111/j.1558-5646.1966.tb03342.x. [DOI] [PubMed] [Google Scholar]
- Soto I, Cortese M, Carreira V, Folguera G, Hasson E. Longevity differences among lines artificially selected for developmental time and wing length in Drosophila buzzatii. Genetica. 2006;127:199–206. doi: 10.1007/s10709-005-3638-y. [DOI] [PubMed] [Google Scholar]
- Stalker HD, Carson HL. An altitudinal transect of Drosophila robusta Sturtevent. Evolution. 1948;2:295–305. doi: 10.1111/j.1558-5646.1948.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Tchuinkam T, Simard F, Lele-Defo E, Tene-Fossog B, Tateng-Ngouateu A, Antonio-Nkondjio C, Mpoame M, Toto JC, Njine T, Fontenille D, Awono-Ambene HP. Bionomics of Anopheline species and malaria transmission dynamics along an altitudinal transect in Western Cameroon. Bmc Infectious Diseases 10. 2010 doi: 10.1186/1471-2334-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanVoorhies WA. Bergmann size clines: A simple explanation for their occurrence in ectotherms. Evolution. 1996;50:1259–1264. doi: 10.1111/j.1558-5646.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Weber K, Eisman R, Higgins S, Morey L, Patty A, Tausek M, Zeng Z. An analysis of polygenes affecting wing shape on chromosome 2 in Drosophila melanogaster. Genetics. 2001;159:1045–1057. doi: 10.1093/genetics/159.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KE. Selection on wing allometry in Drosophila melanogaster. Genetics. 1990;126:975–989. doi: 10.1093/genetics/126.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, McKechnie SW, Hoffmann AA. Dissecting adaptive clinal variation: markers, inversions and size/stress associations in Drosophila melanogaster from a central field population. Ecology Letters. 2002;5:756–763. [Google Scholar]
- Wondji C, Frederic S, Petrarca V, Etang J, Santolamazza F, Della Torre A, Fontenille D. Species and populations of the Anopheles gambiae complex in Cameroon with special emphasis on chromosomal and molecular forms of Anopheles gambiae s.s. Journal of Medical Entomology. 2005;42:998–1005. doi: 10.1093/jmedent/42.6.998. [DOI] [PubMed] [Google Scholar]
- Yadav JP, Singh BN. Population genetics of Drosophila ananassae: inversion polymorphism and body size in Indian geographical populations. Journal of Zoological Systematics and Evolutionary Research. 2003;41:217–226. [Google Scholar]
- Yadav JP, Singh BN. Evolutionary genetics of Drosophila ananassae. I. Effect of selection on body size and inversion frequencies. J. Zool. Syst. Evol. Res. 2006;44:323. [Google Scholar]
- Zhou G, Munga S, Minakawa N, Githeko AK, Yan G. Spatial relationship between adult malaria vector abundance and environmental factors in western Kenya highlands. American Journal of Tropical Medicine and Hygiene. 2007;77:29–35. [PubMed] [Google Scholar]
- Zimmerman E, Palsson A, Gibson G. Quantitative trait loci affecting components of wing shape in Drosophila melanogaster. Genetics. 2000;155:671–683. doi: 10.1093/genetics/155.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Test of repeatability for landmark measurements. All individuals were measured twice. Estimates of landmarks correlation between both measures were obtained by comparing aligned coordinates X and Y (after Procustes analysis) as in (Arnqvist and Martensson, 1998). Size correlation between both measures was analyzed using the centroid size (CS correlation = 0.999). Landmark numbers correspond to positions showed in Figure 1.