Abstract
Between 1/1/02 and 12/31/07, our center performed 1687 adult renal transplants. A retrospective analysis was performed to compare outcomes between patients receiving alemtuzumab (n=632) and those receiving either basiliximab (n=690) or thymoglobulin (n=125). Patients receiving alemtuzumab were younger (49 vs. 51 years, p=0.02), had fewer HLA matches (1.7 vs. 2.0, p<0.0001), were more likely to have a CMV donor(+)/recipient(−) transplant (22% vs. 17%, p=0.03) and were less likely to receive a living donor allograft (32% vs. 37%, p=0.04). Alemtuzumab recipients were less likely to receive tacrolimus (35% vs. 47%, p<0.0001). The 1-, 3-, and 5-year cumulative incidence of antibody-mediated rejection (AMR) in alemtuzumab-treated patients was 19%, 24%, and 27%, versus 11%, 15%, and 18% for the other group (p<0.0001). The 1-, 3-, and 5-year allograft survival in the alemtuzumab group was 88%, 75%, and 67%, versus 91%, 82%, and 74% for the other group (p<0.0001). Patient survival was equivalent. Alemtuzumab was an independent risk factor for living donor allograft loss (HR 2.0, p=0.004), opportunistic infections (HR 1.3, p=0.01), CMV infections (HR 1.6, p=0.001), and AMR (HR 1.5, p=0.002). The significantly worse graft survival in the alemtuzumab cohort may be due to the increased rates of AMR and infectious complications.
Keywords: alemtuzumab, anti-thymocyte globulin, basiliximab, renal transplantation
INTRODUCTION
Alemtuzumab, a humanized rat monoclonal antibody to CD-52, has enjoyed extensive off-label use as an induction agent in solid organ transplantation(1). CD-52 antigen is present on the cell surface of T-cells, B-cells, NK cells, macrophages and monocytes(2). After administration of alemtuzumab, peripheral cells experience near-immediate profound depletion(3). Reconstitution of the varying cell lines follows a disparate course, with T-cell levels depleted for more than one year(4, 5). Both non-human primate and human studies have suggested an immunologic benefit associated with T-cell depletion in terms of decreased rejection and prolonged graft survival(6, 7). Furthermore, non-human primate models have shown tolerance induction in association with lymphocyte depletion(2, 8). These early results prompted us to perform our own investigations of the utility of alemtuzumab as an induction agent in renal transplantation. Favorable results (9, 10) prompted us to adopt alemtuzumab as our standard induction agent. Subsequent internal quality control data prompted us to return to an induction regimen using either the non-depleting CD-25 antibody, basiliximab, or rabbit anti-thymocyte globulin (ATG). CD-25 is an IL-2 receptor on the cell surface on T-cells, and binding of CD-25 eliminates T-cell activation by IL-2. ATG is a polyclonal rabbit anti-human T-cell antibody preparation. Because of perceived efficacy, ATG was preferentially utilized in patients of higher immunologic risk.
Recently, a prospective, open-label, randomized, multicenter, controlled trial which enrolled 501 patients between May 2005 and February 2006 studied the outcomes of alemtuzumab induction versus ATG or basiliximab in a steroid minimization regimen in renal transplantation(11). The authors concluded that the rate of biopsy-confirmed acute rejection was lower in patients receiving alemtuzumab induction and that “adverse event rates were similar” among the treatment groups. As our center had accumulated experience with over 600 patients receiving alemtuzumab induction over a similar time period, and because our clinical experience was not consistent with this report, we retrospectively compared our results using alemtuzumab induction with the alternative regimen consisting of basiliximab or ATG in adult, single-organ, renal transplantation.
PATIENTS AND METHODS
A retrospective review of all adult, single-organ, renal transplants (n=1687) performed between January 1, 2002 and December 31, 2007 was conducted utilizing the University of Wisconsin prospectively-collected transplant database. The study was conducted under Institutional Review Board approval. Patients on minimization or desensitization protocols, as well as those receiving HLA-identical organs, were excluded from review. In general, during the first two years of the study period recipients underwent induction with alemtuzumab (Campath-1H, ILEX, n=632). During the latter half of the study period, patients of higher immunologic risk (high PRA, retransplants, African-American race) underwent induction with anti-thymocyte globulin (Thymoglobulin, Genzyme, n=125), and those of lower risk with basiliximab (Simulect, Novartis, n=690). During the first six months of alemtuzumab use, patients underwent induction with two doses given during the transplant hospitalization (n=125). This was later decreased to a single dose of 30 mg at the time of transplant. ATG was administered at 0.75-1.5 mg/kg/day starting at the time of transplant for a total dose of 6-8 mg/kg. Basiliximab was administered as a 20 mg dose on post-operative day (POD) #0 and POD #4. All patients received dexamethasone or methylprednisilone and mycophenolate mofetil (CellCept, Roche) at the time of transplant. The timing of the institution of calcineurin inhibitors was decided by individual surgeons, but, in general, calcineurin inhibitors were started when the recipient’s creatinine fell below 3.0 mg/dL. During the study period, our group transitioned to a regimen that preferentially utilized tacrolimus (Prograf, Fujisawa, n=608) for a calcineurin inhibitor, although cyclosporine (Neoral, Novartis) continued to be used at the discretion of the surgeon (n=608). Calcineurin inhibitor therapy was not utilized in 231 cases (alemtuzumab group n=118, anti-thymocyte globulin /basiliximab group n=113). Maintenance immunosuppressive therapy typically consisted of a calcineurin inhibitor and either mycophenolate mofetil or mycophenolic acid (Myfortic, Novartis). In the alemtuzumab group, cyclosporine levels were typically maintained 50 ng/ml lower than in the other group, and tacrolimus levels were maintained 2-3 ng/ml lower. In the alemtuzumab group, steroids were rapidly tapered during the transplant hospitalization, and were continued on an outpatient basis at a low dose (5 to 7.5 mg/day). In the other group, steroids were tapered during the transplant hospitalization to prednisone 30 mg/day. This dose was tapered further over the first post-operative months to a baseline of 5-10 mg/day.
Cytomegalovirus (CMV) prophylaxis consisted of valganciclovir for CMV-negative recipients of CMV-positive donor organs and those receiving thymoglobulin induction, and acyclovir for all other donor-recipient combinations for three months. Trimethoprim/sulfamethoxazole 160 mg/800 mg daily for one year was used for pneumocystis carinii prophylaxis. Mucosal candidiasis prophylaxis was with oral nystatin or clotrimazole tablets for three months.
Standard CDC cross-matching was used throughout the study period. Biopsies were performed in patients with a creatinine elevated at least 20% above baseline, and were scored using hematoxylin and eosin staining per the Banff criteria (12). C4d staining was utilized to diagnose antibody-mediated rejection (AMR) when clinically indicated. Renal allograft failure was defined as a return to dialysis, renal retransplantation, or death with a functioning graft. Graft losses ascribed to rejection were confirmed histologically.
Rates of rejection, rates of infection, and patient and graft survival were estimated by Kaplan-Meier analysis. Group comparisons were performed by a log-rank test. Additional factors potentially associated with the outcomes were assessed utilizing a Cox proportional hazards regression model. Statistical analysis was performed with SAS software. P-values <0.05% were considered significant.
Luminex® testing with single-antigen beads was introduced in our center in September, 2004 for patients enrolled in our desensitization program, and was expanded to screen all sensitized patients in May, 2005. Pre-transplant Luminex® screening for all patients began in July, 2008 and does not apply to this study population. For the purpose of this study, AMR was defined as positive staining for C4d, since donor-specific antibody (DSA) was not available in the early time period of the study.
Mean follow-up in the alemtuzumab group was 4.2 years and was 3.2 years in the other group.
RESULTS
Demographics
As shown in Tables 1 and 2, patients receiving alemtuzumab induction (n=632) were similar to the other group receiving either basiliximab (n=690) or anti-thymocyte globulin (n=125) in regards to gender, race, body-mass index (BMI), duration of end-stage renal disease (ESRD), type of dialysis, history of diabetes, number of retransplants, CMV status, peak PRA, and creatinine at discharge. There were no differences in donor age, gender, race, or CMV status.
Table 1.
Demographics
| Alemtuzumab (n=632) |
Other (n=815) |
p-value | |
|---|---|---|---|
| Mean | |||
| Age (years) | 49.2±13.1 | 51.1±13.0 | 0.01 |
| BMI (kg/m2) | 27.4±5.8 | 27.7±5.2 | 0.09 |
| Caucasian (%) | 80.1 | 83.2 | 0.13 |
|
CMV donor+/
recipient− (%) |
22.4 | 17.1 | 0.03 |
| CMV negative (%) | 42.9 | 42.0 | 0.79 |
| Diabetes (%) | 28.6 | 25.3 | 0.17 |
|
Disease duration
(years) |
16.8±11.8 | 16.9±12.4 | 0.87 |
| Living donor (%) | 31.7 | 36.7 | 0.04 |
| Male (%) | 58.1 | 62.3 | 0.10 |
| PRA (peak) | 8.0±19 | 8.7±19 | 0.05 |
| Retransplant (%) | 24.4 | 24.0 | 0.85 |
|
Tacrolimus
maintenance (%) |
35.3 | 47.2 | <0.0001 |
| Total match | 1.7±1.5 | 2.0±1.5 | <0.001 |
Table 2.
Donor and recipient CMV antibody status
| CMV status | Alemtuzumab | Conventional |
|---|---|---|
| Donor−/recipient− (%) | 20.5 | 22.6 |
| Donor−/recipient+ (%) | 25.2 | 25.9 |
| Donor+/recipient− (%) | 22.4 | 17.1 |
| Donor+/recipient+ (%) | 31.9 | 30.1 |
Patients receiving alemtuzumab were slightly younger (49.2 vs. 51.1, p= 0.02), had fewer HLA matches (1.7 vs. 2.0, p<0.0001), were more likely to have a CMV donor(+)/recipient(−) transplant (22.4% vs. 17.1%, p=0.03) and were less likely to receive a living donor allograft(31.7% vs. 36.7%, p=0.04). Although there were no significant differences in steroid or mycophenolate use, alemtuzumab recipients were less likely to receive tacrolimus (35.3% vs.47.2%, p<0.0001).
Patient and graft survival
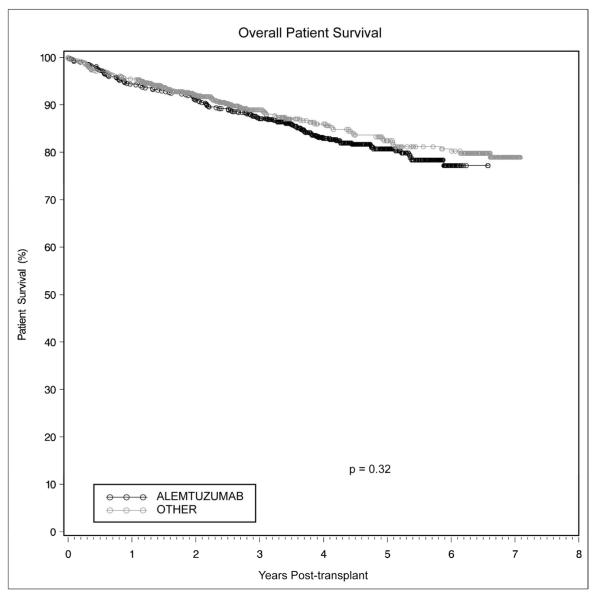
There was no difference noted in overall patient survival between the two groups. Kaplan-Meier estimates of 1-, 3-, and 5-year patient survival in the alemtuzumab group were 94.3%, 87.1% and 80.7%, versus 95.4%, 89.0%, and 82.4% for the other group (Figure 1, p=0.32). There likewise was no difference when the groups were stratified by primary versus non-primary transplants, as well as deceased versus living donor transplants. On multivariate analysis of patient survival, the degree of matching at the DR locus (HR 0.80, p=0.04) and receipt of a living donor allograft (HR0.44, p=0.0002) proved protective. The two most common causes of death were cardiac-related (n=22 in both groups) and infectious complications (n=33 in the alemtuzumab group, n=17 in the other group, p=0.03).
Figure 1.
Patient survival was equivalent in renal transplant recipients receiving alemtuzumab induction versus conventional immunosuppression
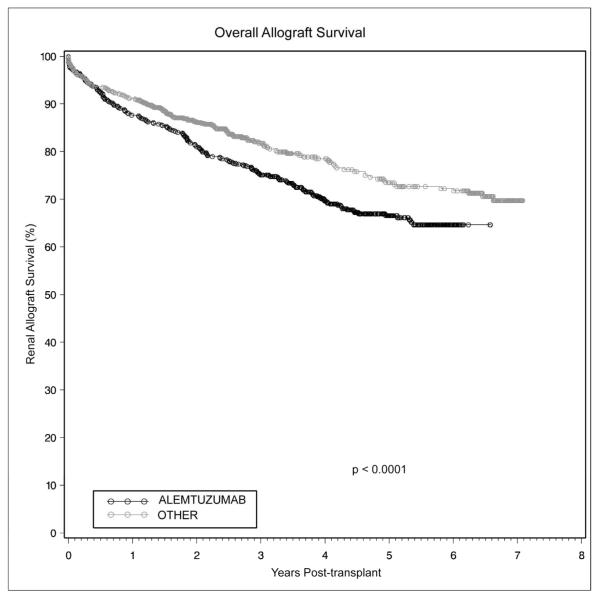
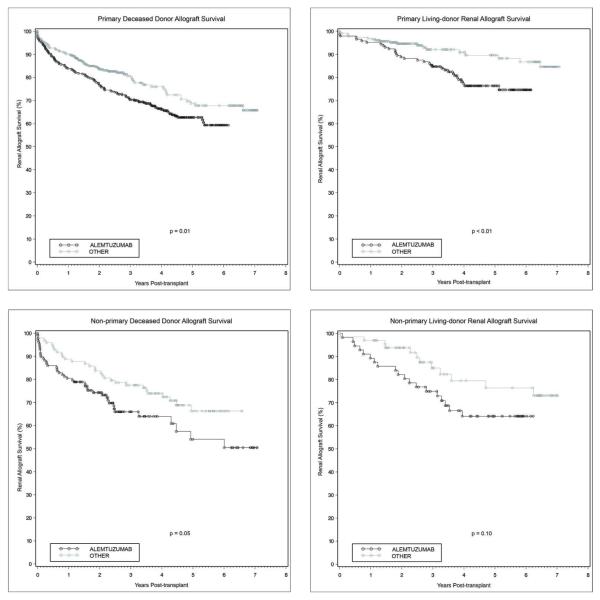
There was significantly lower graft survival in patients receiving alemtuzumab induction at all time points. Kaplan-Meier estimates of 1-, 3-, and 5-year renal allograft survival in the alemtuzumab group were 87.7%, 75.1%, and 66.6%, versus 91.2%, 81.8%, and 73.5% for the other group (Figure 2, p<0.0001). This lower graft survival remained significant when the groups were stratified by primary transplants with deceased versus living donors (Figures 3a and 3b), as well as non-primary transplants with deceased donors (Figure 3c). Although a similar trend was noted with non-primary living donor transplants, this did not reach statistical significance (Figure 3d, p=0.10). On multivariate analysis of overall graft survival, CMV donor(+)/recipient(−) transplantation was associated with an increased risk of graft loss (HR 1.28, p=0.05). Receipt of a living donor allograft (HR 0.55, p= 0.001) and matching at the DR locus (HR 0.82, p=0.02) proved protective. However, on multivariate analysis specific to living donor renal allograft survival, alemtuzumab induction emerged as a significant independent risk factor for graft loss (HR 2.00, p=0.004). In this particular group, CMV donor(+)/recipient(−) transplantation remained a risk factor for graft loss (HR 1.04, p=0.01). Alemtuzumab was not found to be an independent risk factor for allograft loss on multivariate analysis of deceased donor graft survival. The most common cause of graft loss was death with a functioning graft (alemtuzumab n=80, other n=62), followed by chronic rejection (alemtuzumab n=34, other n=20) and acute rejection (alemtuzumab n=31, other n=32). Tacrolimus maintenance immunosuppression was not an independent predictor of renal allograft loss (HR 0.798, p=0.061).
Figure 2.
Overall renal allograft survival was significantly worse with alemtuzumab induction compared to alternative, contemporary immunosuppression
Figures 3.
a-d. Renal allograft survival was significantly worse with alemtuzumab induction in primary deceased donor (a), primary living donor (b), non-primary deceased donor (c), and non-primary living donor (d) renal allografts
Rejection
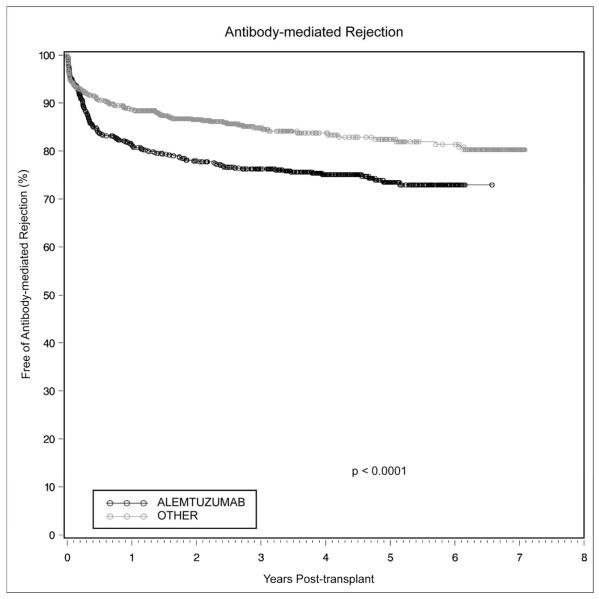
There were no significant differences in overall episodes of biopsy-proven rejection between the two groups (p=0.82). There was a significantly increased incidence of antibody-mediated rejection, diagnosed by C4d staining on biopsy, in patients receiving alemtuzumab induction. As shown in Figure 4, the 1-, 3-, and 5-year cumulative incidence of antibody-mediated rejection in alemtuzumab-treated patients was 18.8%, 23.8%, and 26.5%, versus 11.3%, 15.2%, and 17.6% for the other group (p<0.0001). On multivariate analysis of biopsy-proven antibody-mediated rejection, alemtuzumab was an independent predictor of rejection (HR 1.55, p=0.002).
Figure 4.
Antibody-mediated rejection following renal transplantation occurred more commonly with alemtuzumab induction than with conventional immunosuppression
Infectious complications
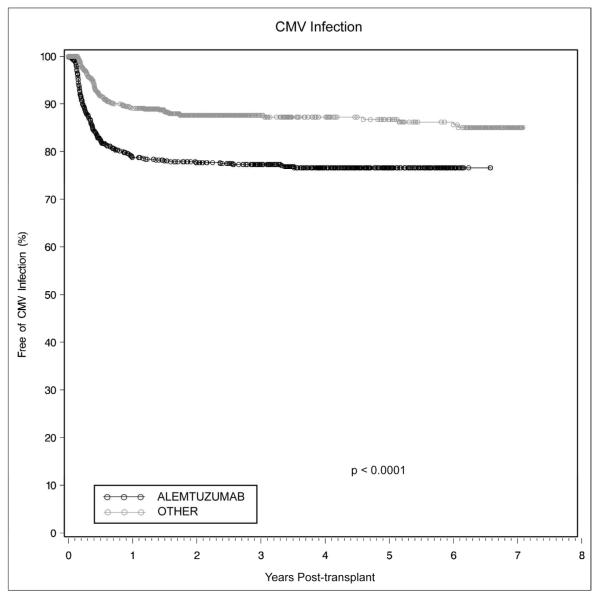
Infectious complications occurred more frequently in patients undergoing alemtuzumab induction. Overall cumulative infection rates (defined as culture, serologic, or pathologic evidence of bacterial, viral, or fungal infection at any site) at 1, 3, and 5 years in alemtuzumab-treated patients was 64.7%, 75.8%, and 80.3%, as compared to 58%, 69.1%, and 77.4% (p=0.04). Opportunistic infections and CMV infections (diagnosed serologically or pathologically) occurred with greater frequency in the alemtuzumab cohort. Cumulative 1-, 3-, and 5-year opportunistic infection rates in alemtuzumab-treated patients were 34.5%, 42.7%, and 45.5%, versus 26.6%, 34.6%, and 37.4% (p<0.001). Cumulative CMV infection rates in alemtuzumab-treated patients were 21.3% and 22.7% at 1 and 3 years versus 10.8% and 12.4% in the other group (Figure 5, p<0.0001). There was no difference in the frequency of non-candidal fungal infections (p=0.99).
Figure 5.
CMV infection occurred more commonly in renal transplant recipients undergoing alemtuzumab induction as compared to those receiving conventional immunosuppression
Leukopenia
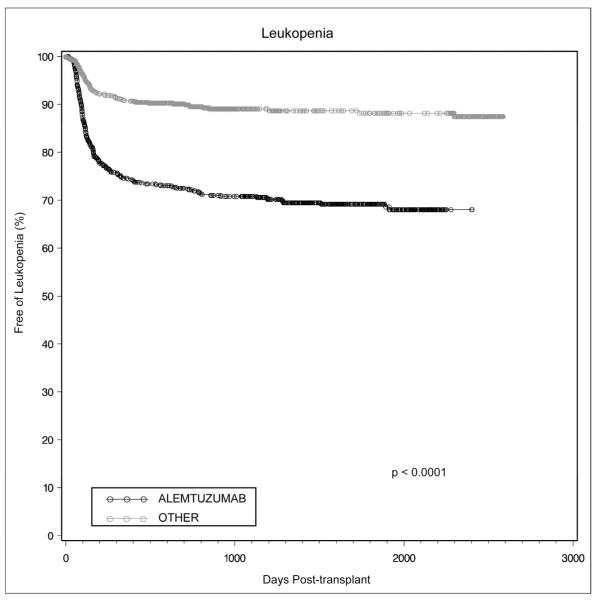
Patients receiving alemtuzumab induction had significantly lower white blood cell counts at 1, 3, 6, 12, and 24 months (p<0.0001). Leukopenia, defined as a white blood cell (WBC) count < 1500 cells/μl, developed more often in patients receiving alemtuzumab induction, with a cumulative incidence of 25.4%, 29.3%, and 30.9% at 1, 3 and 5 years versus 9.2%, 11.0%, and 11.9% for the other group (Figure 6, p<0.0001). Leukopenia developed as late as 3 years following alemtuzumab induction.
Figure 6.
Leukopenia in renal transplant recipients was more common following alemtuzumab induction and continued to develop more than 3 years post-transplant
Maintenance immunosuppression
Patients receiving alemtuzumab induction were less likely to be maintained on tacrolimus than on cyclosporine. In both cases, mean calcineurin inhibitor levels were lower in the alemtuzumab group than in the other group over the first year. The mean cyclosporine level in the alemtuzumab group was 118 ng/ml, versus 141 ng/ml in the other group (p<0.0001). A similar trend was noted with patients maintained on tacrolimus, with mean levels of 6.6 ng/ml in the alemtuzumab group as compared to 6.9 ng/ml in the other group (p=0.01).
Multiple doses of alemtuzumab
There was no difference in patient (p=0.73) or allograft (p=0.59) survival between patients who received one dose of alemtuzumab versus two doses. Similarly, there were no differences in rates of biopsy-proven rejection, antibody-mediated rejection, opportunistic infection, CMV infection, or fungal infection.
DISCUSSION
Alemtuzumab induction has been associated with a durable, sustained suppression of a number of components of the immune system, with a lesser to absent suppression of the humoral response(13-16). However, it is unclear whether this sustained immunosuppressive effect translates into improved patient and allograft survival. A recent randomized multicenter trial demonstrated decreased rates of acute rejection in low risk patients undergoing renal transplantation with alemtuzumab induction as compared to basiliximab(11). No difference in patient or graft survival was noted. Although the group in which there was a perceived benefit of alemtuzumab induction (low risk recipients) had a statistically significant increased rate of serious infections, it was concluded that adverse events rates were similar. The maintenance immunosuppression did not include steroids. Rates of antibody-mediated rejection were not reported.
Due to an institutional shift in the type of induction agent, two cohorts of patients have emerged for analysis in our center. Alemtuzumab was employed as standard induction for renal transplant recipients between 2002 and 2004. After 2004, the standard regimen changed to utilize basiliximab for low-risk recipients and ATG for high-risk recipients.
The sustained immunosuppressive effect of alemtuzumab is a reasonable explanation for the increased infectious complications seen in renal transplant patients undergoing alemtuzumab induction, with overall, opportunistic, and CMV infections all occurring with greater frequency in this group. This occurred despite near-identical prophylactic regimens in both groups, and likely is reflective of the increased rates of leukopenia seen in the alemtuzumab group. The elevated rate of CMV infection may be secondary to inadequate viral prophylaxis in the alemtuzumab group, and may have been lower had all patients undergoing alemtuzumab induction received long-duration valganciclovir. Our protocol limited valganciclovir to CMV-negative recipients of CMV-positive donor organs, or to those patients undergoing induction with thymoglobulin. Another report, in which all recipients were treated with valganciclovir, did not note an increased rate of CMV infection associated with alemtuzumab(17).
More concerning, however, is the patient survival data. Although there was no statistically significant difference in overall patient survival, the rates of infectious causes leading to patient death was notably higher in the alemtuzumab induction group.
Although the infectious complications suggest a net state of over-immunosuppression, the alemtuzumab cohort suffered worse overall graft survival. This cohort also experienced a significantly higher rate of antibody-mediated rejection, a likely explanation for the overall worse allograft survival.
One limitation of this retrospective study was that the cohort of patients undergoing alemtuzumab induction was not identical to the cohort of patients receiving either basiliximab or ATG induction. The alemtuzumab cohort had a somewhat greater HLA mismatch, had a higher ratio of CMV donor(+)/recipient(−) transplants, and was less likely to receive tacrolimus maintenance therapy. The latter represents an institutional time bias, as in the early time points in the study, our preferred calcineurin inhibitor was cyclosporine, and with increasing experience with tacrolimus, this replaced cyclosporine as our routine calcineurin inhibitor. Multivariate analysis, however, demonstrated that tacrolimus maintenance therapy did not prove to be an independent risk factor for patient and graft survival. Graft survival remained superior in the non-alemtuzumab cohort even when controlling for tacrolimus versus cyclosporine. Although overall CMV infection was more common in patients undergoing alemtuzumab induction, when this was stratified by all possible donor-recipient combinations, the only combination to reach statistical significance was in the CMV donor(+)/recipient(+) match. In this case, 30.7% of patients undergoing alemtuzumab induction developed CMV infection versus 12.4% of the other group (p<0.0001). It is possible that this is secondary to inadequate CMV prophylaxis in this particular group, and that treatment with valganciclovir could have prevented this outcome. The differential degree of HLA matching is of unclear etiology, and does not represent any institutional changes in organ allocation, but perhaps is representative of a higher proportion of deceased donors in the alemtuzumab cohort.
A further confounding factor, especially given the high rate of antibody-mediated rejection in patients undergoing alemtuzumab induction, was the absence of adequate class II crossmatch capabilities. Our center relied upon the CDC crossmatch for these patients. Luminex® testing was initially utilized in our desensitization program in 2004, and was gradually expanded to sensitized patients in 2005. Luminex testing was not utilized in pre-transplant patient screening during the study period. Nonetheless, this deficiency would be expected to affect all groups equally.
Although these results must be interpreted within the context of a retrospective study, with some significant demographic differences between the treatment groups, this study presents a large cohort of alemtuzumab-treated recipients with the longest follow-up period reported to date. Furthermore, there is nearly complete follow-up with our recipients.
There are a number of potential explanations for the differences noted in outcomes between our study and those of the multicenter trial cited earlier. The two primary differences between our study and the multicenter trial are the increased rate of allograft loss in patients receiving alemtuzumab and the equivalent rate of acute rejection between groups in our study. It must be noted that the patient groups between studies are slightly different, as our group included donation after cardiac death donors, expanded criteria donors, and overall a higher proportion of deceased donors. These factors would be expected to increase the rate of allograft loss. Importantly, all patients in our study were maintained on triple maintenance immunosuppression. A higher rate of late rejection was noted in patients undergoing alemtuzumab induction in the multicenter trial. This may be due to the lack of maintenance steroids, or could be secondary to antibody-mediated rejection. Their study is consistent with our finding of higher infectious complications in patients receiving alemtuzumab, but this was limited to patients of lower immunologic risk in their analysis.
There are a number of disparate results from other centers utilizing alemtuzumab induction. The Miami group reported on 90 patients, undergoing primary renal transplantation with either alemtuzumab, thymoglobulin, or daclizumab induction(17). Maintenance immunosuppression was limited to mycophenolate and low-dose tacrolimus (4-7 ng/ml) in the alemtuzumab group, and included mycophenolate, higher dose tacrolimus (8-10 ng/ml), and steroids in the other groups. They noted no differences in patient or allograft survival, or in rejection rates, at a median follow-up of 15 months. They did, however, note the association between alemtuzumab and leukopenia. All patients were given intravenous ganciclovir and outpatient valganciclovir for three months, and only one patient developed CMV. This study did not note an association between alemtuzumab induction and antibody-mediated rejection, as only 2-3 patients per group had C4d-positive staining on biopsy.
A larger study in renal transplantation from Pittsburgh utilized tacrolimus monotherapy in 90 patients receiving alemtuzumab and 101 patients receiving thymoglobulin(13). The outcomes from these two groups were compared to 152 historical controls undergoing standard triple immunosuppressive maintenance therapy. No difference was noted in patient or allograft survival. Overall rejection was more common with thymoglobulin induction and, like the recent trial, late rejection was noted to occur more frequently with alemtuzumab. Rejection episodes seemed to correlate with efforts to minimize maintenance immunosuppression. Follow-up in the alemtuzumab group was limited to 12-18 months.
The group from Northwestern performed a retrospective database analysis comparing 123 patients receiving alemtuzumab to 155 patients receiving basiliximab induction in a steroid-free protocol in renal transplantation(18). Maintenance therapy consisted of mycophenolate mofetil and tacrolimus. No difference in patient or graft survival was noted, and rejection rates were equivalent at 1 year (although rejection tended to occur earlier in the basiliximab group). The mean follow-up was 33 months. Although the results did not reach statistical significance, the patient and graft survival curves tended to be lower in the alemtuzumab cohort. Similarly to the above studies, this report did not specifically investigate rates of AMR.
Although the smaller, single-center studies show equivalent results in regards to rejection rates, a review of the Organ Procurement and Transplantation Network (OPTN) database of over 14,000 patients who received deceased donor renal allografts in 2003 and 2004 showed alemtuzumab to be associated with less early rejection, but higher rejection rates at 6 months and 1 year as compared to thymoglobulin, interleukin-2 receptor antibody, or no induction(19). Although the unadjusted graft survival curves showed alemtuzumab to be associated with lower graft survival, it was not an independent risk factor for graft loss on multivariate analysis.
The plethora of minimization studies utilizing alemtuzumab suggest that the durable immunosuppression and leukopenia associated with alemtuzumab induction encourage centers to limit long-term exposure to steroids and high-dose calcineurin inhibitors. In our center, a deliberate attempt was made to limit the dose of both steroids and calcineurin inhibitors. This effort was met with an unacceptably high rate of antibody-mediated rejection. The large sample size and duration of follow-up are one potential explanation for the elevated rate of rejection seen in our study (as well as in the OPTN database review) that was not noted in smaller, single-center reports.
Although we did not note a difference in episodes of acute rejection between groups, there was a markedly decreased graft survival in patient receiving alemtuzumab induction. In multivariate analysis of living donor renal transplant recipients, alemtuzumab proved to be an independent risk factor for graft loss. While there were a number of real factors that disadvantaged the alemtuzumab cohort in terms of graft survival (slightly older donors, minimal differences in HLA-matching, lower numbers of patients receiving tacrolimus, increased CMV mismatches, and fewer living donors), it is likely that the majority of this difference is accounted for by the increased rates of infectious complications (in particular, death from infection causes) and episodes of antibody-mediated rejection.
Alemtuzumab has clear benefits in terms of cost and ease of administration and it has been used with reasonable outcomes in many centers. Nonetheless, as used in our center, there were clearly worse overall outcomes. Based on our internal analysis of this data, we have abandoned the use of alemtuzumab induction in favor of a regimen consisting of basiliximab for low-risk recipients and thymoglobulin for high-risk recipients.
ACKNOWLEDGEMENTS
The authors wish to thank Barbara Voss and Glen Leverson for assistance in the analysis of data and the preparation of this manuscript.
Funding sources: None
Footnotes
Conflicts of interest: The authors of this manuscript have no conflicts of interest to disclose as described by Transplant International.
REFERENCES
- 1.Magliocca JF, Knechtle SJ. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006 Sep;19(9):705–14. doi: 10.1111/j.1432-2277.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 2.Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990 Mar;35(3):118–27. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003 Jul 15;76(1):120–9. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 4.Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006 Jan 15;81(1):81–7. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- 5.Knechtle SJ, Pirsch JD, H. Fechner JJ, Becker BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003 Jun;3(6):722–30. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 6.Knechtle SJ, Vargo D, Fechner J, Zhai Y, Wang J, Hanaway MJ, et al. FN18-CRM9 immunotoxin promotes tolerance in primate renal allografts. Transplantation. 1997 Jan 15;63(1):1–6. doi: 10.1097/00007890-199701150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JM, Neville DM, Contreras JL, Eckhoff DE, Meng G, Lobashevsky AL, et al. Preclinical studies of allograft tolerance in rhesus monkeys: a novel anti-CD3-immunotoxin given peritransplant with donor bone marrow induces operational tolerance to kidney allografts. Transplantation. 1997 Jul 15;64(1):124–35. doi: 10.1097/00007890-199707150-00022. [DOI] [PubMed] [Google Scholar]
- 8.Hale G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy. 2001;3(3):137–43. doi: 10.1080/146532401753174098. [DOI] [PubMed] [Google Scholar]
- 9.Knechtle SJ, Fernandez LA, Pirsch JD, Becker BN, Chin LT, Becker YT, et al. Campath-1H in renal transplantation: The University of Wisconsin experience. Surgery. 2004 Oct;136(4):754–60. doi: 10.1016/j.surg.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Barth RN, Janus CA, Lillesand CA, Radke NA, Pirsch JD, Becker BN, et al. Outcomes at 3 years of a prospective pilot study of Campath-1H and sirolimus immunosuppression for renal transplantation. Transpl Int. 2006 Nov;19(11):885–92. doi: 10.1111/j.1432-2277.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011 May 19;364(20):1909–19. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 12.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999 Feb;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro R, Basu A, Tan H, Gray E, Kahn A, Randhawa P, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg. 2005 Apr;200(4):505–15. doi: 10.1016/j.jamcollsurg.2004.12.024. quiz A59-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson CJ, Bradley JA, Friend PJ, Firth J, Taylor CJ, Bradley JR, et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation--efficacy and safety at five years. Am J Transplant. 2005 Jun;5(6):1347–53. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 15.Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roohipour R, Carreno MR, et al. The use of Campath-1H as induction therapy in renal transplantation: preliminary results. Transplantation. 2004 Aug 15;78(3):426–33. doi: 10.1097/01.tp.0000128625.29654.eb. [DOI] [PubMed] [Google Scholar]
- 16.Cai J, Terasaki PI, Bloom DD, Torrealba JR, Friedl A, Sollinger HW, et al. Correlation between human leukocyte antigen antibody production and serum creatinine in patients receiving sirolimus monotherapy after Campath-1H induction. Transplantation. 2004 Sep 27;78(6):919–24. doi: 10.1097/01.tp.0000134398.86243.81. [DOI] [PubMed] [Google Scholar]
- 17.Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005 Aug 27;80(4):457–65. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman DB, Leventhal JR, Axelrod D, Gallon LG, Parker MA, Stuart FP. Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: comparison with basiliximab induction--long-term results. Am J Transplant. 2005 Oct;5(10):2539–48. doi: 10.1111/j.1600-6143.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang E, Cho YW, Hayashi R, Bunnapradist S. Alemtuzumab induction in deceased donor kidney transplantation. Transplantation. 2007 Oct 15;84(7):821–8. doi: 10.1097/01.tp.0000281942.97406.89. [DOI] [PubMed] [Google Scholar]