Abstract
Objective
Irradiation of the chest or chest wall has been shown to caause calcific aortic stenosis. However, the mechanisms are unknown. Aortic valve interstitial cells (AVICs) have been implicated in the pathogenesis of aortic stenosis; they have been shown to change from the phenotype of a myofibroblast to an osteoblast-like cell. We therefore hypothesized that irradiation of human AVICs induces an osteogenic phenotype. In isolated human AVICs, our purpose was to determine the effect of irradiation on the production of osteogenic factors: (a) bone morphogenetic protein-2 (BMP-2) (b) osteopontin (OPN) (c) alkaline phosphatase (ALP), and (d) the transcription factor Runx2.
Methods
Human AVICs were isolated from normal aortic valves obtained from explanted hearts of patients undergoing cardiac transplantation (n=4) and grown in culture. The cells were grown to confluence, irradiated with 10 Gy using a cesium-137 irradiator and then lysed 24 hours following irradiation. Cell lysates were analyzed via immunoblot and densitometry for BMP-2, OPN, ALP and Runx2. Statistics were by ANOVA. P < 0.05 was significant.
Results
Irradiation induced an osteogenic phenotype in human AVICs. Irradiation induced a 2-fold increase in BMP-2, a 7-fold increase in OPN, a 3-fold increase in ALP, and a 2-fold increase in Runx2.
Conclusions
Radiation induces an osteogenic phenotype in human AVICs. The irradiated cells had significantly increased expression of the osteogenic factors BMP-2, OPN, ALP and Runx2. These data offer mechanistic insight into the pathogenesis of radiation-induced valvular heart disease.
Keywords: aortic stenosis, osteogenesis, radiation
INTRODUCTION
The use of radiation to treat malignancies of the chest and chest wall is associated with increased rates of cardiac disease, including valvular disease. The majority of these valvular defects involve the aortic and mitral valves (1–3). Within 20 years following mantle radiation, as many as 16% of patients will develop calcific aortic stenosis; this is at least three times the incidence found in the general population (4). This is particularly significant because many patients that undergo chest radiation therapy are young adults, leading to the development of aortic stenosis at a younger age than commonly seen. In fact, among patients with aortic stenosis, those with a prior history of mantle irradiation develop aortic stenosis at an age that is approximately 20 years younger than those patients with no prior irradiation (2, 5). Furthermore, following chest radiation therapy, the risks of cardiac surgical operations, including aortic valve replacement, are significantly increased (6, 7, 8).
The mechanisms of radiation-induced aortic stenosis are unknown. However, in other tissues radiation has been shown to be inflammatory. The principal cell type found in the human aortic valve, the aortic valve interstitial cell (AVIC), has been implicated in the pathogenesis of calcific aortic stenosis. When stimulated by pro-inflammatory stimuli, isolated human AVICs have been shown to undergo a change in phenotype from that of a myofibroblast to that of an osteoblast-like cell (9, 10). This osteogenic phenotype is characterized by the production of osteogenic factors such as bone morphogenetic protein-2 (BMP-2, a protein necessary for bone formation), osteopontin (OPN, an important bone remodeling protein), alkaline phosphatase (ALP, an enzyme important for bone mineralization), and the transcription factor Runx2, which is necessary for bone formation.
Given the recognized pro-inflammatory actions of radiation, we hypothesized that radiation-induced aortic stenosis derives from the induction of an osteogenic phenotype in human AVICs. The purpose of this study was to determine the effect of irradiation on the production of BMP-2, OPN, ALP and Runx2 in isolated human AVICs. The results of this study demonstrate that radiation induces an osteogenic phenotype in the human AVICs.
MATERIAL AND METHODS
This study was approved by the Colorado Multiple Institutional Review Board of the University of Colorado School of Medicine. All patients provided written informed consent.
Chemicals and Reagents
Medium 199 was purchased from Lonza (Walkersville, MD). Rabbit polyclonal antibody against human BMP-2 was purchased from Prosci (Poway, CA) and rabbit poly clonal antibody against ICAM-1 was purchased from AbD Serotec (Raleigh, NC). Protein assay reagents and chemiluminescent substrate (ECL) were purchased from Thermo Scientific (Rockford, IL). 4–20% gradient polyacrylamide ready gels, nitrocellulose membranes and 2X Laemmli sample buffer were purchased from Bio-Rad (Hercules, CA). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell Isolation and Culture
Grossly normal aortic valve leaflets (n=4) were obtained from explanted hearts from patients (males, 28–48 years) undergoing cardiac transplantation at the University of Colorado Hospital. On gross examination, all leaflets were thin, pliable and without calcification. Isolation by collagenase digestion was performed as previously described and AVICs were cultured and maintained as independent cultures in medium 199 with penicillin G, streptomycin, amphotericin B, and 10% fetal bovine serum in an incubator supplied with 5% carbon dioxide (9). The aortic valves were placed in 4°C sterile saline immediately after removal. The valves were washed with sterile saline three times and then sectioned. Segments were placed into 4% formaldehyde in PBS, flash frozen, or placed in OCT for frozen sections. The remaining sections were washed five times with Earl’s Balanced Salt Solution (EBSS), placed in 2.5 mg/ml collagenase in full medium 199 for 30 minutes and incubated at 37°C. After discarding the supernatant, the valve sections were washed once with EBSS to remove endothelial cells. The valve segments were then placed in 0.8 mg/ml collagenase in full medium 199 and digested for 3 additional hours. The supernatant from this digestion was centrifuged, the pellet resuspended in full medium 199, and the cells were grown in culture (passage zero). Cells from passages 3–6 grown to 70–90% confluence were used for all experiments.
Radiation
To irradiate the cells in culture, 24-well plates of cells were first prepared. These allowed 24 hours of incubation at 37°C with 5% carbon dioxide for 24 hours prior to transport to a Cesium-137 irradiator. The control cells were transported to the radiation suite as well though they were not treated. The experimental cells underwent 10 Gy total irradiation (175 rad/min for 2.85 minutes twice). The cells were then transported back to the incubator until lysis at 24 hours. The cells were checked for morphologic changes prior to lysis, and none were present. The radiation doses were adapted from Williams et al (13).
Immunofluorescence
Slides were prepared with normal and stenotic valve specimen from frozen OCT preparations. These slides were dried overnight, and then fixed in 30% Acetone: 70% Methanol for ten minutes. After three PBS washes for 10 minutes each, the slides were blocked with 10% non-immune donkey serum in PBS for 1 hour. ICAM-1 (AbD Serotec, Raleigh, NC) and BMP-2 (Abcam, Cambridge, MA) primary antibody was diluted in 1% BSA to concentrations of 1:50 and 1:250 respectively. 100µl of this dilution was placed on the mounted tissue and incubated at 4°C overnight in a box with a moist towel. Negative controls were prepared as well, with rabbit IgG only in the solution at the same concentrations. The following morning, the slides were washed in PBS three times. The secondary antibody solution was then prepared with WGA (Alexa 633) 1:500, donkey anti-rabbit (Alexa 488) 1:100 and donkey anti-mouse (Alexa 555) 1:100. 100µl of this solution was placed on the mounted tissue, and the slides were incubated for 45 minutes at room temperature. After three more PBS washes, antiquenching medium containing DAPI (Invitrogen, Grand Island, NY) was placed on the tissue and the coverslips were put in place. The next morning, the slides were sealed and viewed.
Examination of Aortic Valve Leaflets from a Patient with Prior Irradiation
A 56 year-old male with a prior history of mediastinal irradiation underwent heart transplantation at our institution. This provided the opportunity to examine the aortic valve leaflets from his explanted heart. He had no gradient across the aortic valve on pre-transplant echocardiogram. The valve leaflets were grossly normal. The aortic valve leaflets from the explanted heart were examined for BMP-2 expression (immunofluorescence, methodology described above). For comparison, the leaflets from of explanted irradiated valve were compared with age-matched aortic valve leaflets from a normal aortic valve (control) and a stenotic aortic valve from a patient undergoing aortic valve replacement for aortic stenosis with no history of irradiation.
Immunoblotting
Cell lysates were analyzed for BMP-2, OPN, ALP and Runx2 by immunoblotting. AVICs in culture were lysed in 1X Laemmmli sample buffer with β-mercaptoethanol. Lysates were loaded into a 15-well 4–20% gradient ready gels (Bio-Rad) and run at 200 V for 30 minutes. The gels were transferred to nitrocellulose membranes at 100 V for 70 minutes, and then cross-linked twice using a UV Stratalinker (Stratagene, La Jolla, CA). The membranes were blocked in 5% dry milk in 0.1% Tween in PBS (T-PBS), and then rinsed three times in 0.1% T-PBS. The blocked membranes were incubated with primary antibodies overnight at 4°C (diluted to 1:1000 to 1:10,000 in 5% BSA in 0.1% T-PBS). The membranes were washed in 0.1% T-PBS three times and then incubated in appropriate horseradish peroxidase-conjugated secondary antibodies diluted to 1:5000 in 5% dry milk in 0.1% T-PBS for one hour at room temperature. After decanting the secondary antibody, the wash step was repeated and the membranes were placed in ECL for 5 minutes at room temperature and exposed on X-ray film. The film was scanned using an Epson flatbed scanner (Long Beach, CA) and densitometry was performed using Image J software from the NIH.
Statistical Analysis
Data are represented as means ± standard error mean. Statistical analysis was performed using ANOVA; p < 0.05 was significant.
RESULTS
Increased expression of BMP-2 in the aortic valve of a patient with prior mediastinal radiation
A 56 year-old male with a prior history of mantle radiation (lymphoma) underwent heart transplantation at our institution. While the aortic valve leaflets from his explanted heart were grossly normal, histological examination revealed the increased presence of BMP-2. As demonstrated in Figure 1, immunofluorescence staining revealed no evidence of BMP-2 in normal valve leaflets (control). By comparison, leaflets from a patient with calcific aortic stenosis (no history of radiation) demonstrated increased BMP-2 expression. The leaflets from this patient with a history of prior radiation demonstrated significantly increased BMP-2 expression.
Figure 1.
mmunofluorescent staining of aortic valve leaflets. BMP-2 signal is increased (immunofluorescence) for both stenotic and irradiated aortic valve tissue compared to non-stenotic controls. In the upper panels, the red stain is for BMP-2, the blue is for nuclei (DAPI). In the lower panels, the green stain is for cellular architecture (WGA). The control and irradiated leaflets were from grossly normal valves. The stenotic leaflet was from a calcified aortic valve removed at aortic valve replacement. (40× magnification)
Radiation-induced increase expression of BMP-2, OPN, ALP and Runx in human AVICs
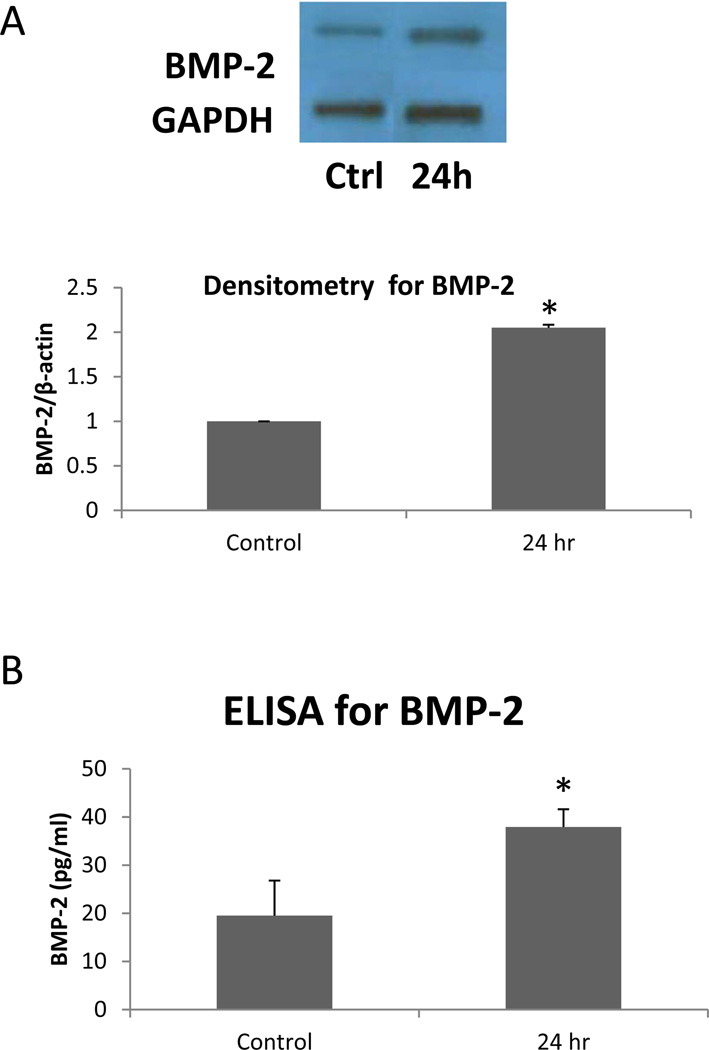
As shown in Figure 2, BMP-2 expression was significantly (two-fold, p<0.05) increased following irradiation. Not only was the protein expression significantly increased, but BMP-2 levels in the cell culture media were significantly increased by two-fold (p<0.05). This is noteworthy because once synthesized within the cell, BMP-2 is secreted by the cell and acts in an autocrine and paracrine fashion. These data indicate that BMP-2 secretion by the AVICs was increased following radiation.
Figure 2.
BMP-2 expression was increased in irradiated human AVICs. A. This representative immunoblot and densitometry shows increased expression in cell lysate 24 hours after irradiation. B. The concentration of BMP-2 is increased at 24 hours following irradiation in cell culture media. * p < 0.05
Other factors associated with osteogenesis were also increased following irradiation. As shown in Figure 3, OPN, ALP and Runx 2 were all significantly increased. Twenty-four hours following irradiation, OPN expression was increased greater than 7-fold in comparison to cells that were not irradiated (p<0.05). The expression of ALP was increased nearly 3-fold (p<0.05). The expression of the transcription factor, Runx2, was increased two-fold following irradiation (p<0.05). Cells that were not removed from the lab showed no difference in comparison with control cells taken to the radiation suite (data not shown).
Figure 3.
The expression of osteopontin, alkaline phosphatase and Runx2 were increased following radiation. Using immunoblotting and densitometry, expression of OPN, ALP and Runx2 was increased at 24 hours following irradiation. * p < 0.05.
DISCUSSION
Radiation-induced valvular disease, particularly aortic stenosis, affects a substantial number of the patients treated with chest radiation (11, 12). The results of the present study demonstrate that irradiation of human AVICs induces an osteogenic phenotype with increased production of the osteogenic factors BMP-2, OPN, ALP and the transcription factor, Runx2. These data offer mechanistic insight into the pathogenesis of radiation-induced calcific aortic stenosis.
In the present study, the effects of radiation were examined in vitro. Others have found that the effects of radiation are even more pronounced in cells in vivo than in culture (13). Such data suggest that the in vivo cellular environment and cell-cell interactions in vivo may augment the effects of irradiation. Additionally, only one dose of radiation was used; it was chosen from an optimization done for cells in culture that does not affect viability (14). It must be acknowledged that different radiation dosages may have other effects. Despite these limitations, however, the results of the present study demonstrate that radiation induces an osteogenic phenotype in isolated human AVICs. Further, the finding of significantly increased expression of BMP-2 in the aortic valve leaflets from a patient with prior mantel irradiation (Figure 1) lends strong support to the in vitro findings of the present study.
Irradiation of isolated cells has previously been shown to have pro-inflammatory effects. Jelonek and colleagues (15) recently examined the effects of radiation on mouse endothelial cells in vitro and in vivo, and demonstrated increases expression of endothelial-specific inflammatory and cell adhesion molecules following irradiation. Ryu and colleagues found that mitogen-activated protein kinases (MAPK), such as c-jun terminal kinase (JNK), extracellular related kinases (ERK) and p38 are up-regulated in the radiation-induced pneumonitis in rats (16). These intracellular signaling cascades have been implicated in the pathogenesis of aortic stenosis (17), and are an example of the mechanistic linkage between inflammation and radiation-induced damage (18).
To our knowledge, this is the first study to examine the effects of radiation on isolated human AVICs. Work from our laboratory has previously demonstrated pro-inflammatory stimulation may induce an osteogenic phenotype in human AVICs (9, 10, 16). Given the pro-inflammatory effects of irradiation (15), if seems possible that the mechanisms by which radiation induces an osteogenic phenotype in human AVICs may involve mechanisms of inflammation.
Cardiac valve disease is a particularly vexing complication of chest radiation therapy. Radiation-associated cardiac valve disease has been described following irradiation of the breast, lung and chest wall. But it is particularly prevalent following mediastinal irradiation: up to 60% of patients develop radiation-associated cardiac valve pathology (1). The increased risks of cardiac surgical operations in patients with a history of prior mediastinal radiation are well-recognized (6, 7). The risks of aortic valve replacement may be increased following mediastinal irradiation in part because the effects of radiation are not confined to the valve. While probably dose-dependent, mediastinal irradiation may result in significant mediastinal fibrosis along with constrictive pericardial disease, restrictive myocardial disease, coronary artery disease and aortic fibrosis. These injuries may culminate in increased cardiac surgical risks in patients with prior mediastinal irradiation. Hence, greater understanding of the mechanisms responsible for radiation-induced aortic stenosis may be an important step in preventing this complication of chest radiation therapy.
In summary, the results of the present study demonstrate that radiation induces an osteogenic phenotype in human aortic valve interstitial cells irradiated in vitro. These findings offer mechanistic insight into the pathogenesis of radiation-induced calcific aortic stenosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 38th Annual Meeting of the Western Thoracic Surgical Association, Maui, Hawaii, June 30, 2012.
REFERENCES
- 1.Heidenriech PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42:743–749. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 2.Wethal T, Lund W-B, Edvardsen T, Fossa SD, Pripp AH, Holte H, Kjekshus J, Fossa A. Br J Cancer. 2009;101:575–581. doi: 10.1038/sj.bjc.6605191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cella L, Liuzzi R, Conson M, Torre G, Caterino M, De Rosa N, Picardi M, Camera L, Solla R, Farella A, Salvatore M, Pacelli R. Dosimetric predictors of asymptomatic heart valvular dysfunction following mediastinal irradiation for Hodgkins lymphoma. Radiother Oncol. 2011;101(2):316–321. doi: 10.1016/j.radonc.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 4.Hull MC, Morris CG, Pepine CJ, Mendenhall NP. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of Hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 5.Poulin F, Seminov A, Romeo P, Demers P, Pressacco J, Basmadjian A. Extensive radiation-induced heart disease in an adult patient treated for lymphoma as a child. Can J Cardiol. 2011;27:390.e1–390.e4. doi: 10.1016/j.cjca.2010.12.048. [DOI] [PubMed] [Google Scholar]
- 6.Crestanello JA, McGregor CGA, Danielson GK, Daly RC, Dearani JA, Orszulak TA, Mullany CJ, Puga FJ, Zehr KJ, Schleck C, Schaff HV. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2004;78:826–831. doi: 10.1016/j.athoracsur.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Suri RM, Burkhart HM, Greason KL, Dearani JA, Schaff HV, Sundt TM. Identifying patients at particular risk of injury during repeat sternotomy: Analysis of 2555 cardiac reoperations. J Thorac Cardiovasc Surg. 2010;140:1028–1045. doi: 10.1016/j.jtcvs.2010.07.086. [DOI] [PubMed] [Google Scholar]
- 8.Gongora E, Dearani JA, Orszulak TA, Schall HV, Zhou L, Sundt TM., III Tricuspid regurgitation in patients undergoing pericardiectomy for constrictive pericarditis. Ann Thorac Surg. 2008;85:163–171. doi: 10.1016/j.athoracsur.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 9.Meng X, Ao L, Song Y, Babu A, Yang X, Wang M, Weyant MJ, Dinarello CA, Cleveland JC, Jr, Fullerton DA. Expression of functional toll-like receptors 2 and 4 in human aortic valve interstitial cells: Potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294:C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Meng X, Weyant MJ, Reece TB, Cleveland JC, Jr, Fullerton DA. Stenotic aortic valves have dysfunctional mechanisms of anti-inflammation: implications for aortic stenosis. J Thorac Cardiovasc Surg. 2011;141(2):481–486. doi: 10.1016/j.jtcvs.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, Fornander T, Gigante B, Jensen MB, Peto R, Rahimi K, Taylor CW, Ewertz M. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Hooning MJ, Botma A, Aleman BMP, Baaijens MHA, Bartelink H, Klijn JGM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 13.Mitra AK, Singh RK, Krishna M. MAP Kinases: Differential activation following in vivo and ex vivo irradiation. Mol Cell Biochem. 2007;294:65–72. doi: 10.1007/s11010-006-9246-z. [DOI] [PubMed] [Google Scholar]
- 14.Williams JR, Zhang Y, Zhou H, Gridley DS, Koch CJ, Dicello JF, Slater JM, Little JB. Tumor response to radiotherapy is dependent on genotype-associated mechanisms in vitro and in vivo. Radiother Oncol. 2010;5:71. doi: 10.1186/1748-717X-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jelonek K, Walaszczyk A, Gabrys D, Pietrowska M, Kanthou C, Widlak P. Cardiac endothelial cells isolated from mouse heart – a novel model for radiobiology. Acta Biochim Pol. 2011;58(3):397–404. [PubMed] [Google Scholar]
- 16.Ryu SY, Do SH, Chung JY, Kim TH, Kim SH, Choi CY, Jeong KS, Park SJ. Activation of MAP kinases during progression of radiation-induced pneumonitis in rats. Hum Exp Toxicol. 2010;30(8):876–883. doi: 10.1177/0960327110382562. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Meng X, Su X, Mauchley DC, Ao L, Cleveland JC, Jr, Fullerton DA. Bone morphogenic protein 2 induces Runx2 and osteopontin expression in human aortic valve interstitial cells: role of Smad1 and extracellular signal-related kinase 1/2. J Thorac Cardiovasc Surg. 2009;138:1008–1015. doi: 10.1016/j.jtcvs.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Jinlian L, Yinbin Z, Chunbo W. p38 MAPK in regulating cellular responses to ultraviolet radiation. J Biomed Sci. 2007;14:303–312. doi: 10.1007/s11373-007-9148-4. [DOI] [PubMed] [Google Scholar]