Abstract
Objective
To evaluate the efficacy and safety of soybean-derived phosphatidylserine (SB-PS) (300 mg/day) in improving cognitive performance in elderly with memory complaints, following a short duration of 12 weeks’ SB-PS administration.
Methods
SB-PS was administered daily for 12 weeks to 30 elderly volunteers with memory complaints (age range 50–90 years). Cognitive performance was determined by a computerized test battery and by the Rey Auditory Verbal Learning Test (Rey-AVLT). Physical examination and blood safety parameters were part of the extensive safety analysis of PS that was performed.
Results
The computerized test results showed that SB-PS supplementation significantly increased the following cognitive parameters: memory recognition (P = 0.004), memory recall (P = 0.006), executive functions (P = 0.004), and mental flexibility (P = 0.01). The Rey-AVLT indicated that, following SB-PS administration, total learning and immediate recall improved significantly (P = 0.013 and P = 0.007, respectively). Unexpected results from the safety tests suggested that SB-PS significantly reduces both systolic (P = 0.043) and diastolic (P = 0.003) blood pressure. SB-PS consumption was well tolerated and no serious adverse events were reported during the study.
Conclusion
This exploratory study demonstrates that SB-PS may have favorable effects on cognitive function in elderly with memory complaints. In addition, the study suggests that SB-PS is safe for human consumption and may serve as a safe alternative to phosphatidylserine extracted from bovine cortex. These results encourage further extended studies in order to establish the safety and efficacy of SB-PS treatment.
Keywords: learning, AAMI, memory, cognitive, phosphatidylserine
Introduction
Since the beginning of the 20th century, life expectancy in most developed countries has been characterized by a steady increase,1 resulting in aging of the population and increasing prevalence of diminished cognitive function and memory performance. Subjective memory complaints are common and strongly associated with age, and are becoming more frequent with age.2 The steady increases in aging and frequency of memory impairment complaints has created the need for substances that will improve cognitive performance and prevent or diminish its deterioration. One possible treatment strategy is based on the association between aging and alterations in brain lipid composition,4 suggesting that phospholipids, which are fundamental components of neuronal membranes, may serve as effective treatment for cognitive deterioration.
Phosphatidylserine (PS) is the major acidic phospholipid in the brain and is mainly present in the inner leaflet of the plasma membrane.5,6 Oral administration of PS has been shown to affect neuronal membranes, cell metabolism, and specific neurotransmitter systems.7–11 Numerous clinical trials6,10,13,14,16 have suggested that PS extracted from bovine cortex (BC-PS) exerts significant benefits to brain functions, especially those functions that tend to decline with age, including memory, learning, and concentration.7 A number of studies have shown that supplementation of BC-PS (300 mg/day) to elderly subjects with intellectual deterioration has resulted in improved cognitive performance.12–16 In addition, BC-PS supplementation has been shown to have positive effects on the cognitive performance of Alzheimer’s disease patients.8,10 However, safety concerns due to the risk of bovine spongiform encephalopathy, commonly known as mad cow disease,17 brought an end to the use of BC-PS and encouraged a search for safe alternative sources.
In the mid-1990s, soybean-derived PS (SB-PS) replaced BC-PS and was examined as a safer alternative.18 A few preclinical studies suggested that administration of SB-PS could benefit cognitive performance in rodents.19–21 To date, some clinical trials have shown that SB-PS is safe for human consumption and may potentially improve cognitive performance. According to Jorissen et al22 and Kato-Kataoka et al,23 Gindin et al was the first to examine the effect of SB-PS on cognitive function of elderly volunteers.24 In addition, based on a preliminary open-label trial,25 Kato-Kataoka et al23 conducted a double-blind clinical trial on 78 elderly Japanese with memory complaints and examined the effect of SB-PS (100 and 300 mg/day) following 6 months of administration and at 3 months’ follow-up. The results of this study demonstrate that SB-PS has a positive effect on cognitive performance, which was especially evident in a subgroup of subjects who had low pretreatment scores. Interestingly, this subgroup demonstrated a significant influence of SB-PS on cognitive function following 6 months’ administration versus baseline, and, at 3-month posttreatment follow-up, there was a significant difference between the SB-PS group and placebo.23 The efficacy of SB-PS was also examined by Schreiber et al.26 In this open-label study, the authors tested the effect of 300 mg/day SB-PS on 18 healthy elderly volunteers, meeting Age Associated Memory Impairment (AAMI) inclusion and exclusion criteria.3 The study showed that those treated with SB-PS had a significant improvement on recall and immediate memory parameters following 6 weeks’ administration, an effect that was maintained following an additional 6 weeks of supplementation.26 In contrast to these clinical trials, a double-blind study conducted by Jorissen et al, which evaluated the effect of SB-PS (300–600 mg/day) administration on 81 subjects with AAMI, failed to find any significant improvement in cognitive skills.22
Obviously, there are very few studies that have examined the efficacy of SB-PS on the improvement of cognition in the elderly population and more information is needed. The present study is an exploratory single-center, open-label study that aimed to evaluate the efficacy and safety of SB-PS on healthy elderly volunteers with memory complaints following a short treatment duration of 12 weeks.
Materials and methods
Subjects
Healthy male and female volunteers (age range 50–90 years) with memory complaints were recruited through advertisements in senior citizens’ homes and in hospitals.
Screening included medical history documentation, physical examination, routine laboratory tests and a computerized neuropsychological assessment tool. Subjects were included if suffering from memory impairment as reflected by scores within or below the normative data ± 1.5 standard deviation for impaired population (a maximum of four scores above the norm was allowed) in the computerized tool.
Volunteers were excluded if they had signs or history of delirium; confusion or other disturbances of consciousness; evidence of dementia, depression (score ≥ 4 determined by the short version of the Geriatric Depression Scale); any neurological disorder that could produce cognitive deterioration (including Parkinson’s disease, stroke, intracranial hemorrhage, local brain lesions including tumors and normal pressure hydrocephalus); any infective or inflammatory brain disease (including those of viral, fungal, or syphilitic etiologies); any uncontrolled medical disorder that could produce cognitive deterioration (including hypertension, renal, respiratory, cardiac, and hepatic disease, diabetes mellitus, endocrine, metabolic or hematological disturbances, and malignancy not in remission for more than 2 years); head injury immediately preceding cognitive deterioration; or history of alcohol or drug abuse. Subjects were also excluded from the study if using a coexisting medication or supplement that influences cognitive performance within 1 month prior to screening or as concomitant therapy, had history of hypersensitivity or allergy to soy, or were unable to perform the cognitive tests in Hebrew.
The study was conducted according to the principals of the Declaration of Helsinki and Good Clinical Practice. The protocol was approved by the ethics committee of the Sourasky Medical Center, Tel-Aviv, Israel, and all volunteers gave written informed consent prior to participation.
Study design and supplement
The study was designed as a single-center, open label study. All participants were examined for cognitive performance evaluation and safety assessment at baseline and after 12 weeks of treatment. Cognitive performance was evaluated by two different methods: a computerized cognitive assessment tool and the Rey Auditory Verbal Learning Test (Rey-AVLT). Safety examination included: physical examination, weight, and vital signs (systolic and diastolic blood pressure and heart rate). Blood safety parameters such as blood lipids, liver function, blood electrolytes, hematological blood parameters, and glucose were examined on nine participants. Additional adverse events documentation and computerized cognitive performance evaluation were done following 6 treatment weeks.
The participants received three capsules (Sharp•PS®; Enzymotec LTD, Migdal HaEmeq, Israel) per day with meals for 12 weeks, for a total of 300 mg SB-PS per day.
Outcome measures
Laboratory analysis
Biochemical safety parameters consisted of glucose, sodium, calcium, phosphorus, chloride, potassium, blood urea nitrogen, and creatinine, as well as alkaline phosphatase, alanine-aminotransferase, aspartate aminotransferase, bilirubin, and total protein. In addition, lipid profile was measured (total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol).
Hematological safety parameters consisted of white blood cell count, red blood cell count, lymphocytes, monocytes, neutrophils, platelet, basophiles, eosinophils, and large unstained cells, in addition to measurement of hematocrit, hemoglobin, mean cell volume, mean hemoglobin volume, and mean cell hemoglobin concentration.
The samples were analyzed by the American Medical Laboratories (AML), Herzeliya, Israel.
Cognitive evaluation
NexAde™27 (NexSig Neurological Examination Technologies Ltd, Herzliya, Israel) is a reliable and validated computerized test battery containing some of the most commonly used neuropsychological tests. The subtests include:
Spot the Plus Symbol, where subjects have to press any keyboard key whenever a “+” symbol appears on the screen.
Identify the Odd Pattern, where subjects have to identify the different picture among three.
Recall a Pattern, where subjects are exposed to a picture (similar to the ones in Identify the Odd Pattern) and then have to identify it among three pictures.
Digit-Symbol Substitution Test, where subjects have to match symbols to digits according to a given template.
Digits span forward and backward, where subjects have to repeat a sequence of digits (more digits every time) in forward, and, later, reversed order.
Delayed Pattern Recall, where subjects have to identify simple patterns that they had been told to remember and which were presented again after about 10 minutes. Each subtest is preceded by a brief training module used to familiarize the subject with the task. Tests are limited to 30–45 minutes, including the training phase.
The subtests are presented in an increasing computer-interaction difficulty order (ie, from pressing any key on the keyboard to typing a set of digits), and each subtest essentially serves as training for the next. All tests have multiple versions so that the effect of learning between different administrations is diminished. The scores’ results are obtained by algorithms permitting the isolation of each set of results and their placement within a statistical model. Based upon that model and the increasing amount of data collected, the software is able to score the test.
Based on the results obtained in the single tasks, eight cognitive composite scores are calculated. These composite scores include: focused attention; sustained attention; memory recognition and recall; visuospatial learning; spatial short-term memory; executive functions; and mental flexibility.
Rey-AVLT28 is an assay consisting of a list of 15 common nouns, which are read to the subject in five consecutive trials (trials 1 through 5); each reading is followed by a free-recall task. In trial 6, an interference list of 15 new common nouns is presented, followed by free recall of these new nouns. In trial 7, without additional reading, subjects are again asked to recall the first list. Twenty minutes later, without an additional reading, subjects are asked to recall once more the first list (trial 8). Five different scores are derived from the test: immediate memory recall (trial 1 score); best learning (trial 5); verbal total learning (sum of scores of trials 1 through 5); delayed recall (trial 8); and memory recognition (trial 9).
Statistical methods
The computerized scores collected at baseline and after 6 weeks and 12 weeks were subjected to repeated measures analysis of variance (RM-ANOVA) model. Student’s t-test contrast analysis for dependent samples was carried out on the tests that were found statistically significant in the RM-ANOVA. This was done to determine the statistical significance of the difference in computerized scores between baseline and 6 weeks and between baseline and 12 weeks.
Student’s t-test for dependent samples was used to test the change between baseline and 12-week treatment in Rey-AVLT scores and in the physical parameters (blood pressure and pulse). Due to the exploratory nature of this study, there was no correction for multiple testing.
Wilcoxon signed-rank test was used to test the change from baseline to 12 weeks in weight and blood parameters. This aparametric test was selected due to the small sample size and deviation from normality.
The values are reported as mean ± standard error (SE), and P < 0.05 was considered statistically significant.
The SPSS statistical package (version 17; IBM Corporation, Armonk, NY, USA) was used for all analyses.
Results
Study population
A total of 30 subjects (8 men and 22 women) fit the inclusion criteria and were included in the study. Four subjects dropped out of the study due to adverse events; two of these reported gastrointestinal discomfort, which was deemed probably related to the study treatment. The two other dropouts reported pneumonia and suspected cardiac arrhythmia, which were both categorized as severe adverse events not related to the study material. An additional two subjects reported adverse events that were categorized as probably not related to the treatment and completed the study. Otherwise, subjects maintained good health throughout the study and the treatment was well tolerated. A total of 26 subjects (7 men and 19 women) completed the study and were eligible for statistical analysis. The mean age of completers was 74.6 ± 1.7 years, 46% were married, and mean education years was 13.8 ± 0.01.
Cognitive outcomes
The computerized cognitive assessment results presented in Table 1 are for the cognitive composite scores at baseline and following 6 and 12 weeks of SB-PS administration. RM-ANOVA demonstrated a significant improvement over time in memory recognition (P = 0.007), memory recall (P = 0.015), executive functions (P = 0.013), and mental flexibility (P = 0.029) scores. An improvement was detected also in the scores of focused attention, sustained attention, visuospatial learning, and spatial short-term memory, although these improvements did not reach statistical significance.
Table 1.
The effect of SB-PS on subjects’ performance in the cognitive computerized tool
| Cognitive composite score (points) | Baseline | 6 weeks | 12 weeks | P-value* |
|---|---|---|---|---|
| Memory recognition | 80.60 ± 2.11 | 86.24 ± 2.07 | 87.36 ± 1.54 | 0.007 |
| Memory recall | 73.52 ± 2.55 | 81.12 ± 2.64 | 82.32 ± 2.30 | 0.015 |
| Executive functions | 72.36 ± 4.62 | 79.16 ± 4.36 | 82.64 ± 3.69 | 0.013 |
| Mental flexibility | 73.12 ± 4.64 | 79.32 ± 4.35 | 82.44 ± 3.53 | 0.029 |
| Focused attention | 85.20 ± 2.46 | 88.24 ± 2.45 | 90.28 ± 1.91 | 0.076 |
| Sustained attention | 82.04 ± 3.18 | 86.00 ± 2.52 | 87.76 ± 1.80 | 0.100 |
| Visuospatial learning | 60.72 ± 2.65 | 66.40 ± 4.08 | 67.88 ± 3.67 | 0.170 |
| Spatial short-term memory | 58.44 ± 2.93 | 63.44 ± 4.52 | 64.32 ± 4.17 | 0.423 |
Notes: Mean cognitive composite scores, at baseline and following 6 and 12 weeks of SB-PS administration are presented. Values are described as mean ± SE; n = 25.
Based on RM-ANOVA of the mean change between baseline and 6 and 12 weeks’ treatment.
Abbreviations: RM-ANOVA, repeated measures analysis of variance; SB-PS, soybean-derived phosphatidylserine; SE, standard error.
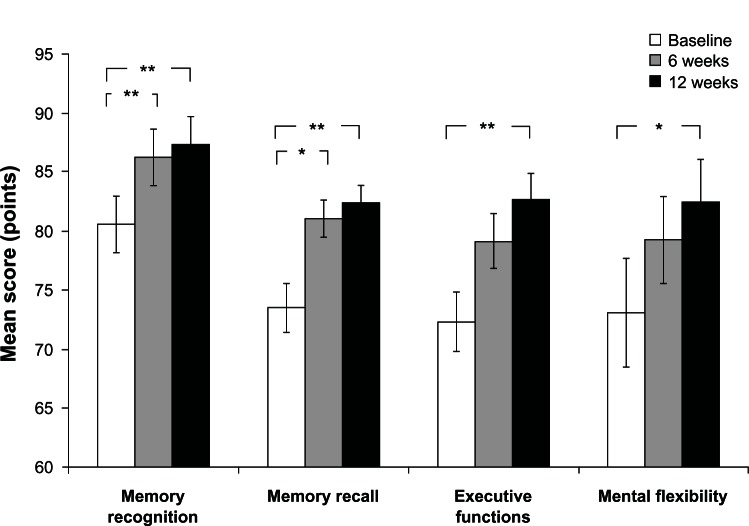
Cognitive composite scores that were found to have a statistically significant change over time following the RM-ANOVA were subjected to a Student’s t-test contrast analysis for dependent samples (Figure 1). The following statistically significant differences in composite scores were found between baseline and 12 weeks: memory recognition 6.76 ± 2.13 (P = 0.004), memory recall 8.80 ± 2.92 (P = 0.006), executive functions 10.28 ± 3.27 (P = 0.004), and mental flexibility 9.32 ± 3.32 (P = 0.01).
Figure 1.
The effect of SB-PS on subjects’ performance in the cognitive computerized tool.
Notes: RM-ANOVA was initially used to test the changes in the cognitive composite scores over the treatment period (results are shown in Table 1). Then, Student’s t-test comparison for dependent samples was applied on the parameters that were found to be statistically significant in the RM-ANOVA analysis. The figure demonstrates the mean ± SE scores at baseline and 6 and 12 treatment weeks and statistical significance as analyzed using Student’s t-test. *P ≤ 0.01; **P ≤ 0.006; n = 25.
Abbreviations: RM-ANOVA, repeated measures analysis of variance; SB-PS, soybean-derived phosphatidylserine; SE, standard error.
In addition, statistically significant improvement was shown for scores of memory recognition (P = 0.003) and memory recall (P = 0.01) between baseline and 6 weeks of SB-PS administration.
Table 2 presents the effect of SB-PS on subjects’ performance as examined by Rey-AVLT assay. Both immediate recall and total learning scores improved significantly from baseline to 12 weeks (mean change from baseline: 1.00 ± 0.34; P = 0.007 and 3.65 ± 1.36; P = 0.013, respectively).
Table 2.
The effect of SB-PS on subjects’ performance in the Rey-AVLT28 assay following 12 weeks’ administration
| Rey-AVLT scores (no of words correctly recalled) | Baseline | 12 weeks | P-value* |
|---|---|---|---|
| Immediate recall | 4.12 ± 0.34 | 5.12 ± 0.31 | 0.007 |
| Total learning | 36.23 ± 1.90 | 39.88 ± 1.82 | 0.013 |
| Best learning | 9.42 ± 0.61 | 9.77 ± 0.47 | 0.502 |
| Delayed recall | 7.15 ± 0.54 | 7.81 ± 0.59 | 0.183 |
| Recognition | 11.81 ± 0.46 | 12.46 ± 0.42 | 0.156 |
Notes: Mean scores at baseline and following 12 weeks of SB-PS administration are presented. Values are described as mean ± SE; n = 26.
Based on Student’s t-test comparison for dependent samples of the mean change between baseline and 12 weeks. Numbers in bold mark significant change from baseline.
Abbreviations: Rey-AVLT, Rey Auditory Verbal Learning Test; SB-PS, soybean-derived phosphatidylserine; SE, standard error.
Scores for best learning, delayed recall, and recognition were also improved (mean change from baseline: 0.35 ± 0.51, 0.65 ± 0.48, and 0.65 ± 0.45, respectively) following 12 weeks of SB-PS; however, these improvements did not reach statistical significance (Table 2).
Safety laboratory tests
No serious adverse events that were classified as treatment related were reported during the course of the study. In addition, no significant findings were observed between baseline and end point in the physical examination, resting heart rate, or weight, nor in the hematological and biochemical blood parameters (data not shown).
Interestingly, following 12 weeks of SB-PS supplementation, both mean systolic and diastolic blood pressure were significantly reduced in comparison to mean baseline values, by 8.9 ± 4.2 mmHg (P = 0.043) and 6.8 ± 2.1 mmHg, (P = 0.003), respectively (Table 3).
Table 3.
The effect of 12 weeks’ SB-PS administration on systolic and diastolic BP
| BP (mmHg) | Baseline | 12 weeks | P-value* |
|---|---|---|---|
| Resting systolic BP | 137.8 ± 3.7 | 128.8 ± 3.5 | 0.043 |
| Resting diastolic BP | 75.9 ± 2.1 | 69.1 ± 2.1 | 0.003 |
Notes: Mean systolic and diastolic BP values at baseline and following 12 weeks of SB-PS administration are presented. Values are described as mean ± SE; n = 25.
Based on Student’s t-test comparison for dependent samples of the mean change between baseline and end point. Numbers in bold mark significant change from baseline.
Abbreviations: BP, blood pressure; SB-PS, soybean-derived phosphatidylserine; SE, standard error.
Discussion
The present study examined the effect of 6 and 12 weeks of SB-PS administration on the cognitive function of 30 healthy volunteers with memory complaints, using a computerized cognitive assessment tool and Rey-AVLT. The main conclusion to be drawn from this study is that SB-PS may have a positive effect on cognitive performance in healthy elders with memory complaints, following a short supplementation period. The study also shows that SB-PS is well tolerated and safe.
Many clinical and preclinical studies carried out in the past 2 decades have helped to substantiate the role of PS in supporting cognitive function. Most of the clinical trials used BC-PS, and many showed improvement in cognitive parameters after short-term supplementation of 12, 6, and even 3 weeks.10,12,14–16 Safety concerns regarding the bovine source of PS brought the use of BC-PS to an end, leading to the development of soybean PS as a safe vegetarian alternative. An open-label clinical trial conducted by Schreiber et al26 tested the effect of SB-PS supplementation on healthy elderly volunteers. The study showed that SB-PS has a significant improvement on recall and immediate memory parameters following 6 weeks’ administration, an effect that was maintained following an additional 6 weeks’ supplementation.26 Consistent with the findings of Schreiber et al, the results of the current study showed that 12 weeks of SB-PS administration resulted in a statistically significant improvement in memory parameters such as memory recognition, memory recall, and immediate recall. In addition, the present study demonstrates significant positive effect of SB-PS on total learning and on activities of daily living (ie, executive function and mental flexibility). Kato-Kataoka et al23 showed a positive effect of SB-PS on cognitive performance and, in particular, on verbal list recall, again substantiating our finding of improved immediate recall in the Rey-AVLT. The last study tested the effect following 24 weeks of administration; however, the authors suggest that it is impossible to deduce from the study if shorter periods of time would have been effective.23
Despite the above findings, the effect of SB-PS in humans has been found to be inconsistent. Jorissen et al did not find any significant improvement in cognitive skills following 12 weeks of SB-PS administration.22 The findings of the Jorissen et al study render the effect of PS controversial; however, there may be explanations for the lack of efficacy of the PS that was tested. One aspect that needs to be addressed is the stability of the active ingredients. This aspect is relevant to the effective dose that remains in the capsule following a defined period of time. Jorissen et al reported that one possibility for the ineffectiveness of SB-PS in their study was due to degradation of the PS with time. Actually, they reported on 50% PS degradation after 15 months.22 Jorissen et al, as well as Kato-Kataoka et al,23 used soft gelatin capsules in their studies, a form of PS that is known to be unstable. The degradation of PS in soft gelatin capsules was also mentioned by Kato-Kataoka et al in their paper. In order to avoid the influence of PS degradation, Kato-Kataoka et al sent a portion of the capsules to the subjects every single month and instructed the participants to keep the samples under refrigerated conditions.23 In the current study, we used special PS soft gelatin capsules (Sharp-PS®) that have been proven to be stable for long term (shelf life of 24 months, data not shown). In contrast, Schreiber et al26 mentioned the use of PS capsules but did not mention soft gelatin capsules, therefore we assume that they used powder PS instead of liquid PS. Powder PS is more stable than liquid, a point that could explain the positive efficacy that Schreiber et al demonstrated in their trial. The quality and stability of the PS should therefore be considered a very important parameter in a trial.
The possible role of PS in the improvement of cognitive parameters is embedded in the understanding that neurological changes, in many cases, can be traced to structural and functional changes of neurological membranes. In neuronal membranes of animals, PS has demonstrated a role in the modulation of neuronal excitability and neurotransmitter activity,29–31 functions related to cerebral processes such as learning and memory. Animal studies reporting increased levels of norepinephrine,32 serotonin and dopamine,10 and acetylcholine33 are evidence of PS effects in the central nervous system. PS is thus considered to be important to the brain, and supplementation of PS is considered effective for proper structure and function of neurological membranes.
The comprehensive safety parameters in the current study indicate that SB-PS administration was well tolerated, and the participants maintained good health throughout the study with no serious adverse events related to the treatment. Resting blood pressure was among the safety parameters tested. Interestingly, a significant reduction in systolic and diastolic blood pressure was observed following 12 weeks of SB-PS supplementation. These results are surprising since they have not been previously reported,18,34 and should therefore be further investigated.
Although the results of this pilot study are encouraging, we acknowledge certain limitations in the study design. First, due to the exploratory nature of this study, we chose not to correct for multiple testing. Additionally, the study is lacking a corresponding placebo-controlled group and, therefore, our findings should be further tested in a double-blind, controlled, confirmatory study.
Conclusion
This exploratory study demonstrates that SB-PS is well tolerated, safe for human consumption, and may have positive effects on cognitive function in healthy elderly with memory complaints, including memory parameters, executive function, and mental flexibility. These results encourage a further, extended, double-blind, placebo-controlled study in order to establish the safety and efficacy of SB-PS treatment.
Footnotes
Disclosure
All authors are either present or former employees of Enzymotec Ltd.
References
- 1.Klenk J, Rapp K, Büchele G, Keil U, Weiland SK. Increasing life expectancy in Germany: quantitative contributions from changes in age- and disease-specific mortality. Eur J Public Health. 2007;17(6):587–592. doi: 10.1093/eurpub/ckm024. [DOI] [PubMed] [Google Scholar]
- 2.Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry. 2011;11:108. doi: 10.1186/1471-244X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien JT. Age-associated memory impairment and related disorders. Advances in Psychiatric Treatment. 1999;5:279–287. [Google Scholar]
- 4.Küllenberg D, Taylor LA, Schneider M, Massing U. Health effects of dietary phospholipids. Lipids Health Dis. 2012;11(1):3. doi: 10.1186/1476-511X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44(4):207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Mozzi R, Buratta S, Goracci G. Metabolism and functions of phosphatidylserine in mammalian brain. Neurochem Res. 2003;28(2):195–214. doi: 10.1023/a:1022412831330. [DOI] [PubMed] [Google Scholar]
- 7.Phosphatidylserine. Monograph. Altern Med Rev. 2008;13(3):245–247. [No authors listed] [PubMed] [Google Scholar]
- 8.Amaducci L. Phosphatidylserine in the treatment of Alzheimer’s disease: results of a multicenter study. Psychopharmacol Bull. 1988;24(1):130–134. [PubMed] [Google Scholar]
- 9.Amaducci L, Crook TH, Lippi A, et al. Use of phosphatidylserine in Alzheimer’s disease. Ann N Y Acad Sci. 1991;640:245–249. doi: 10.1111/j.1749-6632.1991.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 10.Crook T, Petrie W, Wells C, Massari DC. Effects of phosphatidylserine in Alzheimer’s disease. Psychopharmacol Bull. 1992;28(1):61–66. [PubMed] [Google Scholar]
- 11.Richter Y, Herzog Y, Cohen T, Steinhart Y. The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: a pilot study. Clin Interv Aging. 2010;5:313–316. doi: 10.2147/CIA.S13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allegro L, Favaretto V, Ziliotto G. Oral phosphatidylserine in elderly patients with cognitive deterioration. An open study. Clin Trials J. 1987;24(1):104–108. [Google Scholar]
- 13.Cenacchi T, Bertoldin T, Farina C, Fiori MG, Crepaldi G. Cognitive decline in the elderly: a double-blind, placebo-controlled multicenter study on efficacy of phosphatidylserine administration. Aging (Milano) 1993;5(2):123–133. doi: 10.1007/BF03324139. [DOI] [PubMed] [Google Scholar]
- 14.Crook TH, Tinklenberg J, Yesavage J, Petrie W, Nunzi MG, Massari DC. Effects of phosphatidylserine in age-associated memory impairment. Neurology. 1991;41(5):644–649. doi: 10.1212/wnl.41.5.644. [DOI] [PubMed] [Google Scholar]
- 15.Sinforiani E, Agostinis C, Merlo P, Gualtireri S, Mauri M, Mancuso A. Cognitive decline in ageing brain. Therapeutic approach with phosphtadylserine. Clin Trials J. 1987;24(1):115–124. [Google Scholar]
- 16.villardita C, Grioli S, Salmeri G, Nicoletti F, Pennisi G. Multicentre clinical trial of brain phosphatidylserine in elderly patients with intellectual deterioration. Clin Trials J. 1987;24(1):84–93. [Google Scholar]
- 17.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 18.Jorissen BL, Brouns F, Van Boxtel MP, Riedel WJ. Safety of soy-derived phosphatidylserine in elderly people. Nutr Neurosci. 2002;5(5):337–343. doi: 10.1080/1028415021000033802. [DOI] [PubMed] [Google Scholar]
- 19.Blokland A, Honig W, Brouns F, Jolles J. Cognition-enhancing properties of subchronic phosphatidylserine (PS) treatment in middle-aged rats: comparison of bovine cortex PS with egg PS and soybean PS. Nutrition. 1999;15(10):778–783. doi: 10.1016/s0899-9007(99)00157-4. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Yamatoya H, Sakai M, Kataoka A, Furushiro M, Kudo S. Oral administration of soybean lecithin transphosphatidylated phosphatidylserine improves memory impairment in aged rats. J Nutr. 2001;131(11):2951–2956. doi: 10.1093/jn/131.11.2951. [DOI] [PubMed] [Google Scholar]
- 21.Sakai M, Yamatoya H, Kudo S. Pharmacological effects of phosphatidylserine enzymatically synthesized from soybean lecithin on brain functions in rodents. J Nutr Sci Vitaminol (Tokyo) 1996;42(1):47–54. doi: 10.3177/jnsv.42.47. [DOI] [PubMed] [Google Scholar]
- 22.Jorissen BL, Brouns F, Van Boxtel MP, et al. The influence of soy-derived phosphatidylserine on cognition in age-associated memory impairment. Nutr Neurosci. 2001;4(2):121–134. doi: 10.1080/1028415x.2001.11747356. [DOI] [PubMed] [Google Scholar]
- 23.Kato-Kataoka A, Sakai M, Ebina R, Nonaka C, Asano T, Miyamori T. Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints. J Clin Biochem Nutr. 2010;47(3):246–255. doi: 10.3164/jcbn.10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gindin JNM, Dedar D, Walter-Ginzburg A, Naor S, Levi S. The Effect of Plant Phosphatidylserine on Age Associated Memory Impairment and Mood in the Functioning Elderly. The Geriatric Institute for Education and Research, and Department of Geriatrics, Kaplan Hospital, Rehovot, Israel. 1993.
- 25.Asano TK-KA, Sakai M, Tsuji A, Ebina R, Nonaka C, Takamizawa K. The effect of soybean derived phosphatidylserine on the cognitive function of the elderly. Jpn J Nutr Ass. 2005;24:165–170. [Google Scholar]
- 26.Schreiber S, Kampf-Sherf O, Gorfine M, Kelly D, Oppenheim Y, Lerer B. An open trial of plant-source derived phosphatydilserine for treatment of age-related cognitive decline. Isr J Psychiatry Relat Sci. 2000;37(4):302–307. [PubMed] [Google Scholar]
- 27.Aharonson V, Korczyn AD. Human-computer interaction in the administration and analysis of neuropsychological tests. Comput Methods Programs Biomed. 2004;73(1):43–53. doi: 10.1016/s0169-2607(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Vakil E, Blachstein H. Rey Auditory-Verbal Learning Test: structure analysis. J Clin Psychol. 1993;49(6):883–890. doi: 10.1002/1097-4679(199311)49:6<883::aid-jclp2270490616>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler KP, Whitman R. ATPase activity of the sodium pump needs of phosphatidylserine. Nature. 1970;225(5231):449–450. doi: 10.1038/225449a0. [DOI] [PubMed] [Google Scholar]
- 30.Raese J, Patrick RL, Barchas JD. Phospholipid-induced activation of tyrosine hydroxylase from rat brain striatal synaptosomes. Biochem Pharmacol. 1976;25(20):2245–2250. doi: 10.1016/0006-2952(76)90005-8. [DOI] [PubMed] [Google Scholar]
- 31.Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- 32.Toffano G, Leon A, Mazzari S, Savoini G, Teolato S, Orlando P. Modification of noradrenergic hypothalamic system in rat injected with phosphatidylserine liposomes. Life Sci. 1978;23(10):1093–1101. doi: 10.1016/0024-3205(78)90671-9. [DOI] [PubMed] [Google Scholar]
- 33.Maggioni M, Picotti GB, Bondiolotti GP, et al. Effects of phosphatidylserine therapy in geriatric patients with depressive disorders. Acta Psychiatr Scand. 1990;81(3):265–270. doi: 10.1111/j.1600-0447.1990.tb06494.x. [DOI] [PubMed] [Google Scholar]
- 34.Benton D, Donohoe RT, Sillance B, Nabb S. The influence of phosphatidylserine supplementation on mood and heart rate when faced with an acute stressor. Nutr Neurosci. 2001;4(3):169–178. doi: 10.1080/1028415x.2001.11747360. [DOI] [PubMed] [Google Scholar]