Abstract
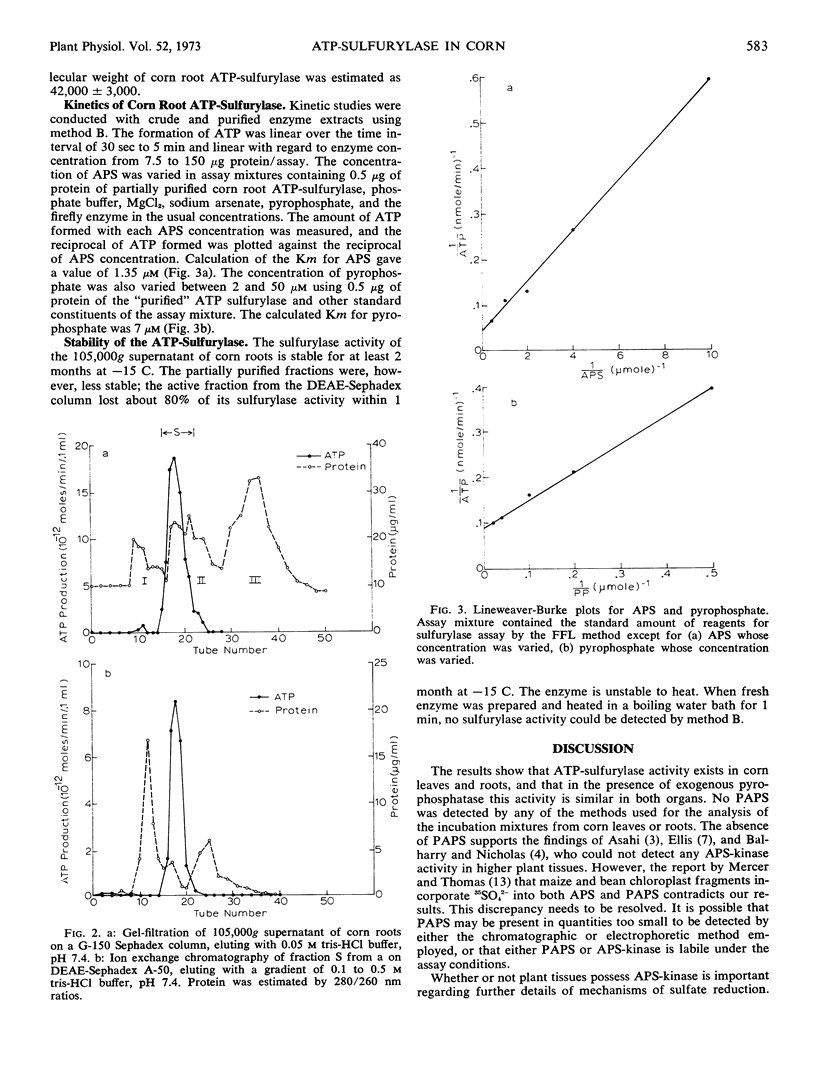
ATP-sulfurylase (ATP-sulfate adenyltransferase, EC 2.7.7.4) was found in nonparticulate fractions of both roots and leaves of Zea mays L. seedlings using two detection methods. Addition of exogenous pyrophosphatase was essential for maximum rates of conversion of 35SO42− to labeled adenosine phosphosulfate in unpurified root extracts, but not in unpurified leaf extracts. In the presence of exogenous pyrophosphatase, the enzyme from roots exhibited specific activities as high as those obtained with the leaf enzyme. The root enzyme was purified 33-fold by centrifugation and column chromatography procedures. Its molecular weight obtained by Sephadex gel filtration was about 42,000. Its Km for pyrophosphate was 7 μm, while for adenosine phosphosulfate, the Km was 1.35 μm. None of the enzyme fractions studied converted adenosine phosphosulfate into detectable amounts of 3′-phosphoadenosine-5′-phosphosulfate. ATP-sulfurylase was also found in roots of corn seedlings grown aseptically. The data suggest that at least the first reaction in sulfate reduction might proceed as effectively in roots as in shoots.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Johnson R. E. ATP Sulfurylase Activity in the Soybean [Glycine max (L.) Merr.]. Plant Physiol. 1968 Dec;43(12):2041–2044. doi: 10.1104/pp.43.12.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balharry G. J., Nicholas D. J. ATP-sulphurylase in spinach leaves. Biochim Biophys Acta. 1970 Dec 16;220(3):513–524. doi: 10.1016/0005-2744(70)90282-2. [DOI] [PubMed] [Google Scholar]
- Bledsoe C., Cole C. V., Ross C. Oligomycin inhibition of phosphate uptake and ATP labeling in excised maize roots. Plant Physiol. 1969 Jul;44(7):1040–1044. doi: 10.1104/pp.44.7.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. V., Ross C. Extraction, separation, and quantitative estimation of soluble nucleotides and sugar phosphates in plant tissues. Anal Biochem. 1966 Dec;17(3):526–539. doi: 10.1016/0003-2697(66)90188-6. [DOI] [PubMed] [Google Scholar]
- GREGORY J. D., ROBBINS P. W. Metabolism of sulfur compounds (sulfate metabolism). Annu Rev Biochem. 1960;29:347–364. doi: 10.1146/annurev.bi.29.070160.002023. [DOI] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A., Scarsella A. J., Levinthal M. Studies of Sulfate Utilization by Algae. 6. Adenosine-3'-Phosphate-5'-Phosphosulfate (PAPS) as an Intermediate in Thiosulfate Formation From Sulfate by Cell-Free Extracts of Chlorella. Plant Physiol. 1968 Apr;43(4):563–569. doi: 10.1104/pp.43.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Schoenhard D. E. Biochemical characterization of sulfur assimilation by Salmonella pullorum. J Bacteriol. 1970 Apr;102(1):142–148. doi: 10.1128/jb.102.1.142-148.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A. S., Wolf G. Purification and properties of the enzyme ATP-sulfurylase and its relation to vitamin A. Biochim Biophys Acta. 1969 Apr 22;178(2):262–282. doi: 10.1016/0005-2744(69)90395-7. [DOI] [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS P. W., LIPMANN F. Enzymatic synthesis of adenosine-5'-phosphosulfate. J Biol Chem. 1958 Sep;233(3):686–690. [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie J. W., Segel I. H. Adenosine triphosphate sulfurylase from Penicillium chrysogenum. II. Physical, kinetic, and regulatory properties. J Biol Chem. 1971 Apr 25;246(8):2438–2446. [PubMed] [Google Scholar]
- WHELDRAKE J. F., PASTERNAK C. A. THE CONTROL OF SULPHATE ACTIVATION IN BACTERIA. Biochem J. 1965 Jul;96:276–280. doi: 10.1042/bj0960276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- Wheldrake J. F. Intracellular concentration of cysteine in Escherichia coli and its relation to repression of the sulphate-activating enzymes. Biochem J. 1967 Nov;105(2):697–699. doi: 10.1042/bj1050697. [DOI] [PMC free article] [PubMed] [Google Scholar]