Abstract
The recognition, diagnosis, and management of sepsis remain among the greatest challenges in pediatric critical care medicine. Sepsis remains among the leading causes of death in both developed and underdeveloped countries and has an incidence that is predicted to increase each year. Unfortunately, promising therapies derived from preclinical models have universally failed to significantly reduce the substantial mortality and morbidity associated with sepsis. There are several key developmental differences in the host response to infection and therapy that clearly delineate pediatric sepsis as a separate, albeit related, entity from adult sepsis. Thus, there remains a critical need for well-designed epidemiologic and mechanistic studies of pediatric sepsis in order to gain a better understanding of these unique developmental differences so that we may provide the appropriate treatment. Herein, we will review the important differences in the pediatric host response to sepsis, highlighting key differences at the whole-organism level, organ system level, and cellular and molecular level.
The Pediatric Host Response to Sepsis
Key Differences at the Whole-organism Level
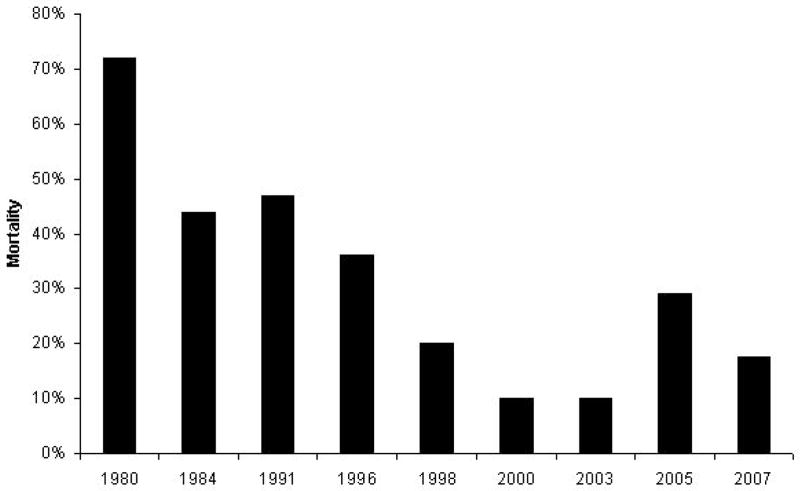
Perhaps one of the most striking differences in pediatric versus adult sepsis is that the mortality rate is much lower in children compared to adults (1–2). Mortality for pediatric sepsis in several recent series ranges between 10–20% (2–7). While the overall mortality has declined significantly since the early 1980’s, the mortality rate for pediatric sepsis has remained more or less constant over the last 10 years (2–4, 6–12) (Figure 1). In comparison, mortality is much higher in adults with sepsis (1), ranging between 35–50% in recent series (1, 13–15). The reduction in the overall mortality rate from sepsis has been less significant compared to the pediatric experience, and further progress has been stagnant over the last decade (Figure 2). Some of these differences in mortality between children and adults with sepsis may relate to the presence of co-morbid conditions in critically ill adults, e.g. atherosclerosis, coronary artery disease, obesity, chronic obstructive pulmonary disease (COPD), diabetes mellitus, and hypercholesterolemia. However, the presence of co-morbid conditions (e.g. prematurity, congenital heart disease, cancer, solid organ and hematopoietic stem cell transplantation) in critically ill children also significantly increases the risk of mortality (2, 16). More importantly, as more and more children survive diseases that were previously fatal, such as leukemia, extreme prematurity, and liver failure, these co-morbid conditions will have an even greater impact on both the incidence and outcome of sepsis in children.
Figure 1. Is the mortality rate decreasing in adults with sepsis?
A PubMed search using the MeSH headings “SIRS”, “sepsis”, “severe sepsis”, and “septic shock” was performed (1980–2009). Landmark and pivotal studies on sepsis (either epidemiologic studies or randomized, controlled clinical trials of therapeutic agents) were reviewed. The mortality rate in each study was recorded and graphed by year (in the case of randomized, controlled clinical trials of therapeutic agents, only the mortality in the placebo group was recorded).
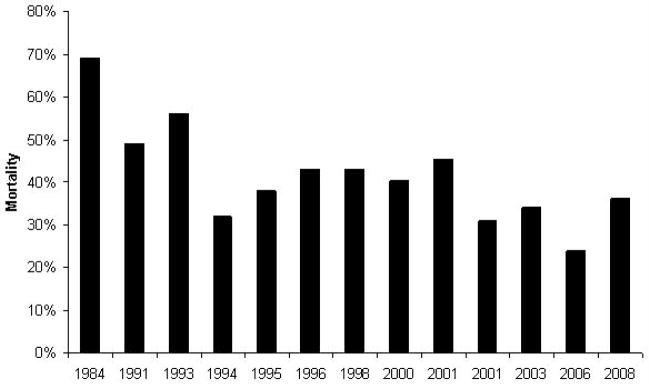
Figure 2. Is the mortality rate decreasing in children with sepsis?
A PubMed search using the MeSH headings “SIRS”, “sepsis”, “severe sepsis”, and “septic shock” was performed (1980–2009). Only studies involving children were included in the review. Landmark and pivotal studies on sepsis (either epidemiologic studies or randomized, controlled clinical trials of therapeutic agents) were reviewed. The mortality rate in each study was recorded and graphed by year (in the case of randomized, controlled clinical trials of therapeutic agents, only the mortality in the placebo group was recorded).
Sepsis is exceedingly more common in children less than 1 year of age, with rates 10-fold higher during infancy compared to childhood and adolescence (2). There are stark differences in the epidemiology and pathophysiology of sepsis during infancy, especially in the neonatal period, that become less distinct with increasing age (16). For example, congenital heart disease and chronic lung disease are the most common underlying co-morbid conditions observed in infants with sepsis. In contrast, the childhood sepsis landscape is dominated by co-morbid conditions such as neuromuscular disease and cancer (2, 16). The site of infection also appears to be age-dependent, as infants with sepsis tend to present with primary bacteremia, while older children with sepsis present with respiratory infection and secondary bacteremia. Importantly, the stark differences between pediatric sepsis and adult sepsis become less distinct with increasing age.
The developmental differences between children and adults have very important implications on the unique epidemiology, pathophysiology, and management of sepsis in children compared to adults (17). Moreover, the differences between children at various stages in development also greatly impact the host response to infection, as well as the host response to therapy. One of the particular challenges in pediatric critical care medicine is dealing with these developmental differences that greatly impact the pathophysiology of disease states, like sepsis. Given the importance of pediatric sepsis to the health care system, the need for further epidemiologic studies to help discern the effects of age on the host response to sepsis are clearly necessary.
Key Differences at the Organ-system Level
Overwhelming sepsis frequently manifest with concurrent derangements in cardiovascular function, intravascular volume status, respiratory function, immune/inflammatory regulation, renal function, coagulation, hepatic function, and metabolic function – sepsis literally affect every organ system to some degree. The degree to which any of these derangements are manifest in any given patient is highly variable and influenced by multiple host and pathogen factors, including the patient’s age, the presence or absence of co-morbid conditions as discussed above, the patient’s underlying immune status, the patient’s genetic background, and even the specific pathogen involved (17–19). Age-specific differences in hemoglobin concentration and composition, heart rate, stroke volume, blood pressure, pulmonary vascular resistance, systemic vascular resistance, metabolic rate, glycogen stores, and protein mass are the basis for many of the age-specific differences in the cardiovascular and metabolic responses to sepsis (16–17, 20–22). For example, newborn infants have comparatively higher heart rates, lower stroke volumes, near systemic pulmonary artery blood pressures, and higher metabolic rates with high energy needs, but the lowest glycogen stores and protein mass for glucose production (20, 22). A complete picture of pediatric sepsis therefore requires a thorough understanding of these developmental differences and how they contribute to organ dysfunction in the critically ill pediatric patient with sepsis. We will primarily concentrate on the developmental differences in the cardiovascular, respiratory, renal, and coagulation systems.
Distribution of Body Fluids
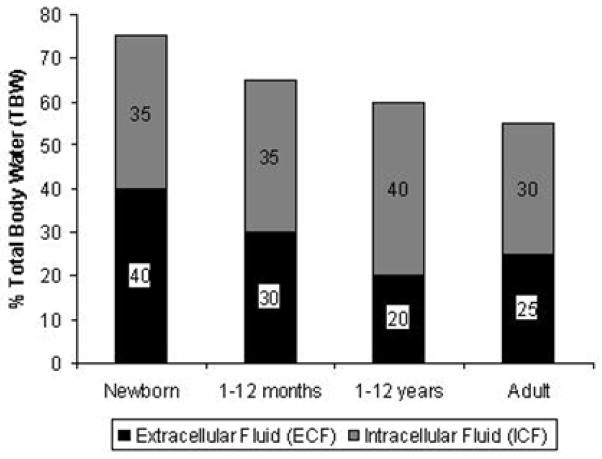
Total body water (TBW) as a percentage of body weight decreases rapidly with age (Figure 3) (20, 22–26). It therefore would be logical to assume that children, due to their relatively greater percentage of TBW, are relatively protected against intravascular volume loss. Unfortunately, this is not the case, primarily because fluid losses are proportionately greater per kilogram of body weight in children versus adults. For example, 10% dehydration in a 6 month-old child weighing 7-kg is equivalent to approximately 700 mL, which is roughly one-tenth the total volume loss required to produce the same degree of dehydration in a 70-kg adult (approximately 7000 mL fluid loss) (20). The normal distribution of TBW is age-dependent as well. TBW consists of the intracellular (ICF) and extracellular (ECF) fluid compartments. The ECF compartment consists of the plasma volume (5% TBW) and the interstitial volume (15% TBW). The ECF volume decreases rapidly during the first year of life, while the ICF volume remains relatively constant (20, 22–26). Infants and young children have a proportionally higher ratio of ECF to ICF (Figure 3), which predisposes them to rapid fluid losses, increasing their vulnerability to hypovolemic shock. In fact, experimental models utilizing radio-labeled albumin demonstrate that the percentage of body weight lost is directly proportional to the percentage of plasma volume lost (e.g., children who lose 5% of their body weight have lost approximately 5% of their plasma volume) (27).
Figure 3. The effect of age on normal distribution of body fluids.
Total body water (TBW), which consists of the intracellular (ICF) and extracellular (ECF) fluid compartments, as a percentage of body weight decreases rapidly with age. The ECF compartment consists of the plasma volume (5% TBW) and the interstitial volume (15% TBW). The ECF volume decreases rapidly during the first year of life, while the ICF volume remains relatively constant. Fluid losses usually affect either the interstitial or intracellular compartments (20, 22–26).
Differences in Cardiovascular Function
While decreased intravascular volume (either absolute or relative, as occurs due to third spacing) is the most frequent cause of shock in children (28), abnormalities in vasoregulation and myocardial dysfunction likely play a greater role in neonates and young infants (29–33). Numerous investigators have failed to demonstrate an increase in blood pressure with expansion of the plasma volume in the neonatal age group – the use of dopamine appears to be much more effective in this regard (28, 31, 34–36). Unfortunately, an understanding of the physiologic basis for these abnormalities in peripheral vasoregulation during the neonatal period is far from complete.
Myocardial dysfunction is likely to play a greater role in the pathophysiology of shock in the pediatric age group (29–32, 36–38). There are significant differences in both myocardial structure and function that compromise the compensatory response to sepsis (38–40). For example, important changes in excitation-contraction coupling occur due to the immaturity of the calcium regulation system (T tubules, sarcoplasmic reticulum, L-type Ca2+ channels). These developmental differences lead to alterations in the normal mechanisms leading to the Ca2+-induced Ca2+ release (CICR) that triggers excitation-contraction coupling, such that the neonatal myocardium is more dependent upon extracellular calcium versus intracellular calcium for contractility compared to the mature heart (41–45). These developmental differences further explain the extreme sensitivity of neonates to calcium channel antagonists (42). Indeed, some authors have suggested that calcium chloride is an effective inotrope in neonates after cardiopulmonary bypass (46). Further notable differences in the neonatal myocardium include decreased expression of ATP-sensitive K+ channels (KATP) (47) and alterations in β-adrenergic receptor signal transduction (48). KATP channels are inhibited by intracellular ATP and activated by intracellular nucleoside diphosphates (e.g. ADP). These channels are activated in response to ischemia or hypoxia and are therefore important for the adaptations that must occur in sepsis (49–52).
Left ventricular systolic performance in neonates and young infants is critically dependent upon afterload (53–54). An abrupt increase in afterload in the setting of shock and vasoconstriction would therefore result in markedly reduced left ventricular systolic performance and myocardial dysfunction. The neonatal myocardium has a relatively decreased left ventricular mass in comparison to the adult myocardium (55–56), as well as an increased ratio of type I collagen (decreased elasticity) to type III collagen (increased elasticity) (57). Of note, the re-modeling that occurs following an acute myocardial infarction (AMI) leads to a similar increased ratio of type I collagen to type III collagen, which may explain in part the decrease in myocardial function that occurs in adults following an AMI (58). Similar changes are observed in the myocardium of patients with dilated cardiomyopathy (59). In addition, the neonatal myocardium in particular functions at a relatively high contractile state, even at baseline (38, 53). Collectively, these developmental changes result in a relatively limited capacity to increase stroke volume during stress (38, 56, 60), and hence neonates and young infants are critically dependent upon an increase in heart rate to generate increased cardiac output during stress. Unfortunately, myocardial perfusion occurs to the greatest degree during diastole and depends directly upon the difference between diastolic blood pressure and left atrial pressure, and inversely with heart rate (as an indirect measure of diastolic filling time). As the heart rate increases, diastolic filling will eventually reach a point at which further increases in cardiac output are limited (22).
Collectively, the differences in myocardial structure and function discussed above may explain the differences in the hemodynamic response, as well as the response to therapeutic agents in children versus adults. Again, myocardial dysfunction appears to play a greater pathophysiologic role in pediatric sepsis. In what is now considered a classic study, Ceneviva and colleagues (61) categorized 50 children with fluid-refractory shock based upon hemodynamic data obtained with a pulmonary artery (PA) catheter into one of three possible hemodynamic derangements (i) a hyperdynamic state characterized by a high cardiac output (> 5.5 L/min/m2 BSA) and low systemic vascular resistance (< 800 dynes sec/cm5) (classically referred to as warm shock); (ii) a hypodynamic state characterized by low cardiac output (< 3.3 L/min/m2 BSA) and low systemic vascular resistance (SVR); or (iii) a hypodynamic state characterized by low cardiac output and high SVR (> 1200 dynes sec-cm5) (classically referred to as cold shock). In contrast to adults in which the early stages of septic shock is characterized by a high cardiac output and low SVR, most of these children were in a hypodynamic state characterized by low cardiac output and high systemic vascular resistance (cold shock) and required the addition of vasodilators to decrease SVR, increase cardiac output, and improve peripheral perfusion (61). Children with low cardiac output (as defined by a cardiac index less than 2.0 L/min/m2 BSA) had the highest risk of mortality. These findings have been confirmed in multiple studies (9, 62–66). For example, Reynolds et al. (62) reported that pediatric burn victims with fluid-refractory shock had decreased left ventricular stroke work (LVSW) and responded to inotropic support with improvements in cardiac output. Feltes et al. (66) reported echocardiographic findings consistent with decreased left ventricular systolic function and increased afterload in 5 out of 10 children with septic shock.
Differences in Respiratory Function
Acute respiratory failure is a major cause of morbidity and mortality in critically ill children with sepsis. Developmental differences contribute to the prevalence and impact the management of acute respiratory failure in the pediatric age group. For example, the pediatric upper airway is markedly different from the adult upper airway. The main differences include (i) a proportionally larger head and occiput (relative to body size), causing neck flexion and potential airway obstruction when lying supine; (ii) a relatively larger tongue, relative to the size of the oral cavity; (iii) decreased muscle tone, resulting in passive obstruction of the airway by the tongue; (iv) a shorter, narrower, horizontally positioned, softer, omega-shaped epiglottis; (v) cephalad and anterior position of the larynx; (vi) shorter, smaller, narrower trachea; (vii) funnel-shaped versus cylindrical airway, such that the narrowest portion of the airway is located at the level of the cricoid cartilage; (viii) prominent adenoidal and tonsillar lymphoid tissue that can contribute to airway obstruction (67). Collectively, these anatomic differences render children more susceptible to acute airway obstruction and may complicate airway management in the critically ill child with septic shock.
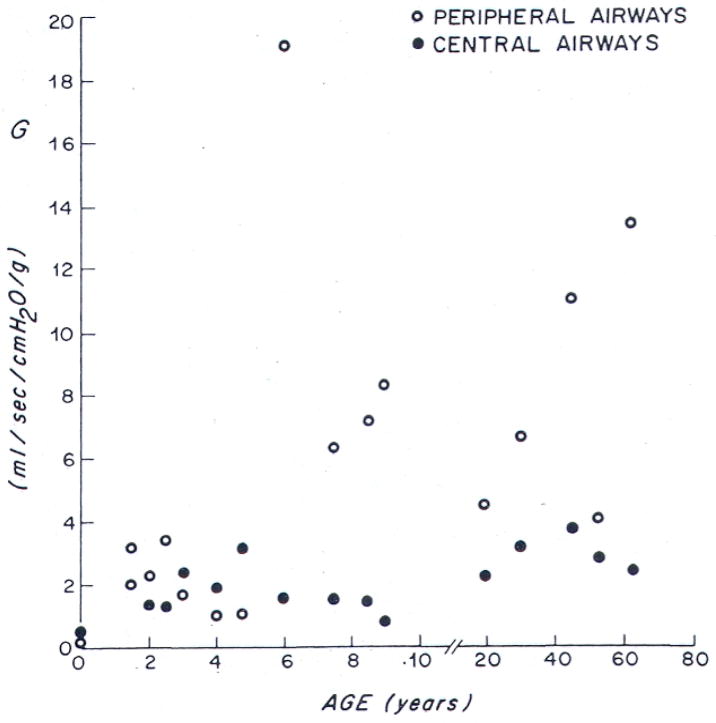
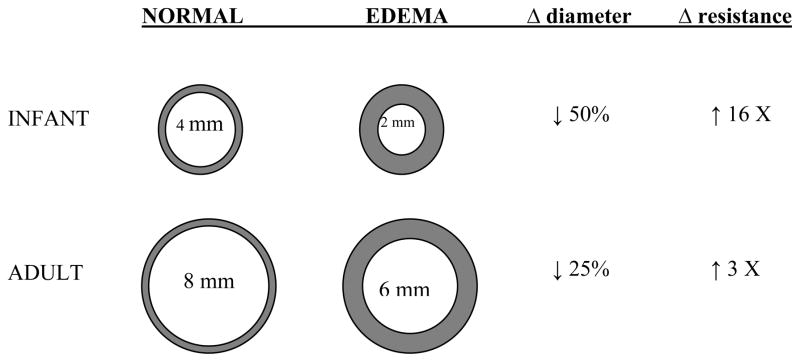
Similarly, there are several key developmental differences that predispose children to acute respiratory failure (68–70). Infants and young children have fewer alveoli compared to adults (approximately 20 million alveoli after birth to 300 million alveoli by the age of 8 years) (71–73). The size of each individual alveolus is smaller in children (150–180 μm diameter versus 250–300 μm diameter) (74). Together, these two anatomic differences markedly decrease the surface area available for gas exchange. The airways enlarge both in length and diameter with age. However, growth of the distal airways lags behind that of the proximal airways during the first 5 years of life, accounting for the increased peripheral versus central airways resistance in children relative to adults (75) (Figure 4). According to Poiseuille’s law, resistance is inversely proportional to the radius of the airway to the fourth power, such that a similar reduction in airway caliber (e.g. by mucus, bronchospasm, edema, etc.) results in a greater decrease in the total cross-sectional area of the airway, as well as a relatively greater increase in resistance in children versus adults (Figure 5). The cartilaginous support of the peripheral airways is less well developed, increasing the risk of dynamic compression with high expiratory flow rates (e.g. as occurs during crying, coughing, or respiratory distress). Finally, the pathways of collateral ventilation (e.g., pores of Kohn) are not fully developed in young children. These pathways allow alveoli to participate in gas exchange even in the presence of an obstructed distal airway. Collectively, these important anatomic differences significantly increase the risk of atelectasis in children (69, 76).
Figure 4. Developmental influences on airway conductance in central versus peripheral airways.
(Note: Airway conductance is the inverse of airway resistance). The growth of the peripheral airways lags behind that of the central airways. As such, peripheral airway conductance increases with age – hence, peripheral airway resistance is higher in children compared to adults.
Copied with permission from Hogg JC, N Eng J Med 1970; 282:1283.
Figure 5. Model of the age-dependent effects of a reduction in airway caliber on the airway resistance and airflow.
Normal airways are represented on the left (top, infants; bottom, adults), edematous airways are represented on the right. According to Poiseuille’s law, airway resistance is inversely proportional to the radius of the airway to the fourth power when there is laminar flow and to the fifth power when there is turbulent flow. One mm of circumferential edema will reduce the diameter of the airway by 2 mm, resulting in a 16-fold increase in airway resistance in the pediatric airway versus a 3-fold increase in the adult (cross-sectional area reduced by 75% in the pediatric airway versus a 44% decrease in the adult airway). Note that turbulent air flow (such as occurs during crying) in the child would increase the resistance by 32-fold.
The developmental influences on respiratory mechanics are also critically important (77). For example, the ribs are more horizontally aligned in young infants and children compared to adults, which makes it difficult to generate a greater negative intrathoracic pressure in the presence of poor lung compliance. The elastic recoil pressure of the alveoli is reduced in children, which increases the risk of alveolar collapse in the presence of altered lung compliance. Similarly, the infant’s chest wall is soft and compliant, providing little opposition to the natural recoil (deflating tendency) of the lungs. This leads to a lower functional residual capacity in pediatric patients than in adults, which in young infants may even approach the critical closing volume of the alveolus (Figure 6). These developmental changes in both chest wall and lung compliance require the infant to perform more work than an adult to generate the same tidal volume. During an episode of respiratory distress, an infant will develop severe retractions in order to maintain acceptable oxygenation and ventilation. Unfortunately, a significant portion of the energy generated is wasted through the distortion of the highly compliant rib cage during negative pressure generation from contraction of the diaphragm (78). More importantly, some infants will stop breathing from fatigue when faced with excessive respiratory demands. This impression of diaphragmatic fatigue and failure has been confirmed through electromyographic measurements of the diaphragms of fatiguing infants who become apneic in the face of increased work of breathing (79–80). Collectively, all of these factors increase the risk of acute respiratory failure in children (69–70, 77).
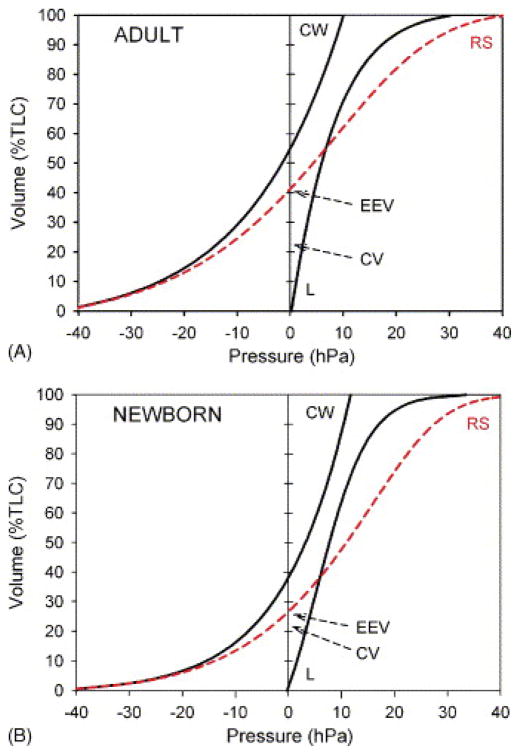
Figure 6. Changes in total respiratory system compliance (RS), chest wall compliance (CW), and lung compliance (L) as a function of age.
Two theoretical pressure-volume curves are provided for comparison – the top curve shows a pressure-volume curve in an adult, while the bottom curve shows a pressure-volume curve in a neonate. The normal elastic properties of the lung and chest wall are such that there is an inward elastic recoil of the lung (lung tends to collapse) and outward elastic recoil of the chest wall (chest wall tends to expand). At functional residual capacity (FRC) (depicted as the volume at which the airway pressure on the respiratory system pressure-volume curve is zero, EEV), these forces are in equilibrium. Note that the FRC is lower in children compared to adults. Also note that at FRC, the corresponding airway pressure on the chest wall curve is negative (i.e. at this volume, the natural tendency of the chest wall is to expand). The chest wall is in equilibrium (i.e. volume at which the corresponding airway pressure is zero) at a higher percentage of total lung capacity (TLC) in adults compared to children (due to increased chest wall compliance in children). Finally, also note that the closing volume (depicted as CC) approaches FRC in children compared to adults.
Adapted from West JB. Ventilation/Blood Flow and Gas Exchange. 3rd edition. Oxford: Blackwell; 1977. p33–52.
Ventilation-perfusion mismatching is one of the most common causes of hypoxemia in pediatric patients with sepsis. Under normal conditions, both ventilation and perfusion decrease significantly from the base of the lung to the apex, though perfusion decreases at a far more rapid rate (81). The effect of gravity on the thorax creates an intrapleural pressure gradient that distributes gas to the alveoli heterogeneously. Thus, the greater gravitational pressure at the base of the lung generates less intrapleural pressure and so expands these alveoli less. This creates a seeming paradox in the normal lung, where lower volume alveoli have greater compliance and are more easily inflated then higher volume alveoli because they are situated on the steeper segment of the pressure-volume curve (81). This results in increased ventilation in the dependent lung regions. Therefore, while the average ventilation/perfusion ratio is equal throughout the entire lung, there is greater ventilation to perfusion at the apex; while at the base, blood flow is in excess of ventilation (Figure 7). Pulmonary edema and inflammation in patients with sepsis worsen compliance and exaggerate the intrapleural pressure gradient. As intrapleural pressure exceeds alveolar pressure, atelectasis or closure of lung units in dependent lung regions will occur during a portion of tidal ventilation. This inverts the normal distribution of ventilation causing the apex of the lung to receive improved ventilation. While there are significant changes in the distribution of alveolar ventilation, perfusion is less affected. Perfusion continues to be greatest at the base of the lungs, such that poorly ventilated lung units continue to be perfused. There is evidence to suggest important differences in children versus adults with regards to regional differences in ventilation during sepsis. For example, in adults with unilateral lung collapse, ventilation and perfusion are better matched if patients are positioned with the “good lung” in a dependent position (“good lung down”). Conversely, in children with unilateral lung collapse, ventilation and perfusion are better matched if patients are positioned with the “good lung” in a non-dependent position (“bad lung down”) (82–85).
Figure 7. Differential distribution of ventilation (VA), perfusion (Q), and ventilation-perfusion ratio in the lung.
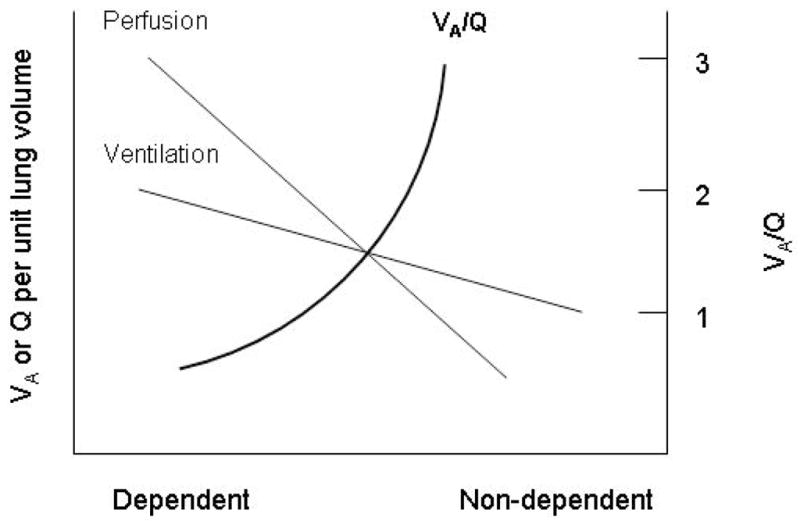
The dependent lung regions preferentially receive better ventilation and perfusion compared to the non-dependent lung regions. However, the perfusion gradient is much steeper than the ventilation gradient, such that the ventilation-perfusion ratio is higher in the non-dependent (apex) regions compared to the dependent (base) regions.
Adapted from West JB. Ventilation/Blood Flow and Gas Exchange. 3rd edition. Oxford: Blackwell; 1977. p33–52.
Sepsis is one of the most common causes of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (86). The Surviving Sepsis Campaign (87) recommends a target tidal volume (VT) of 6 mL/kg predicted body weight in critically ill adults with ALI/ARDS, based on the results of the ARDS Network study of low tidal volume ventilation (88). While a similar study has not been performed in critically ill children, most authorities recommend a similar low VT strategy in critically ill children with sepsis (89). These recommendations are based largely upon extrapolation from studies performed in critically ill adults, as well as the observation that this strategy has been utilized in several recently completed or ongoing multi-center, randomized, controlled trials in critically ill children with acute lung injury with acceptable results (90–92). There have been relatively few studies comparing the susceptibility to ventilator-induced lung injury (VILI) in children versus adults. Adkins and colleagues (93) reported that the lungs of young, newborn rabbits were more susceptible to the development of ventilator-induced lung injury (VILI) due to increased lung and chest wall compliance and larger distending volumes at high peak airway pressures compared to adult rabbits. However, Kavanagh’s group has published several studies using a rodent model of ventilator-induced lung injury (VILI) suggesting that newborns may not be as susceptible to the adverse effects of VILI compared to adults (94–96).
A recent single center, retrospective study compared the mortality in critically ill children with ALI from a time period before low VT ventilation was prevalent—the “past” group (1988–1992)—with that of critically ill children with ALI from an era when low VT ventilation had become well established in adult critical care—the “recent” group (2000–2004) (97). There were no significant differences between the two groups with regard to demographics, severity of illness, or baseline respiratory parameters. Data from the first three days of mechanical ventilation show that the recent group indeed had a lower mean VT/kg (8.1 ± 1.4 mL/kg vs. 10.2 ± 1.7 mL/kg, p<0.001), with resultant lower peak inspiratory pressure (PIP) (27.8 ± 4.2 mm Hg vs. 31.5 ± 7.3 mm Hg, p<0.001) and higher PaCO2 (47.2 ± 11.8 torr vs. 37.0 ± 5.0 torr, p<0.001). The recent group also had a higher positive end-expiratory pressure (PEEP) (7.1 ± 2.4 mm Hg vs. 6.1 ± 2.7 mm Hg, p=0.007), lower PaO2 (78.9 ± 14.9 torr vs. 84.4 ± 14.4 torr, p=0.017), and higher oxygenation index (17.7 ± 5.3 vs. 14.7 ± 5.0, p<0.001). The PaO2/FIO2 ratio trended lower in the recent group, but this was not statistically significant. More importantly, the recent group had significantly lower mortality (21% vs. 35%, p=0.04) and more ventilator-free days (16.0 ± 9.1 days vs. 12.7 ± 10 days, p=0.03). Multi-variate analysis showed that increased tidal volume, increased PRISM III score, and immunodeficiency were each independently associated with increased mortality in the past group. Neither era of treatment, indices of lung function, nor ventilation variables other than tidal volume were independently associated with mortality.
Interestingly, Erickson and colleagues (98) published the results of a prospective, observational study of ALI which included nearly all of the pediatric intensive care units in Australia and New Zeland. Contrary to the results reported above, and perhaps consistent with Kavanagh’s animal data (94–96), this study found that increased tidal volumes were associated with decreased mortality. These investigators noted that the majority of patients in their study received pressure control ventilation, and thus higher tidal volumes may have resulted from increased compliance (implying less severe lung disease.) This point is buttressed by the fact that higher PIP’s were associated with increased mortality in this same study, and (assuming similar inspiratory times) increased tidal volumes would require higher PIP’s in patients whose lung compliance was similar (99–100).
Collectively these studies further highlight the differences between critically ill children and adults, as well as the dangers of extrapolating the results of adult trials to children. In addition, these studies further highlight the need for a randomized, controlled clinical trial of low tidal volume ventilation in pediatric ALI, perhaps comparing tidal volumes of 6 mL/kg to 8 mL/kg. While such a trial would be challenging, given the lack of equipoise based on these conflicting data and the recent success of groups such as the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI), Canadian Critical Care Trials Group (CCCTG), and the Paediatric Study Group of the Australian and New Zealand Intensive Care Society, such a trial is both necessary and feasible.
Differences in Renal Function
Acute kidney injury (AKI), formerly known as acute renal failure, continues to represent a very common and potentially devastating problem in critically ill children and adults (101–105). Unfortunately, the mortality and morbidity associated with AKI remain unacceptably high, with mortality rates approaching 80% in critically ill children and adults with multiple organ dysfunction syndrome (MODS). While this dismal prognosis is partly attributable to other co-morbid conditions, recent studies have revealed that AKI may be an independent risk factor for mortality in both critically ill children (106–108) and adults (109–112). In other words, critically ill patients are not just dying with AKI, but importantly, in many cases, critically ill patients are dying from AKI. Sepsis remains a significant risk factor and one of the leading causes of AKI in critically ill children (104, 106–107, 113–119). While the pathophysiology of AKI in sepsis is somewhat controversial, most experts now believe that the prevailing mechanisms do not involve alterations in renal blood flow, but rather cellular injury arising from immunologic, toxic, and inflammatory factors (120–123). While we are not familiar with any studies that specifically describe the developmental influences on sepsis-induced AKI, there are a few studies suggesting that the renal response to hemorrhagic shock is influenced by stage of maturation (124–126). Moreover, the kidney’s response to prostaglandins, an important regulator of renal blood flow appears to be developmentally regulated (127–128). Clearly more studies need to be done, but collectively these studies offer insight into the potential maturational influences on sepsis-induced AKI.
Differences in the Coagulation Cascade
Disturbances in coagulation are common in both critically ill children and adults with sepsis. Sepsis is one of the most common causes of disseminated intravascular coagulation (DIC). DIC results from uncontrolled thrombin generation and subsequent microvascular thrombosis, resulting in end organ dysfunction and paradoxically, bleeding diathesis due to the consumption of coagulation factors (129–130). As such, DIC represents a major risk factor for developing the multiple organ dysfunction syndrome (MODS) (131) and has been associated with increased risk of mortality in critically ill children (129, 132) and adults with sepsis (133–134). Again, there are important developmental differences in the coagulation and fibrinolytic system that are likely to impact the pathophysiology and management of DIC in children versus adults (135). For example, neonates and infants less than one year of age appear to be at an increased risk for bleeding complications, primarily due to lower circulating levels of vitamin K-dependent procoagulant factors (factor II, VII, IX, and X), a decreased capacity to generate thrombin, and decreased circulating levels of coagulation inhibitors. Importantly, while the neonatal coagulation system contains all of the essential factors necessary for an intact coagulation system, the amounts of the individual coagulation factors are decreased relative to adult levels (135). Similarly, while there are no distinct differences in platelet quantity between children and adults, platelets are relatively hyporesponsive to physiologic agonists, resulting in an increased risk of bleeding, especially in the neonatal period (136). These unique differences may partly explain the increased risk of mortality in younger children with DIC, compared to older children (137).
Key differences in the Immune System
Aside from the important physiologic differences discussed above, there is now a growing body of literature to suggest that there are important differences in the host response to sepsis between children and adults that occur at the cellular and molecular level. There appears to be a bimodal age distribution in sepsis mortality, such that the very young and very old are at a significantly increased risk for death. Young mice (age 4 months - roughly equivalent to a human age of approximately 15 years) subjected to cecal ligation and puncture (CLP) had a lower mortality from sepsis (20% mortality at 10 days) compared to both adult mice (age 12 months – roughly equivalent to a human age of 40 years) (70% mortality at 10 days; p=0.0013 compared to young mice) and aged mice (age 24 months – roughly equivalent to a human age of approximately 80 years) (75% mortality at 10 days; p=0.0001 compared to young mice). Consistent with the increase in mortality in the older mice, the host inflammatory response, as determined by plasma levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-α was more pronounced as well (138). Unfortunately, this particular study did not examine mortality following CLP in immature mice. However, Wynn and colleagues compared the host inflammatory response and subsequent mortality in a fecal slurry model of generalized peritonitis between neonatal mice (age 5–7 days) and young adult mice (age 7–10 weeks). Compared with young adult mice, sepsis was associated with a markedly attenuated systemic inflammatory response and increased mortality (139).
The differences in the neonatal innate and adaptive immune response compared to adults have recently been reviewed (17, 140) (Table 1). Consistent with the studies in animal models described above, important developmental differences in the host inflammatory response have been observed in clinical studies as well. Barsness and colleagues collected peritoneal macrophages during laparoscopic surgery in children (mean age 3.6 years) and adults (mean age 46.9 years) and treated them ex vivo with IL-1β (141) and LPS (142). Both IL-1β- and LPS-induced TNF-α and IL-6 production were markedly increased in the peritoneal macrophage cultures obtained from children versus adults. The anti-inflammatory response, as determined by IL-10 production, was even greater in the cultures obtained from children. Finally, the ratio of IL-10 to TNF-α was much higher in the peritoneal macrophage cultures obtained from children compared to adults, suggesting a predominant anti-inflammatory phenotype (141–142). The compensatory anti-inflammatory response may therefore play a greater role in the pathophysiology of septic shock and multiple organ failure in children versus adults (143).
Table 1.
Deficits in the innate and adaptive immune response in neonates.
Adapted from (140)
| Deficits in Innate Immune Response | Deficits in Adaptive Immune Response |
|---|---|
| Decreased serum complement components | Greater requirement for CD4 T-cell stimulation |
| Defective neutrophil function (decreased chemotaxis, phagocytosis, respiratory burst) | TH2-skewed and attenuated CD4 T-cell cytokine response |
| Impaired Antigen Presenting Cell (APC) function | Poor CD4 T-cell-dependent B cell stimulation |
| Depressed Natural Killer (NK) cell function | Decreased CD8 T-cell cyotoxic activity |
| Immature Dendritic Cells | Weak humoral response (primarily IgM) |
| Impaired cytokine response to pathogens | Poor antibody response to polysaccharide antigens |
| Impaired response to TLR agonists | Immature (underdeveloped) spleen and lymph nodes |
| Decreased MHC Class 2 expression on APC’s | Limited antecedent exposure (no immunologic memory) |
| Decreased opsonin production | Presence of interfering maternal antibodies |
Conclusion
The developmental differences between children and adults have very important implications on the unique epidemiology, pathophysiology, and management of sepsis in children compared to adults. Indeed, there are important developmental differences in the host response to infection and therapy at the whole organism, organ system, cellular, and molecular level. These unique developmental differences further highlight the pitfalls of extrapolating the results of adult studies to children. Additional studies targeting sepsis in the pediatric population are urgently required.
Acknowledgments
Supported by the National Institutes of Health, K08GM077432 (DSW), R03HD058246 (DSW), R01AG027990 (BZ), R01GM067202, R01GM064619 (HRW)
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 3.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, et al. Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 4.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 5.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomized trial. Lancet. 2000;356:961–7. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 6.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, et al. Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet. 2007;369:836–43. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 7.Shanley TP, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, et al. Genome-Level Longitudinal Expression of Signaling Pathways and Gene Networks in Pediatric Septic Shock. Mol Med. 2007 Sep;13(9–10):495–508. doi: 10.2119/2007-00065.Shanley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taqi AM, Macfarlane JT, Morton R, Wali SS, Greenwood BM. Treatment of acute meningococcaemia with chemotherapy and immune plasma. J Infect. 1980;2:145–9. doi: 10.1016/s0163-4453(80)91184-6. [DOI] [PubMed] [Google Scholar]
- 9.Pollack MM, Fields AI, Ruttiman UE. Sequential cardiopulmonary variables of infants and children in septic shock. Crit Care Med. 1984;12:554–9. doi: 10.1097/00003246-198407000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–5. [PubMed] [Google Scholar]
- 11.Proulx F, Fayon M, Farrell CA. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–7. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 12.Arnal LE, Stein F. Pediatric septic shock: Why has mortality decreased? The utility of goal-directed therapy. Semin Pediatr Infect Dis. 2003;14:165–72. doi: 10.1053/spid.2003.127233. [DOI] [PubMed] [Google Scholar]
- 13.Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. Jama. 2000 Apr 5;283(13):1723–30. doi: 10.1001/jama.283.13.1723. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 15.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 16.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;2005:S3–S5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 17.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–41. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler DS, Zingarelli B, Wheeler WJ, Wong HR. Novel pharmacologic approaches to the management of sepsis: Targeting the host inflammatory response. Recent Pat Inflamm Allergy Drug Discov. 2009;3:96–112. doi: 10.2174/187221309788489779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazelzet J, Driessen GJA, Abboud P, Wheeler DS, Shanley TP, Wong HR. Sepsis. In: Wheeler DS, Wong HR, Shanley TP, editors. Pediatric Critical Care Medicine: Basic Science and Clinical Evidence. London, UK: Springer-Verlag London Limited; 2007. pp. 1421–44. [Google Scholar]
- 20.Thomas NJ, Carcillo JA. Hypovolemic shock in the pediatric patient. New Horizons. 1998;6(2):120–9. [PubMed] [Google Scholar]
- 21.Carcillo JA. Pediatric septic shock and multiple organ failure. Crit Care Clin. 2003;19:413–40. doi: 10.1016/s0749-0704(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 22.Carcillo JA, Wheeler DS, Kooy NW, Shanley TP, editors. Shock. London: Springer-Verlag; 2007. [Google Scholar]
- 23.Friss-Hansen BJ, Holiday M, Stapleton T, Wallace WM. Total body water in children. Pediatrics. 1951;7:321–7. [PubMed] [Google Scholar]
- 24.Aloia JF, Vaswani A, Flaster E, Ma R. Relationship of body water compartments to age, race, and fat-free mass. J Lab Clin Med. 1998;132:483–90. doi: 10.1016/s0022-2143(98)90126-3. [DOI] [PubMed] [Google Scholar]
- 25.Ritz P, Vol S, Berrut G, Tack I, Arnaud MJ, Tichet J. Influence of gender and body composition on hydration and body water spaces. Clin Nutrition. 2008;27:740–6. doi: 10.1016/j.clnu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Greenbaum LA, editor. Electrolyte and acid-base disorders. 18. Philadelphia: Saunders; 2007. [Google Scholar]
- 27.Czaczkes JW. Plasma volume as an index of total fluid loss. Am J Dis Child. 1962;102:190–3. doi: 10.1001/archpedi.1961.02080010192006. [DOI] [PubMed] [Google Scholar]
- 28.Perkin RM, Levin DL. Shock in the pediatric patient. J Pediatr. 1982;101:163–9. doi: 10.1016/s0022-3476(82)80110-8. [DOI] [PubMed] [Google Scholar]
- 29.Barr PA, Bailey PE, Sumners J, Cassady G. Relation between arterial blood pressure and blood volume and effect of infused albumin in sick preterm infants. Pediatrics. 1977;60:282–9. [PubMed] [Google Scholar]
- 30.Walther FJ, Siassi B, Ramadan NA, Wu PY. Cardiac output in newborn infants with transient myocardial dysfunction. J Pediatr. 1985;107:781–5. doi: 10.1016/s0022-3476(85)80417-0. [DOI] [PubMed] [Google Scholar]
- 31.Gill AB, Weindling AM. Cardiac function in the shocked very low birth weight infant. Arch Dis Child. 1993;68:17–21. doi: 10.1136/adc.68.1_spec_no.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill AB, Weindling AM. Echocardiographic assessment of cardiac function in shocked very low birthweight infants. Arch Dis Child. 1993;68:17–21. doi: 10.1136/adc.68.1_spec_no.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer K, Linderkamp O, Versmold HT. Systolic blood pressure and blood volume in preterm infants. Arch Dis Child. 1993;69:521–2. doi: 10.1136/adc.69.5_spec_no.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright IMR, Goodall SR. Blood pressure and blood volume in preterm infants. Arch Dis Child. 1994;70:F230–F2. doi: 10.1136/fn.70.3.f230-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill AB, Weindling AM. Randomized controlled trial of plasma protein fraction versus dopamine in hypotensive very low birth weight infants. Arch Dis Child. 1993;69:284–7. doi: 10.1136/adc.69.3_spec_no.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasgupta SJ, Gill AB. Hypotension in the very low birthweight infant: the old, the new, and the uncertain. Arch Dis Child Fetal Neonatal Ed. 2003;88:F450–F4. doi: 10.1136/fn.88.6.F450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz SM, Duffy JY, Pearl JM, Nelson DP. Cellular and molecular aspects of myocardial dysfunction. Crit Care Med. 2001;29:S214–S9. doi: 10.1097/00003246-200110001-00003. [DOI] [PubMed] [Google Scholar]
- 38.Luce WA, Hoffman TM, Bauer JA. Bench-to-bedside review: Developmental influences on the mechanisms, treatment and outcomes of cardiovascular dysfunction in neonatal versus adult sepsis. Critical Care. 2007;11:228. doi: 10.1186/cc6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seri I. Circulatory support of the sick preterm infant. Semin Neonatol. 2001;6:85–95. doi: 10.1053/siny.2000.0034. [DOI] [PubMed] [Google Scholar]
- 40.Noori S, Seri I. Pathophysiology of newborn hypotension outside the transitional period. Early Hum Dev. 2005;81:399–404. doi: 10.1016/j.earlhumdev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Wibo M, Bravo G, Godfraind T. Postnatal maturation of excitation-contraction coupling in rate ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res. 1991;68:662–73. doi: 10.1161/01.res.68.3.662. [DOI] [PubMed] [Google Scholar]
- 42.Brillantes AM, Bezprozvannaya S, Marks AR. Developmental and tissue-specific regulation of rabbit skeletal and cardiac muscle calcium channels involved in excitation-contraction coupling. Circ Res. 1994;75:503–10. doi: 10.1161/01.res.75.3.503. [DOI] [PubMed] [Google Scholar]
- 43.Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Perez CG, Meija-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H971–H8. doi: 10.1152/ajpheart.00308.2003. [DOI] [PubMed] [Google Scholar]
- 44.Huang J, Xu L, Thomas M, Whitaker K, Hove-Madsen L, Tibbits GF. L-type Ca2+ channel function and expression in neonatal rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2006;290:H2267–H76. doi: 10.1152/ajpheart.01093.2005. [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Hove-Madsen L, Tibbits GF. Ontogeny of the Ca2+-induced Ca2+ release in rabbit ventricular myocytes. Am J Physiol. 2008;294:C516–C25. doi: 10.1152/ajpcell.00417.2007. [DOI] [PubMed] [Google Scholar]
- 46.Murdoch IA, Quershi SA, Huggon IC. Perioperative haemodynamic effects of an intravenous infusion of calcium chloride in children following cardiac surgery. Acta Paediatr. 1994;83:658–61. doi: 10.1111/j.1651-2227.1994.tb13103.x. [DOI] [PubMed] [Google Scholar]
- 47.Morrissey A, Parachuru L, Leung M, Lopez G, Nakamura TY, Tong X, et al. Expression of ATP-sensitive K+ channel subunits during perinatal maturation in the mouse heart. Pediatr Res. 2005;58:185–92. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- 48.Kuznetsov V, Pak E, Robinson RB, Steinberg SF. Beta-2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- 49.Findlay I. The ATP sensitive potassium channel of cardiac muscle and action potential shortening during metabolic stress. Cardiovasc Res. 1994;28:760–1. doi: 10.1093/cvr/28.6.760. [DOI] [PubMed] [Google Scholar]
- 50.Chen CC, Lin YC, Chen SA, Luk HN, Ding PY, Chang MS, et al. Shortening of cardiac action potentials in endotoxic shock in guinea pigs is caused by an increase in nitric oxide activity and activation of the adenosine triphosphate-sensitive potassium channels. Crit Care Med. 2000;28:1713–20. doi: 10.1097/00003246-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Buckley JF, Singer M, Clapp LH. Role of KATP channels in sepsis. Cardiovasc Res. 2006;72:220–30. doi: 10.1016/j.cardiores.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: Metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–93. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- 53.Crepaz R, Pitscheider W, Radetti G, Gentili L. Age-related variation in left ventricular myocardial contractile state expressed by the stress velocity relation. Pediatr Cardiol. 1998;19:463–7. doi: 10.1007/s002469900358. [DOI] [PubMed] [Google Scholar]
- 54.Crepaz R, Cemin R, Pedron C, Gentili L, Trevisan D, Pitscheider W. Age-related variations of left ventricular endocardial and midwall function in healthy infants, children, and adolescents. Ital Heart J. 2005;6:634–9. [PubMed] [Google Scholar]
- 55.Ichihashi K, Ewert P, Welmitz G, Lange P. Changes in ventricular and muscle volumes of neonates. Pediatr Int. 1999;41:8–12. doi: 10.1046/j.1442-200x.1999.01008.x. [DOI] [PubMed] [Google Scholar]
- 56.Joyce JJ, Dickson PI, Qi N, Noble JE, Raj JU, Baylen BG. Normal right and left ventricular mass development during early infancy. Am J Cardiol. 2004;93:797–801. doi: 10.1016/j.amjcard.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 57.Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collage and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol. 1994;23:1204–8. doi: 10.1016/0735-1097(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 58.Wei S, Chow LT, Shum IO, Qin L, Sanderson JE. Left and right ventricular collagen type I/III ratios and remodeling post-myocardial infarction. J Card Fail. 1999;5:117–26. doi: 10.1016/s1071-9164(99)90034-9. [DOI] [PubMed] [Google Scholar]
- 59.Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: A quantitative assessment. J Am Coll Cardiol. 1995;25:1263–72. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 60.Rowland DG, Gutgesell HP. Non-invasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am J Cardiol. 1995;75:818–21. doi: 10.1016/s0002-9149(99)80419-6. [DOI] [PubMed] [Google Scholar]
- 61.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid refractory pediatric septic shock. Pediatrics. 1998;102:e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 62.Reynolds EM, Ryan DP, Sheridan RL, Doody DP. Left ventricular failure complicating severe pediatric burn injuries. J Pediatr Surg. 1995;30:264–70. doi: 10.1016/0022-3468(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 63.Pollack MM, Fields AI, Ruttiman UE. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit Care Med. 1985;13:454–9. doi: 10.1097/00003246-198506000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. 1975;51:867–74. doi: 10.1161/01.cir.51.5.867. [DOI] [PubMed] [Google Scholar]
- 65.Mercier J-C, Beaufils F, Hartmann J-F, Azema D. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16:27–33. doi: 10.1097/00003246-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Feltes TF, Pignatelli R, Kleinart S, Mariscalco MM. Quantitated left ventricular systolic mechanics in children with septic shock utilizing noninvasive wall-stress analysis. Crit Care Med. 1994;22:1647–58. [PubMed] [Google Scholar]
- 67.Wheeler DS, Spaeth JD, Mehta R, Hariprakash SP, Cox PN, editors. The pediatric airway. London: Springer-Verlag; 2007. [Google Scholar]
- 68.Stocks J. Respiratory physiology during early life. Monaldi Arch Chest Dis. 1999;54:358–64. [PubMed] [Google Scholar]
- 69.Bateman ST, Arnold JH. Acute respiratory in children. Curr Opin Pediatr. 2000;12:233–7. doi: 10.1097/00008480-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Chase M, Wheeler DS, editors. The Pediatric Chest. London: Springer-Verlag London Limited; 2007. [Google Scholar]
- 71.Boyden EA, Tompsett DH. The changing patterns in the developing lungs of infants. Acta Anat (Basel) 1965;61:164–92. doi: 10.1159/000142692. [DOI] [PubMed] [Google Scholar]
- 72.Reid L. Influence of the pattern of structural growth of lung on susceptibility to specific infectious diseases in infants and children. Pediatr Res. 1977;11:210–5. [PubMed] [Google Scholar]
- 73.Thurlbeck WM. Postnatal growth of the lung and its significance in disease. Hum Pathol. 1978;9:492–3. doi: 10.1016/s0046-8177(78)80130-0. [DOI] [PubMed] [Google Scholar]
- 74.Zeman KL, Bennett WD. Growth of the small airways and alveoli from childhood to the adult lung measured by aerosol-derived airway morphometry. J Appl Physiol. 2006;100:965–71. doi: 10.1152/japplphysiol.00409.2005. [DOI] [PubMed] [Google Scholar]
- 75.Hogg JC, Williams J, Richardson JB, Macklem PT, Thurlbeck WM. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med. 1970;282:1283–7. doi: 10.1056/NEJM197006042822302. [DOI] [PubMed] [Google Scholar]
- 76.Peroni DG, Boner AL. Atelectasis: mechanisms, diagnosis, and management. Paediatr Respir Rev. 2000;1:274–8. doi: 10.1053/prrv.2000.0059. [DOI] [PubMed] [Google Scholar]
- 77.Muller NL, Bryan AC. Chest wall mechanics and respiratory muscles in infants. Pediatr Clin North Am. 1979;26:503–16. doi: 10.1016/s0031-3955(16)33745-2. [DOI] [PubMed] [Google Scholar]
- 78.Guslits BG, Gaston SE, Bryan MH, England SJ, Bryan AC. Diaphragmatic work of breathing in premature human infants. J Appl Physiol. 1987;62:1410–5. doi: 10.1152/jappl.1987.62.4.1410. [DOI] [PubMed] [Google Scholar]
- 79.Muller N, Volgyesi G, Calle D, Whitton J, Froes AB, Bryan MH, et al. Diaphragmatic muscle fatigue in the newborn. J Appl Physiol. 1979;46:688–95. doi: 10.1152/jappl.1979.46.4.688. [DOI] [PubMed] [Google Scholar]
- 80.Muller N, Volgyesi G, Bryan MH, Bryan AC. The consequences of diaphragmatic muscle fatigue in the newborn infant. J Pediatr. 1979;95:793–7. doi: 10.1016/s0022-3476(79)80738-6. [DOI] [PubMed] [Google Scholar]
- 81.West JB. Respiratory physiology: the essentials. 6. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 82.Heaf DP, Helms P, Gordon I, Turner HM. Postural effects on gas exchange in infants. N Engl J Med. 1983;308:1505–8. doi: 10.1056/NEJM198306233082505. [DOI] [PubMed] [Google Scholar]
- 83.Davies H, Kitchman R, Gordon I, Helms P. Regional ventilation in infancy. Reversal of adult pattern. N Engl J Med. 1985;313:1626–8. doi: 10.1056/NEJM198512263132603. [DOI] [PubMed] [Google Scholar]
- 84.Bhuyan U, Peters AM, Gordon I, Davies H, Helms P. Effects of posture on the distribution of pulmonary ventilation and perfusion in children and adults. Thorax. 1989;44:480–4. doi: 10.1136/thx.44.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies H, Helms P, Gordon I. Effect of posture on regional ventilation in children. Pediatr Pulmonol. 1992;12:227–32. doi: 10.1002/ppul.1950120406. [DOI] [PubMed] [Google Scholar]
- 86.Timmons O. Infection in pediatric acute respiratory distress syndrome. Semin Pediatr Infect Dis. 2006;17:65–71. doi: 10.1053/j.spid.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 88.Network TARDS. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 89.Hanson JH, Flori H. Application of the acute respiratory distress syndrome network low-tidal volume strategy to pediatric acute lung injury. Respir Clin North Am. 2006;12:349–57. doi: 10.1016/j.rcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 90.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA. 2002;288:2561–8. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 91.Randolph AG, Forbes PW, Gedeit RG, Arnold JH, Wetzel RC, Luckett PM, et al. Cumulative fluid intake minus output is not associated with ventilator weaning during or extubation outcomes in children. Pediatr Crit Care Med. 2005;6:642–7. doi: 10.1097/01.pcc.0000185484.14423.0d. [DOI] [PubMed] [Google Scholar]
- 92.Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: A randomized controlled trial. JAMA. 2005;294:229–37. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adkins WK, Herndon LA, Coker PJ, Buchanan B, Parker JC. Age effects susceptibility to pulmonary barotrauma in rabbits. Crit Care Med. 1991;19:390–3. doi: 10.1097/00003246-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 94.Copland IB, Martinez F, Kavanagh BP, Engelberts D, McKerlie C, Belik J, et al. High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med. 2004;169:739–48. doi: 10.1164/rccm.200310-1417OC. [DOI] [PubMed] [Google Scholar]
- 95.Kornecki A, Tsuchida S, Ondiveeran HK, Engelberts D, Frndova H, Tanswell AK, et al. Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;171:743–52. doi: 10.1164/rccm.200408-1053OC. [DOI] [PubMed] [Google Scholar]
- 96.Martinez F, Lewis J, Copland I, Engelberts D, Kavanagh BP, Post M, et al. Mechanical ventilation effect on surfactant content, function, and lung compliance in the newborn rat. Pediatr Res. 2004;56:19–25. doi: 10.1203/01.PDR.0000128980.82797.29. [DOI] [PubMed] [Google Scholar]
- 97.Albuali WH, Singh RN, Fraser DD, Seabrook JA, Kavanagh BP, Parshuram CS, et al. Have changes in ventilation practice improved outcome in children with acute lung injury? Pediatr Crit Care Med. 2007;8:324–30. doi: 10.1097/01.PCC.0000269390.48450.AF. [DOI] [PubMed] [Google Scholar]
- 98.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: A prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–23. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 99.Jouvet P, Lacroix J. Acute lung injury: Is volume the question? Pediatr Crit Care Med. 2007;8:397–8. doi: 10.1097/01.PCC.0000269381.43529.92. [DOI] [PubMed] [Google Scholar]
- 100.Nowak J, Wheeler D. Pediatric Critical Care Journal Club - October 2007. New York: American Thoracic Society; 2007. [cited 2009 May 16]; Available from: http://www.thoracic.org/sections/clinical-information/critical-care/journal-club/pediatrics/oct-2007.html. [Google Scholar]
- 101.Brady H, Singer G. Acute renal failure. Lancet. 1995;346:1533–40. doi: 10.1016/s0140-6736(95)92057-9. [DOI] [PubMed] [Google Scholar]
- 102.Thadhani R, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 103.Nolan CR, Anderson RJ. Hospital-acquired acute renal failure. J Am Soc Nephrol. 1998;9:710–8. doi: 10.1681/ASN.V94710. [DOI] [PubMed] [Google Scholar]
- 104.Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005;45:96–101. doi: 10.1053/j.ajkd.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 105.Uchino S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12:538–43. doi: 10.1097/01.ccx.0000247448.94252.5a. [DOI] [PubMed] [Google Scholar]
- 106.Plotz FB, Hulst HE, Twist JW, Bokenkamp A, Markhorst DG, van Wijk JA. Effect of acute renal failure on outcome in children with severe septic shock. Pediatr Nephrol. 2005;20:1177–81. doi: 10.1007/s00467-005-1946-1. [DOI] [PubMed] [Google Scholar]
- 107.Bailey D, Phan V, Litalien C, Ducruet T, Merouani A, Lacroix J, et al. Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8:29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 108.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 109.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: Comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002;62:986–96. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 110.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30:2051–8. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 111.Bagshaw SM, Mortis G, Doig CJ, Godinez-Luna T, Fick GH, Laupland KB. One-year mortality assessment in critically ill patients by severity of kidney dysfunction: A population-based assessment. Am J Kidney Dis. 2006;48:402–9. doi: 10.1053/j.ajkd.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 112.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–94. [PubMed] [Google Scholar]
- 113.Otukesh H, Hoseini R, Hooman N, Chalian M, Chalian H, Tabarroki A. Prognosis of acute renal failure in children. Pediatr Nephrol. 2006;21:1873–8. doi: 10.1007/s00467-006-0240-1. [DOI] [PubMed] [Google Scholar]
- 114.Williams DM, Sreedhar SS, Mickell JS, Chan JCM. Acute kidney failure: A pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002;156:893–900. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 115.Arora P, Kher V, Rai PK, Singhal MK, Gulati S, Gupta A. Prognosis of acute renal failure in children: A multivariate analysis. Pediatr Nephrol. 1997;11:153–5. doi: 10.1007/s004670050247. [DOI] [PubMed] [Google Scholar]
- 116.Lowrie LH. Renal replacement therapies in pediatric multi-organ dysfunction syndrome. Pediatr Nephrol. 2000;14:6–12. doi: 10.1007/s004670050002. [DOI] [PubMed] [Google Scholar]
- 117.Goldstein SL, Currier H, Graf JM, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–12. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 118.Bunchman TE, McBryde KD, Mottes TE, Gardner JJ, Maxvold NJ, Brophy PD. Pediatric acute renal failure: Outcome by modality and disease. Pediatr Nephrol. 2001;16:1067–71. doi: 10.1007/s004670100029. [DOI] [PubMed] [Google Scholar]
- 119.Loza R, Estremadoyro L, Loza C, Cieza J. Factors associated with mortality in acute renal failure (ARF) in children. Pediatr Nephrol. 2006;21:106–9. doi: 10.1007/s00467-005-2038-y. [DOI] [PubMed] [Google Scholar]
- 120.Langenberg C, Bagshaw SM, May CN, Bellomo R. The histopathology of septic acute kidney injury: A systematic review. Crit Care. 2008;12:R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: What do we really know? Crit Care Med. 2008;36:S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 122.Klenzak J, Himmelfarb J. Sepsis and the kidney. Crit Care Clin. 2005;21:211–22. doi: 10.1016/j.ccc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 123.Bellomo R, Wan L, Langenberg C, May C. Septic acute kidney injury: New concepts. Nephron Exp Nephrol. 2008;109:e95–e100. doi: 10.1159/000142933. [DOI] [PubMed] [Google Scholar]
- 124.Thomson JJ, Smith FG. Age-dependent cardiovascular, renal, and endocrine responses to furosemide in conscious lambs. Clin Exp Pharmacol Physiol. 2004;31:70–5. doi: 10.1111/j.1440-1681.2004.03952.x. [DOI] [PubMed] [Google Scholar]
- 125.Smith FG, Sener A, Basati R, Abu-Amarah I. Renal response to hemorrhage are age dependent in conscious sheep. J Appl Physiol. 2004;96:131–6. doi: 10.1152/japplphysiol.00492.2003. [DOI] [PubMed] [Google Scholar]
- 126.Smith FG, Abu-Amarah I. Systemic and renal hemodynamic effects of hemorrhage in conscious lambs. Am J Physiol. 1997;273:H339–H46. doi: 10.1152/ajpheart.1997.273.1.H339. [DOI] [PubMed] [Google Scholar]
- 127.Drukker A, Mosig D, Guignard JP. The renal hemodynamic effects of aspirin in newborn and young adult rabbits. Pediatr Nephrol. 2001;16:713–8. doi: 10.1007/s004670100641. [DOI] [PubMed] [Google Scholar]
- 128.Toth-Heyn P, Drukker A, Guignard JP. The stressed neonatal kidney: From pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol. 2000;14:227–39. doi: 10.1007/s004670050048. [DOI] [PubMed] [Google Scholar]
- 129.Kenet G, Strauss T, Kaplinsky C, Paret G. Hemostasis and thrombosis in critically ill children. Semin Thromb Hemost. 2008;34:451–8. doi: 10.1055/s-0028-1092875. [DOI] [PubMed] [Google Scholar]
- 130.Levi M, Schouten M, Van der Poll T. Sepsis, coagulation, and antithrombin: Old lessons and new insights. Semin Thromb Hemost. 2008;34:742–6. doi: 10.1055/s-0029-1145256. [DOI] [PubMed] [Google Scholar]
- 131.Proulx F, Sebastien J, Mariscalco MM, Leteutre S, Leclerc F, Lacroix J. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 132.Khemani RG, Bart RD, Alonzo TA, Hatzakis G, Hallam D, Newth CJ. Disseminated intravascular coagulation score is associated with mortality for children with shock. Intensive Care Med. 2009;35:327–33. doi: 10.1007/s00134-008-1280-8. [DOI] [PubMed] [Google Scholar]
- 133.Vervloet MG, Thijs LG, Hack CE. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost. 1998;24:33–44. doi: 10.1055/s-2007-995821. [DOI] [PubMed] [Google Scholar]
- 134.Levi M. Pathogenesis and treatment of disseminated intravascular coagulation in the septic patient. J Crit Care. 2001;16:167–77. doi: 10.1053/jcrc.2001.30666. [DOI] [PubMed] [Google Scholar]
- 135.Kuhle S, Male C, Mitchell L. Developmental hemostasis: Pro- and anticoagulant systems during childhood. Semin Thromb Hemost. 2003;29:329–37. doi: 10.1055/s-2003-42584. [DOI] [PubMed] [Google Scholar]
- 136.Israels SJ, Rand ML, Michelson AD. Neonatal platelet function. Semin Thromb Hemost. 2003;29:363–71. doi: 10.1055/s-2003-42587. [DOI] [PubMed] [Google Scholar]
- 137.Hazelzet JA, Risseeuw-Appel IM, Kornelisse RF, Hop WC, Dekker I, Joosten KF, et al. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemost. 1996;76:932–8. [PubMed] [Google Scholar]
- 138.Turnbull IR, Wizorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–3. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 139.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–83. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 140.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009;29:79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, Banerjee A, et al. IL-1beta induces an exagerrated pro- and anti-inflammatory response in peritoneal macrophages of children compared with adults. Pediatr Surg Int. 2004;20:238–42. doi: 10.1007/s00383-003-1118-y. [DOI] [PubMed] [Google Scholar]
- 142.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre J, RC Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg. 2004;39:912–5. doi: 10.1016/j.jpedsurg.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 143.Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin-10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113:1625–31. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]