Abstract
The presence of active developmental angiogenesis and vascular outgrowth in the postnatal brain may differentially affect vascular responses to stroke in newborns and adults, but very little is known about the dynamics of vascular injury and re-growth after stroke during the neonatal period. In this study we used a clinically relevant animal model of ischemic arterial stroke in neonate rats, a transient middle cerebral artery occlusion (MCAO) in postnatal day 7 (P7), to characterize the effects of injury on vascular density and angiogenesis from acute through the chronic phase. A marked vessel degeneration and suppressed endothelial cell proliferation occur in the ischemic regions early after neonatal stroke. In contrast to what has been described in adult animals, endothelial cell proliferation and vascular density are not increased in the peri-ischemic regions during the first week after MCAO in neonates. By two weeks after injury, endothelial cell proliferation is increased in the cortical peri-ischemic region but these changes are not accompanied by an increased vascular density. Suppressed angiogenesis in injured postnatal brain that we report may limit recovery after neonatal stroke. Thus, enhancement of angiogenesis after neonatal stroke may be a promising strategy for the long-term recovery of the affected newborns.
Keywords: neonatal stroke, angiogenesis, blood-brain barrier, endothelial
Introduction
The estimated incidence of neonatal arterial stroke is of 1 in 5000 births [1, 2]. Neonatal stroke leads to permanent disabilities in approximately 57% of the cases, including motor deficits, cerebral palsy, epilepsy and/or learning impairment [3] and requires life-long medical and social care. Risk factors for neonatal arterial stroke are broad, including maternal disorders (pre-eclampsia, chorioamnionitis, autoimmune disorders), placental disorders (abruption, thrombosis), cardiac disorders (congenital heart failure, patent ductus arteriosus, pulmonary valve atresia), systemic infections, and other blood disorders [2]. In stark contrast to adult stroke, studies using age-appropriate animal models of neonatal focal stroke have been very limited, in part contributing to lack of treatments for newborn stroke patients.
To date, only hypothermia (head or body cooling of term human babies) has provided encouraging results for prevention of long-lasting consequences of neonatal encephalopathy [4]. However, hypothermia alone may not be sufficient to consistently prevent the progression of brain damage [5]. The use of thrombolytics, the only approved treatment for a subset of adult stroke patients, is not recommended for treatment of neonatal stroke, since its effectiveness and safety have not been demonstrated in newborns [6]. Thus, a better understanding of the mechanisms of brain injury and repair following neonatal stroke is necessary to identify new potential therapeutic targets for the treatment of newborn stroke patients.
Vascular immaturity or abnormalities in part own to differences in the pathogenesis of stroke in neonates, children and adults. A growing number of studies show that neurovascular integrity is controlled differently in the normal developing and adult brain [7] and that both gene and protein expression of BBB endothelial proteins undergo major changes from embryonic period to adulthood [8-10]. However, these differences are not incremental or continuous, as one would expect based on data on the continuously increasing astrocyte or pericyte coverage during postnatal brain maturation. Using a model of transient middle cerebral artery occlusion (MCAO) in postnatal day 7 (P7) rats and a similar model in the adult, we recently discovered that the neonatal blood brain barrier (BBB) is intrinsically more resistant to ischemic injury than the adult BBB [11]. While somewhat paradoxical, this conclusion is supported by several lines of evidence, including endothelial transcriptome data and data on the functional and structural integrity of the BBB in injured adult and neonatal rats. Although functional intactness of the BBB during acute injury is likely to protect and support recovery, leakiness of the BBB is thought necessary to allow angiogenesis and support migration of neural progenitors [12]. Persistent BBB impermeability in injured neonatal brain over time, if the case, would translate in a reduced capacity of brain vessels to exert the plastic responses necessary for angiogenesis, vascular outgrowth and neurogenesis during the recovery phase. In this study we aimed to characterize the effects of neonatal focal arterial stroke on endothelial proliferation and vascular outgrowth and maturation during both the acute and chronic injury phases. Understanding developmental differences in the CNS responses to injury is a fundamental step for the identification of pharmacologic targets and potential therapies designed for the treatment of neonatal stroke patients.
Materials and Methods
MCAO model in P7 rats
All animal research was approved by the University of California San Francisco Institutional Animal Care and Use Committee and was performed in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. Department of Healt and Human Services, Publication Number 85-23, 1985). Female Sprague-Dawley rats with a 5- to 6-day-old litter (10±1 pups per litter) were given food and water ad libitum and housed in a temperature/light controlled animal care facility. A transient 3-hour MCAO was achieved by inserting a 6-0 coated filament into the internal carotid artery of P7 rats, as previously described [13]. Reperfusion of the MCA was achieved by the retraction of the filament. The pups were then returned to the dam until sacrificed.
BrdU injections
To label proliferating cells 5-bromo-2’-deoxyuridine (BrdU, Roche, 50mg/kg in 0.85% sterile saline) was intraperitoneally injected to the rat pups twice daily during the two days prior to sacrifice at P14 or P21.
Western blot
Protein expression of claudin-1, laminin and PDGFR-β was determined in lysates obtained from P7, P14 and P17 naïve rat brains. After SDS-PAGE, membranes were blocked with 5% non-fat milk in 0.2% Tween TBS 1x (TBST) and incubated with the following primary antibodies: mouse anti-claudin-1 (1:500, Invitrogen), rabbit anti-laminin (1:1000, Novus Biologicals), mouse anti-PDGFR-β (1:500, R&D systems) and mouse anti-β-actin (1:5000, Sigma-Aldrich) in 5% milk TBST overnight at 4°C. Membranes were washed 3 × 10 minutes with TBST and incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 hour at RT. Membranes were washed 3 × 10 minutes with TBST and developed using ECL kit (Thermo Scientific).
Histology and immunofluorescence
Animals were perfused transcardiacally with 4% paraformaldehyde in 0.1M phosphate buffer (PB), the brains postfixed for 24 hours in the same solution and dehydrated in 30% sucrose in 0.1M PB. Brains were then cut in 12 μm-thick slices in a cryostat. Serial sections were processed for double immunofluorescence. For BrdU unmasking, slides were incubated in 2N HCl for 30 minutes at 37°C followed by incubation in boric acid (0.1M, pH 8.5) for 10 minutes and in phosphate buffer saline (PBS) two times for 10 minutes. For unmasking of proliferating cell nuclear antigen (PCNA), slides were boiled in 2.5mM sodium citrate in a microwaves and cooled down at room temperature (RT), then washed 3×10 minutes with PBS 1x. Slides were blocked in 10% normal goat serum (NGS) in PBST (0.1% Triton X-100) and incubated overnight at 4°C in blocking solution with primary antibodies: mouse anti-RECA-1 (rat endothelial cell antigen, for labeling of endothelial cells, 1:200, ABD Serotec), rabbit anti-PECAM-1 (for detection of endothelial cells, 1:500, kind gift from Peter Newman's laboratory, Blood Center of Wisconsin), mouse anti-PCNA (proliferating nuclear cell antigen, 1:100, Santa Cruz Biotechnology), rat anti-BrdU (1:100, ABD Serotec) and mouse anti-rat EBA (endothelial barrier antigen, 1:1000, Covance). Slides were then washed in PBST and incubated with secondary antibodies (mouse or rabbit Alexa-488 or Alexa-568) and Alexa-647 isolectin-B4 for 1 hour at RT. After washing with PBS slides were incubated with DAPI for 2 minutes in PBS. Slides were washed two times in PBS and coverslipped using FluoroGold mounting medium.
Image capturing and quantification
A minimum of three z-stacks (10 μm thick, 1 μm z-spacing) per brain and per region were captured using the 25x objective. The regions of interest included the ischemic core and peri-ischemic region in the cortex and the caudate, regions identified by the presence of abnormally looking DAPI+ nuclei, and matching contralateral regions. Z-stacks were analyzed using Volocity software (Improvision). Vascular density is expressed as the volume percentage occupied by brain vessels in the three different fields of view per region and animal. Vessel number and length are expressed as the sum of the values obtained from three different fields of view per region and animal. Endothelial tip cells were identified and quantified by PECAM-1 immunofluorescence. Endothelial cell proliferation was quantified as the number of PCNA+/PECAM-1+ and BrdU+/RECA-1+ cells, and expressed as the sum of the three fields of view per region and animal. EBA presence in brain vessels was analyzed by colocalization with the vascular marker isolectin-B4.
Statistical analysis
Data are expressed and plotted as the mean ± SD. Data were tested for normality using the Kolmogorov-Smirnoff test and analyzed using t-test and ANOVA, with Bonferroni post-hoc test for multiple comparisons. A p<0.05 was considered statistically significant.
RESULTS
Vascular density and endothelial cell proliferation are reduced in ischemic-reperfused brain regions after neonatal stroke
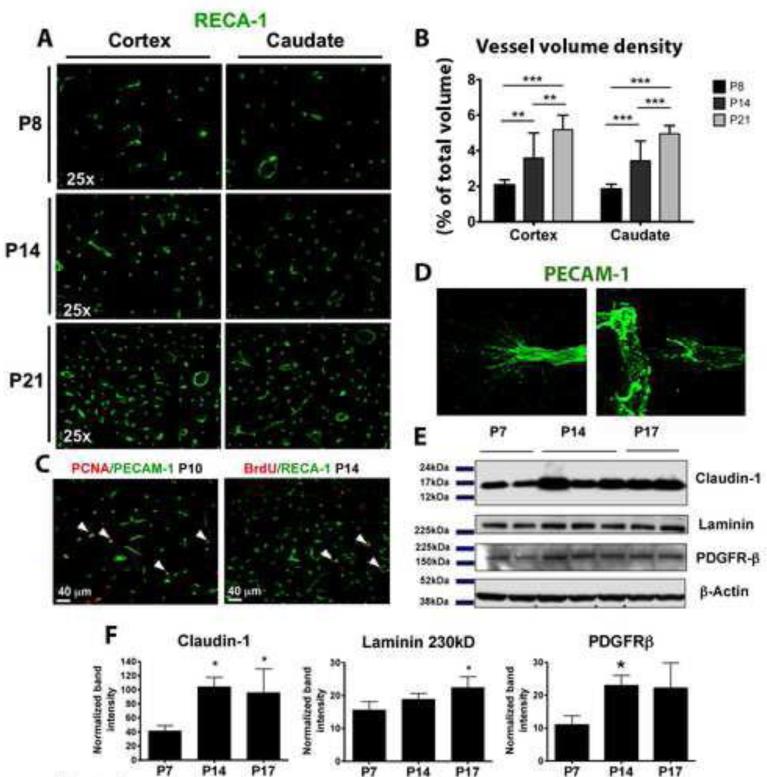
Based on vessel volume and density and endothelial cell proliferation, active vascularization continues at least through the third postnatal week in normal rat brain (figure 1A). Vessel density progressively increases in the cortex and caudate of normally developing brains from P8 to P21 (figure 1A, 1B) and is associated with endothelial cell proliferation in both brain regions during this period (figure 1C), consistently with previous observations [14-16]. Abundant PECAM-1+ endothelial tip cells showing extended filopodia are also observed in these brains (figure 1D). In some cases tip cells are observed in close proximity to adjacent vessels, probably due to vascular anastomosis (figure 1D, left). Protein expression of several vascular markers, such as the tight junction protein claudin-1, the basement membrane protein laminin, and the pericyte marker PDGFR-β, is also increased in naïve brains of P14 and P17 compared to P7 (figure 1E, 1F), further supporting the notion of vascular outgrowth during the first postnatal weeks.
Figure 1. Vascular outgrowth and angiogenesis in normal postnatal brain.
A, B. Vascular volume density is gradually increased in naive brain between P8 and P21 C. Double immunofluorescence showing numerous proliferating endothelial cells (white arrowheads) at P10 (PCNA+/PECAM-1+) and P14 (BrdU+/RECA-1+) brains. D. Examples of PECAM-1+ endothelial tip cells with extended filopodia. E, F. Western blot analysis depicts increased protein expression of claudin-1, laminin and PDGFR-β from P7 to P14 and/or P17. ANOVA (*) p<0.05, (**) p<0.01, (***) p<0.001.
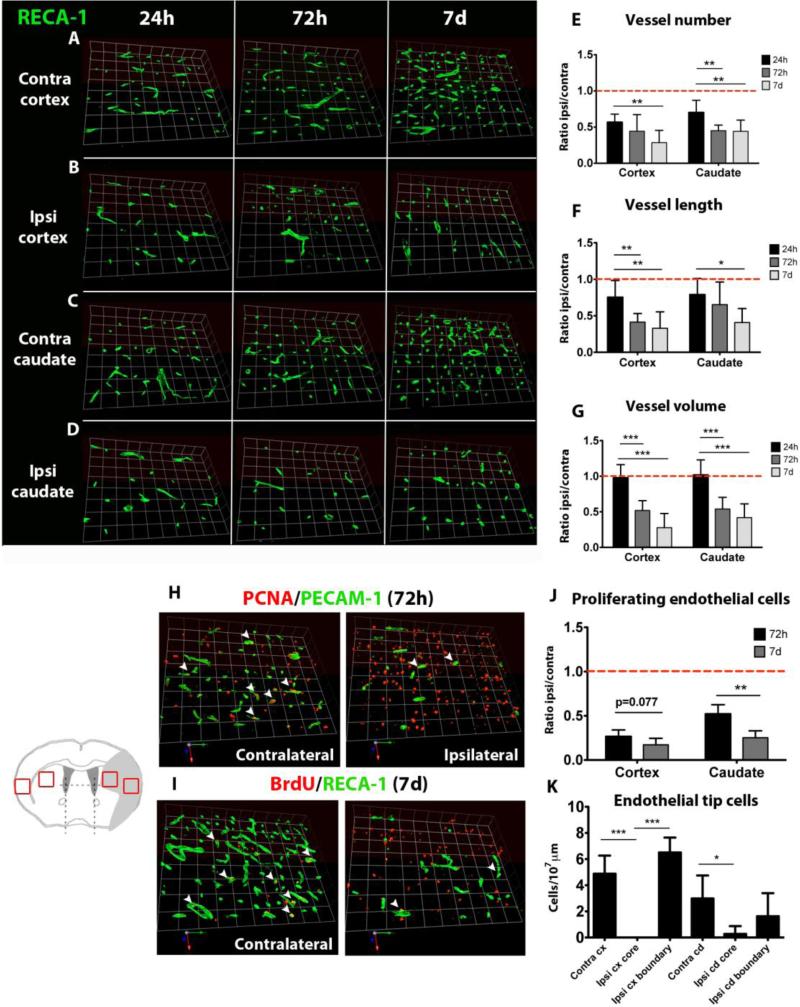
After stroke, vascular density is greatly reduced in the ischemic core regions in both the cortex and the caudate, the most severely and consistently injured regions in our model. Stroke leads to reduced vessel number and length as early as at 24 hours in the cortex (figures 2A vs 2B, 2E, 2F) and in the caudate (figures 2C vs 2D, 2E, 2F). The volume density of vessels remains unchanged at 24 hours (figure 2G), possibly due to vasodilation following reperfusion. The reduction of vascular density is not transient and is sustained through 72 hours, when a decrease in volume density is also observed (figure 2G), and is further decreased at 7 days (figures 2E-2G).
Figure 2. Reduced vascular density and endothelial proliferation in the ischemic core after neonatal stroke.
A-D. Examples of RECA-1 vessel distribution in the ischemic core in the cortex (A, B) and caudate (C, D) 24 hours, 72 hours and 7 days after injury. E-G. Quantification of vessel number (E), vessel length (F) and vessel volume (G) in the ischemic cortex and caudate from 24 hours to 7 days after injury. H, I. Double immunofluorescence showing reduced number of proliferating endothelial cells (white arrowheads) in the ischemic cortex 72 hours (PCNA+/PECAM-1+) and 7 days after injury (BrdU+/RECA-1+). J. Quantification of proliferating endothelial cells in the ischemic cortex and caudate. K. Quantification of the number of endothelial tip cells in the contralateral and ischemic cortex and caudate. ANOVA (*) p<0.05, (**) p<0.01, (***) p<0.001.
Consistently, endothelial proliferation, which was determined by the use of proliferating cell nuclear antigen (PCNA) and by BrdU, is markedly reduced in the same brain regions at 72 hours (figure 2H, 2J) and even further at 7 days after injury (figure 2I, 2J). Also, endothelial tip cells are essentially absent in the injured regions as early as 72 hours after stroke (figure 2K). Together, these data show that neonatal stroke leads to severe vascular degeneration and impairment of postnatal angiogenesis in the injured regions.
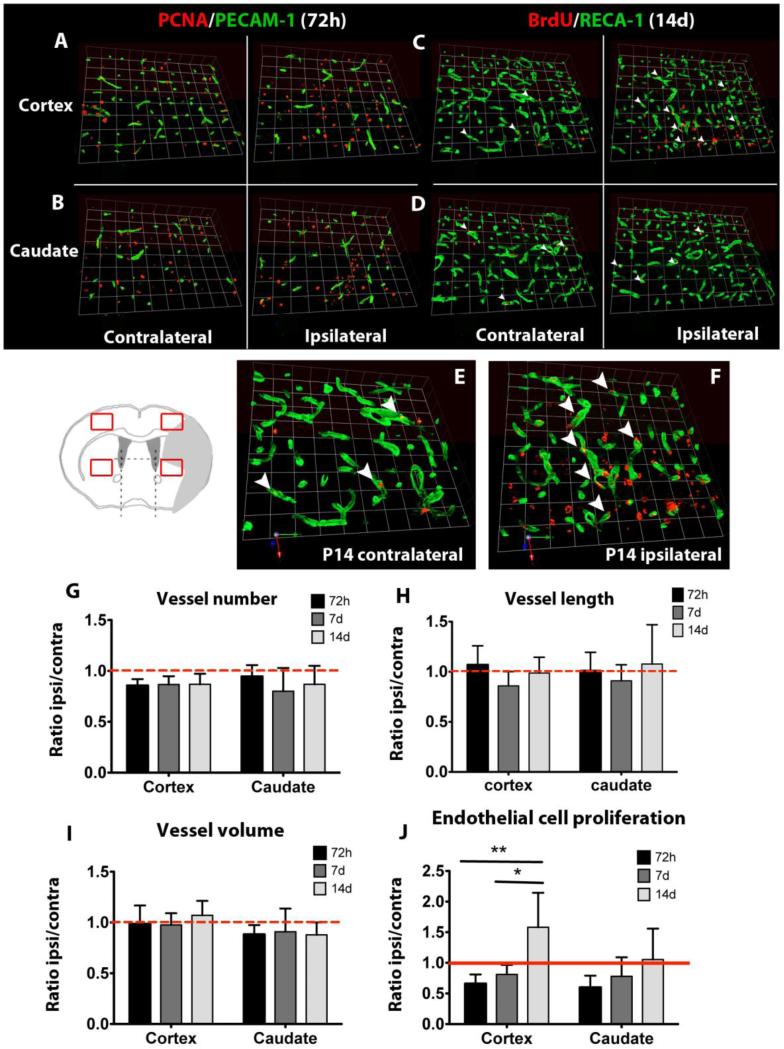
Endothelial cell proliferation is increased but vascular density is unaffected in the peri-ischemic in the cortex and caudate two weeks after neonatal stroke
We then analyzed the presence of vascular responses in peri-ischemic regions, in which angiogenesis and vascular sprouting have been described in animal models of adult stroke [17-19]. Peri-ischemic regions were defined as uninjured regions immediately adjacent to the ischemic core regions, and were identified by the observation of cell nuclear morphology based on DAPI staining. Quantification of vascular density in the peri-ischemic regions in the cortex and caudate revealed unchanged number (figure 3G), length (figure 3H) and volume density of vessels (figure 3I) compared to the corresponding regions in the contralateral hemisphere at 72 hours, 7 days and 14 days after stroke (figure 3A-3D). Interestingly, endothelial cell proliferation is partially decreased compared to the corresponding contralateral regions at 72 hours and 7 days after injury (figure 3J). Together with our previous observations, these data indicate that neonatal stroke reduces endothelial cell proliferation in the ischemic core and more discretely in the peri-ischemic regions in the cortex and caudate. In contrast, 14 days after injury the number of proliferating endothelial cells (BrdU+/RECA-1+) cells is significantly increased in the peri-ischemic region in the cortex, reaching levels higher than in the contralateral cortex (figure 3C, 3E vs 3F, 3J). At the same time, no increase on the number of proliferating endothelial cells is observed in the peri-ischemic region of the caudate (figure 3D, 3J).
Figure 3. Vessel distribution and endothelial proliferation in the ischemic boundaries.
A-D. BrdU/RECA-1 immunofluorescence showing unchanged vascular density in the peri-ischemic cortex and caudate 72 hours and 14 days after injury. E, F. High-magnification images from C showing increased presence of proliferating endothelial cells (white arrowheads) in the peri-ischemic cortex 14 days after injury. G-I. Quantification of vessel number (G), vessel length (H) and vessel volume (I) in the peri-ischemic cortex and caudate. J. Quantification of proliferating endothelial cells in the peri-ischemic cortex and caudate. ANOVA (*) p<0.05, (**) p<0.01.
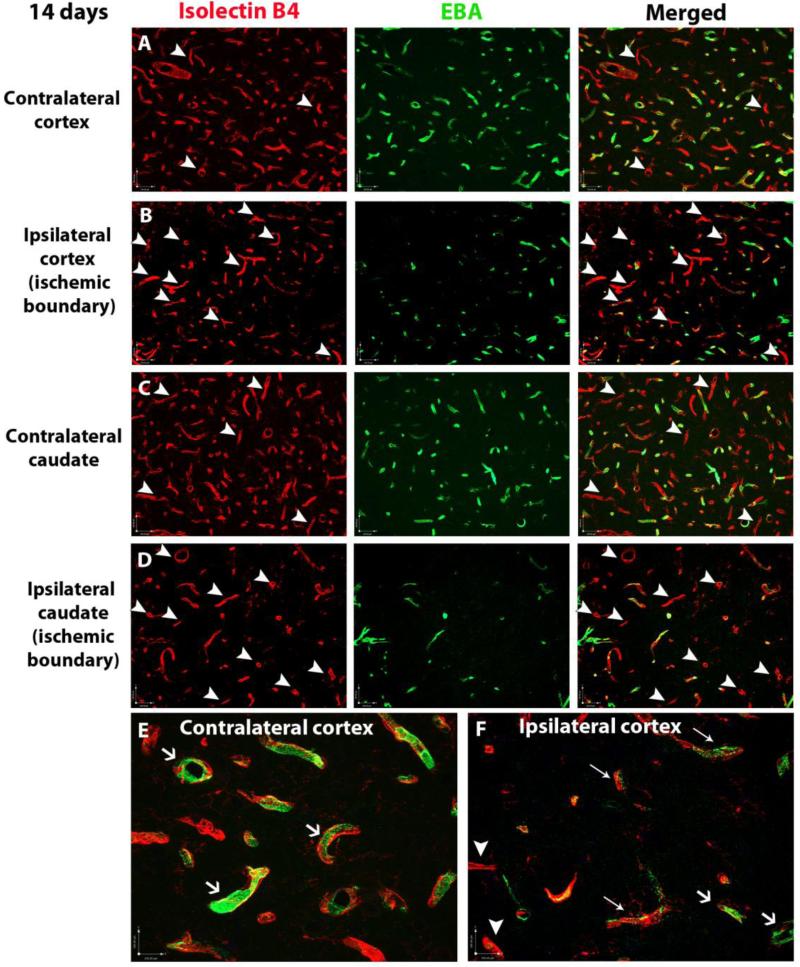
Distribution of the rat endothelial barrier antigen, EBA, is altered in the peri-ischemic regions in the cortex
To characterize functional maturation of proliferating vessels in peri-ischemic regions, we analyzed the distribution of the EBA, a protein present in functionally integrant vessels in mature brain [20, 21]. At P21 (14 days after stroke) most vessels in the contralateral regions express EBA (figure 4A, 4C, arrows in figure 4E), consistent with previous reports that showed presence of this barrier antigen in most brain vessels at this age [22]. However, EBA expression was absent in a small proportion of vessels (figure 4A, 4C, arrowheads). In contrast, in peri-ischemic regions in the cortex and caudate EBA expression is absent in numerous vessels (figure 4B, 4D, 4F white arrowheads), while other vessels showing reduced EBA expression (figure 4F, thin arrows). The altered spatial expression of this barrier antigen may indicate abnormalities in the vascular maturation of vessels surrounding the severely injured regions.
Figure 4. EBA distribution in vessels in the ischemic boundaries after neonatal stroke.
Double immunofluorescence showing the presence of EBA (green) in brain vessels (Isolectin B4, red). In uninjured regions only a small proportion of vessels lack EBA (A, C white arrowheads), while EBA staining is present in most brain vessels (E, white arrows). In contrast, EBA staining is absent in most vessels in the ischemic boundaries in the cortex (B, F, white arrowheads) and the caudate (D, white arrowheads) and is reduced and irregular in other vessels (F, thin arrows).
DISCUSSION
In this study we demonstrate that neonatal focal arterial stroke leads to both severe and long-lasting vascular degeneration and to impairment of angiogenesis in the ischemic core regions. We also show that in the peri-ischemic regions endothelial cell proliferation is reduced during the acute and sub-acute injury phases, but is restored in the caudate and significantly increased in the cortex during the chronic recovery phase. Increased endothelial cell proliferation, however, is not associated with increased vascular density. Altered patterns of expression of the endothelial barrier antigen, EBA, in the peri-ischemic regions suggest an abnormal BBB structure.
During the first 3 postnatal weeks there is a gradual increase in the complexity of the neurovascular network in the rat brain [14-16, 23] that coincides with progressive increase in the cerebral blood flow and metabolic rate of glucose, as reported in the rat, rabbit, dog and human during postnatal brain maturation [24-29]. We recently showed a significant increase in the capillary network from P7 to adulthood [11]. In this study we observed increased vascular density between P8 and P21, which was also associated with higher expression of BBB and perivascular protein markers.
In our study we focused on the cortex and the caudate, two brain regions supplied by the MCA. Although both regions are injured after transient MCAO in neonatal rats, the consequences of injury in these individual regions differ and injury in the caudate is more severe and consistent. Thus, a parallel study of vascular responses in these two regions may prove as a good tool to uncover the mechanisms of long-term injury after neonatal stroke. Following neonatal focal arterial stroke, we have observed a severe and long-lasting vascular degeneration in the injured hemisphere. Similar observations have been reported in animal models of neonatal hypoxia-ischemia (HI), describing a significant rapid (24 hours) and persistent (up to 14 days) reduction in the number of perfused brain vessels in the peri-lesional regions [14], and decreased vessel length in the injured hemisphere 7 days after HI [30].
Angiogenesis may protect by restoring blood supply to the injured brain and enhance recovery by supporting neurogenesis after injury. In uninjured adult brain, where angiogenesis is low, stroke induces a prominent, fast and long-lasting proliferative response in endothelial cells in the ischemic and peri-ischemic regions [17, 18, 31]. In vessels surrounding ischemic brain regions, endothelial cells begin to proliferate as soon as 12 to 24 hours after stroke, coinciding with induction of gene and protein expression of several known pro-angiogenic mediators, such as VEGF [32, 33], VEGF receptor type-2 [34], angiopoietins and Tie receptors [35, 36], the PDGF-B/PDGFR-β axis [37], erythropoietin [38] and TGF-β [39]. As a result, higher vascular density in those areas was seen 3 days after stroke [17, 18, 31], and vessel formation lasted even up to 21 days after injury [17]. In adult human stroke patients, angiogenesis was reported 3-4 days after injury, also concomitant to the induction of several known pro-angiogenic factors [40], but it is still a matter of discussion whether newly generated brain vessels maturate correctly and translate into functional vessels in the adult brain.
The most striking observation in our study is the absence of an angiogenic response early after injury and only a limited induction of angiogenesis during the chronic phase, but the underlying mechanisms of such a different response in post-ischemic adult and neonatal brains are unknown. In neonatal brain, developmental angiogenesis is still ongoing [15, 16]. Consistently, using endothelial transcriptome, we recently reported that gene expression of at least some regulators of angiogenesis, VEGFR2 and angiopoientin 2, is approximately 3-fold higher in endothelial cells in uninjured hemisphere of P7 rats than in uninjured hemisphere of adult rats [11]. In the same study we observed a significant increase in VEGFR2 and angiopoientin-2 gene expression in acutely injured adult brain at 24 hours, but unchanged expression of these genes and several other angiogeneic regulators in acutely injured P7 brain [11].
At the same time, in our prior immunofluorescence study in P10 rats subjected to MCAO, rapid up-regulation of VEGF was evident in ischemic regions between 4 and 24 hours after reperfusion, but expression was increased in neurons early whereas reactive astrocytes were the major source of VEGF 7 days later [41]. VEGF, which is a known critical mediator of neuronal and endothelial function as well as a mediator of angiogenesis and neurogenesis in the adult [42], is important in recovery after neonatal stroke [43]. Blockade of VEGF signaling through VEGFR2 inhibition led to enhanced injury and decreased endothelial cell proliferation in the injured caudate 7 days after neonatal stroke. As in this study, endothelial cell proliferation in the injured caudate of non-treated animals was only marginally reduced compared to the contralateral caudate [43]. Here we extended the characterization of endothelial cell proliferation up to 14 days after reperfusion, observing a recovery of normal proliferative levels in the caudate and an increase in the cortex.
It has been shown that in adult stroke models angiogenesis and neurogenesis occur simultaneously and that newly-generated neuroblasts migrate along proliferating blood vessels in the peri-ischemic brain areas [12, 44]. Endothelial cells can indeed modulate neural stem cell migration and process outgrowth when subjected to oxygen and glucose deprivation [45]. In the neonatal brain stroke also induces neural stem cell proliferation in the subventricular zone [46] but the interaction of neural progenitors with brain vessels during their migration into the ischemic regions has not been determined after neonatal focal stroke. Hence, it remains unclear whether the limited angiogenic responses observed after neonatal stroke provide sufficient support for the survival and migration of neural progenitors into the injured brain.
We then showed that angiogenesis occurring by two weeks after injury affects properties of vessels in peri-infract regions, as evident from altered EBA expression. EBA is specifically expressed in brain endothelial cells and its loss [47, 48] or neutralization [49, 50] induces a major rapid and transient BBB disruption in the adult. EBA is not detected in embryonic brain, is expressed in only a few microvessels in early postnatal brain, but is widely expressed in brain vessels in a juvenile brain [20, 21], when the BBB becomes remarkably sensitive to inflammation-triggered injury. Based on data that BBB is integrant at birth in most brain regions [51], EBA is clearly not required for proper BBB assembly or function during embryonic development or during the first two postnatal weeks. While lack of this protein is used as a marker of BBB breakdown in immunohistochemistry, surprisingly little is known about its exact function. It has been suggested that the “mosaic” pattern of EBA expression is linked to tight junction function [47]. Although there are no studies that directly demonstrate such a link, the dynamic nature of the BBB opening/closure in response to an anti-EBA antibody makes such explanation plausible, in which case EBA likely modulates tight junctions. Here we observed that, in contrast to vessels in uninjured regions, in P21 rats, where EBA distribution is almost ubiquitous, vessels in the peri-ischemic regions show altered expression of this protein, suggesting an impaired barrier function. While we demonstrate that stroke prior to postnatal EBA expression reshapes EBA distribution at P21 (14 days after injury), the underlying mechanisms and functional consequences of its lack or altered localization to vascular integrity are yet to be uncovered.
Functional BBB integrity depends not only on endothelial properties, but also on adequate structure and interaction between different components of the neurovascular unit (endothelial cells, basal lamina proteins, pericytes and astrocyte endfeet) [52]. The selective permeability of the BBB also relies on the activity of specific endothelial transporters that limit the trafficking of circulating molecules to the brain parenchyma [52]. The presence of different inflammatory mediators (cytokines, chemokines and others) in the ischemic brain may affect both the disruption of the BBB during acute injury and the maturation of the BBB in new vessels generated during the recovery phase. It is known that some mechanisms of stroke-induced neuroinflammation are differentially regulated in neonates and adults [53]. In particular, the cross-talk and physical interactions of resident and/or peripheral immune cells with vessels may determine the time-course of vascular degeneration and angiogenesis in a different way in the neonatal and adult brain.
In summary, our temporo-spatial characterization of the acute and long-term vascular responses to neonatal stroke has shown that stroke-induced angiogenesis is more limited in the neonatal brain compared to the adult brain. Therefore, strategies that enhance angiogenesis after neonatal stroke may represent an effective therapeutic approach for long-term recovery of the affected newborns.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grant RO1 NS44025 (Z.S.V.), National Institutes of Health grant RO1 NS55915 (Z.S.V.) and Postdoctoral Fellowship Program from the Ramón Areces Foundation, Madrid, Spain (D.F.L.). We acknowledge Peter Newman, PhD, Blood Center of Winconsin, for the kindly provided anti-PECAM-1 antibody, Erin Oswald, BS, for her support with experiments and Richard Daneman, PhD, University of California San Francisco, for useful discussions and advice.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Lee J, Croen LA, Lindan C, Nash KB, Yoshida CK, Ferriero DM, Barkovich AJ, Wu YW. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58(2):303–308. doi: 10.1002/ana.20557. [DOI] [PubMed] [Google Scholar]
- 2.Kamath BD, Todd JK, Glazner JE, Lezotte D, Lynch AM. Neonatal outcomes after elective cesarean delivery. Obstet Gynecol. 2009;113(6):1231–1238. doi: 10.1097/AOG.0b013e3181a66d57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch JK, Nelson KB. Epidemiology of perinatal stroke. Curr Opin Pediatr. 2001;13(6):499–505. doi: 10.1097/00008480-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Harbert MJ, Tam EW, Glass HC, Bonifacio SL, Haeusslein LA, Barkovich AJ, Jeremy RJ, Rogers EE, Glidden DV, Ferriero DM. Hypothermia is correlated with seizure absence in perinatal stroke. J Child Neurol. 2011;26(9):1126–1130. doi: 10.1177/0883073811408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cilio MRFD. Synergistic neuroprotective therapies with hypothermia. Semin Fetal Neonatal Med. 2010;15(5):293–298. doi: 10.1016/j.siny.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cnossen MH vOC, Appel IM. Etiology and treatment of perinatal stroke; a role for prothrombotic coagulation factors? Semin Fetal Neonatal Med. 2009;14(5):311–317. doi: 10.1016/j.siny.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 9.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106(2):641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Lopez D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, Wendland MF, Vexler ZS. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 2012;32(28):9588–9600. doi: 10.1523/JNEUROSCI.5977-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derugin N, Dingman A, Wendland MF, Fox C, Bollen A, Vexler ZS. Magnetic resonance imaging as a surrogate measure for histological sub-chronic endpoint in a neonatal rat stroke model. Brain Res. 2005;1066(1-2):49–56. doi: 10.1016/j.brainres.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):2795–2803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 15.Robertson PL, Du Bois M, Bowman PD, Goldstein GW. Angiogenesis in developing rat brain: an in vivo and in vitro study. Brain Res. 1985;355(2):219–223. doi: 10.1016/0165-3806(85)90044-6. [DOI] [PubMed] [Google Scholar]
- 16.Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119(1):139–153. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23(2):166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 18.Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156(3):965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276(17):4644–4652. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sternberger NH, Sternberger LA. Blood-brain barrier protein recognized by monoclonal antibody. Proc Natl Acad Sci U S A. 1987;84(22):8169–8173. doi: 10.1073/pnas.84.22.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenstein JM, Krum JM, Sternberger LA, Pulley MT, Sternberger NH. Immunocytochemical expression of the endothelial barrier antigen (EBA) during brain angiogenesis. Brain Res Dev Brain Res. 1992;66(1):47–54. doi: 10.1016/0165-3806(92)90138-m. [DOI] [PubMed] [Google Scholar]
- 22.Argandona EG, Bengoetxea H, Lafuente JV. Lack of experience-mediated differences in the immunohistochemical expression of blood-brain barrier markers (EBA and GluT-1) during the postnatal development of the rat visual cortex. Brain Res Dev Brain Res. 2005;156(2):158–166. doi: 10.1016/j.devbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Michaloudi H, Batzios C, Grivas I, Chiotelli M, Papadopoulos GC. Developmental changes in the vascular network of the rat visual areas 17, 18 and 18a. Brain Res. 2006;1103(1):1–12. doi: 10.1016/j.brainres.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 24.Ohata M, Sundaram U, Fredericks WR, London ED, Rapoport SI. Regional cerebral blood flow during development and ageing of the rat brain. Brain. 1981;104(2):319–332. doi: 10.1093/brain/104.2.319. [DOI] [PubMed] [Google Scholar]
- 25.Tuor UI, Grewal D. Autoregulation of cerebral blood flow: influence of local brain development and postnatal age. Am J Physiol. 1994;267(6 Pt 2):H2220–2228. doi: 10.1152/ajpheart.1994.267.6.H2220. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy C, Grave GD, Juhle JW, Sokoloff L. Changes in blood flow in the component structures of the dog brain during postnatal maturation. J Neurochem. 1972;19(10):2423–2433. doi: 10.1111/j.1471-4159.1972.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M, Syrota A. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33(5):696–703. [PubMed] [Google Scholar]
- 28.Kennedy C, Sakurada O, Shinohara M, Miyaoka M. Local cerebral glucose utilization in the newborn macaque monkey. Ann Neurol. 1982;12(4):333–340. doi: 10.1002/ana.410120404. [DOI] [PubMed] [Google Scholar]
- 29.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 30.Svedin P, Guan J, Mathai S, Zhang R, Wang X, Gustavsson M, Hagberg H, Mallard C. Delayed peripheral administration of a GPE analogue induces astrogliosis and angiogenesis and reduces inflammation and brain injury following hypoxia-ischemia in the neonatal rat. Dev Neurosci. 2007;29(4-5):393–402. doi: 10.1159/000105480. [DOI] [PubMed] [Google Scholar]
- 31.Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. Am J Pathol. 2000;157(5):1473–1483. doi: 10.1016/S0002-9440(10)64786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobbs CS, Chen J, Greenberg DA, Graham SH. Vascular endothelial growth factor expression in transient focal cerebral ischemia in the rat. Neurosci Lett. 1998;249(2-3):79–82. doi: 10.1016/s0304-3940(98)00377-2. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. VEGF and flt. Expression time kinetics in rat brain infarct. Stroke. 1996;27(10):1865–1872. doi: 10.1161/01.str.27.10.1865. discussion 1872-1863. [DOI] [PubMed] [Google Scholar]
- 34.Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol. 1998;57(9):874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Lin TN, Wang CK, Cheung WM, Hsu CY. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2000;20(2):387–395. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Lee C, Shen F, Du R, Young WL, Yang GY. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36(7):1533–1537. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]
- 37.Renner O, Tsimpas A, Kostin S, Valable S, Petit E, Schaper W, Marti HH. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res Mol Brain Res. 2003;113(1-2):44–51. doi: 10.1016/s0169-328x(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 38.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19(6):643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Haqqani AS, Nesic M, Preston E, Baumann E, Kelly J, Stanimirovic D. Characterization of vascular protein expression patterns in cerebral ischemia/reperfusion using laser capture microdissection and ICAT-nanoLC-MS/MS. FASEB J. 2005;19(13):1809–1821. doi: 10.1096/fj.05-3793com. [DOI] [PubMed] [Google Scholar]
- 40.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25(9):1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 41.Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14(3):524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimotake J, Derugin N, Wendland M, Vexler ZS, Ferriero DM. Vascular endothelial growth factor receptor-2 inhibition promotes cell death and limits endothelial cell proliferation in a neonatal rodent model of stroke. Stroke. 2010;41(2):343–349. doi: 10.1161/STROKEAHA.109.564229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 45.Plane JM, Andjelkovic AV, Keep RF, Parent JM. Intact and injured endothelial cells differentially modulate postnatal murine forebrain neural stem cells. Neurobiol Dis. 2010;37(1):218–227. doi: 10.1016/j.nbd.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang YS, Mu D, Wendland M, Sheldon RA, Vexler ZS, McQuillen PS, Ferriero DM. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58(1):106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- 47.Saubamea B, Cochois-Guegan V, Cisternino S, Scherrmann JM. Heterogeneity in the rat brain vasculature revealed by quantitative confocal analysis of endothelial barrier antigen and P-glycoprotein expression. J Cereb Blood Flow Metab. 2012;32(1):81–92. doi: 10.1038/jcbfm.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Ran R, Khatri P, Tomsick T, Sharp FR. Reperfusion activates metalloproteinases that contribute to neurovascular injury. Exp Neurol. 2008;210(2):549–559. doi: 10.1016/j.expneurol.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghabriel MN, Zhu C, Hermanis G, Allt G. Immunological targeting of the endothelial barrier antigen (EBA) in vivo leads to opening of the blood-brain barrier. Brain Res. 2000;878(1-2):127–135. doi: 10.1016/s0006-8993(00)02721-9. [DOI] [PubMed] [Google Scholar]
- 50.Lu H, Demny S, Zuo Y, Rea W, Wang L, Chefer SI, Vaupel DB, Yang Y, Stein EA. Temporary disruption of the rat blood-brain barrier with a monoclonal antibody: a novel method for dynamic manganese-enhanced MRI. Neuroimage. 2010;50(1):7–14. doi: 10.1016/j.neuroimage.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314(1):119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 52.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 53.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31(5):378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]