Abstract
The influence of incubation and auxin (2,4-D) on polyribosome level in soybean hypocotyl was studied.
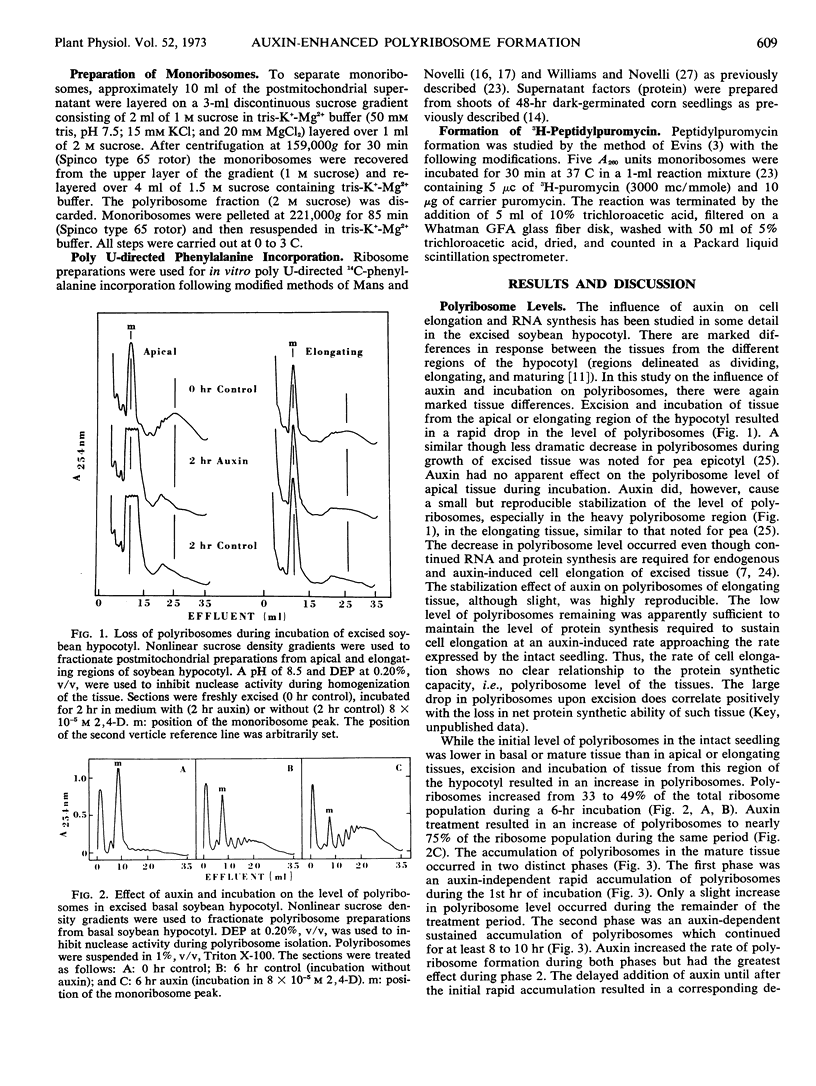
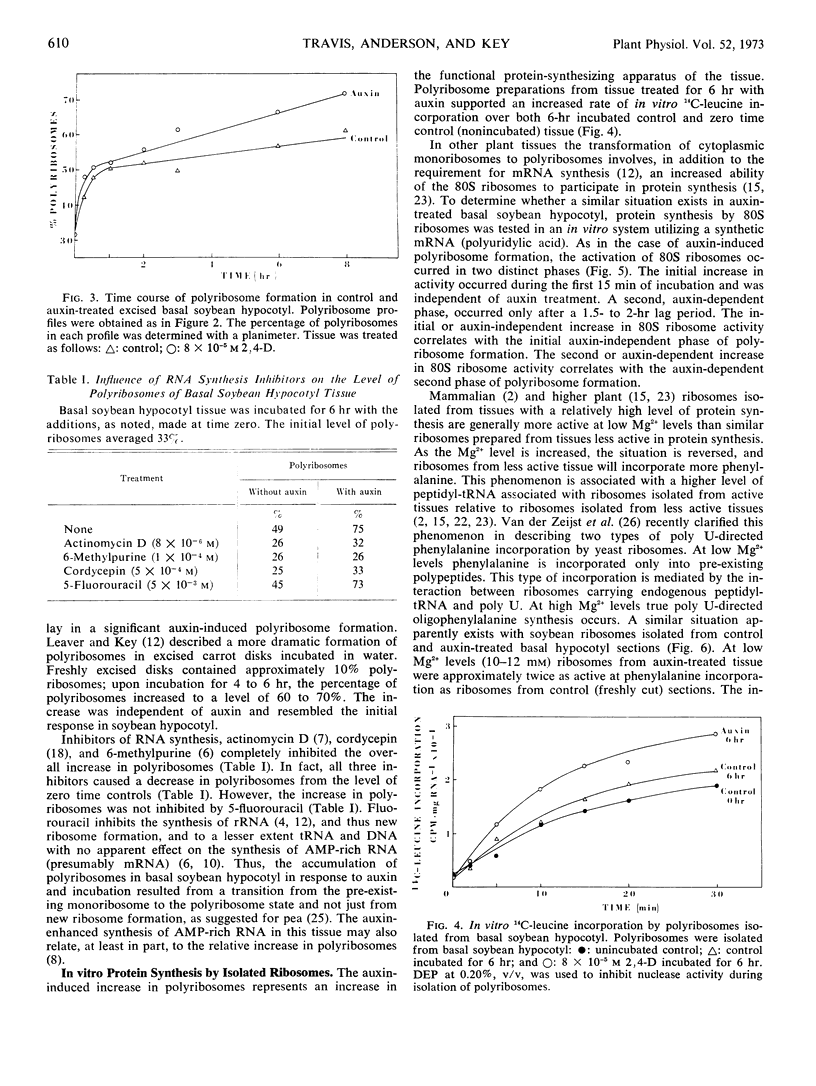
A marked drop in the relative level of polyribosomes in excised apical or meristematic tissue (0 to 5 millimeters below the cotyledons) occurred during incubation. The addition of auxin to the incubation medium did not affect polyribosome level. A similar decrease in polyribosome level occurred in excised elongating tissue (5 to 15 millimeters below cotyledons) during incubation. Auxin, however, caused a small but highly reproducible stabilization of polyribosomes in this tissue. There was a rapid, but small, auxin-independent increase in polyribosomes of basal or nongrowing hypocotyl (from 20 to 40 millimeters below cotyledons) during incubation, followed by a larger auxin-dependent increase in polyribosomes. While auxin is known to cause an increase in total ribosomes during incubation of the excised basal hypocotyl, the observed transformation from monoribosomes to polyribosomes was not dependent on new ribosome synthesis.
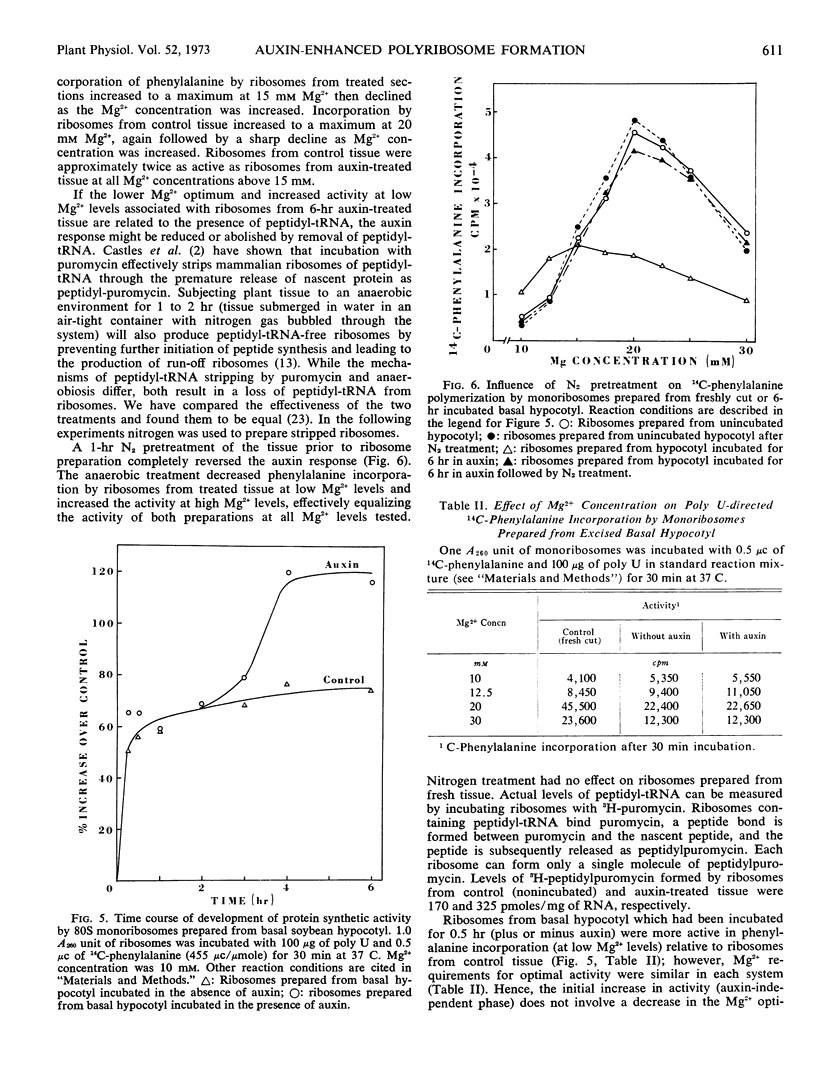
Protein synthetic activity (poly U-directed phenylalanine incorporation) of the 80S monoribosomes at low Mg2+ levels increased during incubation of the excised basal hypocotyl. The increase in ribosome activity was biphasic (an initial auxin-independent phase followed by an auxin-dependent increase in activity) correlating with the biphasic increase in polyribosomes. The enhanced activity of 80S monoribosomes was related, at least in part, to an increase in the level of peptidyl-tRNA associated with the ribosome population. Removal of peptidyl-tRNA from the ribosomes reversed the auxin effect.
The hypothesis is advanced that the increase in polyribosomes in response to incubation and to auxin is preceded by and dependent upon the activation of 80S monoribosomes. This activation is in addition to a requirement for continued RNA synthesis, at least in part mRNA, for the transition from monoribosomes to polyribosomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Key J. L. The effects of diethyl pyrocarbonate on the stability and activity of plant polyribosomes. Plant Physiol. 1971 Dec;48(6):801–805. doi: 10.1104/pp.48.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles J. J., Rolleston F. S., Wool I. G. Polyphenylalanine synthesis and binding of phenylalanyl transfer ribonucleic acid by ribosomes from muscle of normal and diabetic rats. J Biol Chem. 1971 Mar 25;246(6):1799–1805. [PubMed] [Google Scholar]
- Evins W. H. Enhancement of polyribosome formation and induction of tryptophan-rich proteins by gibberellic acid. Biochemistry. 1971 Nov;10(23):4295–4303. doi: 10.1021/bi00799a022. [DOI] [PubMed] [Google Scholar]
- Evins W. H., Varner J. E. Hormonal control of polyribosome formation in barley aleurone layers. Plant Physiol. 1972 Mar;49(3):348–352. doi: 10.1104/pp.49.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Key J. L. A comparative evaluation of the synthesis of DNA-like RNA in excised and intact plant tissues. Plant Physiol. 1965 Nov;40(6):1212–1219. doi: 10.1104/pp.40.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L. Effect of purine and pyrimidine analogues on growth and RNA metabolism in the soybean hypocotyl-the selective action of 5-fluorouracil. Plant Physiol. 1966 Oct;41(8):1257–1264. doi: 10.1104/pp.41.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Leaver C. J., Cowles J. R., Anderson J. M. Characterization of Short Time Labeled Adenosine Monophosphate-rich Ribonucleic Acids of Soybean. Plant Physiol. 1972 May;49(5):783–788. doi: 10.1104/pp.49.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Key J. L. Polyribosome formation and RNA synthesis during aging of carrot-root tissue. Proc Natl Acad Sci U S A. 1967 May;57(5):1338–1344. doi: 10.1073/pnas.57.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L., Bracker C. E. Association of D-RNA with Polyribosomes in the Soybean Root. Plant Physiol. 1966 Jun;41(6):976–982. doi: 10.1104/pp.41.6.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Patterson B. D., Trewavas A. J. Changes in the pattern of protein synthesis induced by 3-indolylacetic Acid. Plant Physiol. 1967 Aug;42(8):1081–1086. doi: 10.1104/pp.42.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson R., Beevers L. Effects of growth regulators on ribonucleic acid metabolism of barley leaf segments. Plant Physiol. 1970 Dec;46(6):782–785. doi: 10.1104/pp.46.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota A. E., Leaver C. J., Key J. L. A detailed evaluation of the possible contribution of bacteria to radioactive precursor incorporation into nucleic acids of plant tissues. Plant Physiol. 1968 Jun;43(6):907–913. doi: 10.1104/pp.43.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis R. L., Lin C. Y., Key J. L. Enhancement by light of the in vitro protein synthetic activity of cytoplasmic ribosomes isolated from dark-grown maize seedlings. Biochim Biophys Acta. 1972 Sep 14;277(3):606–614. doi: 10.1016/0005-2787(72)90105-0. [DOI] [PubMed] [Google Scholar]
- van der Zeijst B. A., Engel K. J., Bloemers J. P. In vitro protein synthesis in yeast. Polyuridylic acid-dependent incorporation of phenylalanine into endogenous peptidyl transfer RNA. Biochim Biophys Acta. 1973 Feb 4;294(1):517–526. [PubMed] [Google Scholar]



