Abstract
Aims
To report and compare the cap thickness predictability of small incision lenticule extraction (SMILE) and flap thickness of femtosecond laser-assisted in situ keratomileusis (femto-LASIK).
Settings and design
Beyoglu Eye Training and Research Hospital, Refractive Surgery Department, Istanbul, Turkey. Retrospective pilot study.
Materials and methods
Medical records of patients who had SMILE in one eye and femto-LASIK in the other eye were reviewed. Visante corneal Optical Coherence Tomography (OCT) images at 1 week and 1 month post-surgery were analyzed. Both cap and flap thickness at the temporal edge and the nasal edge were measured and compared to each other.
Statistical analyses used
PAWS Statistics 18 and unpaired student t-test were used to compare the groups.
Results
The study included 66 eyes of 33 patients (24.7 ± 3.8 years, 20 females and 13 males). Mean flap thickness was 114.88 μm ± 4.96 μm, and mean cap thickness was 114.63 μm ± 5.18 μm. In group 1 (SMILE), cap thickness values were 115.84 μm ± 6.84 μm, 114.75 μm ± 7.36 μm, 113.66 μm ± 6.88 μm, and 114.27 μm ± 6.90 μm in measurement zones 1, 2, 3, and 4, respectively. In group 2 (FemtoLASIK), flap corneal thickness values were 115.96 mmHg ± 7.01 mmHg, 114.72 mmHg ± 7.17 mmHg, 113.54 mmHg ± 6.45 mmHg, and 115.30 mmHg ± 6.64 mmHg in measurement zones 1, 2, 3, and 4, respectively. In both groups, no statistically significant change within the measurement zones was observed.
Conclusion
The predictability of cap thickness in SMILE surgery does not differ from the femto-LASIK flaps created using the same femtosecond laser platform.
Keywords: SMILE, small incision lenticule extraction
Introduction
Femtosecond lenticule extraction is a new method for refractive correction of myopia and myopic astigmatism.1 The only available femtosecond laser platform to perform this surgery is Visumax (Carl Zeiss Meditec AG, Jena, Germany). During the femtosecond lenticule extraction procedure, an intrastromal lenticule is created between two photodisruption planes (ie, cuts) and mechanically removed for refractive correction. The anterior lamellar cut is parallel to the corneal surface at a preset depth. The depth and shape of the posterior cut determine the thickness, shape, and refractive power of the removed lenticule. Corneal cuts are performed from posterior to anterior, as once a photodisruption plane is created the bubbles make deeper structures untreatable for the femtosecond laser platform.
In the small incision lenticule extraction (SMILE) procedure, the anterior lamellar cut is similar to the flap cut in a femtolaser-assisted laser in situ keratomileusis (LASIK) procedure, and the stromal tissue anterior to the anterior lamellar cut is called the “cap”. However, the side cut is only 3–4 mm (50°–60°) in length in contrast to the approximate 310° side cut of a LASIK flap. However in femtosecond laser lenticule extraction (FLEX), the side cut is elongated similar to a LASIK flap, and the cap is lifted as a femtolasik flap to remove the underlying lenticule. In the current study, we investigated the accuracy of cap thickness in patients treated with SMILE and compared it with the accuracy of flap thickness in patients treated with femtolaser-assisted LASIK.
Materials and methods
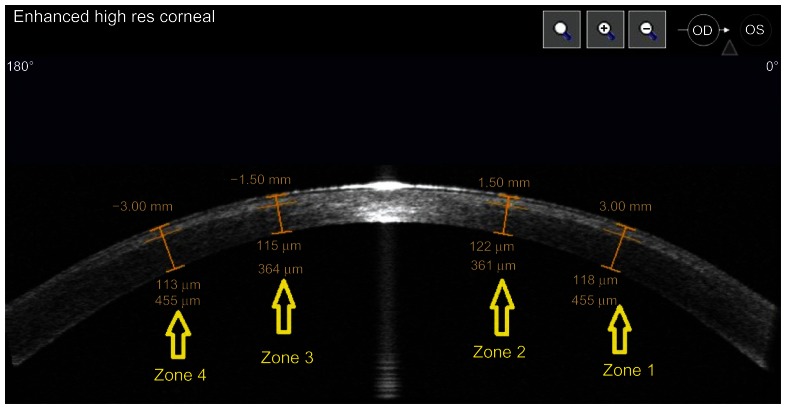
Medical records of patients who had refractive surgery in Beyoglu Eye Training and Research Hospital, Refractive Surgery Department, Istanbul, Turkey, between May 2012 and February 2013, retrospectively reviewed. This retrospective study was approved by the hospital’s ethics committee. Only patients who had SMILE in one eye and femtolasik in the other eye were included in the study. Other inclusion criteria were; uncomplicated surgery on both eyes and the availability of enhanced high resolution corneal Visante optical coherens tomography (OCT) (Carl Zeiss Meditec AG) images of both eyes 1 week, and 1 month post-surgery. The first month image data were evaluated in the study. Thirty-three patients who had SMILE in one eye and femtolasik in the other eye were identified. One of two surgeons performed each surgery. In our clinic, refractive surgery patients are seen routinely at postoperative day 1, month 1, month 3, and month 6. For all patients, corneal topography, ocular and corneal wavefront analysis, as well as ocular response analyzer (ORA) and corneal OCT measurements, are routinely performed by a technician (except at the postoperative day 1 visit) before examination by an ophthalmology resident and, eventually, one of the study authors. As a result, enhanced high resolution corneal Visante OCT images at postoperative week 1 and month 1 visits are acquired for all patients. The central light reflex, which represents the corneal vertex in the Visante images, was used as a standard reference point. The “flap tool” of the Visante software was used to measure flap thickness at four points, which were 3 mm and 6 mm from the central light reflex. These points were named from 0° to 180° as zone 1, 2, 3, and 4. The same zones from the other eye of the patient were compared. The measurement zones are shown in Figure 1. Preoperative intended flap and cap thicknesses were recorded from the femtosecond laser platform’s printouts in patients’ medical files.
Figure 1.
Flap and cap thickness measurement zones.
The Visumax femtosecond laser platform was used for the SMILE surgeries. In all patients, the spot distance was 3 μm for lamellar cuts and 2 μm for side cuts. The spot energy was set to 140 nanojoules in all patients. The minimum lenticule side cut thickness was set to 15 μm. Lenticule side cut angle was 120°, and the optical zone was 6.5 mm as SMILE is not performed in patients with a mesopic pupillary diameter greater than 6.5 mm. A small-sized (S) patient interface was used in all patients, cap diameter was set to 7.5 mm, and the cap thickness was set to 110 μm.
A drop of 0.5% proparacaine hydrochloride (Alcaine®, Alcon Co. Inc., Mississauga, ON, Canada) was instilled in each eye at the beginning of the procedure. This was followed by povidone-iodine (Betadine®, Stamford, CT, USA) preparation of the lids and periocular skin. Sterile draping was followed by insertion of a lid speculum. The patient’s eye was then positioned under the patient interface of the laser platform. The patient was instructed to look at the blinking fixation light. The patient bed was elevated and positioned using the joystick of the platform to achieve centralized and full contact between the eye and the patient interface. Suction was activated and the procedure was initiated by depressing the foot-pedal of the device.
After the lenticule and the side cut for removal were created, the surgeon positioned the eye under the operating microscope of the laser platform using the joystick. Under the operating microscope, a blunt spatula was inserted into the anterior lamellar photodisruption plane to perform dissection of any remaining attachments. The same maneuver was performed in the posterior lamellar photodisruption plane. After the lenticule was completely dissected from the overlying and underlying stroma, it was extracted from the side cut using forceps. Vigamox® drops (0.5% moxifloxacin, Alcon Co. Inc.) was instilled at the end of the operation.
The Visumax femtosecond laser platform was used to create the flaps in all of the femtosecond laser-assisted LASIK patients. The spot distance was 3 μm for the lamellar flap cut and 2 μm for side cuts in all patients. The side cut angle was 90°, and the spot energy was set to 140 nanojoules. Flap diameter was set to 8.5 mm, and flap thickness was set to 110 μm in all patients. A medium-sized (Size M) patient interface was used for all patients. After the flap was created, the patient was transported to a Schwind Amaris 750S (Schwind-eye-tech solutions, Kleinostheim, Germany) excimer laser platform. The flap was lifted using a blunt spatula then excimer laser photoablation was performed. The residual stromal bed was washed with balanced salt solution, and the flap was repositioned. An antibiotic drop was instilled at the end of the operation.
The main outcome measures were the specific thickness zone measurements on the flap or cap. Repeated measures analysis of variance (rANOVA) was used to compare the measurement zones in the flap or cap. Unpaired student t-test was used to compare the similar measurement zones between groups. The data were analyzed using PAWS® Statistics 18 (IBM Corporation Armonk, NY, USA).
Results
Sixty-six eyes of 33 patients were included in the study. The mean age was 24.7 ± 3.8 years. Twenty patients (60%) were female and 13 patients (40%) were male. In both group 1 and group 2, no statistically significant differences were found in the measurement zones within the groups (group 1, P = 0.627; group 2, P = 0.337) or the similar measurement zones between the groups, 1 month postoperative. Measurement zone 1, P = 0.944; measurement zone 2, P = 0.987; measurement zone 3, P = 0.941; measurement zone 4, P = 0.539 (Table 1).
Table 1.
Flap and cap thickness measurements
| Group 1 | Group 2 | P values | |
|---|---|---|---|
| Measurement zone 1 | 115.84 ± 6.84 | 115.96 ± 7.01 | 0.944* |
| Measurement zone 2 | 114.75 ± 7.36 | 114.72 ± 7.17 | 0.987* |
| Measurement zone 3 | 113.66 ± 6.88 | 113.54 ± 6.45 | 0.941* |
| Measurement zone 4 | 114.27 ± 6.90 | 115.30 ± 6.64 | 0.539* |
| P values | 0.627** | 0.337** |
Notes: Group 1: SMILE, Group 2: FemtoLASIK.
Unpaired student t-test was used to compare the similar measurement zones between the groups;
repeated measures Analyses of Variance (rANOVA) test was used to compare the measurement zones within groups.
Abbreviations: SMILE, small incision lenticule extraction; femto-LASIK, Femtosecond laser-assisted laser in situ keratomileusis.
Discussion
SMILE is a good option for patients who have moderate to high levels of myopia, but do not like the idea of living the rest of their lives without a completely healed flap in their eye. In our retrospective analysis, we identified 33 patients who had SMILE in one eye and femtolasik in the other eye. Operations were performed by two different surgeons (AD and OFY). For all patients, the primary reason for performing femtolasik surgery in one eye was the presence of astigmatism greater than 1.50 diopters (D) in that particular eye. Although we believe SMILE has advantages over LASIK or photorefractive keratectomy (PRK), in our clinic it is preferred not to perform SMILE in eyes with astigmatism greater than 1.50 D because the Visumax platform does not have an eye tracker, or static/dynamic cyclotorsion control. No study investigating the astigmatic achievements of the SMILE surgery in larger patient groups has been published yet. SMILE may be equally successful on astigmatic eyes. However, at this time, we prefer to treat astigmatic eyes using the excimer laser platform (Schwind Amaris 750S), which has an active eye tracker, including static and dynamic cyclotorsion control. The results of lenticule extraction using the Visumax platform in astigmatic eyes should be analysed and compared with results of excimer laser photoablation using excimer laser platforms. However, this issue is beyond the scope of this article.
In SMILE, corneal cuts are performed from posterior to anterior because, once a photodisruption plane is created, bubbles make deeper structures untreatable for the femtosecond laser. Furthermore, resulting gas bubbles at the dissection plane may penetrate into anterior or posterior stromal tissue, resulting in an opaque bubble layer (OBL). Increasing spot energy or decreasing spot separation increases the formation of an OBL when the other parameters are constant. Creating all the femtosecond photodisruption planes to create the lenticule takes approximately 30 seconds. This time depends on the pulse repetition rate (500 kHz in the Visumax), diameter of the lenticule and distance between the femtosecond laser spots (ie, spot separation), but not on the amount of intended refractive correction. The device lacks an eye tracker that centers the treatment zone to the entrance pupilla, thus, we believe that the optical zone (lenticule diameter) should be at least 6.5 mm in SMILE to compensate for small amounts of decentration. As a result, the only way to decrease surgery time is to increase spot separation. At this time, it is not possible to further increase the spot separation in SMILE due to software restrictions. However, we know that Zeiss allows some surgeons working to optimize parameters to use Visumax platforms that do not have this restriction (personal communication, 2012). Shah et al have shown that scanning pattern affects the visual results and suggested that accumulation of gas at the interface during the first lamellar cut affects the precision of the second lamellar cut.2 Prior to this study, we had a concern that 30 seconds was a long time and that accumulating bubbles at the interface, or the OBL in the stromal tissue, could lead to a decrease in predictability and accuracy of the most anterior lamellar cut, which may have clinical implications. It has been suggested that flaps thinner than 90 μm can increase the risk for haze and striae, while flaps thicker than 150 μm can reduce the range of refractive correction stability of the cornea.3–5 In the current study, we measured the cap thickness in multiple locations. We found that in group 1, the stromal cap had a uniform thickness in all these locations with no statistically significant differences. There was also a very high correlation between the intended and achieved thickness. For all patients included in this study, the intended flap or cap thickness was 110 μm because this was a standard parameter in our clinic. Since the intended thickness was the same for both groups, mean achieved thicknesses were compared directly instead of comparing the differences between the intended and achieved thicknesses. There was no statistically significant difference between the achieved cap thickness in SMILE versus the achieved flap thickness in femtolasik in any location when the intended depth was 110 μm. In other words, SMILE cap depth was not only uniform throughout the diameter of the lenticule, but it was also as accurate as the conventional femtolasik flap depth of the Visumax platform.
Conclusion
Our study found the Visumax femtosecond laser platform to be an equally reliable platform for creating lamellar cuts at the intended depths in both SMILE and femtolasik surgery.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sekundo W, Kunert K, Russman C, et al. First efficacy and safety study of femtosecond lenticule extraction for the correction of myopia: six month results. J Cataract Refract Surg. 2008;34:1513–1520. doi: 10.1016/j.jcrs.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Shah R, Shah S. Effect of scanning patterns on the results of femtosecond laser lenticule extraction refractive surgery. J Cataract Refract Surg. 2011;37:1636–1647. doi: 10.1016/j.jcrs.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 3.Rocha KM, Kagan R, Smith SD, Krueger RR. Thresholds for interface haze formation after thin-flap femtosecond laser in situ keratomileusis for myopia. Am J Ophthalmol. 2009;147:966–972. doi: 10.1016/j.ajo.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Muallem MS, Yoo SY, Romano AC, Schiffman JC, Culbertson WW. Corneal flap thickness in laser in situ keratomileusis using the Moria M2 microkeratome. J Cataract Refract Surg. 2004;30:1902–1908. doi: 10.1016/j.jcrs.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Slade SG. The use of the femtosecond laser in the customization of corneal flaps in laser in situ keratomileusis. Curr Opin Ophthalmol. 2007;18:314–317. doi: 10.1097/ICU.0b013e3281bd88a0. [DOI] [PubMed] [Google Scholar]