Abstract
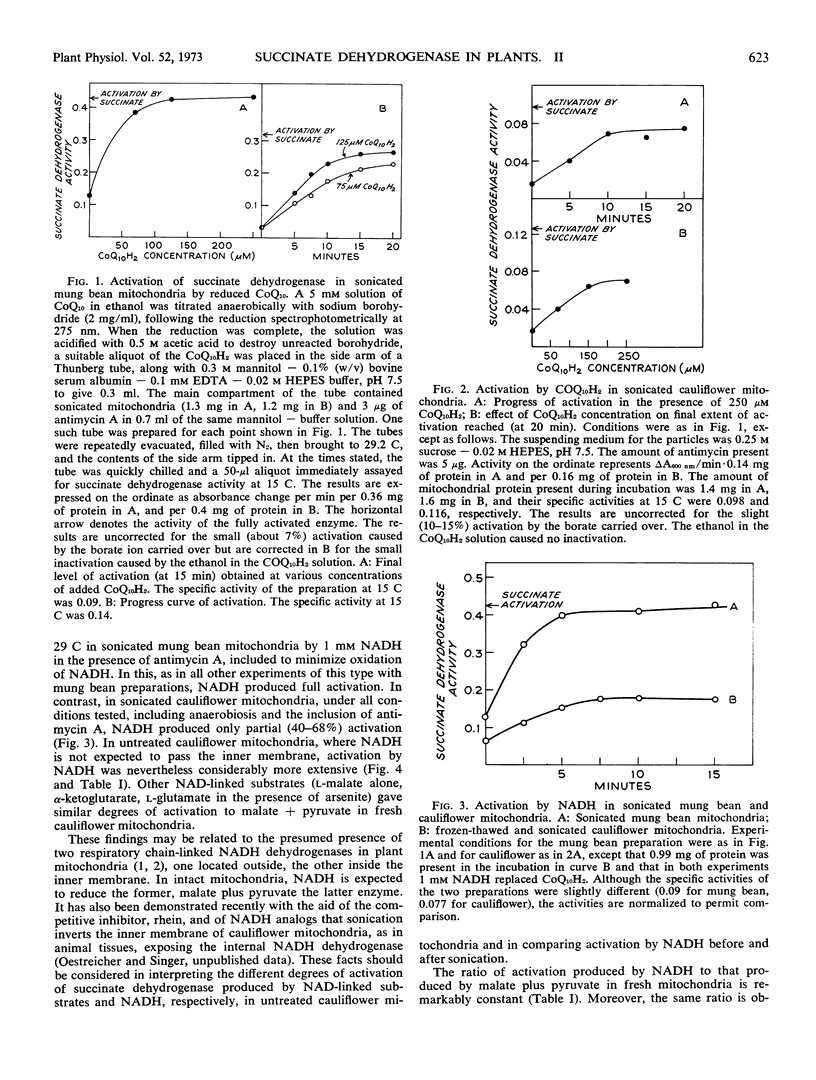
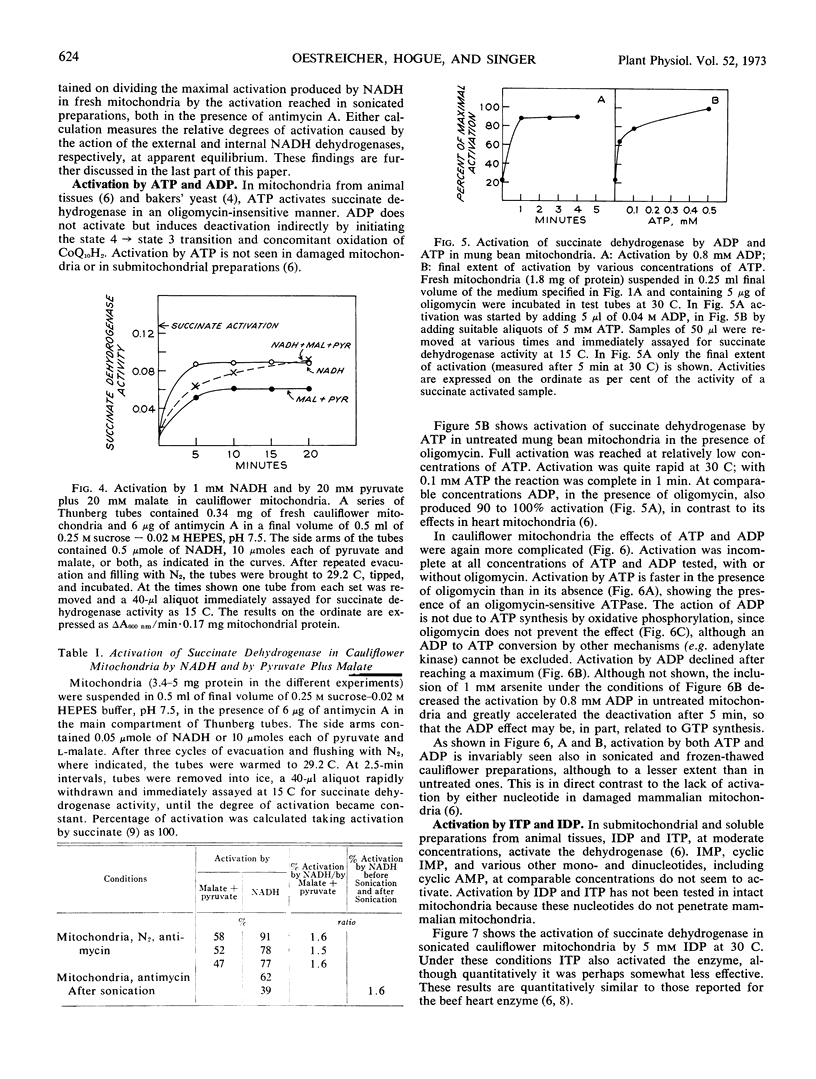
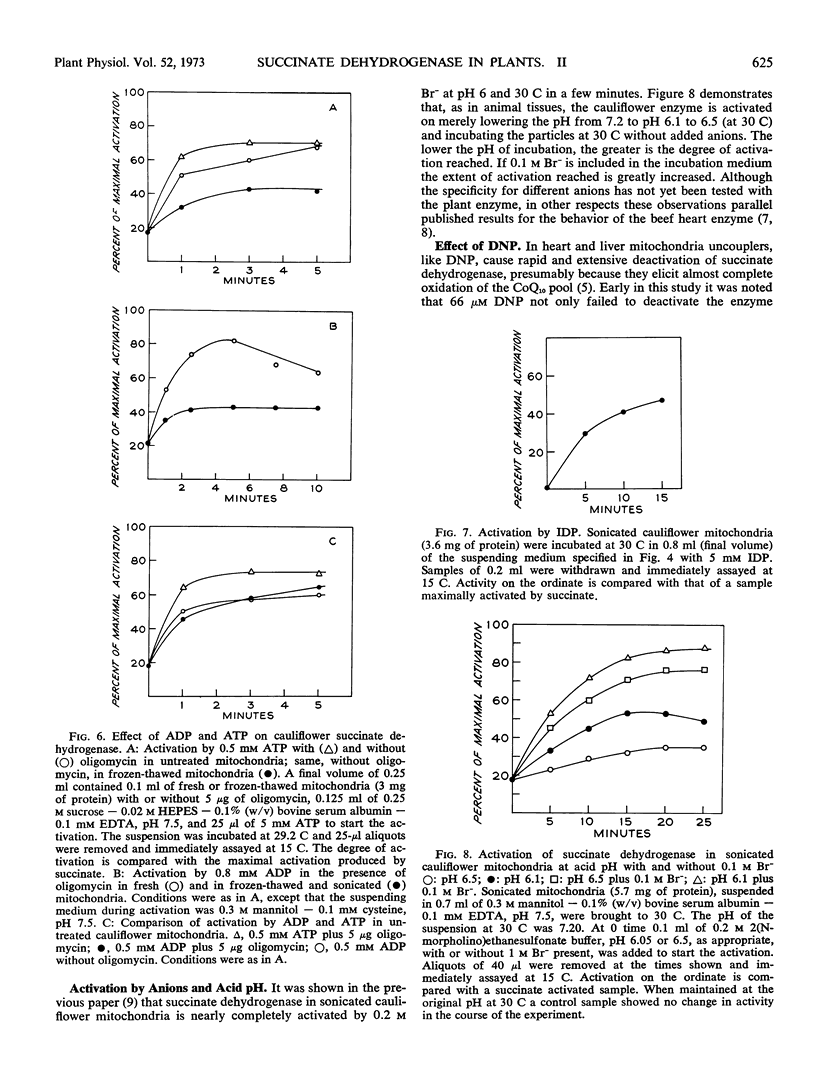
The effect of various agents on the activation of succinate dehydrogenase in cauliflower (Brassica oleracea) and mung bean (Phaseolus aureus) mitochondria and in sonicated particles has been investigated. Reduced coenzyme Q10, inosine diphosphate, inosine triphosphate, acid pH, and anions activate the enzyme in mitochondria from higher plants in the same manner as in mammalian preparations. Significant differences have been detected in the behavior of plant and animal preparations in the effects of ATP, ADP, NADH, NAD-linked substrates, and of 2, 4-dinitrophenol on the state of activation of the dehydrogenase. In mammalian mitochondria ATP activates, whereas ADP does not, and the ATP effect is shown only in intact mitochondria. In mung bean and cauliflower mitochondria, both ATP and ADP activate and the effect is also shown in sonicated and frozen-thawed preparations. In sonicated mung bean mitochondria NADH causes complete activation, as in mammalian submitochondrial particles, but in sonicated cauliflower mitochondria activation by NADH is incomplete, as is also true of intact, anaerobic cauliflower mitochondria. Moreover, neither NAD-linked substrates nor a combination of these with NADH can fully activate the enzyme in cauliflower mitochondria. In contrast to mammalian mitochondria, succinate dehydrogenase is not deactivated in cauliflower or mung beam mitochondria under the oxidized conditions brought about by uncoupling of oxidative phosphorylation by 2,4-dinitrophenol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coleman J. O.D., Palmer J. M. Role of Ca(2+) in the oxidation of exogenous NADH by plant mitochondria. FEBS Lett. 1971 Oct 1;17(2):203–208. doi: 10.1016/0014-5793(71)80148-5. [DOI] [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Gregolin C., Scalella P. Activation of the oxidation of succinate by adenosine triphosphate in respiratory particles of yeast. Biochim Biophys Acta. 1965 Apr 26;99(1):185–187. doi: 10.1016/s0926-6593(65)80024-8. [DOI] [PubMed] [Google Scholar]
- Gutman M., Kearney E. B., Singer T. P. Control of succinate dehydrogenase in mitochondria. Biochemistry. 1971 Dec 7;10(25):4763–4770. doi: 10.1021/bi00801a025. [DOI] [PubMed] [Google Scholar]
- Gutman M., Kearney E. B., Singer T. P. Regulation of succinate dehydrogenase activity by reduced coenzymes Q10. Biochemistry. 1971 Jul 6;10(14):2726–2733. doi: 10.1021/bi00790a011. [DOI] [PubMed] [Google Scholar]
- Kearney E. B., Mayr M., Singer T. P. Regulatory properties of succinate dehydrogenase: activation by succinyl CoA, pH, and anions. Biochem Biophys Res Commun. 1972 Jan 31;46(2):531–537. doi: 10.1016/s0006-291x(72)80171-2. [DOI] [PubMed] [Google Scholar]
- Singer T. P., Oestreicher G., Hogue P. Regulation of Succinate Dehyrogenase in Higher Plants: I. Some General Characteristics of the Membrane-bound Enzyme. Plant Physiol. 1973 Dec;52(6):616–621. doi: 10.1104/pp.52.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]


