Abstract
A major focus in the field of orthopaedic tissue engineering is the development of tissue engineered bone and soft tissue grafts with biomimetic functionality to allow for their translation to the clinical setting. One of the most significant challenges of this endeavor is promoting the biological fixation of these grafts with each other as well as the implant site. Such fixation requires strategic biomimicry to be incorporated into the scaffold design in order to re-establish the critical structure-function relationship of the native soft tissue-to-bone interface. The integration of distinct tissue types (e.g. bone and soft tissues such as cartilage, ligaments, or tendons), requires a multi-phased or stratified scaffold with distinct yet continuous tissue regions accompanied by a gradient of mechanical properties that mimics that of the multi-tissue transition between bone and soft tissues. This review discusses tissue engineering strategies for regenerating common tissue-to-tissue interfaces (ligament-to-bone, tendon-to-bone or cartilage-to-bone), and the strategic biomimicry implemented in stratified scaffold design for multi-tissue regeneration. Potential challenges and future directions in this emerging field will also be presented. It is anticipated that interface tissue engineering will enable integrative soft tissue repair, and will be instrumental for the development of complex musculoskeletal tissue systems with biomimetic complexity and functionality.
Keywords: Insertion site, enthesis, Interface Tissue Engineering, Strategic Biomimicry, co-culture, stratified scaffold, multi-phased scaffold
Introduction
Trauma and degeneration of orthopaedic tissues are commonly associated with injuries to soft tissues such as cartilage which cover the surface of articulating joints, as well as ligaments and tendons, which connect bone to bone, and muscle to bone, respectively. Tissue-to-tissue interfaces such as those that connect soft tissue (e.g. ligament, tendon, or cartilage) to bone are ubiquitous in the body, and they are essential for facilitating synchronized joint motion and musculoskeletal function. These critical junctions between distinct tissue types are however prone to injury and unfortunately not re-established following standard surgical repair methods. Failure to regenerate the intricate tissue-to-tissue interface has been reported to compromise graft stability and long term clinical outcome19,40,61, consequently the biological fixation or integrative repair of soft tissues remain a significant clinical challenge.
In the past decade, tissue engineering32,70 has emerged as a promising approach to orthopaedic repair. Utilizing a combination of cells, growth factors and/or biomaterials, the principles of tissue engineering have been readily applied to the formation of a variety of connective tissues such as bone, cartilage, ligament or tendon in vitro and in vivo. More recently, the emphasis in the field of orthopaedic tissue engineering has shifted from tissue formation to tissue function7, with a concentration on imparting biomimetic functionality to orthopaedic grafts and enabling their translation to the clinic. Presently, a significant barrier to clinical translation is how to achieve biological fixation or functional integration of the tissue engineered orthopaedic grafts, be it bone, ligaments or cartilage, either with each other and/or with the host environment.
This review focuses on current biological fixation strategies aimed at engineering tissue-to-tissue interfaces, as the elegant design methodologies developed from tissue engineering can be readily applied to regenerate the aforementioned critical junction between soft tissue and bone. The nature of this interface tissue engineering challenge is rooted in the complexity of the musculoskeletal system and the structural intricacy of both hard and soft tissues. These tissues, each with a distinct cellular population, must operate in unison to facilitate physiologic function and maintain tissue homeostasis. It is thus not surprising that the transition between various tissue types is characterized by a high level of heterogeneous structural organization that is crucial for joint function. For example, ligaments or tendons with direct insertions into subchondral bone exhibit a multi-tissue transition consisting of three distinct, yet continuous, regions of ligament, fibrocartilage, and bone 4,13,82. The fibrocartilage interface is further divided into non-calcified and calcified regions. This interface with a gradient of mechanical properties has a number of functions, from mediating load transfer between two distinct types of tissue to sustaining the heterotypic cellular communications required for interface function and homeostasis4,40,85. In light of this complexity, functional tissue engineeering must incorporate strategic biomimicry in order to facilitate the formation of the tissue-to-tissue interface and enable seamless graft integration.
The detailed mechanisms that drive the development of the tendon-to-bone and ligament-to-bone interface are not fully understood. Published studies have investigated the change of cell type and collagen fiber composition of the interface as a function of age, which have yielded valuable clues to its development. Immunohistologial evaluation of the ligament-to-bone interface of postnatal rats revealed that at birth, the majority of proliferating cells at the interface were near the ligament region of the insertion site52. These cells produced type I and II collagen and slowly developed into fibrochondrocyte-like cells until a month after birth. After that time, rapid longitudinal growth of the ACL took place. These observations suggest that fibrochondrocytes at the ligament-to-bone interface may originate from the ligament. In the case of injury, it has been well established that the native interface is not regenerated between soft tissue and bone. Studies of tendon-to-bone healing post ACL reconstruction have provided insights into the neotissue formed when soft tissue is juxtaposed against bone. Liu et al. examined the morphology and matrix composition of the interface during the early tendon-to-bone healing process37, and found that by two weeks after reconstruction, the tendon attached to the bone with scar tissue filling the tendon-to-bone junction. This scar tissue had reorganized into a dense connective tissue matrix by one month, with predominantly fibroblasts present. After six weeks, contraction of the interface was prominent and significantly less type I collagen was found in the remodelling matrix, however type II collagen became detectable. As such, no well defined fibrocartilage interface was observed over time. This study correlates with the biomechanical studies of Rodeo et al. and demonstrates that surgically juxtaposing ligament and bone does not spontaneously result in the regeneration of the fibrocartilaginous interface62. Collectively, these studies suggest that cell source is a significant consideration in interface regeneration, and moreover, the differentiation of these cells in interface-relevant populations is likely driven by both biochemical and mechanical factors both during development and healing.
In additional to developmental cues, characterization studies4,6,45,49,54,60,71,79,85 of the structure-function relationship inherent at the soft tissue-to-bone insertion have revealed remarkable organizational similarities between many tissue-to-tissue interfaces, as they often consist of a multi-tissue, multi-cell transition as described above for ligaments or tendons, as well as being associated with a controlled distribution of non-mineralized and mineralized cartilaginous interface regions that along with other structural parameters such as collagen fiber organization, are reported to be responsible for engineering a gradient of mechanical properties progressing from soft tissue to bone. These observations have provided invaluable clues for the design of biomimetic scaffolds for engineering the tissue-to-tissue interface. Specifically, a stratified or multi-phased scaffold will be essential for recapturing the multi-tissue organization observed at the soft tissue-to-bone interface. In order to minimize the formation of stress concentrations, the scaffold should exhibit phase-specific structural and mechanical properties, with a gradual increase in mechanical properties across the scaffold phases, similar to that of the native tissue. To this end, introducing spatial control over mineral distribution on a stratified scaffold can impart controlled mechanical heterogeneity similar to that of the native interface. Compared to a homogenous structure, a scaffold with pre-designed, tissue-specific matrix inhomogeneity can better sustain and transmit the distribution of complex loads inherent at the multi-tissue interface. It is emphasized that while the scaffold is stratified or consists of different phases, a key criteria is that these phases must be interconnected and pre-integrated with each other, thereby supporting the formation of distinct yet continuous multi-tissue regions. Furthermore, interactions between interface-relevant cells serve important functions in the formation, maintenance, and repair of interfacial tissue. Therefore, precise control over the spatial distribution of these cell populations is also critical for multi-tissue formation and interface regeneration. Consideration of these biomimetic parameters will collectively enable the design of stratified scaffolds optimized for promoting the formation and maintenance of controlled matrix heterogeneity and interface regeneration.
This review will highlight current tissue engineering efforts in the regeneration of three characteristic connective tissue interfaces, namely the ligament-to-bone, tendon-to-bone or cartilage-to-bone interface, focusing on biomimetic scaffold design and biomaterial- as well as cell-based strategies to engineer a functional gradient of mechanical properties that approximates that of the native interface. Each section will begin with a brief description of the current understanding of the requirements for biomimetic and functional interface scaffold design, which have been distilled through characterization and structure-function understandings of the native interface. This is followed by a brief review of stratified scaffold and gradient-based scaffold design currently researched in soft tissue-to-bone interface tissue engineering. Lastly, potential challenges and future direction in this rapidly expanding area of functional tissue engineering will be discussed.
Stratified Scaffold Design for Ligament-to-Bone Interface Tissue Engineering
The site of anterior cruciate ligament (ACL) insertion into bone (Fig. 1A) is a classic example of a complex soft tissue-to-bone interface consisting of spatial variations in cell type and matrix composition resulting in three distinct tissue regions of ligament, fibrocartilage, and bone4,13,82, whereby the fibrocartilage region is further divided into mineralized and non-mineralized regions. From a structure-function perspective, the complex organization of this interface is likely related to the nature and distribution of mechanical stress experienced at the region. It has been reported that matrix organization at this site is optimized to sustain both tensile and compressive stresses45,85. For example, fibrocartilage is often localized in anatomical regions subjected to compressive loading45. These region-specific mechanical properties facilitate a gradual transition in strain across the insertion and provide valuable cues for ligament-to-bone interface scaffold design.
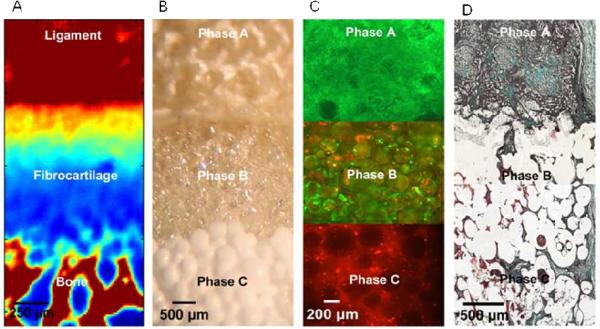
Fig.1.
Biomimetic Stratified Scaffold Design for Ligament-to-Bone Interface Tissue Engineering.
(A) Fourier Transform Infrared Spectroscopic Imaging (FTIR-I) revealed that relative collagen content is the highest in the ligament and bone regions, with a decrease in collagen across the fibrocartilage interface from ligament to bone (neonatal bovine, bar 250 μm, with blue to red representing low to high collagen content, respectively). (B) A tri-phasic stratified scaffold designed to mimic the three interface regions (ligament, fibrocartilage and bone, bar 200 μm). (C) In vitro co-culture of fibroblasts and osteoblasts on the tri-phasic scaffold resulted in region-specific cell distribution and cell-specific matrix deposition. Fibroblasts (Calcein AM, green) were localized in Phase A and osteoblasts (CM-DiI, red) in Phase C, and both osteoblasts and fibroblasts migrated into Phase B over time (bar 200 μm). (D) In vivo evaluation of the tri-phasic scaffold tri-cultured with fibroblasts (Phase A), chondrocytes (Phase B), and osteoblasts (Phase C) showed abundant host tissue infiltration and matrix production (wk 4, Modified Goldner Masson Trichrome Stain, bar 500 μm).
The multi-tissue transition from ligament to bone at the ACL-to-bone interface represents a significant challenge for functional interface tissue engineering. Initial attempts to improve ligament graft to bone fixation focused on augmenting the surgical graft with a material that would encourage bone tissue growth. For example, Tien et al. used calcium phosphate cements to fill the tendon-to-bone junction in a rabbit ACL reconstruction study and found that the addition of this ceramic helped to improve bone tissue growth and organization81. In a similar study, the addition of injectable tricalcium phosphate (TCP) cement to the interface region in a canine ACL reconstruction model resulted in more organized bone tissue formation than the uncemented control25. Using a different approach, Mutsuzaki et al. soaked tendon grafts in a series of solutions which coated the tendons with a calcium phosphate layer prior to surgery51. The modified graft was tested using a rabbit ACL reconstruction model, and it was found that the precoated tendons enhanced healing and promoted integration. Additional approaches to improve bone tunnel osteointegration have included the addition of periosteum grafts to the region of the graft that interacts with the bone8,31,56,87 and growth factors such as rhBMP-263. Although these methods have improved osteointegration between the ACL graft and the bone tunnel, these efforts do not result in the regeneration of the fibrocartilage interface. Moreover, single-factor approaches do not fully mimic the complexity of events at the healing interface, thus a more systematic and controlled approach which uses a biomimetic stratified scaffold to direct the growth of the multi-tissue interface may overcome these shortcomings as it can be designed to recapitulating the inherent complexity of this multi-layered ligament-to-bone interface (Fig. 1).
The ideal scaffold has several functions including supporting the growth and differentiation of the relevant cell populations, directing cellular interactions, and promoting the formation and maintenance of controlled matrix heterogeneity. The scaffold must also exhibit a gradation in mechanical properties, mimicking the native insertion site, with magnitudes comparable to those of the ligament-to-bone interface. Additionally, the scaffold must be biodegradable in order to be gradually replaced by living tissue. Lastly, for in vivo integration, the engineered graft must be easily adaptable within current ACL reconstruction grafts, or pre-incorporated into the design of the ligament replacement grafts.
Traditional efforts for developing tissue engineered grafts for ACL reconstruction have centered on regenerating the ligament proper3,15,16, with more recent studies focusing on the restoration of the anatomic ACL-bone interface11,12,27,38. Cooper et al. reported on a multi-phased design of a synthetic ACL graft fabricated from 3-D braiding of polylactide-co-glycolide fibers, with a ligament proper as well as two bony regions11. In vitro38 and in vivo12 evaluation demonstrated scaffold biocompatibility, healing and mechanical strength in a rabbit model. Recently, Altman et al. developed a multi-region, porous knitted silk ACL graft which was evaluated in a goat model with promising results24. Using a cell-based approach, Ma et al. reported that it is possible to form bone-ligament-bone constructs by introducing engineered bone segments to ligament monolayers43. Upon introduction of bone segments into a ligament monolayer, the monolayer rolled up around the bone pieces and self assembled into a ligament-bone-ligament construct. Paxton et al. utilized a similar methodology with promising results when evaluating the use of a poly(ethylene glycol) hydrogel incorporating HA and the RGD cell-adhesion peptide to engineer functional ligament-to-bone attachments57. These novel ACL graft designs represent significant improvement over single-phased ACL grafts, and the next step is to address the challenge of graft integration with bone, by considering the fibrocartilage interface in the ACL scaffold design.
To this end, Spalazzi et al. pioneered the design of a tri-phasic scaffold (Figure 1) for the regeneration of the ACL-to-bone interface72,73. Modeled after the native insertion, the scaffold consists of three distinct yet continuous phases, each engineered for a specific tissue region found at the interface: Phase A is designed with PLGA (10:90) mesh for fibroblast culture and soft tissue formation, Phase B consists of PLGA (85:15) microspheres and is the interface region intended for fibrochondrocyte culture, and Phase C is comprised of sintered PLGA (85:15) and 45S5 bioactive glass composite microspheres for bone formation39. The scaffold is innovative in that it is essentially a “single” scaffold system with three distinct yet continuous phases, intended to support the formation of the multi-tissue regions observed across the ACL-bone junction.
Interactions between interface relevant cell types (e.g. fibroblasts, chondrocytes, osteoblasts) on the tri-phasic scaffold have been evaluated both in vitro73 and in vivo72. To form the ligament and bone regions, fibroblasts and osteoblasts were seeded onto Phase A and Phase C, respectively. This controlled cell distribution resulted in the elaboration of cell type-specific matrix on each phase of the scaffold, with a mineralized matrix detected only on Phase C, and an extensive type I collagen matrix found on both Phases A and B. In vivo evaluation72 of co-culture of fibroblasts and osteoblasts on the tri-phasic scaffold revealed extensive tissue infiltration and abundant matrix deposition on Phase A and Phase C. Cell migration and increased matrix production and vascularization were observed on Phase B, the interface region. Moreover, tissue continuity was maintained across all three scaffold phases. Interestingly, extracellular matrix production compensated for the decrease in mechanical properties accompanying scaffold degradation, and the phase-specific controlled matrix heterogeneity was maintained in vivo72.
To form a fibrocartilage interface-like tissue at the interface phase, Spalazzi et al. extended the in vivo evaluation of the above scaffold system to tri-culture73,74, including chondrocytes along with fibroblasts and osteoblasts72. Specifically, articular chondrocytes were encapsulated in a hydrogel matrix and loaded into Phase B of the scaffold, while ligament fibroblasts and osteoblasts were pre-seeded onto Phase A and Phase C, respectively. At two months post-implantation, an extensive collagen-rich matrix was prevalent in all three phases of the tri-cultured scaffolds (Fig. 1D). Moreover, a fibrocartilaginous region of chondrocyte-like cells embedded within a matrix containing types I and II collagen as well as glycosaminoglycans was observed. Interestingly, both cell shape and matrix morphology of the neo-fibrocartilage resembled that of the neonatal ACL-bone interface83. Moreover, the neo-fibrocartilage formed was continuous with the ligament-like tissue observed in Phase A as well as the bone-like tissue found in Phase C74.
These promising results demonstrate that biomimetic stratified scaffold design coupled with spatial control over the distribution of interface relevant cell populations can lead to the formation of cell type- and phase-specific matrix heterogeneity in vitro and in vivo, with a fibrocartilage-like interface formed in tri-culture. These observations not only validate the feasibility of the stratified scaffold for promoting biological fixation of ACL grafts to bone, but also highlight the potential for continuous multi-tissue regeneration on a single scaffold system. In terms of clinical application, the tri-phasic scaffold can be used to guide the re-establishment of an anatomic fibrocartilage interfacial region directly on soft tissue grafts. Specifically, the scaffold can be used as a graft collar during ACL reconstruction surgery, and the feasibility of such an approach was recently demonstrated in a study by Spalazzi et al., where a mechanoactive scaffold system was formed based on a composite of poly-α-hydroxyester nanofibers and sintered microspheres74,75. It was observed that scaffold-induced compression of tendon grafts resulted in significant matrix remodeling and the expression of fibrocartilage interface-related markers such as type II collagen, aggrecan, and transforming growth factor-β3 (TGF-β3). These results suggest that the stratified scaffold can be used to induce the formation of an anatomic fibrocartilage interface directly on biologically derived ACL reconstruction grafts.
In summary, current strategies in ligament-to-bone interface tissue engineering first tackles the difficult problem of soft tissue-to-bone integration ex vivo by pre-engineering the soft tissue bone interface through stratified scaffold design for mutli-tissue regeneration, and then focuses on the relatively less challenging task of bone-to-bone integration in vivo. Moreover, functional and integrative ligament repair may be achieved by coupling both cell-based and scaffold-based approaches.
Stratified Scaffold Design for Tendon-to-Bone Interface Tissue Engineering
Similar to the ligament-to-bone interface, the tendon-to-bone interface displays a zonal distribution of extracellular matrix components4,5. Thus, the biomimetic scaffold design and multi-lineage cell culture methods previously discussed for the ligament-to-bone interface are also applicable for the regeneration of tendon-to-bone insertions, such as that of the rotator cuff tendons and bone. However, while tendon-to-bone and ligament-to-bone insertions are physiologically and biochemically similar, the tissue engineering strategy applied is expected to differ as the two interfaces do vary in terms of loading environment, mineral distribution, surgical repair methods which would also influence the subsequent healing response.
The debilitating effect of rotator cuff tears coupled with the high incidence of failure associated with existing repair techniques10,14,26 underscore the clinical need for functional solutions for integrative tendon-to-bone repair. To address this challenge, several groups have evaluated the feasibility of integrating tendon grafts with bone or biomaterials through the formation of an anatomic insertion site. By surgically reattaching the Achilles tendon to bone, Fujioka et al. reported that cellular reorganization occurred at the reattachment site, along with the formation of non-mineralized and mineralized fibrocartilage-like regions20. Additionally, Inoue et al. were able to successfully promote supraspinatus tendon integration with a metallic implant using a bone marrow-infused bone graft27. These promising results indicate that the tendon-to-bone interface may be regenerated and underscore the need for functional grafting solutions that can promote biological fixation.
Based on the aforementioned characteristics of the interface, nanofiber scaffolds have been explored for tendon and tendon-to-bone interface tissue engineering applications44. The biomimetic potential and physiological relevance of nanofibers makes them advantageous for orthopaedic tissue engineering. These scaffolds can be tailored to resemble the native tendon extracellular matrix as they exhibit a high surface area to volume ratio, permeability, and porosity34,35,44,58. Additionally, nanofiber organization and alignment can be modulated during fabrication and used to guide cell response50. Recently, the potential of a degradable PLGA nanofiber-based scaffold system for rotator cuff repair was evaluated in vitro48. Moffat et al. examined the effects of nanofiber organization on fibroblast attachment and alignment as well as gene expression and matrix deposition47. It was reported that nanofiber orientation (aligned vs. unaligned) was the primary factor guiding tendon fibroblast morphology, alignment, and integrin expression. Types I and III collagen, the dominant collagen types of the supraspinatus tendon, were synthesized on the nanofiber scaffolds and it was shown that their deposition was also controlled by the underlying fiber orientation. Furthermore, scaffold mechanical properties, directly related to fiber alignment, decreased as the polymer degraded but remained within range of those reported for the native supraspinatus tendon28. Building upon these promising results, Moffat et al. also designed a composite nanofiber system of PLGA and hydroxyapatite (HA) nanoparticles in an effort to regenerate both the non-mineralized and mineralized fibrocartilage regions of the supraspinatus insertion site46. The responses of interface-relevant cell populations such as osteoblasts, chondrocytes and fibroblasts have been examined on the polymer-ceramic composite nanofibers with promising results (Fig. 2).
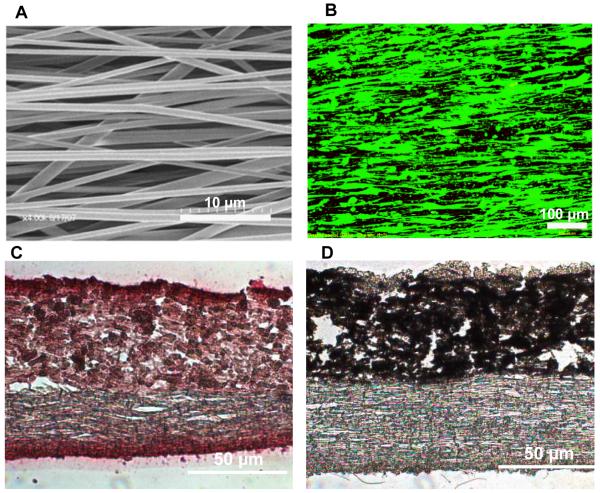
Fig.2.
Nanofiber-Based Scaffold for Tendon-to-Bone Integration.
(A) SEM micrograph depicting aligned fiber organization (4000x, bar 10 μm). (B) Fluorescence microscopy of human rotator cuff fibroblasts cultured on aligned nanofibers, cellular attachment and alignment is directed by the underlying substrate morphology. Histological analysis of a bi-phasic scaffold after 3 weeks of subcutaneous implantation, exhibiting (C) region-dependent distribution of mineral (von Kossa, 20x, bar 50 μm) and (D) collagen matrix deposition and in-growth (picrosirius red, 20x, bar 50 μm).
In addition to engineering the tendon-to-bone interface, the muscle-tendon interface is another critical research area for integrative tendon repair, although to date, it has been relatively under-explored. As the tendon joins the muscle to bone, thus the myotendinous junction (MTJ), which connects muscle to tendon, acts as a bridge to distribute mechanical loads86. This interface consists of a band of fibroblast-laden, interdigitating tissue that connects the dense collagen fibers of the tendon to the more elastic muscle fibers while displaying a gradient of structural properties80. Current tissue engineering approaches, as demonstrated by Swasdison et al., include the culturing of myoblasts in collagen gels in vitro to form contractile muscle constructs with fibrils that terminate in a manner similar to the native MTJ76,77. Adopting a cell-based approach, Larkin et al. evaluated a novel self-organizing system for in vitro myotendinous junction formation by co-culturing skeletal muscle constructs with engineered tendon constructs. Interestingly, upregulation of paxillin was observed at the neo-interface, and the MTJ formed was able to sustain tensile loading beyond the physiological strain range33.
The aforementioned studies demonstrate the promise of the biodegradable scaffold system for tendon-to-bone interface tissue engineering as well as the potential of harnessing cellular interaction for engineering both tendon-to-bone and muscle-to-tendon interface and ultimately, functional and integrative tendon repair.
Stratified Scaffold Design for Cartilage-to-Bone Interface Tissue Engineering
Another common tissue-to-tissue interface of the musculoskeletal system is the osteochondral interface which acts as a barrier between articular cartilage and subchondral bone. The ultrastructure of articular cartilage can be divided into three regions: the tangential (surface) zone, the transitional (middle) zone and the radical (deep) zone. Directly below the deep zone is the calcified cartilage region containing hypertrophic chondrocytes embedded in a densely mineralized matrix, which constitutes the osteochondral interface6,18,42,55. As with the ligament-to-bone and tendon-to-bone interfaces, the heterogeneity at the cartilage-to-bone interface is important for load bearing and force distribution54 (Fig. 3). Thus, the regeneration of this controlled heterogeneity is a critical component of integrative and functional cartilage tissue engineering strategies.
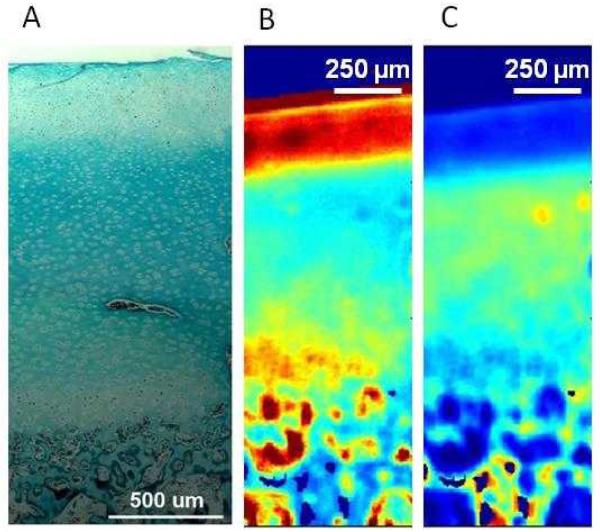
Fig.3.
Native Structure of the Osteochondral Interface.
(A) Histological evaluation of the osteochondral interface region revealed a collagen rich tissue interface region (neonatal bovine, bar 500 μm, Goldner’s Masson Trichrome Stain). (B) Fourier Transform Infrared Spectroscopic Imaging (FTIR-I) revealed that relative proteoglycan content is the highest on the articular surface, with a decrease in proteoglycan content across the interface region from cartilage to bone (neonatal bovine, bar 250 μm, with blue to red representing low to high proteoglycan content, respectively). (C) Fourier Transform Infrared Spectroscopic Imaging (FTIR-I) revealed that relative collagen content is the highest in the interface region with a decrease in proteoglycan content on the articular surface and the subcondral bone (neonatal bovine, bar 250 μm, with blue to yellow representing low to high proteoglycan content, respectively)30.
Stratified scaffold design has been researched for osteochondral tissue engineering21,23,53,64,88, with the first generation of scaffolds consisting of two distinct cartilage or bone regions joined together using either sutures or sealants21,64. Schaefer et al. seeded bovine articular chondrocytes on polyglycolic acid (PGA) meshes and periosteal cells on poly(lactic-co-glycolic acid) (PLGA)/polyethylene glycol foams, and subsequently sutured the separate constructs together at one or four weeks after seeding64. Integration between the two scaffolds was observed to be superior when brought together at week one instead of four, suggesting the importance of immediate osteoblast-chondrocyte interactions for phase-to-phase integration. Similarly, Gao et al. seeded mesenchymal stem cells (MSCs) stimulated with TGF-β1 for chondrogenic differentiation into a hyaluronan sponge and MSCs stimulated with osteogenic media into a porous calcium phosphate scaffold21. These scaffolds were then joined by a fibrin sealant and implanted subcutaneously in syngeneic rats, with continuous collagen fibers observed between the two scaffolds at six weeks following implantation. Shortly after, Sherwood et al. designed a continuous biphasic scaffold and evaluated chondrocyte response on the scaffold66. Utilizing a sequential photo-polymerization technique, Alhadlaq et al. formed a bi-layered human mandibular condyle-shaped osteochondral construct based on polyethylene glycol-diacrylate hydrogel. The top hydrogel layer contained MSC-derived chondrocytes while the bottom layer contained MSC-derived osteoblasts1. After 12 weeks in vivo, distinct cartilaginous and osseous regions were observed in a subcutaneous SCID mouse model, with histological integration between the two layers. Swieszkowski et al. also reported similar results from hyaluronan/ceramic and PCL/tricalcium phosphate composite scaffolds seeded with MSCs21,78, and these observations have been confirmed by other studies with MSC culture on biphasic scaffolds9,65.
Collectively, these pioneering studies demonstrate the feasibility of engineering multi-tissue formation (cartilage and bone) on a multi-phased scaffold; the next step is to incorporate the osteochondral interface into this scaffold design. To this end, several groups have reported on stratified scaffold designs that mimic the structural organization of the native osteochondral interface. Lu et al. and later Jiang et al. evaluated 3-D osteoblast-chondrocyte co-culture on a biomimetic, continuous multi-phased osteochondral construct consisting of a hydrogel-based cartilage region, a polymer-ceramic composite microsphere bone region, and an interfacial region consisting of a hybrid of the hydrogel and polymer-ceramic composite29,41. It was found that osteoblast and chondrocyte co-culture on this scaffold system supported the formation of distinct yet continuous cartilaginous and osseous matrices, with pre-designed integration between these regions achieving a mineralized interfacial region within which direct osteoblast-chondrocyte interactions are encouraged. In this tri-phasic scaffold system, the calcified interface region was pre-incorporated into scaffold design by the inclusion of a mineralized scaffold phase consisting of osteoblasts seeded on the polymer-ceramic microsphere-based scaffold infused with chondrocyte-laden agarose hydrogel. The formation of a calcified cartilage-like zone has also been investigated by directly seeding deep zone articular chondrocytes on a calcium polyphosphate scaffold2. As the cartilaginous tissue forms, the chondrocytes infiltrated into the superficial region of the calcium polyphosphate scaffold, resulting in three distinct regions – cartilage, mineralized cartilage, and a proteoglycan-rich layer directly above the bone scaffold. More recently, Harley et al. reported on design strategies associated with the formation of an osteochondral scaffold with cartilage and bone regions as well as a continuous osteochondral interface-like region in between these two phases22. While it remains to be validated in vitro and in vivo, this multi-phased design is intended to promote scaffold-mediated integration within an osteochondral defect site.
In summary, stratified scaffold design in conjunction with the use of interface relevant cell populations as well as mesenchymal stem cells is a promising approach to engineer the osteochondral interface. Results from the studies highlighted above demonstrate the importance of the cartilage-to-bone interface, thus advanced scaffold design aimed at the formation of integrated osteochondral grafts must take into consideration the regeneration of a functional and stable interface region between these distinct tissue types.
Gradient Scaffold Design for Interface Tissue Engineering
The stratified scaffolds described above have been designed to mimic the multi-tissue transition inherent at many tissue-to-tissue interfaces. As such, given the well documented changes in tissue type in the interface structural organization and the need to support the related tissue-specific cell phenotypes, there is emerging interest in designing scaffolds with a gradient of properties67. These novel scaffolds with either a compositional17,69 or chemical factor36,59 gradient have the potential to address the need to recapitulate the complex transition of mechanical and chemical properties that occurs in interface regions, offering direct regional control and allow for scaffold heterogeneity that can mimic the complex native interface. In contrast to the previously discussed stratified designs, these scaffolds consist of relatively gradual and continuous transition in either compositional or mechanical properties.
Utilizing an ethanol-based solvent evaporation technique, Singh et al. developed 3D multi-phased PLGA microsphere scaffolds with continuous macroscopic gradients in stiffness. Structural gradients were produced by incorporating a high stiffness nano-phase material (CaCO3 or TiO2) into portions of the microspheres during the scaffold fabrication process69. Preliminary in vitro studies showed that structurally homogenous scaffolds fabricated using this method can support the attachment of human umbilical cord-derived MSCs69. Utilizing a novel extrusion method, Erisken et al. fabricated nanofiber scaffolds containing a mineral gradient or a region-dependent calcium phosphate concentration. In vitro studies showed that culturing MC3T3 cells on these scaffolds led to the formation a gradient of calcified matrix within four weeks17. More recently, Li et al. formed a variable calcium phosphate coating on a nonwoven mat of gelatin-coated PCL nanofibers by incubating the scaffolds in a simulated body fluid (SBF) with physiologic or supra-physiologic calcium and/or phosphate ion concentrations. The results showed that this gradient in mineral content resulted in spatial variations in scaffold stiffness and affected the number of MC3T3 cells that adhered to the scaffold36. Exploring a novel alternative approach, Phillips et al. achieved a gradient of mineralized matrix deposition by seeding fibroblasts onto collagen scaffolds with a composition gradient of retrovirus coating for the osteogenic transcription factor RUNX2. It was found that by controlling the spatial patterning of transcription factor expression, fibroblasts were stimulated to deposit a gradient of mineralized matrix on the collagen scaffold and this matrix heterogeneity was maintained in vivo59.
In addition to scaffolds with compositional gradient, Singh et al. have also designed microsphere scaffolds with spatially controlled growth factor release profiles68. This method offers a unique advantage by avoiding the relative high temperatures required in classic sintering techniques which can compromise growth factors bioactivity as they are incorporated. In addition, Singh et al. also formed cell-encapsulating microsphere scaffolds using a subcritical CO2 sintering technique. Both human umbilical cord MSCs and porcine chondrocytes were used to demonstrate potential of this fabrication metod in cartilage tissue engineering applications69. It is promising that this technique resulted in high initial cell viability, although long term cell response is still been evaluated.
When comparing stratified and gradient scaffold systems, the primary advantage of the aforementioned compositional gradient-based scaffolds resides in their ability to more closely mimic the native transition in composition and potentially result in a gradation of functional properties which facilitates load transfer between distinct tissue types. As such, given the structural complexity and relatively small scale of the interface, which averages from 50 μm to 1 mm in length depending on species and age13,13,82,82,84, it remains a significant design challenge for the gradient scaffolds to recapitulate the micro- to nano-scale gradients that have been reported at the interface. In other words, design parameters for interface regeneration must be prioritized and strategic biomimicry be adopted in functional interface scaffold design. To this end, the multi-phased scaffold represents a simpler approach, whereby a gradation of composition and functional properties is established by engineering the specific tissue region of interest and pre-integrating these tissue regions through stratified design. For these scaffolds, it is anticipated that cellular contributions will play a pivotal role in mediating the regeneration and homeostasis of the gradation of compositional and mechanical properties inherent at the interface. Consequently, controlling cellular response via co-culture, tri-culture or growth factor distribution on the multi-phased scaffolds is a critical strategy to enable the development of local gradients on a physiologically relevant scale.
Summary and Future Directions
This review has provided an overview of current concepts in interface tissue engineering, focusing on strategies for the design of scaffolds with a gradation of mechanical and structural properties aimed at the regeneration of the complex tissue-to-tissue interface. Specifically, these multi-phased scaffolds have been designed to mimic the structure and function of the native soft tissue-to-bone interface while employing spatial control over heterotypic cell interactions and supporting the formation of integrated multi-tissue systems. The vast potential of stratified scaffold systems is evident from the in vitro and in vivo evaluations described here for the integrative repair of cartilage, ligament and tendon injuries. Moreover, these novel scaffolds are capable of multi-tissue regeneration by mediating heterotypic cellular interactions, and can be further refined by incorporating well controlled compositional and growth factor gradients, as well as the use of biochemical and biomechanical stimulation to encourage tissue growth and maturation. Furthermore, functional and integrative soft tissue repair may be achieved by coupling both cell-based and scaffold-based approaches.
Clinically, it is envisioned that stratified scaffolds would significantly improve current soft tissue repair strategies by attaching to existing tissues and stimulating the formation of native insertion tissue in a controlled manner. While integration of osteochondral grafts with host tissue could be accomplished via simple layering techniques or press-fit implants to repair focal defects, the incorporation of stratified scaffolds with tendon and ligament tissue would require more complex methods. Specifically, in terms of ACL-to-bone integration, multi-phased scaffolds could be fabricated as a cylinder, so that it is inserted into the bone tunnels while encasing the ACL graft and promoting interface formation directly on the graft. In addition, fully integrative ACL grafts with distinct yet continuous ligament, interface and bone regions can be developed. In terms of rotator cuff repair, scaffold patches could be fabricated to bridge the gap between torn tendon tissue and bone through interface generation, while surgically sutured to the tendon and potentially augment tendon repair as well.
It is emphasized that interface tissue engineering will be instrumental for the ex vivo development and in vivo regeneration of integrated musculoskeletal tissue systems with biomimetic functionality; however there remains a number of challenges in this exciting area. These include the need for a greater understanding of the structure-function relationship existing at the native tissue-to-tissue interface as well as the mechanisms governing interface development and regeneration. Furthermore, the in vivo host environment plus the precise effects of biological, chemical, and physical stimulation on interface regeneration must be thoroughly evaluated to enable the formation and homeostasis of the neo-interface. Physiologically relevant in vivo models are also needed to determine the clinical potential of the designed scaffolds.
Additional challenges remain to be addressed for successful clinical translation of stratified scaffolds including the integration of these scaffolds with the native tissue post surgical reconstruction. This will require determining how existing tissues will attach to stratified scaffolds in order to direct tissue growth and reform the precise native interfacial organization. Additionally, as is evident in many of the reported studies, selection of multiple cell sources is typically necessary to ensure or enhance heterogeneous tissue formation. Clinical implementation of these scaffolds will require identifying an optimal cell source which is readily available, such as an adult stem cell source which can be quickly isolated and cultured. Translation of these findings may also require reassessing measurable outcomes in order to ensure adequate interface tissue formation following surgical intervention. Finally, to extend clinical translation of stratified scaffolds, methods for sterilization and potentially long-term shelf storage are needed.
In summary, regeneration of tissue-to-tissue interfaces through interface tissue engineering represents a promising strategy for achieving biological fixation for integrative soft tissue repair, using either biological or tissue engineering grafts. It is anticipated that these efforts will lead to the development of a new generation of functional fixation devices for orthopaedic repairs as well as augmenting the clinical translation potential of tissue engineered orthopaedic grafts. Moreover, by bridging distinct types of tissue, interface tissue engineering will be instrumental for the development of integrated musculoskeletal tissue systems with biomimetic complexity and functionality.
Acknowledgements
The authors gratefully acknowledge funding support from the National Institutes of Health (AR056459 and AR055280), the Wallace H. Coulter Foundation, the National Science Foundation Graduate Fellowship (SDS) and the National Sciences and Engineering Research Council of Canada (XZX).
References
- 1.Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005;87:936–44. doi: 10.2106/JBJS.D.02104. [DOI] [PubMed] [Google Scholar]
- 2.Allan KS, Pilliar RM, Wang J, et al. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13:167–77. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 3.Altman GH, Horan RL, Lu HH, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–41. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 5.Blevins FT, Djurasovic M, Flatow EL, Vogel KG. Biology of the rotator cuff tendon. Orthop Clin North Am. 1997;28:1–16. doi: 10.1016/s0030-5898(05)70260-1. [DOI] [PubMed] [Google Scholar]
- 6.Bullough PG, Jagannath A. The morphology of the calcification front in articular cartilage. Its significance in joint function. J Bone Joint Surg Br. 1983;65:72–8. doi: 10.1302/0301-620X.65B1.6337169. [DOI] [PubMed] [Google Scholar]
- 7.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–5. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Chen WJ, Shih CH, et al. Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: A biomechanical and histologic study in rabbits. Arthroscopy. 2003;19:290–6. doi: 10.1053/jars.2003.50014. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Sato T, Tanaka J, Tateishi T. Preparation of a biphasic scaffold for osteochondral tissue engineering. Materials Science and Engineering: C. 2006;26:118–23. [Google Scholar]
- 10.Coons DA, Alan BF. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc. 2006;14:185–90. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Cooper JA, Lu HH, Ko FK, et al. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–32. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JA, Jr., Sahota JS, Gorum WJ, et al. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci U S A. 2007;104:3049–54. doi: 10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970;52:1–20. [PubMed] [Google Scholar]
- 14.Derwin KA, Baker AR, Spragg RK, et al. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–72. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 15.Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29:1363–71. doi: 10.1002/jbm.820291107. [DOI] [PubMed] [Google Scholar]
- 16.Dunn MG, Tria AJ, Kato YP, et al. Anterior cruciate ligament reconstruction using a composite collagenous prosthesis. A biomechanical and histologic study in rabbits. Am J Sports Med. 1992;20:507–15. doi: 10.1177/036354659202000504. [DOI] [PubMed] [Google Scholar]
- 17.Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and beta-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065–73. doi: 10.1016/j.biomaterials.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Fawns HT, Landells JW. Histochemical studies of rheumatic conditions. I. Observations on the fine structures of the matrix of normal bone and cartilage. Ann Rheum Dis. 1953;12:105–13. doi: 10.1136/ard.12.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman MJ, Sherman OH, Fox JM, et al. Autogeneic anterior cruciate ligament (ACL) anterior reconstruction of the knee. A review. Clin Orthop. 1985:9–14. [PubMed] [Google Scholar]
- 20.Fujioka H, Thakur R, Wang GJ, et al. Comparison of surgically attached and non-attached repair of the rat Achilles tendon-bone interface. Cellular organization and type X collagen expression. Connect Tissue Res. 1998;37:205–18. doi: 10.3109/03008209809002440. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Dennis JE, Solchaga LA, et al. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7:363–71. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 22.Harley BA, Lynn AK, Wissner-Gross Z, et al. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A. 2010;92:1078–93. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 23.Hollister SJ, Maddox RD, Taboas JM. Optimal design and fabrication of scaffolds to mimic tissue properties and satisfy biological constraints. Biomaterials. 2002;23:4095–103. doi: 10.1016/s0142-9612(02)00148-5. [DOI] [PubMed] [Google Scholar]
- 24.Altman GH, Horan RL, Weitzel P, et al. The use of long-term bioresorbable scaffolds for anteroir cruciate ligament repair. J Am Acad Orthop Surg. 2008;16:177–87. doi: 10.5435/00124635-200804000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Huangfu X, Zhao J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy. 2007;23:455–62. doi: 10.1016/j.arthro.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Iannotti JP, Codsi MJ, Kwon YW, et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–44. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 27.Inoue N, Ikeda K, Aro HT, et al. Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res. 2002;20:957–66. doi: 10.1016/S0736-0266(02)00037-2. [DOI] [PubMed] [Google Scholar]
- 28.Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13:578–84. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Hung CT, Guo XE, et al. Multiphased polymer-ceramic-hydrogel scaffold for osteochondral repair. 7th Annual Biomaterials World Congress; Sydney, Australia. 2004. [Google Scholar]
- 30.Khanarian NT, Haney NM, Burga RA, Lu HH. Evaluation of Zonal Cartilage Homeostasis Using 3D Explant Model. 2010.
- 31.Kyung HS, Kim SY, Oh CW, Kim SJ. Tendon-to-bone tunnel healing in a rabbit model: the effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg Sports Traumatol Arthrosc. 2003;11:9–15. doi: 10.1007/s00167-002-0317-8. [DOI] [PubMed] [Google Scholar]
- 32.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 33.Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149–58. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li WJ, Laurencin CT, Caterson EJ, et al. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 35.Li WJ, Mauck RL, Cooper JA, et al. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech. 2007;40:1686–93. doi: 10.1016/j.jbiomech.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li XR, Xie JW, Lipner J, et al. Nanofiber Scaffolds with Gradations in Mineral Content for Mimicking the Tendon-to-Bone Insertion Site. Nano Letters. 2009;9:2763–8. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu SH, Panossian V, al Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997:253–60. doi: 10.1097/00003086-199706000-00034. [DOI] [PubMed] [Google Scholar]
- 38.Lu HH, Cooper JA, Jr., Manuel S, et al. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805–16. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 39.Lu HH, El Amin SF, Scott KD, Laurencin CT. Three-dimensional, bioactive, biodegradable, polymer-bioactive glass composite scaffolds with improved mechanical properties support collagen synthesis and mineralization of human osteoblast-like cells in vitro. J Biomed Mater Res. 2003;64A:465–74. doi: 10.1002/jbm.a.10399. [DOI] [PubMed] [Google Scholar]
- 40.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. [PubMed] [Google Scholar]
- 41.Lu HH, Jiang J, Tang A, et al. Development of controlled heterogeneity on a polymer-ceramic hydrogel scaffold for osteochondral repair. Bioceramics. 2005;17:607–10. [Google Scholar]
- 42.Lyons TJ, Stoddart RW, McClure SF, McClure J. The tidemark of the chondro-osseous junction of the normal human knee joint. J Mol Histol. 2005;36:207–15. doi: 10.1007/s10735-005-3283-x. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Goble K, Smietana M, et al. Morphological and functional characteristics of three-dimensional engineered bone-ligament-bone constructs following implantation. J Biomech Eng. 2009;131:101017. doi: 10.1115/1.4000151. [DOI] [PubMed] [Google Scholar]
- 44.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–9. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 45.Matyas JR, Anton MG, Shrive NG, Frank CB. Stress governs tissue phenotype at the femoral insertion of the rabbit MCL. J Biomech. 1995;28:147–57. doi: 10.1016/0021-9290(94)00058-c. [DOI] [PubMed] [Google Scholar]
- 46.Moffat KL, Levine WN, Lu HH. In Vitro Evaluation of Rotator Cuff Tendon Fibroblasts on Aligned Composite Scaffold of Polymer Nanofibers and Hydroxyapatite Nanoparticles. Transactions of the 54th Orthopaedic Research Society. 2008 [Google Scholar]
- 47.Moffat KL, Chahine NO, Hung CT, et al. Characterization of the mechanical properties of the ACL-Bone insertion. Proceedings of the ASME Summer Bioengineering Conference; 2005. [Google Scholar]
- 48.Moffat KL, Kwei AS, Spalazzi JP, et al. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15:115–26. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moffat KL, Sun WH, Pena PE, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105:7947–52. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murugan R, Ramakrishna S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007;13:1845–66. doi: 10.1089/ten.2006.0078. [DOI] [PubMed] [Google Scholar]
- 51.Mutsuzaki H, Sakane M, Nakajima H, et al. Calcium-phosphate-hybridized tendon directly promotes regeneration of tendon-bone insertion. J Biomed Mater Res A. 2004;70:319–27. doi: 10.1002/jbm.a.30084. [DOI] [PubMed] [Google Scholar]
- 52.Nawata K, Minamizaki T, Yamashita Y, Teshima R. Development of the attachment zones in the rat anterior cruciate ligament: changes in the distributions of proliferating cells and fibrillar collagens during postnatal growth. J Orthop Res. 2002;20:1339–44. doi: 10.1016/S0736-0266(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 53.Niederauer GG, Slivka MA, Leatherbury NC, et al. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21:2561–74. doi: 10.1016/s0142-9612(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 54.Oegema TR, Jr., Thompson RC., Jr. The zone of calcified cartilage. Its role in osteoarthritis. In: Kuettner KE, Schleyerbach R, Peyron JG, Hascall VC, editors. Articular Cartilage and Osteoarthritis. Raven Press; New York, NY: 1992. [Google Scholar]
- 55.Oegema TR, Jr., Thompson RC, Jr., K.Brandt C-G. Cartilage Changes in Osteoarthritis. Indiana School of Medicine Publ.; Indianapolis: 1990. Cartilage-Bone Interface (Tidemark) [Google Scholar]
- 56.Ohtera K, Yamada Y, Aoki M, et al. Effects of periosteum wrapped around tendon in a bone tunnel: A biomechanical and histological study in rabbits. Crit Rev Biomed Eng. 2000;28:115–8. doi: 10.1615/critrevbiomedeng.v28.i12.190. [DOI] [PubMed] [Google Scholar]
- 57.Paxton JZ, Donnelly K, Keatch RP, Baar K. Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A. 2009;15:1201–9. doi: 10.1089/ten.tea.2008.0105. [DOI] [PubMed] [Google Scholar]
- 58.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 59.Phillips JE, Burns KL, Le Doux JM, et al. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A. 2008;105:12170–5. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ralphs JR, Benjamin M, Waggett AD, et al. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat. 1998;193(Pt 2):215–22. doi: 10.1046/j.1469-7580.1998.19320215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robertson DB, Daniel DM, Biden E. Soft tissue fixation to bone. Am J Sports Med. 1986;14:398–403. doi: 10.1177/036354658601400512. [DOI] [PubMed] [Google Scholar]
- 62.Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Rodeo SA, Suzuki K, Deng XH, et al. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–88. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 64.Schaefer D, Martin I, Shastri P, et al. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599–606. doi: 10.1016/s0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 65.Shao X, Goh JC, Hutmacher DW, et al. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12:1539–51. doi: 10.1089/ten.2006.12.1539. [DOI] [PubMed] [Google Scholar]
- 66.Sherwood JK, Riley SL, Palazzolo R, et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–51. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 67.Simon CG, Jr., Khatri CA, Wight SA, Wang FW. Preliminary report on the biocompatibility of a moldable, resorbable, composite bone graft consisting of calcium phosphate cement and poly(lactide-co-glycolide) microspheres. J Orthop Res. 2002;20:473–82. doi: 10.1016/S0736-0266(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 68.Singh M, Morris CP, Ellis RJ, et al. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng Part C. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh M, Sandhu B, Scurto A, et al. Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent. Acta Biomater. 2010;6:137–43. doi: 10.1016/j.actbio.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skalak R. Tissue engineering: proceedings of a workshop, held at Granlibakken, Lake Tahoe, California; February 26-29, 1988; New York, NY: Liss; 1988. [Google Scholar]
- 71.Spalazzi JP, Costa KD, Doty SB, Lu HH. Characterization of the mechanical properties, structure, and composition of the anterior cruciate ligament-bone insertion site. Transactions of the 54th Orthopaedic Research Society. 2005 [Google Scholar]
- 72.Spalazzi JP, Dagher E, Doty SB, et al. In Vivo Evaluation of a Tri-Phasic Composite Scaffold for Anterior Cruciate Ligament-to-Bone Integration. Proceedings of the IEEE Engineering in Medicine and Biology Society. 2006 doi: 10.1109/IEMBS.2006.259296. [DOI] [PubMed] [Google Scholar]
- 73.Spalazzi JP, Doty SB, Moffat KL, et al. Development of Controlled Matrix Heterogeneity on a Triphasic Scaffold for Orthopedic Interface Tissue Engineering. Tissue Eng. 2006;12:3497–508. doi: 10.1089/ten.2006.12.3497. [DOI] [PubMed] [Google Scholar]
- 74.Spalazzi JP, Moffat KL, Lu HH. Design of a novel stratified scaffold for ACL-to-bone interface tissue engineering. 8th International Symposium on Ligaments and Tendons; 2008. [Google Scholar]
- 75.Spalazzi JP, Vyner MC, Jacobs MT, et al. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers. Clin Orthop Rel Res. 2008;466:1938–48. doi: 10.1007/s11999-008-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swasdison S, Mayne R. In vitro attachment of skeletal muscle fibers to a collagen gel duplicates the structure of the myotendinous junction. Exp Cell Res. 1991;193:227–31. doi: 10.1016/0014-4827(91)90561-8. [DOI] [PubMed] [Google Scholar]
- 77.Swasdison S, Mayne R. Formation of highly organized skeletal muscle fibers in vitro. Comparison with muscle development in vivo. J Cell Sci. 1992;102(Pt 3):643–52. doi: 10.1242/jcs.102.3.643. [DOI] [PubMed] [Google Scholar]
- 78.Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomolec Eng. 2007;24:489–95. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 79.Thomopoulos S, Williams GR, Gimbel JA, et al. Variations of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–9. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 80.Tidball JG. Myotendinous junction injury in relation to junction structure and molecular composition. Exerc Sport Sci Rev. 1991;19:419–45. [PubMed] [Google Scholar]
- 81.Tien YC, Chih TT, Lin JH, et al. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J Bone Joint Surg Br. 2004;86:1072–6. doi: 10.1302/0301-620x.86b7.14578. [DOI] [PubMed] [Google Scholar]
- 82.Wang IE, Mitroo S, Chen FH, et al. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745–55. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 83.Wang IE, Shan J, Choi R, et al. Role of osteoblast-fibroblast interactions in the formation of the ligament-to-bone interface. J Orthop Res. 2007;25:1609–20. doi: 10.1002/jor.20475. [DOI] [PubMed] [Google Scholar]
- 84.Woo SL, Buckwalter JA. AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18-20, 1987. J Orthop Res. 1988;6:907–31. doi: 10.1002/jor.1100060615. [DOI] [PubMed] [Google Scholar]
- 85.Woo SL, Maynard J, Butler DL, et al. Ligament, Tendon, and Joint Capsule Insertions to Bone. In: Woo SL, Bulkwater JA, editors. Injury and Repair of the Musculosketal Soft Tissues. American Academy of Orthopaedic Surgeons; Savannah, Georgia: 1988. [Google Scholar]
- 86.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15:127–41. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Youn I, Jones DG, Andrews PJ, et al. Periosteal augmentation of a tendon graft improves tendon healing in the bone tunnel. Clin Orthop Relat Res. 2004:223–31. doi: 10.1097/00003086-200402000-00037. [DOI] [PubMed] [Google Scholar]
- 88.Yu H, Grynpas M, Kandel RA. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials. 1997;18:1425–31. doi: 10.1016/s0142-9612(97)00071-9. [DOI] [PubMed] [Google Scholar]