Abstract
The complex biological relationships underlying malaria transmission make it difficult to predict the impact of interventions. Mathematical models simplify these relationships and capture essential components of malaria transmission and epidemiology. Models designed to predict the impact of control programs generally infer a relationship between transmission intensity and human infectiousness to the mosquito, requiring assumptions about how infectiousness varies between individuals. A lack of understanding of human infectiousness precludes a standard approach to this inference, however, and field data reveal no obvious correlation between transmission intensity and human population infectiousness. We argue that model assumptions will have important consequences for predicting the impact of control programs.
Keywords: malaria, elimination, mathematical model, heterogeneity, infectiousness, transmission, human infectious reservoir
Mathematical models and malaria control
Renewed interest in the global eradication of malaria has led to an increase in the number of mathematical modeling frameworks developed to guide national malaria elimination strategies [1, 2]. These models attempt to distill the complex interactions between the Plasmodium parasite and its human and mosquito hosts into a single conceptual framework in order to predict the efficacy of particular interventions, as well as to provide general insights into how elimination strategies may be applied across a range of transmission settings. The technical difficulties associated with measuring malaria transmission intensity, however, create challenges for mathematical modelers. The lack of unambiguous, consistent data with which to parameterize key components of the transmission cycle generates variable model outcomes and limits the predictive capacity of these frameworks. Furthermore, field data provide only point estimates of transmission intensity or infection prevalence for a particular time and place. In order to generate a dynamic framework, mathematical models must make assumptions about the mechanisms linking the human and mosquito components of transmission based on this limited data (Box 1).
Malaria transmission results from heterogeneous processes occurring on multiple biological levels. Studies examining heterogeneity of mosquito biting rates, parasite virulence and spatial patterns of infection have shown that substantial variation exists, and this can have important impacts on model behavior [3-14]. Heterogeneities associated with human infectiousness to mosquitoes have received less attention, although there is considerable evidence that variation in human infectiousness occurs between individuals and over the course of a single infection [15-25]. The sources of heterogeneity in individual infectiousness remain unclear (reviewed in [26]). Gametocytes, the sexual parasite stage responsible for human-to-mosquito transmission, occur at low densities and are notoriously difficult to detect by microscopy. Even with sensitive measurement of gametocytes by polymerase chain reaction (PCR), the relationship between gametocyte density in the blood and the successful infection of mosquitoes and subsequent parasite development is complex and nonlinear [27, 28]. Some of the most detailed information about individual infectiousness comes from malaria therapy data from the 1940s and 1950s [20, 21, 23, 24]. Although this data provides extensive information about the relationships between asexual parasitemia, gametocytemia, and infectiousness to mosquitoes in naive adults with neurosyphilis, it remains unclear how applicable these results are in a natural setting with heterogeneous human, mosquito, and parasite populations. These issues make it difficult to measure population-level patterns of infectiousness in endemic regions, and complicate the quantitative description of the human infectious reservoir within mathematical frameworks.
Here we discuss the methods used to incorporate human infectiousness in malaria transmission models, highlighting the implications of model assumptions for the dynamics of transmission and the efficacy of control programs. We have chosen a common model framework to illustrate our points, but these issues apply more generally to any transmission model. We argue that our lack of understanding of human infectiousness may lead to inaccurate model predictions and misdirected policy recommendations. More data are needed on the determinants of human infectiousness, as well as longitudinal studies in areas with changing transmission intensity.
Modeling individual infectiousness
Mathematical models that describe malaria infection dynamics must explicitly define relationships between different components of the transmission cycle. Here we focus on how assumptions about individual infectiousness are scaled up to define an average population infectiousness, which determines the probability that a mosquito bite on a random individual will infect the mosquito. Two frequently used methods to define individual infectiousness are (i) linking individual infectiousness to parasite density (either sexual or asexual stage parasites) [29-34] and (ii) linking individual infectiousness to disease class [12, 13, 35-48]. Both methods are motivated by the notion that asexual parasite density is linked to gametocyte density, which in turn determines an individual's infectiousness. This assumption is primarily based on studies in neurosyphilis patients, which show that a peak in asexual parasite density is followed after some delay by a peak in gametocyte density, and that this peak is associated with increased infectiousness to mosquitoes [21, 24]. Several field studies, however, suggest that this relationship may be more complex in natural settings. Boudin et al. [18] uncovered no correlation between asexual density and infectiousness and multiple studies have found that subpatent infections efficiently transmit gametocytes to mosquitoes [15, 19, 25]. Even the density of gametocytes in the blood is only a mildly good indicator of infectiousness in natural settings [17, 18], in part because transmission-blocking immunity impacts parasite development in the mosquito rather than affecting gametocyte numbers [26]. Thus, the molecular determinants of human infectiousness are poorly understood, making it difficult to delineate meaningful categories of infectiousness for modeling purposes.
The second method - linking individual infectiousness to disease class - avoids explicit assumptions about the link between asexual parasite density, gametocytemia and infectiousness. It assumes instead that disease severity is linked to parasite density and makes a direct association between disease class and infectiousness. The simplest version of this framework involves a single class of infectious individuals [12, 41, 44, 45, 47]. More intricate versions further subdivide infected individuals into groups that are expected to have different average levels of infectiousness. For example, Griffin et al. [40] group infectious individuals into three different classes: (i) highly infectious individuals with clinical symptoms (symptomatic), (ii) mildly infectious asymptomatic individuals with patent parasitemia (asymptomatic) and (iii) slightly infectious asymptomatic individuals with subpatent parasitemia (subpatent). Although this hierarchy of infectiousness is most common, asymptomatic infections are sometimes assumed to be more infectious than symptomatic infections [35], reflecting the many uncertainties regarding the relative infectiousness of different disease classes [16, 22, 49]. Because of these complications, there is no standard approach for incorporating infectiousness within model frameworks. Here we have chosen to focus on a simple model framework for clarity, but it is important to emphasize that the same kinds of decisions must be made regardless of the level of individual detail included in a model.
Modeling the overall population infectiousness
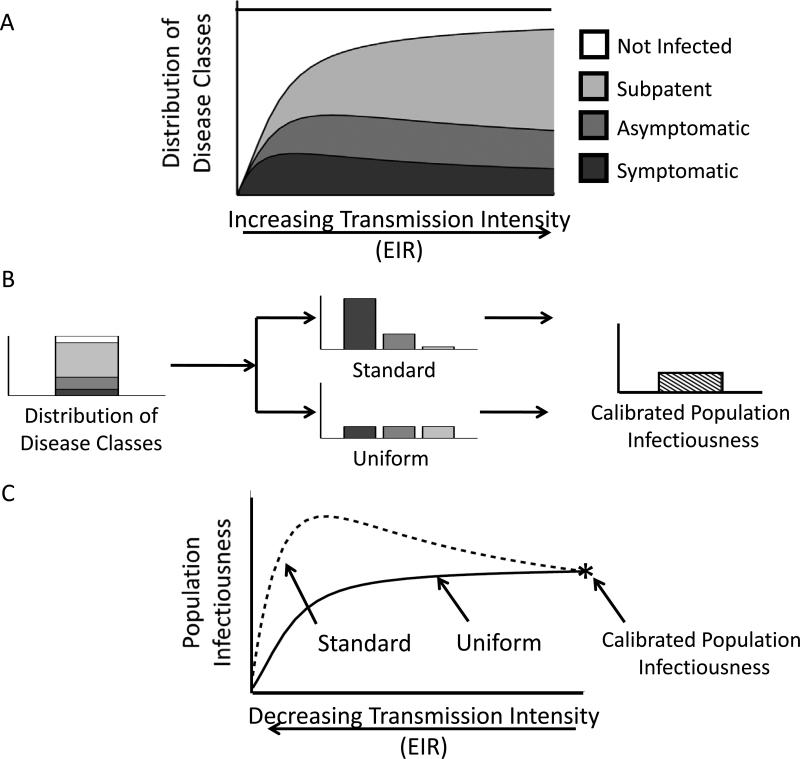
Once individual infectiousness has been defined, the model must then be calibrated to ensure that it reflects a particular transmission setting. Studies comparing EIR to patterns of infection in endemic regions provide data relating transmission intensity to the distribution of disease classes (see hypothetical example in Figure 1a). Population infectiousness (κ), the probability that a mosquito bite on a random individual will result in mosquito infection, is often adjusted to reflect this relationship for a specific location. Figure 1b compares this process for two different models of relative infectiousness that result in the same population infectiousness: (i) the “standard” assumption that symptomatic infections are highly infectious, asymptomatic infections are mildly infectious and subpatent infections are only slightly infectious and (ii) the “uniform” assumption that all infections are equally infectious. At equilibrium, these differences will not impact model results, but as EIR changes – for example following an intervention – assumptions about the distribution of individual infectiousness will have an impact (Figure 1c). In the case of the “standard” assumption, for example, population infectiousness in the model may even increase when transmission intensity drops because more people become symptomatic.
Figure 1. The impact of changes in transmission intensity (EIR) on population infectiousness depends on infectiousness assumptions.
(a) An example of the relationship between disease status and transmission intensity. When EIR is low most infected individuals have little immunity and can suffer from disease (dark grey), but as EIR increases and overall prevalence rises, increasing proportions of infected individuals have asymptomatic or subpatent infections (medium and light grey, respectively). (b) Calculating population infectiousness (κ) from the prevalence of infection and distribution of disease classes. For a given parasite prevalence, two different assumptions about infectiousness among different disease classes (“standard” and “uniform”) are calibrated to yield the same population infectiousness. (c) The relationship between EIR and population infectiousness (κ) for two different models. The functional form of the EIR- κ relationship depends on the distribution of disease classes and the assumptions about how infectiousness is distributed between these classes. The two models are calibrated for a particular setting (shown in (b)), which is the point where the solid and dashed curves intersect (the calibrated population infectiousness). As transmission intensity is decreased following control, the relationship between the new equilibrium EIR and κ depends on assumptions about how infectiousness is distributed among the human population.
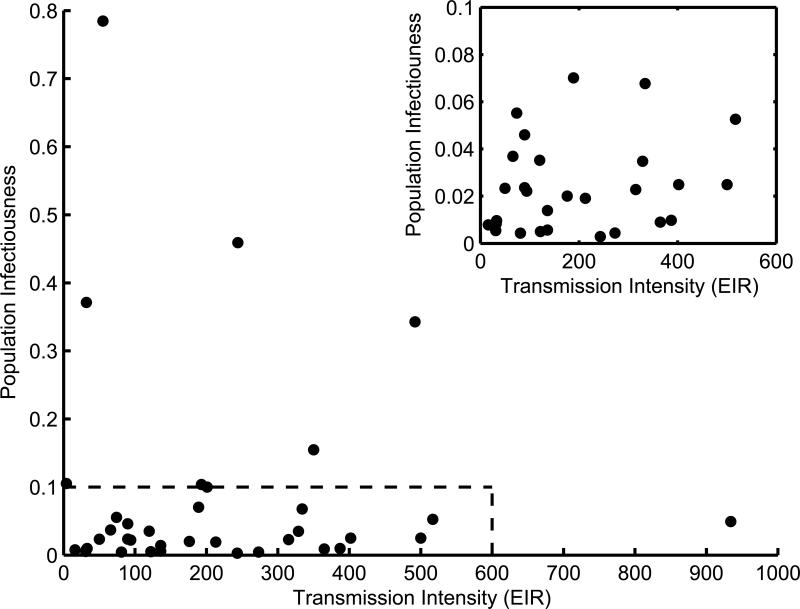
Regardless of how individual infectiousness is encoded, every malaria transmission model implicitly or explicitly specifies a relationship between EIR and population infectiousness. Data from field studies, however, show no discernible pattern between EIR and population infectiousness (Figure 2), making it very difficult to validate modeled EIR-κ relationships. Even if we allow for measurement error and lack of standardized techniques, there is no reason to expect a single setting-transcending relationship between EIR and population infectiousness. The dominant determinants of infectiousness will likely differ between settings due to a multitude of factors including spatial and temporal heterogeneity in transmission and interventions, variable levels of transmission-blocking immunity, human genetic background and parasite genetic diversity. More data is needed that directly measures the EIR-κ relationship longitudinally in areas where transmission intensity is changing, providing insights into how setting specific variables modulate the underlying determinants of infectiousness.
Figure 2. Estimates of annual transmission intensity (EIR) and population infectiousness (κ).
Data from a published survey [50] representing estimates of transmission intensity (annual EIR) and population infectiousness (κ) for different settings. Transmission intensity (EIR) is estimated using standard approaches that combine estimates of the human biting rate and infection rates in the mosquito population. Estimates of population infectiousness were attained directly using feeding experiments and/or indirectly using infection rates in the mosquito population. If multiple estimates of population infectiousness were available for the same site then the geometric mean was used. Inset: magnification of area inside dashed box in main panel.
The effect of model assumptions on the predicted impact of control
The modeling decisions about how to represent the human infectious reservoir have important implications for predicting the impact of control programs. Box 2 illustrates the amount of mosquito control that would be required to reduce transmission by a prescribed amount under the two hypothetical models shown in Figure 1. Mosquito control will cause a direct reduction in EIR and lead to a change in the population infectiousness - as specified by the EIR-κ relationship - which will further modify the EIR. We use mosquito control as an example because it does not directly modify the EIR- κ relationship specified by the human model and thus allows us to separate the direct and indirect impacts of the control. Importantly, these issues occur for both simple deterministic models and in complex individual-based formulations, but the impact of model uncertainties are less transparent and harder to interpret as model complexity increases.
Conclusions
Mathematical models can identify key knowledge gaps and examine the implications of biological relationships that are heterogeneous and hard to measure in the field, providing qualitative insights into the mechanisms of malaria transmission. Making quantitative predictions is much more challenging. Understandably, the quality of a malaria transmission model is often measured by its ability to reproduce observed patterns of clinical disease and infection prevalence. As a result, poorly understood aspects of transmission like human infectiousness are often adjusted to ensure internal model consistency. Here we have shown that assumptions about heterogeneities in human infectiousness can have important implications on predictions about the impact of control programs. Clearly communicating model assumptions and sources of uncertainty is critical, not only for the interpretation of model results by policy makers, but also to drive an iterative process between the development of theory and data collection. Basic research aimed at elucidating the main drivers of infectiousness in different transmission settings will be critical to understanding the potential impact of different control interventions.
Highlights.
- Mathematical models of malaria control must define human-to-mosquito infectiousness.
- Uncertainty about human infectiousness can lead to inaccurate policy predictions.
- More longitudinal data is needed on human infectiousness in endemic regions.
Box 1. Integrating data within models of malaria transmission dynamics.
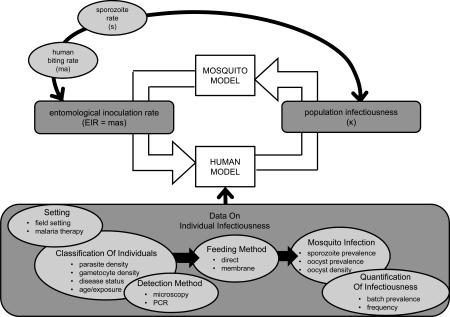
The malaria transmission cycle links mosquito-to-human transmission and human-to-mosquito transmission via the human and mosquito infection dynamics. Although field data from the mosquito component of the transmission cycle can be used to estimate parameters from the human component, point estimates do not explain how changes in mosquito infections translate to changes in human infections or how these changes will impact transmission overall. Mathematical models aim to track these interactions by mechanistically linking dynamics in the direction that transmission actually occurs. This means that whereas field data can use the prevalence of mosquito infection to estimate human population infectiousness without directly considering the human population dynamics, mathematical models must use the current state of the modeled human population to arrive at human population infectiousness. This involves specifying how individual infectiousness is defined and then scaling up to population infectiousness. An important consequence of this is that data used to estimate population infectiousness is often different from data used to inform how population infectiousness is modeled (Figure I).
Malaria transmission terms
population infectiousness (κ): The probability that a mosquito becomes infected after biting a random human.
sporozoite: The parasite form responsible for transmission from mosquito to human.
sporozoite rate (s): The fraction of the mosquito population that has sporozoites. The sporozoite rate is often used as a proxy for the fraction of mosquitoes that are infectious to humans.
mosquito density (m): The average number of mosquitoes per human.
mosquito biting rate (a): The average number of times a mosquito will bite humans per unit time.
human biting rate (ma): The expected number of mosquito bites received by an average human per unit time. The human biting rate can be mathematically expressed as the product of the mosquito density and the mosquito biting rate.
entomological inoculation rate (EIR): The expected number of infectious mosquito bites received by an average human per unit time. The EIR can be expressed mathematically as the product of the human biting rate and the sporozoite rate (EIR = mas).
Figure I. Estimating parameters versus modeling the malaria transmission cycle. Field data on the sporozoite rate (s) can be used to estimate both the entomological inoculation rate (EIR) and the population infectiousness (κ). The EIR is estimated using the sporozoite rate and the human biting rate (black arrow following the counterclockwise direction of the transmission cycle). The population infectiousness can be estimated using the sporozoite rate together with other information about the mosquito dynamics (black arrow flowing opposite to direction of transmission cycle). Mathematical models, however, must map information about the sporozoite rate forward along the direction of the transmission cycle to determine population infectiousness. This involves making assumptions about individual infectiousness within the human population. Mathematical models will often use available data on individual infectiousness to inform this choice. There is no standardized method for collecting data on individual infectiousness and a sample of the types of data available are depicted (bottom shaded area).
Box 2. The role of heterogeneity in human infectiousness on the modeled impact of control.
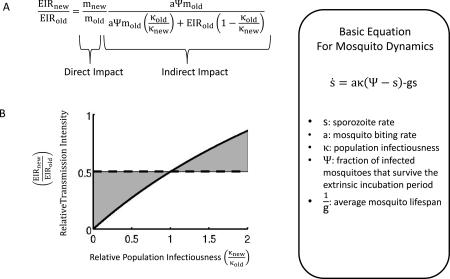
The specific assumptions about how infectiousness is distributed within the population will impact how a population responds to an intervention. Consider for example how a reduction in mosquito density translates to a change in EIR (Figure I). Decreasing mosquito density via the parameter m will cause a direct reduction in the EIR. This change in EIR will have downstream impacts dependent on the specific form of the modeled EIR-κ relationship. In general, models producing different EIR- κ relationships will require different levels of control to achieve a specified change in EIR (Figure II). In this specific example of mosquito control, a uniform distribution of infectiousness (filled circles) predicts a lower required reduction in mosquito density than a more heterogeneous distribution (filled squares). Here we focus on the impact of reducing mosquito density because it allows a clear separation between the direct impact of the intervention and the indirect impact that occurs through the resulting modulation of the population infectiousness. More generally, control measures, such as treatment, can target the human population and directly modify the EIR- κ relationship. In this case however, the resulting EIR- κ relationship will still depend on the specific assumptions of how infectiousness is distributed within the population.
Figure I. Deconstructing direct and indirect impacts of mosquito control. (a) The relative EIR after a reduction in mosquito density consists of the direct impact and an indirect impact caused by the induced change in population infectiousness. This expression does not depend on the specific details of the human model but instead only on the EIR-κ relationship produced by the human model. (b) Consider an intervention that halves the mosquito density. If the population infectiousness does not respond to this change then the overall impact will be to halve the EIR (dashed horizontal line). Conversely, if population infectiousness changes in response to the reduction in mosquito density then the overall impact on EIR will be modulated by this effect. Namely, if population infectiousness decreases the overall reduction in EIR will be greater (lower shaded region) and if the population infectiousness increases the overall reduction in EIR will be less (upper shaded region). Inset: The equation in (a) was computed using the equilibrium sporozoite rate determined by a simple model for mosquito dynamics [51].
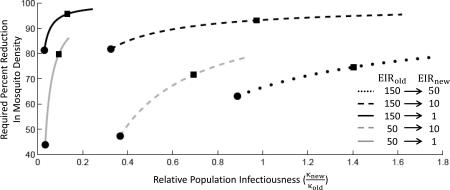
Figure II. Required mosquito control depends on EIR-κ relationships Each curve corresponds to a different initial and target EIR (as described in inset) and shows the required percent reduction in mosquito density as a function of relative population infectiousness. The relative population infectiousness is determined by the EIR-κ relationship. For example each filled circle corresponds to the EIR-κ relationship as specified by the “uniform” assumption and each filled square corresponds to the EIR-κ relationship specified by the “standard” assumption (see Figure 1c).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alonso PL, et al. A research agenda to underpin malaria eradication. PLoS Medicine. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modeling T.m.C.G.o. A research agenda for malaria eradication: Modeling. PLoS Med. 2011;8:1000403. doi: 10.1371/journal.pmed.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousema T, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Medicine. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooker S, et al. Spatial clustering of malaria and associated risk factors during an epidemic in a highland area of western Kenya. Tropical Medicine & International Health. 2004;9:757–766. doi: 10.1111/j.1365-3156.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 5.Carter R, et al. Spatial targeting of interventions against malaria. Bulletin-World Health Organization. 2000;78:1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 6.Cottrell G, et al. Modeling the influence of local environmental factors on malaria transmission in Benin and its implications for cohort study. PLoS One. 2012;7:e28812. doi: 10.1371/journal.pone.0028812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst KC, et al. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malaria Journal. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreuels B, et al. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. Journal of Infectious Diseases. 2008;197:85–93. doi: 10.1086/524066. [DOI] [PubMed] [Google Scholar]
- 9.Pullan RL, et al. Spatial parasite ecology and epidemiology: a review of methods and applications. Parasitology. 1:1–18. doi: 10.1017/S0031182012000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dye C. Vectorial capacity: must we measure all its components? Parasitology Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, et al. Parasite virulence and disease patterns in Plasmodium falciparum malaria. Proceedings of the National Academy of Sciences. 1994;91:3715–3719. doi: 10.1073/pnas.91.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DL, et al. The Risk of a Mosquito-Borne Infectionin a Heterogeneous Environment. PLoS Biology. 2004;2:e368. doi: 10.1371/journal.pbio.0020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DL, et al. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biology. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolhouse ME, et al. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proceedings of the National Academy of Sciences. 1997;94:338–342. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Wahab A, et al. Dynamics of gametocytes among Plasmodium falciparum clones in natural infections in an area of highly seasonal transmission. Journal of Infectious Diseases. 2002;185:1838. doi: 10.1086/340638. [DOI] [PubMed] [Google Scholar]
- 16.Alves FP, et al. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. Journal of medical entomology. 2005;42:777–779. doi: 10.1093/jmedent/42.5.777. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet S, et al. Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in south Cameroon: evaluation and comparison of several indices. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:53–59. doi: 10.1016/s0035-9203(03)90022-8. [DOI] [PubMed] [Google Scholar]
- 18.Boudin C, et al. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. The American journal of tropical medicine and hygiene. 1993;48:700–706. doi: 10.4269/ajtmh.1993.48.700. [DOI] [PubMed] [Google Scholar]
- 19.Bousema JT, et al. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malaria Journal. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chernin E. The malariatherapy of neurosyphilis. The Journal of Parasitology. 1984;70:611–617. [PubMed] [Google Scholar]
- 21.Collins WE, Jeffery GM. A retrospective examination of mosquito infection on humans infected with Plasmodium falciparum. The American journal of tropical medicine and hygiene. 2003;68:366–371. [PubMed] [Google Scholar]
- 22.Gouagna LC, et al. Plasmodium falciparum malaria disease manifestations in humans and transmission to Anopheles gambiae: a field study in Western Kenya. Parasitology. 2004;128:235–243. doi: 10.1017/s003118200300444x. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery GM, Eyles DE. The duration in the human host of infections with a Panama strain of Plasmodium falciparum. The American journal of tropical medicine and hygiene. 1954;3:219. doi: 10.4269/ajtmh.1954.3.219. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery GM, Eyles DE. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. The American journal of tropical medicine and hygiene. 1955;4:781. doi: 10.4269/ajtmh.1955.4.781. [DOI] [PubMed] [Google Scholar]
- 25.Schneider P, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. The American journal of tropical medicine and hygiene. 2007;76:470–474. [PubMed] [Google Scholar]
- 26.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews. 24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul R, et al. Aggregation in malaria parasites places limits on mosquito infection rates. Infection, Genetics and Evolution. 2007;7:577–586. doi: 10.1016/j.meegid.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan JA. Population dynamics of Plasmodium sporogony. Trends in parasitology. 2007;23:63–70. doi: 10.1016/j.pt.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Gatton ML, Cheng Q. Interrupting Malaria Transmission: Quantifying the Impact of Interventions in Regions of Low to Moderate Transmission. PLoS One. 5:e15149. doi: 10.1371/journal.pone.0015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maire N, et al. Predictions of the epidemiologic impact of introducing a pre-erythrocytic vaccine into the expanded program on immunization in sub-Saharan Africa. The American journal of tropical medicine and hygiene. 2006;75:111–118. doi: 10.4269/ajtmh.2006.75.111. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie FE, Bossert WH. An integrated model of Plasmodium falciparum dynamics. Journal of Theoretical Biology. 2005;232:411–426. doi: 10.1016/j.jtbi.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 32.Ross A, et al. Relationships between host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. The American journal of tropical medicine and hygiene. 2006;75:32–37. doi: 10.4269/ajtmh.2006.75.32. [DOI] [PubMed] [Google Scholar]
- 33.Tediosi F, et al. An approach to model the costs and effects of case management of Plasmodium falciparum malaria in sub-Saharan Africa. The American journal of tropical medicine and hygiene. 2006;75:90–103. doi: 10.4269/ajtmh.2006.75.90. [DOI] [PubMed] [Google Scholar]
- 34.Carneiro A. A group decision support system for strategic alternatives selection. Management Decision. 2001;39:218–226. [Google Scholar]
- 35.Águas R, et al. Prospects for malaria eradication in sub-Saharan Africa. PLoS One. 2008;3:e1767. doi: 10.1371/journal.pone.0001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chitnis N, et al. Bifurcation analysis of a mathematical model for malaria transmission. SIAM Journal on Applied Mathematics. 2006;67:24. [Google Scholar]
- 37.Chiyaka C, et al. Effects of treatment and drug resistance on the transmission dynamics of malaria in endemic areas. Theoretical population biology. 2009;75:14–29. doi: 10.1016/j.tpb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Filipe JAN, et al. Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Computational Biology. 2007;3:e255. doi: 10.1371/journal.pcbi.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghani AC, et al. Loss of population levels of immunity to malaria as a result of exposure-reducing interventions: consequences for interpretation of disease trends. PLoS One. 2009;4:e4383. doi: 10.1371/journal.pone.0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffin JT, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Medicine. 7:e1000324. doi: 10.1371/journal.pmed.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu W, et al. An individual-based model of Plasmodium falciparum malaria transmission on the coast of Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:43–50. doi: 10.1016/s0035-9203(03)90018-6. [DOI] [PubMed] [Google Scholar]
- 42.Karl S, et al. A Sub-Microscopic Gametocyte Reservoir Can Sustain Malaria Transmission. PLoS One. 6:e20805. doi: 10.1371/journal.pone.0020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kern S, et al. Community screening and treatment of asymptomatic carriers of Plasmodium falciparum with artemether-lumefantrine to reduce malaria disease burden: a modelling and simulation analysis. Malar J. 10:210. doi: 10.1186/1475-2875-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawpoolsri S, et al. Optimally timing primaquine treatment to reduce Plasmodium falciparum transmission in low endemicity Thai-Myanmar border populations. Malaria Journal. 2009;8:159. doi: 10.1186/1475-2875-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Menach A, et al. An elaborated feeding cycle model for reductions in vectorial capacity of night-biting mosquitoes by insecticide-treated nets. Malaria Journal. 2007;6:10. doi: 10.1186/1475-2875-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okell LC, et al. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One. 6:e20179. doi: 10.1371/journal.pone.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruan S, et al. On the delayed Ross Macdonald model for malaria transmission. Bulletin of mathematical biology. 2008;70:1098–1114. doi: 10.1007/s11538-007-9292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White LJ, et al. The role of simple mathematical models in malaria elimination strategy design. Malaria Journal. 2009;8:212. doi: 10.1186/1475-2875-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okell LC, et al. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nature communications. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Killeen GF, et al. Infectiousness of malaria-endemic human populations to vectors. The American journal of tropical medicine and hygiene. 2006;75:38–45. doi: 10.4269/ajtmh.2006.75.2_suppl.0750038. [DOI] [PubMed] [Google Scholar]
- 51.Smith D, McKenzie FE. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria Journal. 2004;3:13. doi: 10.1186/1475-2875-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]