Abstract
Protein Phosphatase 2A (PP2A) is an important and ubiquitously expressed serine threonine phosphatase and regulates the function by dephosphorylating many critical cellular molecules like Akt, p53, c-Myc and β-catenin. It plays a critical role in cellular processes, such as cell proliferation, signal transduction and apoptosis. Structurally, it is multifarious as it is composed of catalytic, scaffold and regulatory subunits. The catalytic and scaffold subunits have two isoforms and the regulatory subunit has four different families containing different isoforms. The regulatory subunit is the most diverse with temporal and spatial specificity. PP2A undergoes post-translational modifications (i.e. phosphorylation and methylation), which in turn, regulates its enzymatic activity. Aberrant expression, mutations and somatic alterations of the PP2A scaffold and regulatory subunits have been observed in various human malignancies, including lung, breast, skin and colon cancer, highlighting its role as a ‘tumor suppressor’. This review is focused on the structural complexity of serine/threonine phosphatase PP2A and summarizes its expression pattern in cancer. Additionally, the PP2A interacting and regulatory proteins and substrates are also discussed. Finally, the mouse models developed to understand the biological role of PP2A subunits in an in vivo model system are also reviewed in this article.
1. Introduction - Kinases and Phosphatases
In a typical cell, the functions of nearly one-third of the proteins are regulated via phosphorylation and it controls various biological functions like cell division, growth and development, survival, proliferation, and apoptosis. Depending upon the physiological necessity of the cell, proteins transiently shift from a phosphorylated to a dephosphorylated state and vice versa, and are specifically controlled by protein kinases and protein phosphatases [1]. Hence, kinases and phosphatases act as important checkpoint regulators and switches. Upon studying the various kinases and phosphatases, researchers have identified many protein kinases comprising two families - protein tyrosine kinases and protein serine threonine kinases [2], while a comparable number of phosphatases are not present in the biological system. Phosphatases are classified in two major classes, protein tyrosine phosphatases (PTPs) and protein serine/threonine phosphatases (PSPs) [3]. In general, the phosphatases may exist in active monomeric unit (having the catalytic subunit alone-PTP) or may contain active dimeric form (comprising of catalytic and regulatory subunit-PTP/PSP) or active holoenzyme complex of catalytic, regulatory and scaffold subunits, as observed in certain family members of protein serine phosphatase. Each subunit also has many different isoforms. Since the isoforms of a subunit for a single phosphatase can form complexes in many possible combinations with isoforms of other subunits, different variants of a specific phosphatase are present in nature. Thus, the variant based on the composition of its particular isoform of the subunits obtain specificity toward their physiological substrates. It will thus explain how phosphatases, although present in a smaller number, can balance the activity of many kinases. Thus, both the kinases and phosphatases act concurrently to control the amplitude, rate and duration of signaling response [4]. To further highlight the unique property of protein serine/threonine kinases and phosphatases, protein tyrosine kinases and protein tyrosine specific phosphatases in S. cerevisiae, C. elegans, D. melanogaster and humans were elaborated in Table.1.
Table 1.
Total number of coding proteins and number of kinases and phosphatases in the eukaryotic genome.
| Proteins | S.cerevisiae | D.melanogaster | C.elegans | Human | Reference |
|---|---|---|---|---|---|
| Total number of proteins | 6122 | 13600 | 18988 | 25000 | [99] |
| Total Protein kinases | 124 | 236 | 493 | 518 | |
| Protein Kinases-411 Protein Kinases like proteins -82 |
Protein serine/threonine kinase -385 Protein tyrosine kinase (PTK) - 90 PTK like protein - 43 |
||||
| Total Protein phosphatases | 37 | 93 | 185 | 119 | |
| Protein serine/threonine phosphatase-21 Protein tyrosine specific phosphatase-98 |
2. Protein Phosphatases
Protein modification via phosphorylation mainly occurs on the hydroxyl-group containing amino acid residues, namely serine (Ser), threonine (Thr) and tyrosine (Tyr). Upon comparing the degree of phosphorylation between these three phosphorylated amino acids, phosphoserine (pSer) predominates with 86.4%, then phosphothreonine (pThr) at 11.8%, followed by phosphotyrosine (pTyr) with 1.8% [5]. Protein phosphatases nucleophilically attack the phosphate group for catalysis in the presence of a water molecule [6] and dephosphorylate these three phosphorylated amino acids. The members of the protein phosphatase super family are broadly divided into two major groups - protein serine/threonine phosphatases (PSPs) and phosphotyrosine phosphatase (PTPs). PSPs are further divided into three sub-families: phosphoprotein phosphatases (PPPs), metal dependent protein phosphatases (PPMs) and aspartate based phosphatase. PPPs are diverse and contain subfamilies known as PP1, PP2A, calcium-activated PP2B (also known as calcineurin), PP4, PP5, PP6 and PP7. The PPM family is comprised of PP2C and pyruvate dehydrogenase phosphatases; they catalyze the reaction in the presence of Mg2+/Mn2+. Aspartate-based phosphatase has an aspartic acid signature (DXDXT/V). Examples of this group of phosphatases are transcription initiation factor II: TFIIF-associated C-terminal domain phosphatase/small CTD phosphatases, and the halo acid dehydrogenases (HAD) enzyme family [7]. Protein tyrosine phosphatases (PTP) specifically remove the phosphate group from post transnationally modified tyrosine residues. These PTPs have multiple functions encountering protein tyrosine kinases; one of the best examples for PTP target is the regulation of AKT signaling by dual specificity phosphatase, phosphatase and tensin homolog protein (PTEN). Furthermore, a sub group of dual specificity phosphatase family member MAP kinase phosphatases (MKPs) are the major regulator of MAP kinase pathway. The distinct complementary function of PTPs in the regulation of signal transduction and the etiology of human disease was best described in the review by Nicolas et al, 2006 [4].
2.1. Protein Phosphatases 2A: PP2A
PP2A is an important player in many cellular functions. It controls cell metabolism by regulating the activity of the enzymes involved in glycolysis, lipid metabolism and catecholamine synthesis [8]. It also regulates various biological processes such as the cell cycle (by mediating cdc2 kinase activation), DNA replication, transcription and translation, signal transduction, cell proliferation, cytoskeleton dynamics and cell mobility and apoptosis. It has also been shown to play a role in cell transformation and cancer [9-12]. In eukaryotes, the predicted amino acid sequence establishes a high degree of sequence conservation (78-93%) of PP2A between yeast, drosophila and mammals [13]. It is ubiquitously expressed and contributes to 0.3 - 1% of the total cellular protein in the mammalian cell [14]. The majority of the soluble phosphatases activity at phospho serine and phospho threonine is catalyzed by PP2A. PP2A is structurally complex and exists in two different forms: dimeric form (PP2AD) [15] and a trimeric form (PP2AT) [16]. The dimeric form is known as the core enzyme and is composed of the catalytic and scaffold subunit, while the trimeric form is an active holoenzyme complex which consists of three subunits: catalytic (PP2Ac), scaffold (PP2AA) and regulatory subunits (PP2AB) [17](Figure.1).
Figure 1. Schematic representation of the structural diversity of the PP2A holoenzyme complex.
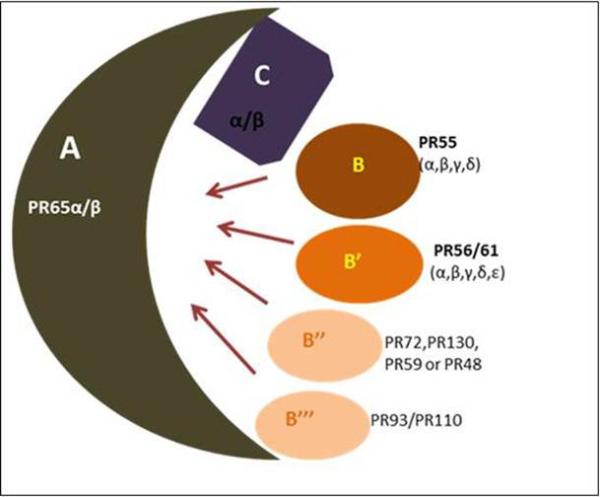
PP2A enzymes are heterotrimers consisting of core dimer sscaffold (A) and a ccatalytic (C) subunit that is associated with one of the rregulatory (B) subunits. The scaffold A and catalytic C are encoded by two distinct genes α and β. The α and β isoforms of the catalytic subunit share 97% homology. However, in the typical cell, the catalytic α isoform predominates which is 10 times more abundant than the β isoform. Similar to catalytic subunit α and β isoforms of the scaffold subunit also share 86% identity in their primary sequence. The B-type subunits were further categorized into four unrelated families: B (PR55), B’ (PR56/61), B” (PR72/130) and B’” (PR93/110). Within each B-type family, distinct genes encode various structurally related isoforms such as α, β, γ and δ.
2.2. PP2A: Catalytic subunit (PP2Ac/PPP2C)
PP2A catalytic subunit (PP2Ac) is globular in structure, ubiquitously expressed in almost every tissue, and it is most abundant in the heart and brain. The protein expression level of PP2Ac in the cell is regulated translationally to maintain a constant level [18]. The amino acid sequence of PP2Ac (37kDa) is 86% identical in human and yeast and it also shares 50% amino acid sequences with PP1 and 40% identical sequences with PP2B. PP2Ac exists in two isoforms Cα and Cβ. They are encoded by two separate genes each comprised of seven exons and six introns. Exon 2-6 participates in substrate binding and catalysis, whereas exon 1 and 7 aid in regulation. Both isoforms consist of 309 amino acids and share 97% sequence similarity. The isoform Cα is predominantly expressed in the plasma membrane and Cβ in the cytoplasm and nucleus. Cα is expressed in higher abundance than Cβ due to its strong promoter activity as well as due to differences in the rate of mRNA turnover [19].
PP2Ac C-terminal tail is uniquely conserved (304TPDYEL309) and binds to the scaffold and regulatory subunits (PR61γ). Toward the N-terminal of PP2A, Cα and Cβ differ by eight amino acids and these amino acids are also not conserved in PP1 and PP2B, suggesting that this part of the protein may not play a role in catalysis [20]. Despite sharing 97% similarity, PP2Acα knockout mice are not viable and die at embryonic day 6.5, indicating that PP2Acβ is unable to substitute for PP2Acα loss, underscoring the non-redundant function of Cα in embryonic development and gastrulation [21].
2.3. PP2A: Scaffold subunit (PPP2AR1/PR65/PP2A-A)
Similar to PP2Ac, the PP2A scaffold subunit is encoded by two distinct genes, PPP2R1A and PPP2R1B, resulting in two isoforms, Aα and Aβ. Both are ubiquitously expressed and share 86% sequence similarity [22]. In about 90% of the PP2A assemblies, the core and/or holoenzyme is composed of the Aα scaffold subunit and is highly abundant in all normal tissues, accounting for 0.1% of the total cell protein. On the other hand, Aβ is found in 10% of PP2A assembly leading to differential preference of interaction with the catalytic and regulatory subunits [23]. Both isoforms are fractionated in the cytoplasm. Uniquely, in Xenopus oocytes, Aβ is highly expressed during stage 35 of embryogenesis in the ovary for meiotic maturation and fertilization. Further, from that stage, it gradually decreases and Aα expression increases [24].
In its heterotrimeric form, PP2A-A acts as a structural assembly base to escort the catalytic subunit and to facilitate interaction with the regulatory subunit and other substrates. Whereas in the dimeric form it acts as a regulator by changing the catalytic specificity [25]. Structurally, the scaffold subunit is comparatively different from other subunits. PP2A-A is composed of HEAT sequence (Huntington/elongation/A-subunit/TOR) having 15 tandem repeats of 39 amino acids each. Each repeat is composed of two α-helices and they are connected by inter- and intra-repeat loops. The catalytic subunit binds to 11-15 repeats and the regulatory subunit binds to 1-10 repeats of the HEAT sequence. The scaffold subunit escorting the catalytic subunit bends to form a base and shows a horseshoe shape-like structure. This flexible bending helps to recruit the regulatory subunit and other PP2A substrates [26].
2.4. PP2A: Regulatory subunit (PP2A-B/PR55- B/PR56or61-B’/PR72-B”/PR93or110-B’”)
2.4.1. PP2A-B/PR55
The PP2A regulatory subunit is structurally diverse and has a minimum of 26 different transcript and splice variants encoded by 15 different genes in the human genome. PP2A-B is thought to be the master regulator of the PP2A holoenzyme and it is likely to act as a targeting modulator to provide temporal and spatial specificity [27]. Even though all the different family members and isoforms of PP2A-B bind to similar recognition sequences of PP2A Aα, they do not possess similar gene sequences. Regulatory subunits are multiform and are classified into four different families: B (commonly known as B55/PR55), B′ (B56/PR61), B″ (PR48/PR72/PR130), and B″′ (PR93/PR110). These numbers represent the approximate molecular weight of each peptide in kDa. The protein secondary structure predicts that they form β-sheets and turns. PP2A-B family members are expressed distinctly at various developmental stages in a tissue-specific manner. B55 has four different isoforms (α, β, γ and δ). It is observed that the expression of B55γ increases and B55β decreases gradually after birth and are developmentally regulated [28]. Structural recognition of the B55 family is the presence of the tryptophan-aspartate repeat (40-WD repeats) involved in protein-protein interaction to facilitate substrate binding. In neurons, B55α and B55β are localized in the cytoplasm, whereas B55γ is localized in the cytoskeletal fraction. It has been shown in yeast that CDC55 (B55 in human) is essential for cytokinesis. Mutation and/or deletion of CDC55 produce abnormal unbudded cells and display a partial blockage of cell separation [29]. In mammals, B55 is associated with cytoskeletal dynamics and nuclear translocation. Conditional B55 knockout in fibroblasts are unable to dephosphorylate vimentin, leading to alteration in the interphase dynamics differentiation and migration [30].
2.4.2. PP2A-B’/PR56/PR61
B56, another regulatory family has five different isoforms (α, β, γ, δ, and ε ). The unique feature of this family is that they can be phosphorylated and are mostly α helical. They can directly bind to the core enzyme and enhance the reaction. These isoforms show 80% identical sequences in their central region but differ in their N and C terminals, leading to different expression levels in tissues. Intracellular localization of B56 isoforms vary, as B56γ is expressed in the nucleus while B56α, B56β, and B56ε are expressed in the cytoplasm, and PR61δ appears to be expressed in both the nucleus and cytoplasm [20]. It is surprising to find that the nuclear localization signal (KRTVETEAVQMLKDIKK) is only present in B56δ and B56γ3 and absent in B56γ1 and B56γ2, yet all these isoforms are efficiently targeted to the nucleus. In the late G1 phase of the cell cycle, B56α regulates p53 via Cyclin G and in Wnt signaling via the APC component [31]. Studies showing differential phosphorylation, sequence alignment and spatial intracellular localization indicate that B56 governs several functions, which are yet to be understood.
2.4.3. PP2A-B”: PR72 and PP2A-B’”/PR93 or110
B” and B’” families were discovered by yeast two hybrid screening. B” family contains PR72 and PR130 isoforms, which differ at the N-terminal region, indicating that the origin of these variants may be due to alternate splicing. PR72 is expressed only in the heart and skeletal muscle, whereas PR130 is ubiquitously expressed in all tissues and is abundant in the heart and muscle. PR72 requires calcium binding in two EFX domains (Domain required for Nkd to interact with the basic/PDZ domains of fly Dsh or vertebrate Dvl proteins in the yeast two-hybrid assay) and this binding facilitates conformational changes in protein structure to interact with the PR65 scaffold subunit [32]. PP2A-B inhibits simian virus 40 (SV40) replication, whereas PP2A-B” containing holoenzyme activates its replication. It was also found that B” regulates Rb phosphorylation at p107 during UV-radiation exposure and they excite the DNA damage response gene [33]. In vitro overexpression of B” can arrest cells at the G1 stage and inhibit cell cycle progression. B’” family is a calmodulin binding protein (CaM) and it interacts with the core enzyme to enhance calcium-dependent signaling. It contains eight splice variants but only four are actively translated. This group also requires ATP and Mg2+ to activate its own activity. Striatin and S/G2 nuclear auto antigen (SG2NA) is an example of the B’” family. It is a nuclear protein expressed during the S and G2 phases of the cell cycle [34]. Different combinations of multifarious PP2A subunits (catalytic: C, scaffold: A, and regulatory: B), including their isoforms, assemble to form more than 200 types of biochemically distinct heterotrimeric PP2A holoenzymes. Comparing all of the three subunits, PP2A-A provides substantial structural flexibility (HEAT region) and can undergo various conformational changes [35]. Its three dimensional architecture allows binding of various molecules.
3. PP2A regulators, interacting proteins, substrates and inhibitors
The major hallmark of PP2A is its protein assembly or stable complex formation, through which it interacts with vital intermediate signaling molecules that are present in the cytoplasm. It also modulates their function by interacting and transforming the phosphorylation status of the partner [36]. During meiosis I, Shugoshins binds to PP2A and dephosphorylates cohesion, which prevents spindle microtubules disassembly [37]. PP2APR55 also dephosphorylates vimentin (intermediate filament) and protects cytoskeleton disassembly [38].
Various cellular proteins such as α4, I1PP2A and I2PP2A directly interact with the AC core dimer or with the free C subunit and regulate the phosphatase regulatory mechanism of PP2A [39]. Studies by McConnell et al 2007 have led to the identification of a novel regulator of PP2A, type 2 A interacting protein (TIP) in the mammalian system which highlights the differently regulated function of PP2A between yeast and metazoan system. The TIP protein directly interacts and retains the catalytic activity of PP2A and PP2A-like enzymes, such as PP4 and PP6, and further highlighted phosphatase as an additional therapeutic target for cancer prevention [40]. Another experimental approach by Lee et al 2007 led to the identification of various PP2A interacting partners that are specific to the catalytic subunit. Apart from the known interacting partners (Axin and CaMK iV), PP2Ac-specific interacting proteins, such as tuberous sclerosis complex 2 (TSC2), R-Ras, Nm23H2 and RhoB, were confirmed by pull down experiments in the presence of the Wnt3a as a ligand, a stimulus that can activate the growth regulatory signaling pathway of Wnt/beta catenin and ERK [41]. Initial studies by Seeling et al 1999 have shown through the yeast two hybrid system that a regulatory subunit of PP2A, B56, interacts with adenomatous polyposis coli (APC) protein, implicating the inhibition of Wnt/betacatenin signaling and an alteration in B56-specific PP2A function is essential for early xenopus development [31, 42, 43]. Recently, a novel complex assembly consisting of RAC1 kinase, PP2A and AKT in NSCLC has been shown to result in reduced phosphorylation of AKT and inhibition of cancer cell metastasis in response to Eph3 activation [44]. The phosphoprotein SET is a potent inhibitor of PP2A activity [45]. It is localized both in the nucleus and cytoplasm. SET interacts with PP2Ac via its highly acidic C-terminal domain and completely inhibits the phosphatases activity of PP2A. SET is overexpressed in various cancers like chronic myelogeneous leukemia and Wilm's tumor [46]. In Alzheimer's disease, inhibition of PP2A activity by SET leads to hyper phosphorylation of the Tau protein [47].
Potent tumor promoter, Okadaic acid (a microbial toxin), inhibits the enzymatic activity of PP2Ac and thereby has facilitated various studies to understand the functional aspects of PP2A and other phosphatases [12]. Other than Okadaic acid, calyculin A, microcystin, cantharidin, nodularm, fostriecin and tautomycin are able to inhibit PP2A activity at different IC50 values [48]. In addition to microbial toxin, viral protein SV40 (potent oncogene) also inactivates PP2A action by binding to the AC dimer and displacing the PR56 (PP2A-B’γ) subunit [35]. Endogenous CIP2A (cancerous inhibitor of PP2A) inhibits PP2Ac activity via interacting with c-Myc (Ser62) and stabilizes it from proteolytic degradation [49]. Recently, a major indirect activation of PP2A by inhibiting CIP2A at both the transcriptional and translational levels through the drug bortezomib was shown in triple negative breast cancer cells [50]. Further, carnosic acid, a ployphenolic diterpene, has been shown to induce apoptosis in prostate cancer cells by modulating AKT/NFkB pathways [51]. Thus, inhibitors of CIP2A and activators of PP2A that modulate the anti-apoptotic (Both intrinsic and extrinsic) pathway may serve as potential agents to be used in the prevention and/or treatment of various cancers.
4. PP2A: Regulation and Modification
PP2A is being regulated by post translation modification, auto-regulation, subunit diversity and substrate protein interaction. Methylation [52] and phosphorylation [53] are two major modifications that have been shown to modulate PP2A catalytic efficiency. As mentioned previously TPDYFL is a conserved sequence in PP2Ac. Tyrosine (Y) and leucine (L) undergo phosphorylation and methylation, respectively. Phosphorylation of Y307 by receptor associated tyrosine kinases effectively decreases the PP2A activity by inhibiting the interaction of PP2Ac with the PP2A-PR55/PR61 subunit [54]. PP2A also possesses the auto protein tyrosine phosphatases activity, which is inhibited by auto-phosphorylated–activated protein kinases to alter PP2A efficacy [55]. Another modification is methylation of PP2A at L309 by PP2A-methyltransferase (PPMT). It is also known as leucine carboxyl methyltransferase 1 or LCMT1. The demethylation occurs by PP2A-methylesterase (PPME). The addition of a methyl group by LCMT1 at L309 enhances the binding affinity of the core dimer (A&C subunit) toward distinct regulatory subunits and provides specific activity to the holoenzyme [56]. In addition to modification, constitutive and constant PP2Ac expression is controlled by auto-regulation. PP2Ac expression is tightly regulated in the cell at the translational level but not at the transcription level [18] (Figure 2).
Figure 2. Post transitional modification of PP2A.
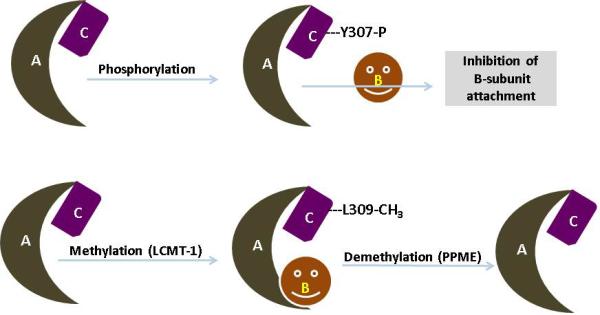
PP2A is being regulated by post translation modification, auto-regulation, subunit diversity and substrate protein interaction. Phosphorylation and methylation are two major modifications that have been shown to modulate PP2A subunit associations and catalytic efficiency. Upon reversible phosphorylation of tyrosine residue 307 (located in the C-terminal part of PP2A catalytic subunit) by receptor associated tyrosine kinases (pp60v-src, pp56lck, EGF and insulin receptors) results in decreased phosphatase activity of PP2A. In addition to tyrosine phosphorylation, PP2A can also be auto-phophorylated on threonine residues by activated protein kinases. Apart from the PP2A catalytic subunit, the regulatory subunits are also subjected to phosphorylation. Notably, PR61δ by PKA and PR 61α by PKR are the best evidence for the change in the PP2A activity. Similarly, methylation of PP2A occurs at leucine 309 residue by PP2A-methyltransferase and enhances the binding affinity of the AC core dimer toward a distinct regulatory subunit.
5. Regulation of PP2A in cellular pathways
PP2A activity is indispensable for every cell and takes part in the majority of the cellular pathways. Dysfunction or deregulation of PP2A will affect various physiological processes. As mentioned previously, PP2A dephosphorylation activity is essential during embryogenesis and PP2Acα knockout mice are embryonically lethal. In Drosophila, Sex Combs Reduced (SCR) Hox protein determines the identity of the labial and prothoracic segments. SiRNA-mediated PR56/B’ knock down resulted in embryos without salivary glands and demonstrated dPP2AB’ directly interacting with SCR as a positive modulator [57], indicating the importance of PP2A during cell survival and maintenance.
PP2A not only controls the cell cycle but it also controls cell death (apoptosis). Akt plays an important role in cell growth, proliferation and apoptosis. Phosphorylation at Thr-308 and Ser-473 leads to activation of Akt and it was found that deregulation of Akt is associated with various human malignancies. Co-immunoprecipitation and in-vitro pull down assay using pro-lymphoid FL5.12 cells showed a direct association of the PP2A-B55 holoenzyme with Akt, which selectively regulates phosphorylation of Akt at Thr-308 and regulates cell proliferation and survival [58]. It suggests that PP2A acts as a negative regulator for the Akt pathway. PP2A also plays a major role in the Wnt signaling pathway. In Xenopus, PP2A-B56 is involved in β-catenin dephosphorylation and degradation and its phosphorylation directs activation of the Wnt pathway [43]. Key players of programmed cell death like BAD (pro-apoptotic) and Bcl-2 are also regulated by PP2A. Phosphorylation of BAD suppresses, and its dephosphorylation by PP2A promotes pro-apoptotic activity [59]. Additionally, phosphorylation of Bcl-2 activates, and its dephosphorylation by PP2A suppresses anti-apoptotic activity. In Drosophila, knock down or loss of either of the subunits A, C, or B56 leads to hyper phosphorylation of Bcl-2, thus initiating p53-mediated apoptosis [60].
The enzymatic activity of PP2A depends upon its stability in the cell. PP2A stability could be dependent upon the half-life of its monomeric subunits, their association with each other and interaction of the dimeric and trimeric subunits. For example, PR56/B’ family members are present throughout the process of mitosis and there is a nuclear and cytoplasmic shuttling between B’α, B’β and B’ε during the interphase [61] with C and A subunits. It has been shown that knockdown of the PP2A-Aα subunit results in a loss of C, B and B’ subunits, whereas B” and striatin remained stable. Likewise, Bγ mutants are rapidly cleared, although B″/PR72 mutants are stable. These monomeric subunits are rapidly degraded via the ubiquitin/proteasome degradation pathway [62].
PP2A dephosphorylation activity maintains cell adhesion and cytoskeleton dynamics. PP2A is co-localized with β1-integrin and is an important regulator of FAK (focal adhesion kinase) complex [63]. Inhibition of PP2A enhances FAK/Src/paxillin hyper phosphorylation, leading to disorganization of focal adhesion sites and increased cell migration in endothelial cells [64]. Its continuous disruption is linked to increased cell motility, invasiveness and loss of cell polarity. Moreover, association of increased cell motility and invasiveness due to altered PP2A activity was observed in BL6 mouse melanoma cells [65], lung carcinoma [66] and head and neck squamous carcinoma [67].
6. Tumor suppressor PP2A in transformation and cancer
Several aberrant biological and environmental factors in cancer evoke alteration in various cellular pathways during the cell cycle, cell growth and maintenance, proliferation and differentiation. Genetic instability, like mutation, amplification, rearrangement, chromosomal insertion/ deletion, frame shift, and homozygous whole gene deletion [68], favors oncogenesis by either activating constitutively certain oncogenes and/or inactivating tumor suppressor genes [69, 70]. Other ways of inactivation of tumor suppressor genes include methylation and acetylation [71].
PP2A is considered as a tumor suppressor and is thought to be functionally inactivated in cancer. Therefore, the PP2A inhibitor, okadaic acid, when injected into mice, caused tumor development [72]. Another tumor antigen-SV40 small T antigen, polyoma small T antigen replaces the B subunit of PP2A and alters the phosphatase activity of PP2A, leading to transformation [73]. These two pieces of evidence established the tumor suppressive nature of PP2A [74]. The PP2A scaffold and regulatory subunit were shown to be mutated or aberrantly expressed in many different types of cancer. Mutation in PR65α/Aα was found in lung carcinoma and melanoma [75] and PR65β/Aβ mutation was seen in colon and lung cancer [76]. Other than lung and colon cancer, loss of function of scaffold subunits was also observed in cancers of the breast, skin, cervix and ovary. Mutations of scaffold PR65α/Aα, such as Glu64 to Asp in lung carcinoma, Glu64 to Gly in breast carcinoma, and Arg418 to Trp in melanoma, had been observed. Mutagenesis of Glu64 to Asp/Gly disturbed the binding ability of PR65α/Aα to PR56/B’ [77]. A PR65β/Aβ missense mutation, like Pro65 to Ser, Leu 101 to Pro, Lys343 to Glu, Asp504 to Gly, and Val545 to Ala, are detected in lung and colon cancers [76]. Recently, unique mutations of Arg183 to Gly/Trp and Arg182 to Trp of PPP2R1A of the regulatory subunit in ovarian cancer have been identified. In vitro mutation of these amino acids showed a defective binding ability of B, B’ subunits, indicating reduced tumor suppressor function of PP2A in ovarian cancer [78]. Interestingly, about 43% of human glioma PP2A-Aα levels were reduced to 10-fold, indicating a decreased expression of core and holoenzyme and high levels of unregulated catalytic C subunit [79].
In HEK293 cells, a 50% reduction in PP2A-Aα levels leads to a reduction in PP2A-B’γ holoenzyme and enhanced tumor formation in immunodeficient mice due to activation of the Akt pathway [80]. Like Akt, GTPase RalA participates during transcription, migration, transport, apoptosis and cell proliferation. It was found that PP2A-Aβ binds and regulates the activity of RalA. Transformation associated with knockdown or loss of PP2A-Aβ showed hyperphosphorylation of RalA [81]. Three splice variants of PP2A-Aβ due to gene skipping were observed in B-cell chronic lymphocytic leukemia (B-CLL). These splice variants were unable to assemble C and B subunits and resulted in a loss of PP2A activity [82]. In breast cancer, there is an alteration of Gly90 to Asp in PP2A-Aβ. Mutation of Gly90 to Asp inhibits the interaction of Aβ with B56γ, although the binding of the PR72/B” subunit was not affected [83]. Very recently, Nagendra et al 2011 confirmed a high frequency of the PP2A-Aα mutation in 32% uterine serous carcinomas. With the help of high throughput sequencing, mutation in Pro179 to Arg in the ACI-158 serous carcinoma cell line, a Pro to Leu in a primary serous carcinoma as well as a Arg to His mutation in the codon 258 of poorly differentiated endometrium cancer were also observed [84].
Like the scaffold subunit, the regulatory subunit of PP2A (mainly B55 and B56) was also mutated in many types of cancers. In acute myeloid leukemia (AML) patients, PP2AB55α expression was found to be lower than normal. As mentioned earlier, B55α dephosphorylates Akt at T308. When B55α is suppressed, it leads to consistent activation of Akt to enhanced proliferation [85]. In metastatic melanoma cells, B56γ has been found to be overexpressed as compared to normal cells [86] and hyperphosphorylation of paxillin promotes cell motility due to the truncation of B56γ in these cells [65]. In lung cancer, B56γ was shown to be mutated from Phe395 to Cys. They also found that as B56γ interacts with p53 and dephosphorylates Thr55, it suppresses tumorigenecity. But due to mutations in B56γ it is no longer able to interact with p53 causing cancer cell proliferation [87]. A study using Affymetrix SNP array in prostate cancer reported the deletion of PP2AB55/PR55 at a frequency of 67.1% in tumor samples with 2.1% homozygous deletion. Somatic copy number changes or germ line sequence variation was thought to be the reason for the deletion [88]. In various species, including humans, it was known that alteration and mutation of scaffold and regulatory subunits lead to transformation of normal cells to cancer cells, but until now there has been no report of mutation or deletion of the catalytic subunit in any type of cancer (Table 2).
Table 2.
Various isoforms of different subunits of PP2A and their nomenclature, chromosomal location and normal/aberrant expression in various cancer and normal tissues.
| Subunit | Gene | Isoform | Other name | Chromosome number | Normal tissue distribution | Subcellular distribution | Aberrant tissue distribution |
|---|---|---|---|---|---|---|---|
| Scaffold (A) | PPP2R1A | α | PR65α,PP2A-Aα | 19q13.33 | Ubiquitously expressed in all the tissues [22]. | Cytosol | Lung, breast, melanoma, endometrial and ovarian cancer [75]. |
| PPP2R1B | β | PR65β,PP2A-Aβ | 11q23.2 | Ubiquitously expressed and highly expressed in ovary (oogenesis) [22]. | Cytosol | Lung, Breast, colon, ovarian, B-CLL [75]. | |
| Catalytic (C) | PPP2CA | α | PP2Acα | 5q31.1 | Brain and Heart (HE) [19, 100]. | Cytoplasm and Nucleus | Prostate [101]. |
| PPP2CB | β | PP2Acβ | 8p12 | Brain and Heart (HE) [19, 100]. | Cytoplasm and Nucleus | - | |
| Regulatory (B) | PPP2R2A | α | PR55α,PP2ABα | 8p21.1 | Widely distributed in all tissues [16, 24, 30]. | Membranes, cytoplasm, Microtubules Nucleus. Golgi complex, endoplasmic reticulum and neurofilaments | AML, prostate P [85, 88]. |
| PPP2R2B | β | PR55β,PP2ABβ | 5q31-5q32 | Brain and testis(HE)[16]. | Cytosol | - | |
| PPP2R2C | γ | PR55γ, PP2ABγ | 4p16.1 | Brain (SE) [27]. | Mainly in Cytoskeletal fraction | Lung cancer, melanoma [102]. | |
| PPP2R2D | δ | PR55δ, PP2ABδ | 10q26.3 | Wide spread distribution in tissues, Testis (HE) [28]. | Cytosol | - | |
| Regulatory(B’) | PPP2R5A | α | PR56/61α,PP2AB’α | 1q32.2-q32.3 | Cardiac tissues and skeletal muscles (HE)[103]. | Cytoplasm | - |
| PPP2R5B | β | PR56/61β,PP2AB’β | 11q12-q13 | Brain (HE) [103, 104]. | Cytoplasm | - | |
| PPP2R5C | γl, 2,3 | PR56/61γ, PP2AB’γ | 14q32 | Cardiac tissues and skeletal muscles (HE) [103-105]. | Cytoplasm and Nucleus | Lung cancer (HE) reduced level in melanoma cells [86, 87]. | |
| PPP2R5D | δ | PR56/61δ, PP2AB’δ | 6p21.1 | Primarily exist in brain [103, 106]. | Cytoplasm, Nucleus, Mitochondria, Microsomes | - | |
| PPP2R5E | ε | PR56/61ε, PP2AB’ε | 14q23.1 | Primarily exist in brain [103] | Cytoplasm | Breast cancer, Soft tissue sarcoma [107]. | |
| Regulatory(B”) | PPP2R3A | α | PR130,B”α1 | 3q22.1 | Brain(HE), heart, Lung, kidney and muscle [16]. | Centrosome and Golgi complex | - |
| PPP2R3A | α | PR72, B”α2 | 3q22.1 | Heart(HE) and skeletal muscle [16]. | Cytosol and nucleus | - | |
| PPP2R3B | β | PR70, PR48, B”β | Xp22.33, Y11.3 | Placenta [108]. | Nucleus | - | |
| PPP2R3C | γ | G5PR, G4-1 | 14q13.2 | During developmental process expressed in fetal brain [109]. | Nucleus | - | |
| PPP2R3D | δ | PR59, B”δ | Cardiac tissue, Kidney and Lungs [33]. | Nucleus | - | ||
| Regulatory(B’”) | STRN | Striatin, PR110 | 2p22.2 | Brain [110]. | Membrane and cytoplasm | - | |
| STRN3 | SG2NA | 14q13-q21 | Neurons [110]. | Nucleus | - | ||
| PPP2R4 | PTPA, PR53 | 9q34 | Widely expressed [20, 111, 112]. | Cytosol, Nucleus | - |
HE-Highly expressed, SE –Specific expression
7. Mouse models relevant to PP2A subunits
To understand the tumor suppressive function of PP2A and its biological attributes, several studies have been carried out using cell line models in various cancers, such as breast, ovary, skin, endometrium and colon. Gaining knowledge about PP2A at a molecular level requires the development of mouse models that recapitulate the human cancer progression, a valuable tool in current scientific research. As discussed previously, PP2A is a highly complex molecule made of trimeric holoenzyme which is comprised of catalytic, structural and regulatory subunits and can form around 70 different combinations of holoenzyme complex [20]. The complexity of this multi-subunit enzyme makes it difficult to identify and propose mouse models suitable for the identification of the tumor suppressor function of PP2A in cancer initiation, progression and metastasis.
The two-point mutations, E64G and E64D, in the PP2A scaffold subunit (PR65α/Aα) were identified in breast and lung cancer that leads to loss of function of the enzyme, provided the platform to study the PP2A tumor suppressive function [75]. As a result of these two mutations, the resulting mutant proteins had defective binding of the A subunit with regulatory B and catalytic C subunit. Among the B’ subunits, which isoforms have a higher affinity for scaffold subunit (PP2A-Aα) is a critical question to be answered. Walter et al 2012 have stated that the composition of the holoenzyme complex of the B’ subunit varies with tissue type and is largely dependent upon the abundance of a particular isoform in that specific tissue. The second aspect to be considered is the differential binding affinity of various B subunits to the Aα scaffold subunit [89]. Based on the mutations E64G and E64D, four different mouse strains were developed by Ruediger et al 2011: knock-in models E64D/+ and E64G/+; conditional Aα knock-out F5-6/+ with exon 5 and 6 floxed for Aα allele; and Δ5-6/+ was harboring exon 5 and 6 deletion in the Aα allele. The main objective for generating these strains was to determine the tumor suppressive function of PP2A. The lung cancer incidence in the heterozygous mutant mice, Δ5-6/+, E64D/+ and Δ5-6/E64D were 77%, 72%, and 75%, respectively. Through immunoprecipitation experiments Ruediger et al 2011 demonstrated that mice with the E64D/E64G mutation are found to have defective binding capacity to the B’γ subunit specifically. The authors concluded that the B’ holoenzyme complex of PP2A functions as tumor suppressor, unlike the PP2A holoenzymes consisting of B and B” subunits [90]. Thus, the in vivo models highlight the importance of the Aα E64D mutation in lung carcinoma development through inactivation of PP2A tumor suppressor activity and pointed out the importance of b’ subunit for the tumor suppressor function.
PP2A catalytic C subunits are expressed ubiquitously and they exist in two isoforms α and β with 97% homology in their amino acid sequence. However, Gotz et al 1998 had shown that a complete loss of Ppp2cα leads to an embryonically lethal phenotype [21] suggesting the non-redundant functions of α and β isoforms. It has been previously identified that the phosphatase activity of PP2A resides on the highly conserved histidine 118 residue of the catalytic subunit and this histidine118 is present in the RGNHE motif of exon 3 [91, 92]. Therefore, a conditional null Ppp2ca knockout mouse was generated having replaced Ppp2ca exon3, 4 and 5 with the Ppp2ca gene targeting vector. Overall, the loss of Ppp2ca led to abnormal embryonic development at day E6.5, whereas heterozygous Ppp2ca fl/Δ mice embryos were normal. Interestingly, Ppp2ca Δ/Δ mice embryos were found to be smaller than normal control embryos at day E7.5 and they contained both the ectoderm and endoderm but no mesodermal layer. Thus, the loss of Ppp2ca results in the absence of mesodermal germ layer, indicating defect in embryo differentiation. Interestingly, Ppp2cb knockout mice model showed no abnormal phenotype [93]. Thus, the results from these knock-out mouse models indicated that while Ppp2ca is essential for the developmental process in the mouse, Ppp2cb is not.
Despite the functional and biochemical studies in various regulatory subunits of PP2A obtained from cellular models, there are limited publications about the knock out models of the B-type subunits. The reasons for this are the complexity of the holoenzyme as a whole and a lack of characterization of the promoters and splice variants of the regulatory subunits [94]. Of the many different regulatory subunits identified so far, the transgenic mice expressing B’/PR56γ has been initiated by Evertt et al in 2002. In order to produce mice that have PR56 γ overexpression in the lung, the full-length cDNA fragment of human PR56γ was cloned into the expression vector that has the lung-specific human surfactant protein promoter and a SV40 small T-antigen poly A cassette. However, the mice obtained through this approach die neonatally. Further, it was observed that those embryos lack normal lung structure and β-catenin expression in lung tissue, suggesting a potential role of PR56γ in association with Wnt signaling during lung development [95]. Recently, the functional consequence of another B-type specific regulatory subunit in the mice model was reported where it reveals the role of PR61/B’δ in the central nervous system [96]. The in vivo model systems exploring the functional consequences of the B-type specific regulatory subunit (particularly in cancer) remains unexplored. Thus the animal models described previously may set the stage for future studies on animal models relevant to the role of PP2A in cancer.
8. Conclusion
PP2A is an essential and multifarious phosphatase in the cellular system. A growing body of evidence indicates that PP2A is known to regulate the activity of more than 30 different kinases. The important kinases, like protein kinase B (AKT), PKC, p70 S6 kinase, cAMP dependent kinases, CAM-kinases, ERK/MAP kinase are the major substrates for PP2A [97]. During cell cycle progression and apoptosis, various proteins like Cdc25, Cdc6, Wee 1, DNA polymerase primase, TAU, and cyclin G2 are also controlled by PP2A [98]. In general, the biology of PP2A is more intricate to understand due to the more diverse regulatory subunits that are differentially expressed in various tissues. The tumor suppressive role of PP2A is mainly governed by these regulatory subunits that play different roles in various cells. Since the isoforms of regulatory subunits are differentially expressed in different tissues, more elaborate studies on each isoform will precisely delineate the understanding of the expression pattern and help us comprehend their role in PP2A controlled signal transduction. Further, the PP2A scaffold subunit is also deregulated in both cancer and neurodegenerative diseases.
Earlier studies have proposed that the use of PP2A as a therapeutic target for cancer was mainly based on the oncogenic potential of the microbial toxin Okadaic acid (PP2Ac Inhibitor). However, this functional loss is not the true representation of the specific holoenzyme complex comprised of the scaffold, catalytic and regulatory subunits (with respective isoform). Thus, the future prospective will be to elucidate the status of the catalytic and other individual subunits and their precise role and potential functions in cells during transformation need to be investigated further. Also, genetic manipulation of PP2A, its inhibitors and substrates will help develop therapeutic agents against cancer and other diseases. It is interesting to note that when scaffold and regulatory subunits of PP2A are deregulated in cancer cells, the cell tries to maintain a constant level of functional catalytic subunit activity (observation from our unpublished data). Further, the involvement of specific PP2A subunits in the tumor initiation, progression and metastasis with its clinical relevance will be explored using the development of genetically-engineered mouse models. In the immediate future, the genetic and epigenetic changes associated with the PP2A holoenzyme complex in cancer cells remain to be explored to find out their impact on oncogenic signaling and therapy response. How PP2A regulates various cancer signaling pathways such as PI3K/Akt, mammalian target of rapamycin (mTOR), FAK (Focal adhesion kinase) and ERK enabling cancer cells addicted for growth is still far from being completely determined for drug validation/targeting PP2A activation (Figure 3). The ultimate goal of this review is to significantly contribute to the understanding of the deregulation of PP2A-regulated signaling pathways contributing to cancer development. The mouse model system discussed in this review will explore the molecular mechanism behind cancer progression, which will enable us to understand PP2A and its clinical relevance and new therapies to be tested that can prevent cancer progression.
Figure 3. Representation of PP2A regulation and signaling pathways.

Methylation and phosphorylation are two major post translation modifications of PP2A. The conserved sequence TPDYFL in PP2Ac undergoes phosphorylation and methylation, respectively. Phosphorylation of Y307 by receptor associated tyrosine kinases effectively decreases the PP2A activity by inhibiting the interaction of the catalytic subunit with the scaffold subunit. On the other hand, the addition of the methyl group to PP2Ac by LCMT1 at L309 by PP2A-methyltransferase (PPMT) will enhance the specific binding of the AC dimer to the distinct B regulatory subunit providing the enzymatic activity. PP2A activity is indispensable for every cell and takes part in the majority of the cellular pathways. Phosphorylation at Thr-308 and Ser-473 leads to activation of Akt and is associated with PP2A activation. PP2A also possesses a vital role in the Wnt signaling pathway and phosphorylation of PP2A-B56 directs the activation of the Wnt pathway. In addition to these pathways, PP2A activity is indispensable in apoptosis. BAD (pro-apoptotic) and Bcl-2 are also regulated by PP2A. Phosphorylation of BAD suppresses, and its dephosphorylation by PP2A promotes pro-apoptotic activity. In addition, phosphorylation of Bcl-2 activates, and its dephosphorylation by PP2A suppresses anti-apoptotic activity. Thus, this diagrammatic representation will elucidate the importance of PP2A during cell growth survival and apoptosis.
Acknowledgments
The authors on this work are supported, in part, by grants from the Department of Defense (PC074289) and the National Institutes of Health (R01 CA 138791).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mumby MC, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 2.Johnson SA, Hunter T. Kinomics: methods for deciphering the kinome. Nat. Methods. 2005;2:17–25. doi: 10.1038/nmeth731. [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 5.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem. Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- 7.Moorhead GB, De, V W, Templeton G, Kerk D. Evolution of protein phosphatases in plants and animals. Biochem. J. 2009;417:401–409. doi: 10.1042/BJ20081986. [DOI] [PubMed] [Google Scholar]
- 8.Tung HY, Alemany S, Cohen P. The protein phosphatases involved in cellular regulation. 2. Purification, subunit structure and properties of protein phosphatases-2A0, 2A1, and 2A2 from rabbit skeletal muscle. Eur. J. Biochem. 1985;148:253–263. doi: 10.1111/j.1432-1033.1985.tb08833.x. [DOI] [PubMed] [Google Scholar]
- 9.Alberts AS, Thorburn AM, Shenolikar S, Mumby MC, Feramisco JR. Regulation of cell cycle progression and nuclear affinity of the retinoblastoma protein by protein phosphatases. Proc. Natl. Acad. Sci. U. S. A. 1993;90:388–392. doi: 10.1073/pnas.90.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenn GM, Eckhart W. Mutation of a cysteine residue in polyomavirus middle T antigen abolishes interactions with protein phosphatase 2A, pp60c-src, and phosphatidylinositol-3 kinase, activation of c-fos expression, and cellular transformation. J. Virol. 1993;67:1945–1952. doi: 10.1128/jvi.67.4.1945-1952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronne H, Carlberg M, Hu GZ, Nehlin JO. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol. Cell Biol. 1991;11:4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schonthal AH. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 2001;170:1–13. doi: 10.1016/s0304-3835(01)00561-4. [DOI] [PubMed] [Google Scholar]
- 13.Orgad S, Brewis ND, Alphey L, Axton JM, Dudai Y, Cohen PT. The structure of protein phosphatase 2A is as highly conserved as that of protein phosphatase 1. FEBS Lett. 1990;275:44–48. doi: 10.1016/0014-5793(90)81435-q. [DOI] [PubMed] [Google Scholar]
- 14.Ruediger R, Van Wart Hood JE, Mumby M, Walter G. Constant expression and activity of protein phosphatase 2A in synchronized cells. Mol. Cell Biol. 1991;11:4282–4285. doi: 10.1128/mcb.11.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem. Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 16.Mayer-Jaekel RE, Hemmings BA. Protein phosphatase 2A--a ‘menage a trois’. Trends Cell Biol. 1994;4:287–291. doi: 10.1016/0962-8924(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 17.Kamibayashi C, Estes R, Lickteig RL, Yang SI, Craft C, Mumby MC. Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem. 1994;269:20139–20148. [PubMed] [Google Scholar]
- 18.Baharians Z, Schonthal AH. Autoregulation of protein phosphatase type 2A expression. J. Biol. Chem. 1998;273:19019–19024. doi: 10.1074/jbc.273.30.19019. [DOI] [PubMed] [Google Scholar]
- 19.Khew-Goodall Y, Hemmings BA. Tissue-specific expression of mRNAs encoding alpha- and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett. 1988;238:265–268. doi: 10.1016/0014-5793(88)80493-9. [DOI] [PubMed] [Google Scholar]
- 20.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gotz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc. Natl. Acad. Sci. U. S. A. 1998;95:12370–12375. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmings BA, Adams-Pearson C, Maurer F, Muller P, Goris J, Merlevede W, Hofsteenge J, Stone SR. alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990;29:3166–3173. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Pham HT, Ruediger R, Walter G. Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: differences in expression, subunit interaction, and evolution. Biochem. J. 2003;369:387–398. doi: 10.1042/BJ20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix P, Turowski P, Mayer-Jaekel RE, Goris J, Hofsteenge J, Merlevede W, Hemmings BA. Analysis of subunit isoforms in protein phosphatase 2A holoenzymes from rabbit and Xenopus. J. Biol. Chem. 1993;268:7330–7337. [PubMed] [Google Scholar]
- 25.Price NE, Mumby MC. Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry. 2000;1939:11312–11318. doi: 10.1021/bi0008478. [DOI] [PubMed] [Google Scholar]
- 26.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 27.Zolnierowicz S, Csortos C, Bondor J, Verin A, Mumby MC, DePaoli-Roach AA. Diversity in the regulatory B-subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry. 1994;33:11858–11867. doi: 10.1021/bi00205a023. [DOI] [PubMed] [Google Scholar]
- 28.Strack S, Chang D, Zaucha JA, Colbran RJ, Wadzinski BE. Cloning and characterization of B delta, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 1999;460:462–466. doi: 10.1016/s0014-5793(99)01377-0. [DOI] [PubMed] [Google Scholar]
- 29.Healy AM, Zolnierowicz S, Stapleton AE, Goebl M, DePaoli-Roach AA, Pringle JR. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell Biol. 1991;11:5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turowski P, Myles T, Hemmings BA, Fernandez A, Lamb NJ. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell. 1999;10:1997–2015. doi: 10.1091/mbc.10.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 32.Janssens V, Jordens J, Stevens I, Van HC, Martens E, De SH, Engelborghs Y, Waelkens E, Goris J. Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B”/PR72 subunit of protein phosphatase 2A. J. Biol. Chem. 2003;278:10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- 33.Voorhoeve PM, Watson RJ, Farlie PG, Bernards R, Lam EW. Rapid dephosphorylation of p107 following UV irradiation. Oncogene. 1999;18:679–688. doi: 10.1038/sj.onc.1202289. [DOI] [PubMed] [Google Scholar]
- 34.Janssens V, Van HC, Martens E, de, I B, Merlevede W, Goris J. Identification and characterization of alternative splice products encoded by the human phosphotyrosyl phosphatase activator gene. Eur. J. Biochem. 2000;267:4406–4413. doi: 10.1046/j.1432-1327.2000.01486.x. [DOI] [PubMed] [Google Scholar]
- 35.Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Structural basis of PP2A inhibition by small t antigen. PLoS. Biol. 2007;5:e202. doi: 10.1371/journal.pbio.0050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yumoto N, Yu X, Hatakeyama M. Expression of the ErbB4 receptor causes reversal regulation of PP2A in the Shc signal transduction pathway in human cancer cells. Mol. Cell Biochem. 2006;285:165–171. doi: 10.1007/s11010-005-9075-5. [DOI] [PubMed] [Google Scholar]
- 37.Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi N, Reiser J, Schwarz K, Sakai T, Kriz W, Mundel P. Process formation of podocytes: morphogenetic activity of microtubules and regulation by protein serine/threonine phosphatase PP2A. Histochem. Cell Biol. 2001;115:255–266. doi: 10.1007/s004180000242. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Peterson RT, Schreiber SL. Alpha 4 associates with protein phosphatases 2A, 4, and 6. Biochem. Biophys. Res. Commun. 1998;247:827–832. doi: 10.1006/bbrc.1998.8792. [DOI] [PubMed] [Google Scholar]
- 40.McConnell JL, Gomez RJ, McCorvey LR, Law BK, Wadzinski BE. Identification of a PP2A-interacting protein that functions as a negative regulator of phosphatase activity in the ATM/ATR signaling pathway. Oncogene. 2007;26:6021–6030. doi: 10.1038/sj.onc.1210406. [DOI] [PubMed] [Google Scholar]
- 41.Lee WJ, Kim DU, Lee MY, Choi KY. Identification of proteins interacting with the catalytic subunit of PP2A by proteomics. Proteomics. 2007;7:206–214. doi: 10.1002/pmic.200600480. [DOI] [PubMed] [Google Scholar]
- 42.Baek S, Seeling JM. Identification of a novel conserved mixed-isoform B56 regulatory subunit and spatiotemporal regulation of protein phosphatase 2A during Xenopus laevis development. BMC. Dev. Biol. 2007;197:139. doi: 10.1186/1471-213X-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Yost HJ, Virshup DM, Seeling JM. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001;20:4122–4131. doi: 10.1093/emboj/20.15.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li G, Ji XD, Gao H, Zhao JS, Xu JF, Sun ZJ, Deng YZ, Shi S, Feng YX, Zhu YQ, Wang T, Li JJ, Xie D. EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex. Nat. Commun. 2012;3:1–10. doi: 10.1038/ncomms1675. [DOI] [PubMed] [Google Scholar]
- 45.Neviani P, Santhanam R, Trotta R, Notari M, Blaser BW, Liu S, Mao H, Chang JS, Galietta A, Uttam A, Roy DC, Valtieri M, Bruner-Klisovic R, Caligiuri MA, Bloomfield CD, Marcucci G, Perrotti D. The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell. 2005;8:355–368. doi: 10.1016/j.ccr.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Carlson SG, Eng E, Kim EG, Perlman EJ, Copeland TD, Ballermann BJ. Expression of SET, an inhibitor of protein phosphatase 2A, in renal development and Wilms’ tumor. J. Am. Soc. Nephrol. 1998;9:1873–1880. doi: 10.1681/ASN.V9101873. [DOI] [PubMed] [Google Scholar]
- 47.Arnaud L, Chen S, Liu F, Li B, Khatoon S, Grundke-Iqbal I, Iqbal K. Mechanism of inhibition of PP2A activity and abnormal hyperphosphorylation of tau by I2(PP2A)/SET. FEBS Lett. 2011;585:2653–2659. doi: 10.1016/j.febslet.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Honkanen RE, Golden T. Regulators of serine/threonine protein phosphatases at the dawn of a clinical era? Curr. Med. Chem. 2002;9:2055–2075. doi: 10.2174/0929867023368836. [DOI] [PubMed] [Google Scholar]
- 49.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, Lu SL, Lin S, Chan EK, Wang XJ, Grenman R, Kast J, Kallunki T, Sears R, Kahari VM, Westermarck J. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 50.Tseng LM, Liu CY, Chang KC, Chu PY, Shiau CW, Chen KF. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012;14:R68. doi: 10.1186/bcr3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kar S, Palit S, Ball WB, Das PK. Carnosic acid modulates Akt/IKK/NF-kappaB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis. 2012;17(7):735–747. doi: 10.1007/s10495-012-0715-4. [DOI] [PubMed] [Google Scholar]
- 52.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brautigan DL. Flicking the switches: phosphorylation of serine/threonine protein phosphatases. Semin. Cancer Biol. 1995;6:211–217. doi: 10.1006/scbi.1995.0028. [DOI] [PubMed] [Google Scholar]
- 54.Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, Janssens V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic Subunit. J. Biol. Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 55.Damuni Z, Xiong H, Li M. Autophosphorylation-activated protein kinase inactivates the protein tyrosine phosphatase activity of protein phosphatase 2A. FEBS Lett. 1994;352:311–314. doi: 10.1016/0014-5793(94)00981-3. [DOI] [PubMed] [Google Scholar]
- 56.Leulliot N, Quevillon-Cheruel S, Sorel I, de La Sierra-Gallay Li, Collinet B, Graille M, Blondeau K, Bettache N, Poupon A, Janin J, van TH. Structure of protein phosphatase methyltransferase 1 (PPM1), a leucine carboxyl methyltransferase involved in the regulation of protein phosphatase 2A activity. J. Biol. Chem. 2004;279:8351–8358. doi: 10.1074/jbc.M311484200. [DOI] [PubMed] [Google Scholar]
- 57.Berry M, Gehring W. Phosphorylation status of the SCR homeodomain determines its functional activity: essential role for protein phosphatase 2A,B'. EMBO J. 2000;19:2946–2957. doi: 10.1093/emboj/19.12.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J. Biol. Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 59.Chiang CW, Harris G, Ellig C, Masters SC, Subramanian R, Shenolikar S, Wadzinski BE, Yang E. Protein phosphatase 2A activates the proapoptotic function of BAD in interleukin- 3-dependent lymphoid cells by a mechanism requiring 14-3-3 dissociation. Blood. 2001;97:1289–1297. doi: 10.1182/blood.v97.5.1289. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Scuderi A, Letsou A, Virshup DM. B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Mol. Cell Biol. 2002;22:3674–3684. doi: 10.1128/MCB.22.11.3674-3684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flegg CP, Sharma M, Medina-Palazon C, Jamieson C, Galea M, Brocardo MG, Mills K, Henderson BR. Nuclear export and centrosome targeting of the protein phosphatase 2A subunit B56alpha: role of B56alpha in nuclear export of the catalytic subunit. J. Biol. Chem. 2010;285:18144–18154. doi: 10.1074/jbc.M109.093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strack S, Cribbs JT, Gomez L. Critical role for protein phosphatase 2A heterotrimers in mammalian cell survival. J. Biol. Chem. 2004;279:47732–47739. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- 63.Young MR, Liu SW, Meisinger J. Protein phosphatase-2A restricts migration of Lewis lung carcinoma cells by modulating the phosphorylation of focal adhesion proteins. Int. J. Cancer. 2003;103:38–44. doi: 10.1002/ijc.10772. [DOI] [PubMed] [Google Scholar]
- 64.Young MR, Kolesiak K, Meisinger J. Protein phosphatase-2A regulates endothelial cell motility and both the phosphorylation and the stability of focal adhesion complexes. Int. J. Cancer. 2002;100:276–282. doi: 10.1002/ijc.10491. [DOI] [PubMed] [Google Scholar]
- 65.Ito A, Kataoka TR, Watanabe M, Nishiyama K, Mazaki Y, Sabe H, Kitamura Y, Nojima H. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 2000;19:562–571. doi: 10.1093/emboj/19.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson J, Meisinger J, Patel S, Lim ZC, Vellody K, Metz R, Young MR. Protein phosphatase-2A associates with the cytoskeleton to maintain cell spreading and reduced motility of nonmetastatic Lewis lung carcinoma cells: the loss of this regulatory control in metastatic cells. Invasion Metastasis. 1997;17:199–209. [PubMed] [Google Scholar]
- 67.Meisinger J, Patel S, Vellody K, Bergstrom R, Benefield J, Lozano Y, Young MR. Protein phosphatase-2A association with microtubules and its role in restricting the invasiveness of human head and neck squamous cell carcinoma cells. Cancer Lett. 1997;111:87–95. doi: 10.1016/s0304-3835(96)04517-x. [DOI] [PubMed] [Google Scholar]
- 68.Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21:497–503. doi: 10.1093/carcin/21.3.497. [DOI] [PubMed] [Google Scholar]
- 69.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 70.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 72.Nagao M, Shima H, Nakayasu M, Sugimura T. Protein serine/threonine phosphatases as binding proteins for okadaic acid. Mutat. Res. 1995;333:173–179. doi: 10.1016/0027-5107(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 73.Campbell KS, Auger KR, Hemmings BA, Roberts TM, Pallas DC. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J. Virol. 1995;69:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janssens V, Goris J, Van HC. PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 2005;15:34–41. doi: 10.1016/j.gde.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Calin GA, di Iasio MG, Caprini E, Vorechovsky I, Natali PG, Sozzi G, Croce CM, Barbanti-Brodano G, Russo G, Negrini M. Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene. 2000;19:1191–1195. doi: 10.1038/sj.onc.1203389. [DOI] [PubMed] [Google Scholar]
- 76.Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, Evans GA. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 77.Ruediger R, Pham HT, Walter G. Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the A alpha subunit gene. Oncogene. 2001;20:10–15. doi: 10.1038/sj.onc.1204059. [DOI] [PubMed] [Google Scholar]
- 78.Jones S, Wang TL, Shih I, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr., Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Colella S, Ohgaki H, Ruediger R, Yang F, Nakamura M, Fujisawa H, Kleihues P, Walter G. Reduced expression of the Aalpha subunit of protein phosphatase 2A in human gliomas in the absence of mutations in the Aalpha and Abeta subunit genes. Int. J. Cancer. 2001;93:798–804. doi: 10.1002/ijc.1423. [DOI] [PubMed] [Google Scholar]
- 80.Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC. Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 2005;65:8183–8192. doi: 10.1158/0008-5472.CAN-05-1103. [DOI] [PubMed] [Google Scholar]
- 81.Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, DeCaprio JA, Hahn WC. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalla C, Scheuermann MO, Kube I, Schlotter M, Mertens D, Dohner H, Stilgenbauer S, Lichter P. Analysis of 11q22-q23 deletion target genes in B-cell chronic lymphocytic leukaemia: evidence for a pathogenic role of NPAT, CUL5, and PPP2R1B. Eur. J. Cancer. 2007;43:1328–1335. doi: 10.1016/j.ejca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Esplin ED, Ramos P, Martinez B, Tomlinson GE, Mumby MC, Evans GA. The glycine 90 to aspartate alteration in the Abeta subunit of PP2A (PPP2R1B) associates with breast cancer and causes a deficit in protein function. Genes Chromosomes. Cancer. 2006;45:182–190. doi: 10.1002/gcc.20284. [DOI] [PubMed] [Google Scholar]
- 84.Nagendra DC, Burke J, III, Maxwell GL, Risinger JI. PPP2R1A mutations are common in the serous type of endometrial cancer. Mol. Carcinog. 2011;51(10):826–831. doi: 10.1002/mc.20850. [DOI] [PubMed] [Google Scholar]
- 85.Ruvolo PP, Qui YH, Coombes KR, Zhang N, Ruvolo VR, Borthakur G, Konopleva M, Andreeff M, Kornblau SM. Low expression of PP2A regulatory subunit B55alpha is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients. Leukemia. 2011;25:1711–1717. doi: 10.1038/leu.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Francia G, Poulsom R, Hanby AM, Mitchell SD, Williams G, Mckee P, Hart IR. Identification by differential display of a protein phosphatase-2A regulatory subunit preferentially expressed in malignant melanoma cells. Int. J. Cancer. 1999;82:709–713. doi: 10.1002/(sici)1097-0215(19990827)82:5<709::aid-ijc14>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 87.Shouse GP, Nobumori Y, Liu X. A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A. Oncogene. 2010;29:3933–3941. doi: 10.1038/onc.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng Y, Liu W, Kim ST, Sun J, Lu L, Sun J, Zheng SL, Isaacs WB, Xu J. Evaluation of PPP2R2A as a prostate cancer susceptibility gene: a comprehensive germline and somatic study. Cancer Genet. 2011;204:375–381. doi: 10.1016/j.cancergen.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walter G, Ruediger R. Mouse model for probing tumor suppressor activity of protein phosphatase 2A in diverse signaling pathways. Cell Cycle. 2012;11:451–459. doi: 10.4161/cc.11.3.19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruediger R, Ruiz J, Walter G. Human cancer-associated mutations in the Aalpha subunit of protein phosphatase 2A increase lung cancer incidence in Aalpha knock-in and knockout mice. Mol. Cell Biol. 2011;31:3832–3844. doi: 10.1128/MCB.05744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myles T, Schmidt K, Evans DR, Cron P, Hemmings BA. Active-site mutations impairing the catalytic function of the catalytic subunit of human protein phosphatase 2A permit baculovirus-mediated overexpression in insect cells. Biochem. J. 2001;357:225–232. doi: 10.1042/0264-6021:3570225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhuo S, Clemens JC, Stone RL, Dixon JE. Mutational analysis of a Ser/Thr phosphatase. Identification of residues important in phosphoesterase substrate binding and catalysis. J. Biol. Chem. 1994;269:26234–26238. [PubMed] [Google Scholar]
- 93.Gu P, Qi X, Zhou Y, Wang Y, Gao X. Generation of Ppp2Ca and Ppp2Cb conditional null alleles in mouse. Genesis. 2012;50:429–436. doi: 10.1002/dvg.20815. [DOI] [PubMed] [Google Scholar]
- 94.Sents W, Ivanova E, Lambrecht C, Haesen D, Janssens V. The biogenesis of active protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase specificity. FEBS J. 2013;280:644–661. doi: 10.1111/j.1742-4658.2012.08579.x. [DOI] [PubMed] [Google Scholar]
- 95.Everett AD, Kamibayashi C, Brautigan DL. Transgenic expression of protein phosphatase 2A regulatory subunit B56gamma disrupts distal lung differentiation. Am. J. Physiol Lung Cell Mol. Physiol. 2002;282:L1266–L1271. doi: 10.1152/ajplung.00262.2001. [DOI] [PubMed] [Google Scholar]
- 96.Louis JV, Martens E, Borghgraef P, Lambrecht C, Sents W, Longin S, Zwaenepoel K, Pijnenborg R, Landrieu I, Lippens G, Ledermann B, Gotz J, Van LF, Goris J, Janssens V. Mice lacking phosphatase PP2A subunit PR61/B'delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6957–6962. doi: 10.1073/pnas.1018777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 98.Bennin DA, Don AS, Brake T, McKenzie JL, Rosenbaum H, Ortiz L, DePaoli-Roach AA, Horne MC. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B’ subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J. Biol. Chem. 2002;277:27449–27467. doi: 10.1074/jbc.M111693200. [DOI] [PubMed] [Google Scholar]
- 99.Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem. Pharmacol. 2000;60:1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
- 100.Arino J, Woon CW, Brautigan DL, Miller TB, Jr., Johnson GL. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhardwaj A, Singh S, Srivastava SK, Honkanen RE, Reed E, Singh AP. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol. Cancer Ther. 2011;10:720–731. doi: 10.1158/1535-7163.MCT-10-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Banerjee AK, Read CA, Griffiths MH, George PJ, Rabbitts PH. Clonal divergence in lung cancer development is associated with allelic loss on chromosome 4. Genes Chromosomes. Cancer. 2007;46:852–860. doi: 10.1002/gcc.20472. [DOI] [PubMed] [Google Scholar]
- 103.McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 104.Csortos C, Zolnierowicz S, Bako E, Durbin SD, DePaoli-Roach AA. High complexity in the expression of the B’ subunit of protein phosphatase 2A0. Evidence for the existence of at least seven novel isoforms. J. Biol. Chem. 1996;271:2578–2588. doi: 10.1074/jbc.271.5.2578. [DOI] [PubMed] [Google Scholar]
- 105.Tehrani MA, Mumby MC, Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J. Biol. Chem. 1996;271:5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- 106.Tanabe O, Nagase T, Murakami T, Nozaki H, Usui H, Nishito Y, Hayashi H, Kagamiyama H, Takeda M. Molecular cloning of a 74-kDa regulatory subunit (B” or delta) of human protein phosphatase 2A. FEBS Lett. 1996;379:107–111. doi: 10.1016/0014-5793(95)01500-0. [DOI] [PubMed] [Google Scholar]
- 107.Dupont WD, Breyer JP, Bradley KM, Schuyler PA, Plummer WD, Sanders ME, Page DL, Smith JR. Protein phosphatase 2A subunit gene haplotypes and proliferative breast disease modify breast cancer risk. Cancer. 2010;116:8–19. doi: 10.1002/cncr.24702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yan Z, Fedorov SA, Mumby MC, Williams RS. PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol. Cell Biol. 2000;20:1021–1029. doi: 10.1128/mcb.20.3.1021-1029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamnasaran D, Chen CP, Devriendt K, Mehta L, Cox DW. Defining a holoprosencephaly locus on human chromosome 14q13 and characterization of potential candidate genes. Genomics. 2005;85:608–621. doi: 10.1016/j.ygeno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 110.Moreno CS, Park S, Nelson K, Ashby D, Hubalek F, Lane WS, Pallas DC. WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 2000;275:5257–5263. doi: 10.1074/jbc.275.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cayla X, Van HC, Bosch M, Waelkens E, Vandekerckhove J, Peeters B, Merlevede W, Goris J. Molecular cloning, expression, and characterization of PTPA, a protein that activates the tyrosyl phosphatase activity of protein phosphatase 2A. J. Biol. Chem. 1994;269:15668–15675. [PubMed] [Google Scholar]
- 112.Van HC, Cayla X, Bosch M, Merlevede W, Goris J. The phosphotyrosyl phosphatase activator of protein phosphatase 2A. A novel purification method, immunological and enzymic characterization. Eur. J. Biochem. 1994;226:899–907. doi: 10.1111/j.1432-1033.1994.00899.x. [DOI] [PubMed] [Google Scholar]


