Abstract
Currently available infectious disease imaging techniques cannot differentiate between infection and sterile inflammation or between different types of infections. Recently, radiolabeled FIAU was found to be a substrate for the thymidine kinase (TK) enzyme of multiple pathogenic bacteria, leading to its translational use in the imaging of bacterial infections. Patients with immunodeficiencies, however, are susceptible to a different group of pathogenic bacteria when compared to immunocompetent subjects. In this study, we wanted to predict the usefulness of radiolabeled FIAU in the detection of bacterial infections commonly occurring in patients with immunodeficiencies, in vitro, prior to attempting in vivo imaging with 124I-FIAU-PET.
Methods
We obtained representative strains of bacterial pathogens isolated from actual patients with genetic immunodeficiencies. We evaluated the bacterial susceptibility of different strains to the effect of incubation with FIAU, which would implicate the presence of the thymidine kinase (TK) enzyme. We also incubated the bacteria with 14C-FIAU and consequently measured its rate of incorporation in the bacterial DNA using a liquid scintillation counter.
Results
Unlike the other bacterial strains, the growth of Pseudomonas aeruginosa was not halted by FIAU at any concentration. All the tested clinical isolates demonstrated different levels of 14C-FIAU uptake, except for P. aeruginosa.
Conclusion
Radiolabeled FIAU has been successful in delineating bacterial infections, both in preclinical and pilot translational studies. In patients with immunodeficiencies, Pseudomonas infections are commonly encountered and are usually difficult to differentiate from fungal infections. The use of radiolabeled FIAU for in vivo imaging of those patients, however, would not be useful, considering the apparent lack of TK enzyme in Pseudomonas. One has to keep in mind that not all pathogenic bacteria possess the TK enzyme and as such will not all retain FIAU. Our technique is simple, and can be easily used to assess whether a certain bacterial strain of interest can or cannot be visualized using radiolabeled FIAU.
Keywords: Bacterial imaging, PET, FIAU, thymidine kinase
Introduction
Structural imaging techniques, such as Computed Tomography, Magnetic Resonance Imaging and ultrasound are often used in the initial diagnosis and follow-up of patients with suspected infection. Their use is limited, however, by their inherent inability to differentiate between infection and sterile inflammation, or between different types of infection, such as bacterial versus fungal. Even clinically used functional imaging techniques such as radiolabeled leukocytes and gallium citrate scans suffer from the same limitation, since their mechanism of uptake relies upon increased vascular permeability, which occurs in most infections as well as in sterile inflammation. These limitations have created an urgent need for more specific imaging techniques capable of differentiating infection from sterile inflammation, and potentially differentiating between different types of infections, which would provide early non-invasive diagnosis and allow accurate follow-up of the infectious burden.
One recently described probe that is thought to target bacteria directly is radiolabeled 1-(2-deoxy-2-fluoro-1-D-arabinofuranosyl)-5-iodouracil (FIAU). FIAU is a thymidine analog, known to act as a substrate for the thymidine kinase enzyme (TK) of Herpes simplex virus-1 (HSV1-TK), and as such is frequently used in genetic molecular imaging as a radiolabeled reporter probe. It was recently found that FIAU can also be a substrate for the TK of multiple pathogenic bacterial strains, as demonstrated both by in vitro and in vivo bacterial phosphorylation of FIAU and secondary entrapment of the radioactive compound. This preclinical discovery was soon successfully translated into a pilot human application for the evaluation of musculoskeletal infections.
The ability to distinguish bacterial infection from other opportunistic infections and sterile inflammation would be especially beneficial in immunodeficient patients. Immunodeficiency can be either innate such as with chronic granulomatous diseases (CGD) and Autosomal Dominant Hyper-IgE syndrome (AD-HIES)or can be acquired such as with bone marrow and solid organ transplants, treated hematologic malignancies and Human Immunodeficiency Virus. Depending on the type of immunodeficiency, different types and different rates of bacterial and fungal infections are seen. For example, CGD is characterized by abnormal NADPH oxidase activity which renders patients susceptible to catalase-positive bacteria and fungi, including Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia and Aspergillus species. In AD-HIES (Job’s syndrome), which is caused by STAT3 mutations and characterized by recurrent skin and lung infections, Staphylococcus aureus is the most frequent pathogen causing pneumonia. This often results in pneumatocele formation and bronchiectasis, which then become secondarily infected with Gram negative bacteria, namely Pseudomonas, and molds, resulting in significant morbidity and mortality. Similar infections are also common in patients with acquired immunodeficiencies. Pseudomonas, for example, frequently causes pneumonias in subjects whose immunodeficiency has led to bronchiectasis, and is a frequent blood stream infection in patients with neutropenia.
Due to the detrimental consequences of uncontrolled infection in immunocompromised hosts, obtaining a definitive diagnosis is of utmost importance. This frequently involves invasive procedures such as lung biopsy or bronchoscopy, and results may not be available in time to guide clinical decisions. If an imaging agent, such as radiolabeled FIAU, can provide a diagnosis of the infectious burden early in the course of disease, it could save those vulnerable patients a lot of invasive procedures, and improve their prognosis.
The goal of our study was to predict the potential usefulness of radiolabeled FIAU in diagnosing bacterial infections in patients with genetic immunodeficiencies such as CGD and AD-HIES. Towards this goal, our study was designed to predict, in vitro, the presence or absence of the TK enzyme in specific pathogenic bacteria known to affect immunocompromised patients.
Materials and Methods
Bacterial Strains
We obtained representative strains of bacterial pathogens (S. aureus, B. cepacia, S. marcescens, Nocardia species and Pseudomonas aeruginosa) isolated from actual CGD and AD-HIES patients from the Department of Microbiology at the Clinical Center of the National Institutes of Health, Bethesda, Md. A wild type reference strain of Escherichia coli with intact TK (w3110 CGSC strain#4474, TK+ E. coli) and a TK negative mutant strain (KY895 CGSC strain #4842, TK− E. coli) were obtained from the Yale University E. coli Genetic Stock Center, New Haven, CT.
In Silico Analysis
We attempted to identify the TK amino acid sequences of clinically relevant microorganisms in immunodeficient patients, including the target organisms named above, in the gene bank. When we were able to identify the gene sequences, we compared the amino acid sequences of the catalytic domain of TK (the central 100 amino acids) to the TK sequence of a representative bacterial species (E. coli W3110) using in silico amino acid sequence alignments (BLAST). The reference microorganism (E. coli W3110) is the same one we used as a positive control bacterial strain (TK+ E. coli) in incubation studies. We also queried the gene bank for the presence of TK sequences in multiple fungi known to infect immunocompromised hosts, including Aspergillus fumigatus, Aspergillus nidulans and Paecilomyces species. For those organisms where a TK gene sequence could be identified, we calculated the percentage match of the selected amino acid sequence to that of the representative E. coli W3110 sequence.
Bacterial Susceptibility Assays
Clinical isolates and reference strains of TK+ and TK− E. coli were grown in 3mL Luria broth (LB) at 37°C without and with FIAU (Moravek Biochemicals, Brea, California) over a range of FIAU concentrations (0, 0.25, 0.49, 0.98, 1.95 and 100 μg/mL). Bacterial survival in the presence of FIAU was evaluated by measuring the optical density (OD, 600 nm) at roughly 2-hour intervals over 30 hours, or until stationary growth phase was established.
Uptake of Radiolabeled FIAU Assays
14C-FIAU was purchased from Moravek Biochemicals, with reported radiochemical purity of 99.9%, specific activity of 55mCi/mmol (2.035 GBq/mmol) and concentration of 100 μCi/ml (3700 KBq/ml; 676.5 μg/ml). Bacterial isolates in 1 mL LB were then incubated either with 4 μl (2.72 μg, 0.4 μCi) 14C-FIAU, or without 14C-FIAU, for 24 hours. The pelleted cells were washed in 100μL 50mM EDTA TRIS HCl –10% glycerol, 2 mg lysozyme per mL and 10% Triton X-100 – to remove un-phosphorylated 14C-FIAU. Bacteria were then lysed by two freeze-thaws, and the lysate was washed again to remove un-incorporated cytosolic phosphorylated 14C-FIAU. Bacterial DNA was isolated using the QIAamp DNA Mini Kit, and DNA concentrations from each isolate were determined using ultraviolet (UV) light absorbance spectroscopy and micro-volume spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific Inc). 1μg of DNA from each isolate was diluted and assessed for radioactivity in counts per minute (CPM) using a liquid scintillation counter (LSC). An additional 10μg sample of DNA from S. aureus was also assayed.
Statistics
We performed linear regression analysis to determine the correlation between the degree of incorporation of 14C-FIAU in the DNA of assayed bacterial species and the percentage match of their amino acid sequence to the reference E. coli W3110 sequence.
Results
In Silico Analysis
Significant homology was identified between the E. coli W3110 reference species and Serratia, Staphylococcus, Streptococcus, Burkholderia, and Haemophilus species (Figure. 1). No TK gene sequence could be identified in the gene bank, however, for Pseudomonas or Nocardia species. Similarly, no TK gene sequence could be identified for fungi known to infect immunocompromised hosts, including A. fumigatus, A. nidulans, and Paecilomyces species.
FIGURE 1.
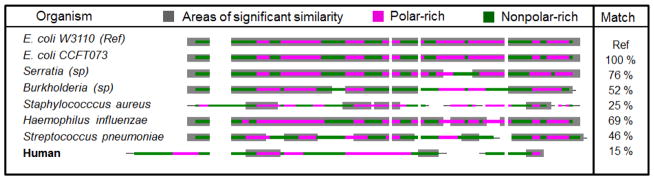
Amino acid sequences of the catalytic domain of the thymidine kinase of various microorganisms based on data extracted from the gene bank and analyzed using BLAST. The last column on the right shows the degree of match between the reference sequence (E. coli W3110) and the different bacterial species. Notice that there is only 15% match between the humanTK1 AA sequence and that of the reference microorganism.
Bacterial Susceptibility Assays
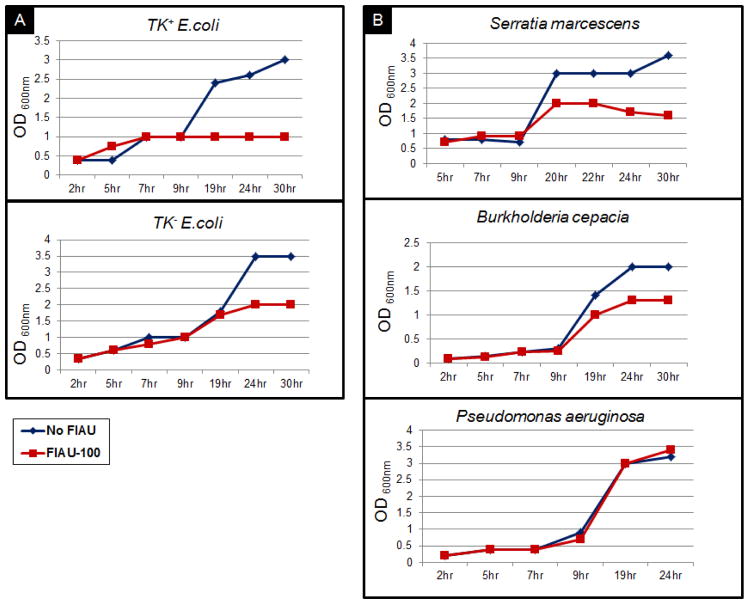
The OD values for TK+ E. coli were decreased by increasing concentrations of FIAU, as expected (Figure. 2A). Although TK− E. coli should exhibit no susceptibility to FIAU, there was in fact a moderate reduction in growth of the bacteria at the high concentrations of FIAU. Because the growth media were not selective for the TK− E. coli mutant, we attributed this result to partial recovery of TK activity in the mutant strain (Figure. 2A). Among the other strains under investigation, S. aureus, B. cepacia, and S. marcescens exhibited decreased OD with increasing concentration of FIAU (Figure. 2B), suggesting that the thymidine analog (FIAU) was incorporated into replicating DNA and negatively affected the growth.
FIGURE 2.
Bacterial survival in the presence of FIAU evaluated by measuring the optical density (OD, 600 nm) at roughly 2-hour intervals over 30 hours, or until stationary growth phase was established. OD values for A. TK+E. coli (W3110) and TK− E. coli and B. S. marcescens, B. cepacia and P. aeruginosa in the absence (blue lozenges) and presence of 100 μg/mL (red squares).
Optical Density values for P. aeruginosa, in contrast, were not affected by FIAU at any concentration (Figure. 2B). The OD values for Nocardia across all concentrations were inconclusive, and were also inconsistent with normal growth. We believe this was due to the inherent difficulty in growing Nocardia species using non-selective growth media. This is why we decided to exclude the results from the Nocardia experiments.
Uptake of Radiolabeled FIAU
TK+ E. coli demonstrated remarkable levels of 14C-FIAU uptake, estimated in CPM, in comparison with TK− E. coli (Figure. 3A). The 14C-FIAU uptake of TK− E. coli, however, was not equal to background as we observed a modest 1.7 fold increased uptake with 14C-FIAU, suggesting the possibility of partial recovery of TK activity in the TK− E. coli strain. Pseudomonas, as expected from its growth in the presence of FIAU, showed CPM values equivalent to background at all concentrations of FIAU.
FIGURE 3.
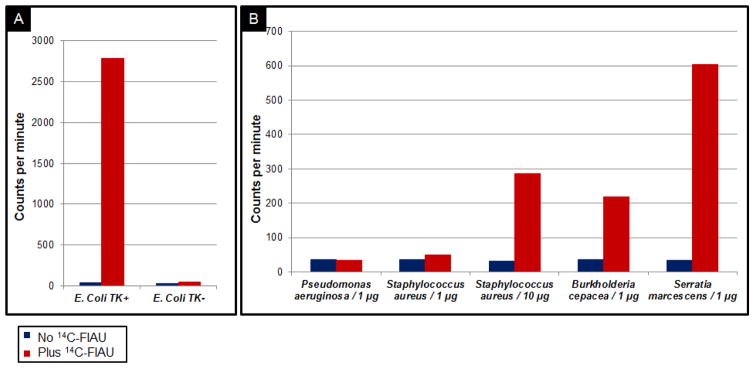
CPM values reflecting incorporation of 14C-FIAU in DNA extracts from A. TK+E. coli (W3110) and TK− E. coli, and B. P. aeruginosa, S. aureus (1μg and 10μg), B. cepacia and S. marcescens, with and without 14C-FIAU (background values) present.
The other clinical isolates demonstrated levels of FIAU uptake intermediate between the TK+ E. coli and P. aeruginosa values. In particular, S. aureus (10μg), B. cepacia, and S. marcescens, showed substantially greater CPM counts than background values, indicative of the presence of 14C-FIAU in the DNA sample. Figure 3B shows CPM values in the bacterial isolates grown with and without 14C-FIAU present.
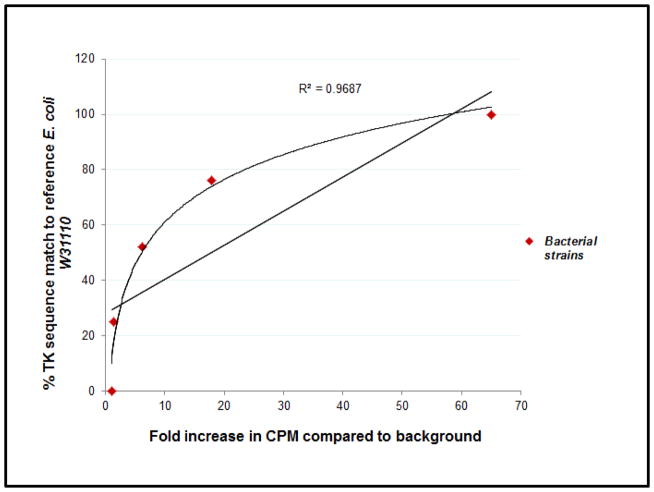
Notably, 10μg S. aureus DNA were required to produce comparable results to 1 μg of S. marcescens and B. cepacia DNA. When only 1μg of S. aureus DNA was analyzed for the incorporation of 14C-FIAU, CPM values were higher than background, but to a lesser extent (1.4-fold increase versus 8.8-fold increase corresponding to 10μg and 1μg of DNA, respectively). These results were expected from the in silico analysis, in which the match between S. aureus and our reference TK+ E. coli W3110 was relatively lower (25%) than other species capable of phosphorylating FIAU. In fact, the degree of 14C-FIAU incorporation in the DNA of the different assayed microorganisms with respect to background values significantly correlated (R2=0.968) with the degree of match of the TK sequence of those microorganisms with that of the reference E. coli W3110 sequence (Figure 4).
FIGURE 4.
Linear regression analysis shows significant correlation (R2=0.968) between the degree of 14C-FIAU incorporation in the DNA of the different assayed microorganisms (described in fold increased CPM compared to background values) and the degree of match of the TK sequence of those microorganisms with that of the reference E. coli W3110 sequence.
Discussion
Accurate microbiologic diagnoses are essential for immunocompromised hosts with clinical evidence of infection. Identifying imaging techniques and probes that target the microorganism directly has been attempted repeatedly using a variety of approaches, including radiolabeled antibiotics or Ubiquicidin, with variable but limited success.
Recently, it has been demonstrated that the TK enzyme of many bacterial strains, similar to the Herpes Simplex Virus type-1 TK enzyme (HSV-TK), is capable of phosphorylating the nucleoside analogue FIAU. This major preclinical discovery was demonstrated through successful in vivo imaging of bacterial infection in an animal mouse model using radiolabeled FIAU, and was translated shortly thereafter in successful imaging of human musculoskeletal infections using 124I-FIAU.
The mechanism of retention of FIAU in the presence of the appropriate TK enzyme is similar in some ways to the mechanism of FDG accumulation. Once inside the cell, FIAU gets phosphorylated by TK, becomes trapped within the microorganisms and can then be imaged using Positron Emission Tomography (PET) or Single Photon Emission Computed Tomography (SPECT). An assessment of TK protein sequences in 53 pathogenic bacteria has previously revealed striking homology with a clear consensus within the TK catalytic domain. Each one of those bacteria contained at least 25 residues that were identical to those of the consensus while, at the same time, this consensus sequence was not found in mammalian TKs, presumably accounting for the differential capacities of the mammalian enzymes to phosphorylate substrates such as FIAU.
However, not all bacteria possess the TK enzyme and as a result not all bacterial infections can be visualized using radiolabeled FIAU. In our study, we wanted to evaluate the usefulness of FIAU as a bacterial TK substrate in the setting of genetic immunodeficiency. If FIAU entrapment is proven to occur in bacteria commonly affecting immunosuppressed patients, radiolabeled FIAU can then be used to differentiate sterile inflammation from infection, and possibly differentiate fungal from bacterial infections in this patient population.
Our prospective patient population consisted of subjects with genetic immunodeficiencies. Towards this goal we selected five representative clinical isolates that were cultured from actual patients at the NIH Clinical Center. Those included Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia and Pseudomonas aeruginosa. Due to problems with suboptimal growth of Nocardia, however, we are not presenting the results from those cultures. As positive and negative controls we decided to use the wild type TK+ E. coli (W3110) along with a mutant TK− E. coli strain generously provided to us by the Yale University E. coli Genetic Stock Center, New Haven, CT.
Our results suggested that FIAU is phosphorylated by S. aureus, B. cepacia, and S. marcescens, while it did not appear to be phosphorylated by P. aeruginosa. There was also lower and more delayed phosphorylation associated with TK− E. coli. Although one would expect no effect of FIAU on TK− E. coli growth and no uptake of 14C-FIAU, we observed partial effect which we believe is due to reversed mutation (“leaky mutation”) of the single gene mutant TK− E. coli into TK+ E. coli during its 24-hour culture in nonselective media. A similar phenomenon was observed in a recently published paper where the same mutant TK− E. coli strain was incubated with 125I-FIAU. Rather than complete lack of radioactivity accumulation in association with the mutant strain as would be expected in the absence of TK, some accumulated radioactivity was detected over time.
The fact that FIAU does not seem to be a substrate for P. aeruginosa is of major importance for potential use of radiolabeled FIAU in vivo for detection of infectious foci. While FIAU accumulation in the lungs is minimal, providing low background activity, the lack of FIAU uptake by Pseudomonas would make imaging for bacterial/fungal distinction futile, since Pseudomonas is a frequent infectious organism in patients with bronchiectasis complicating immunodeficiency and those with neutropenia. Fungi in general do not have TK, and would not be expected to phosphorylate FIAU either.
While this does not invalidate the huge potential of radiolabeled FIAU as a specific bacterial imaging agent, it may preclude using it to visualize infections in patient populations where Pseudomonas infection is likely. A negative result in that case could be misinterpreted as sterile inflammation or non-bacterial infection if one has to rely solely on non-invasive PET diagnosis.
Conclusion
Among all clinically available infectious agents, radiolabeled FIAU promises to be one of the long-sought “specific” imaging agents, i.e. agents capable of differentiating infection from sterile inflammation, and potentially differentiating between various types of pathogens, such as bacteria versus fungi. One has to keep in mind however that not all bacteria possess the TK enzyme and as such, will not all retain FIAU. Our technique is simple, and can be easily adapted to assess whether TK is present in specific bacterial strains versus others.
Acknowledgments
Source of Funding: National Institutes of Health, Bench to Bedside intramural research grant (2011-2012).
Footnotes
Conflicts of interest: No conflicts of interest were reported by any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diaz LA, Jr, Foss CA, Thornton K, Nimmagadda S, Endres CJ, Uzuner O, et al. Imaging of musculoskeletal bacterial infections by [124I]FIAU-PET/CT. PLoS One. 2007;2:e1007. doi: 10.1371/journal.pone.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chopra A. [99mTc]-Acetyl-arg-arg-arg-arg-arg-cys-gly-cys-gly-gly-pro-leu-tyr-arg-arg-ile-il e-arg-arg-leu-leu-glu-ser-dermatan sulfate. Molecular Imaging and Contrast Agent Database (MICAD); Bethesda MD: 2004. [PubMed] [Google Scholar]
- 3.Zhao M, Zhu X, Ji S, Zhou J, Ozker KS, Fang W, et al. 99mTc-labeled C2A domain of synaptotagmin I as a target-specific molecular probe for noninvasive imaging of acute myocardial infarction. J Nucl Med. 2006;47:1367–1374. [PubMed] [Google Scholar]
- 4.Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 5.Lupetti A, Welling MM, Pauwels EK, Nibbering PH. Radiolabelled antimicrobial peptides for infection detection. Lancet Infect Dis. 2003;3:223–229. doi: 10.1016/s1473-3099(03)00579-6. [DOI] [PubMed] [Google Scholar]
- 6.Mahfouz T, Miceli MH, Saghafifar F, Stroud S, Jones-Jackson L, Walker R, et al. 18F-fluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: a study of 165 infectious episodes. J Clin Oncol. 2005;23:7857–7863. doi: 10.1200/JCO.2004.00.8581. [DOI] [PubMed] [Google Scholar]
- 7.Bettegowda C, Foss CA, Cheong I, Wang Y, Diaz L, Agrawal N, et al. Imaging bacterial infections with radiolabeled 1-(2′-deoxy-2′-fluoro-beta-D-arabinofuranosyl)-5-iodouracil. Proc Natl Acad Sci U S A. 2005;102:1145–1150. doi: 10.1073/pnas.0408861102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman AF, Holland SM. Persistent bacterial infections and primary immune disorders. Curr Opin Microbiol. 2007;10:70–75. doi: 10.1016/j.mib.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Freeman AF, Holland SM. Clinical manifestations, etiology, and pathogenesis of the hyper-IgE syndromes. Pediatr Res. 2009;65:32R–37R. doi: 10.1203/PDR.0b013e31819dc8c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, Anderson V, et al. Causes of death in hyper-IgE syndrome. J Allergy Clin Immunol. 2007;119:1234–1240. doi: 10.1016/j.jaci.2006.12.666. [DOI] [PubMed] [Google Scholar]
- 11.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 12.Liese JG, Jendrossek V, Jansson A, Petropoulou T, Kloos S, Gahr M, et al. Chronic granulomatous disease in adults. Lancet. 1996;347:220–223. doi: 10.1016/s0140-6736(96)90403-1. [DOI] [PubMed] [Google Scholar]
- 13.Freeman AF, Holland SM. Hyper IgE syndrome: review and future directions. Expert Rev Clin Immunol. 2005;1:645–651. doi: 10.1586/1744666X.1.4.645. [DOI] [PubMed] [Google Scholar]
- 14.Freeman AF, Holland SM. The hyper-IgE syndromes. Immunol Allergy Clin North Am. 2008;28:277–291. viii. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aryana K, Hootkani A, Sadeghi R, Davoudi Y, Naderinasab M, Erfani M, et al. 99mTc-labeled ubiquicidin scintigraphy. A promising method in hip prosthesis infection diagnosis. Nuklearmedizin. 2012:51. doi: 10.3413/Nukmed-0444-11-11. [DOI] [PubMed] [Google Scholar]
- 16.Nazari B, Azizmohammadi Z, Rajaei M, Karami M, Javadi H, Assadi M, et al. Role of 99mTc-ubiquicidin 29–41 scintigraphy to monitor antibiotic therapy in patients with orthopedic infection: a preliminary study. Nucl Med Commun. 2011;32:745–751. doi: 10.1097/MNM.0b013e3283483964. [DOI] [PubMed] [Google Scholar]
- 17.Petruzzi N, Shanthly N, Thakur M. Recent trends in soft-tissue infection imaging. Semin Nucl Med. 2009;39:115–123. doi: 10.1053/j.semnuclmed.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Zhang W, Wang Y, Jin Z, Wang X, Zhang J, et al. Synthesis and biodistribution of a novel ((9)(9)m)TcN complex of norfloxacin dithiocarbamate as a potential agent for bacterial infection imaging. Bioconjug Chem. 2011;22:369–375. doi: 10.1021/bc100357w. [DOI] [PubMed] [Google Scholar]
- 19.MICAD. 2 -Deoxy-2 -[18F]fluoro-5-fluoro-1-beta-D-arabinofuranosyluracil. Molecular Imaging and Contrast Agent Database (MICAD) 2004 [Google Scholar]
- 20.Igarashi K, Hiraga S, Yura T. A deoxythymidine kinase deficient mutant of Escherichia coli. II. Mapping and transduction studies with phage phi 80. Genetics. 1967;57:643–654. doi: 10.1093/genetics/57.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullambhatla M, Tessier J, Beck G, Jedynak B, Wurthner J, Pomper M. [125I]FIAU imaging in a preclinical model of lung infection: quantification of bacterial load. Am J Nucl Med Mol Imaging. 2012;2:260–270. [PMC free article] [PubMed] [Google Scholar]