Abstract
The use of opioid analgesics for pain has always been hampered by their many side effects; in particular, the addictive liability associated with chronic use. Recently, attempts to develop analgesic agents with reduced side effects have targeted either the putative opioid receptor splice variants or the receptor heterooligomers. This review discusses the potential for receptor splice variant- and the hetero-oligomer-based discovery of new opioid analgesics. We also examine an alternative approach of using receptor mutants for pain management. Finally, we discuss the role of the biased agonism observed and the recently reported opioid receptor crystal structures in guiding the future development of opioid analgesics
Keywords: receptor splice variants, heteroligomers, receptor mutants as therapeutic targets
Receptors as targets for developing opioid analgesics without side effects
Opioids are the most potent and prevalently used analgesic agents for the treatment of severe acute, surgical and cancer pain, as indicated by the continued increase in global consumption of morphine from 7.2 tons in 1990 to 41 tons in 20101. Still, the therapeutic use of morphine has been limited by its frequent side effects, such as nausea (30% of patients), constipation (23%), dizziness (20%), somnolence (18%), and vomiting (13%) observed in patients within pain studies (1). The side effects that have the highest impact on a physician’s or patient’s decision to use morphine for chronic pain treatment are the high addictive liability and drug-induced respiratory depression, which could lead to death. In 2006, opioids were involved in almost 40% of all poisoning fatalities (2). Therefore there is an urgency to develop compounds or treatment paradigms that will minimize the side effects of opioid analgesic agents.
From the initial identification of the high affinity stereo-selective binding sites, it was already apparent that the actions of the opioids are mediated by non-homogeneous receptor populations. The multiple opioid receptors were named by Martin and colleagues for the prototypic drugs that produced the physiological responses: mu (OPRM1), for morphine, and kappa (OPRK1), for ketocyclazocine (3). Subsequent analyses of the opioid activities in mouse vas deferens led to the discovery of the third or delta receptor (OPRD1, δ for deferens) (4). In the years leading to the molecular cloning of the μ-, κ- and δ-opioid receptors, pharmacological studies based on antagonists’ selectivities and agonists’ affinities led to the hypothesis that there are multiple opioid receptor subtypes, namely: μ1-3, κ1-3 and δ1,2 (5). Among these multiple opioid receptor subtypes, the ones that gather the most attention are the μ1 and μ2 subtypes, as the majority of the in vivo morphine responses, including antinociception (pain relief), are absent in oprm1-null mice (6-8). Adding to their interest, the activation of μ1 resulted in antinociceptive responses while μ2 activation led to side effects such as respiratory depression (9, 10). An attractive hypothesis to eliminate side effects of the drugs involves identifying which responses are due to various receptor subtypes and developing ligands that are selective for particular subtypes. However, the molecular cloning of the receptor genes did not yield corresponding genes for various receptor subtypes. Importantly, the ablation of a single receptor gene such as oprm1 eliminates all μ-opioid responses, as the deletion of oprd1 or oprk1 eliminates all δ- or κ-opioid responses.
In the past several years, two hypotheses have gained momentum in the field of opioid drug development: that receptor splice variants, or receptor heterodimers, create the receptor subtypes that exhibit distinct pharmacological responses. This review evaluates the supporting evidence for these hypotheses. We also examine alternative approaches in reducing the side effects of opioid drugs. Also, we discuss the probable implication of the recently resolved crystal structures of the opioid receptors and opioid-biased agonism on future attempts to separate the analgesic activities from the side effects of these drugs. Additional discussion on opioid receptor heterodimers as drug targets has been made in recent reviews (11-15).
Alternative splicing of the receptor genes
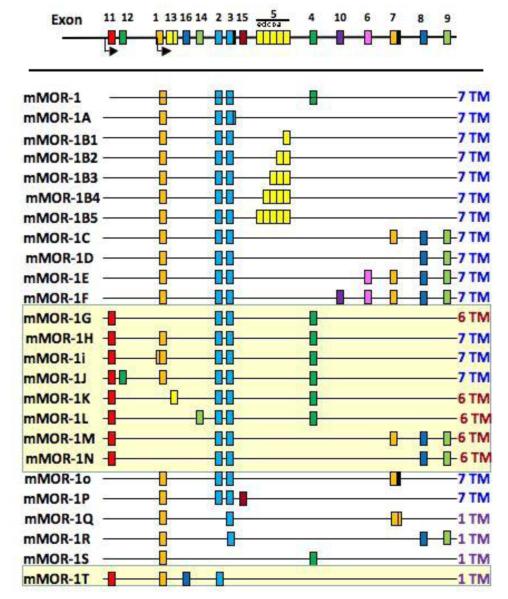
In contrast to some G protein-coupled receptors (GPCRs) such as ß2-adrenergic receptor, all three opioid receptor genes have multiple exons, and splice variants of the receptors can be generated accordingly. For OPRM1, the mRNA is composed of exons 1, 2, 3, and 4. The first splice variant of OPRM1 was identified by Zimprich et al. (16), in which the final receptor protein exhibited a truncated carboxyl terminus and diminished ability to desensitize. Since then, numerous alternative splice variants of OPRM1 have been reported due to the identification of additional exons within the putative receptor gene (17). These splice variants are too numerous to be detailed individually, but they are generated through two main mechanisms: they are either expressed from an alternative promoter at exon 11, resulting in variants with different N terminus sequences or truncated receptors with 6 or 1 transmembrane (TM) region(s); or, they are created from the alternative splicing of exon 3 with exons other than exon 4 (Figure 1). Some of these splice variants are expressed at much lower levels than OPRM1, and have a brain region expression distribution that is distinct from that of OPRM1. For instance, OPRM1C and 1D are presynaptically located in the dorsal horn of the spinal cord whereas OPRM1 is located both pre- and post-synaptically (18-20). Furthermore, when some splice variants with different carboxyl terminus sequences were heterologously expressed in cell models they exhibited distinct pharmacological responses (21, 22), suggesting that the carboxyl tail sequences, or the cellular proteins associated with the carboxyl tail, influence the eventual receptor conformation. However, none of the splice variants can account for the μ1 or μ2 subtypes.
Figure 1.
Schematic representation of various mouse OPRM1 splice variants. The mouse gene is presented with various exons (color boxes) in schematic form and not to scale, with splice variants shown underneath. The splice variants generated by the exon11 promoter (designated by the yellow background) are distinct from those generated by the promoter associated by exon1, which are traditional 7-TM GPCRs. The exon11-associated variants include several full length 7-TM receptors, and both 6-TM and 1-TM variants as indicated. Reproduced with permission of Figure 1 in an article by Majumdar et al, Proc. Natl. Acad. Sci. USA 108: 19778-19783 (2011).
Nevertheless, the in vivo significance of some of these oprm1 splice variants was illustrated using gene deletion studies. In mice lacking the exon 1 of oprm1, morphine was inactive while the antinociceptive activity of the morphine metabolite: morphine-6ß-glucuronide (M6G) and heroin were unexpectedly observed (23). The activities of M6G and heroin were demonstrated subsequently to involve the exon 11 splice variants; that is, those receptors having different N-termini or a truncated N-terminus relative to OPRM1 (Figure 1). Mice with exon 11 knocked out exhibited diminished heroin and M6G antinociceptive responses, while morphine and methadone activity was normal (24). Similarly, the inhibition of gastrointestinal transit by heroin and M6G was greatly diminished in exon 11 knockout mice while inhibition of gastrointestinal transit by morphine and methadone was not affected.
Such drug selectivity for the various splice variants represents an opportunity to identify or design compounds that could selectively activate a splice variant or its complex. This seems to the case with a recently reported compound: iodobenzoylnaltrexamide (IBNtxA). IBNtxA is a very potent analgesic; 10-fold more potent than morphine. However, in contrast to morphine, IBNtxA does not produce respiratory depression, and it inhibits gastrointestinal transit only modestly (25), two of the most concerning side-effects in the clinical use of opioid analgesics. Most interestingly, chronic administration of IBNtxA did not result in dependence or addictive responses, as demonstrated by the absence of withdrawal signs and the drug’s motivational effects. Although the exact target of IBNtxA remains to be identified, the absence of IBNtxA responses in exon 11 knockout mice and the presence of IBNtxA activity in the triple knockout mice (i.e., the absence of OPRM1, OPRD1 and OPRK1 in these mice) suggest that IBNtxA must activate one of the exon 11 splice variants, as illustrated by the ability of IBNtxA to bind to the membrane of cells expressing the MOR1G variant (see Fig 1) and the orphanin F/Q receptor (ORL1), and not to cell membranes that express either of these receptors alone (25). These studies suggest that the separation of the side effects from the analgesic response can be achieved by targeting a subset of opioid receptor variants.
Receptor heterodimerization results in distinct pharmacological profiles
The ability of opioid receptors to homo- or heterodimerize has been the subject of intense investigation. Most of the data supporting hetero-dimerization are based on the apparent pharmacological profiles in cells expressing multiple opioid receptors. The heterodimerization of opioid receptors has been reported to alter ligand selectivity (26), switching signals from being Gi/Go- to Gz- (27) or ß-arrestin-dependent (28). Opioid receptor heterodimerization has resulted in the appearance of ligand activity, as demonstrated by the ability of 6-guanidionaltrindole (6-GNTI)a designed receptor antagonist to induce increase in the intracellular [Ca2+] level in cells that express both OPRD1 and OPRK1, but not either alone (29).
In OPRM1/OPRD1 dimers agonist binding was shown to cause allosteric enhancement through inducing a cross-conformational switch among individual receptors (30). The conformation changes, as reflected in the intra- and intermolecular fluorescence resonance energy transfer (FRET), led to a decrease in activity within the putative OPRM1 and the α2-adrenergic receptor heterodimers (31). These and other in vitro cell model studies, such as those showing that the ligand of one receptor can induce internalization of another receptor (see review by Rijn et al (32)), have implicated the probable distinct heterodimer pharmacological profiles. However, the reported opioid receptor subtypes’ profiles have not been fully replicated in cells expressing various receptor heterodimers.
OPRM1/OPRD1 heterodimer’s role in tolerance development
Nevertheless, there is some encouraging in vivo evidence in support of distinct activities of opioid receptor heterodimers. The heterodimer that attracts the most attention is OPRM1/OPRD1. Interaction between OPRM1 and OPRD1 is best exemplified by the classic studies which showed that administration of a δ-antagonist could block the development of tolerance to chronic morphine treatment (33), and also showed that mice with oprd1 ablated did not develop morphine tolerance (34) and had altered addictive responses (35), (36). Such observations have prompted the designs of ligands or treatment paradigms that could alter the putative OPRM1/OPRD1 heterodimer activities. One such example is a class of bivalent ligands with 2 distinct pharmacophores: an OPRM1-selective agonist, oxymorphone, with the OPRD1-selective antagonist, naltrindole, joined together with flexible spacers between 16 and 21 atoms in length. Such bivalent ligands were shown to exhibit in vivo agonist activities ranging from 1.6- to 45-fold higher than morphine. Furthermore, mice treated chronically with bivalent ligands that had spacers of 19 atoms or higher did not exhibit tolerance or physical dependence, suggesting a role for these heterodimers in these chronic side-effects (37, 38). Accordingly, He et al. (39) interfered with the formation of OPRM1/OPRD1 heterodimers using a cell-permeable OPRM1 TM1-TAT peptide, and demonstrated that morphine antincociception was enhanced while tolerance development was reduced. It is their hypothesis that the activation of OPRD1 by its agonists such as deltorphin II or SNC80 will lead to internalization of heterodimers and OPRM1 degradation (39). Thus, the chronic side effects of morphine can be manipulated by regulating the heterodimers’ formation.
Whether OPRM1/OPRD1 heterodimers are indeed the source of adverse chronic effects is not without controversy. The concurrent internalization of both OPRM1 and OPRD1 upon activation by OPRD1 (39) was not uniformly observed by others with different agonists (40). In order for the heterodimers to exhibit distinct pharmacological profiles, co-localization of both OPRM1 and OPRD1 in the same neuron and at the same sub-cellular location is a prerequisite. The 71-79% of small diameter dorsal root ganglion (DRG) neurons co-expressing OPRM1 and OPRD1 as reported (39) (41) (42) was not observed in the OPRD1-GFP knockin mice, in which only 36% of the non-myelinated DRG neurons contained both OPRD1 and OPRM1 (43). Whether the observed difference was the result of differential distribution of OPRD1 due to fusion with GFP remains to be resolved. Electron microscopy ultrastructural analyses of receptor distribution in the striatum and superficial layers of the cervical spinal cord did not reveal a high percentage of intracellular co-localization of OPRM1 and OPRD1 (44, 45). However, by developing a monoclonal antibody that recognizes only the OPRM1/OPRD1 heterodimers, Gupta et al. (42) reported the presence of these heterodimers in membranes from the cortex, nucleus accumbens, hypothalamus and ventral tegmental area. Furthermore, heterodimer levels in the medial nucleus of the trapezoid body (MNTB, an auditory relay nucleus) and the rostral ventral medulla (RVM) within the descending pain pathway increased after morphine treatment. These data clearly support the in vivo existence of heterodimers, but they appear to contradict the reported ability of morphine to internalize the heterodimers within the in vitro cell model studies (32). Hence, the significance of the observed changes in heterodimer level and the role of heterodimers in the chronic drug effects remain to be resolved in future studies.
Drugs targeting the receptor heterodimers
Despite the unresolved in vivo existence of heterodimers, the development of drug molecules that target heterodimers has already found some success. For example, in addition to the above mentioned 6-GNTI, which appears to be selective for OPRD1/OPRK1 heterodimers and is a very potent spinal analgesic (29), the molecule IBNtxA is devoid of side effects and might target the MOR1G/ORL1 heterodimers, as suggested by Majumdar et al. (25). Another example of targeting a specific heterodimer, and thereby eliminating side effects, is the recent observation that MOR1D and gastrin-releasing peptide receptor (GRPR) can form heterodimers and mediate the itch sensation. Pruritus (itching) is one of the side effects observed with morphine administration, and is especially noticeable with intraspinal administration. In their studies, Li et al. (46) reported that MOR1D and GRPR formed heterodimers, and morphine induced the internalization of both MOR1D and GRPR. Itch sensation was blocked by GRPR inhibitors and was absent in GRPR knockout mice. However, morphine-mediated analgesia was retained in GRPR-null mice. The injection of a Tet peptide containing the MOR1D C-terminal sequence RNEEPSS into the spinal cord reduced heterodimer formation and the itch sensation. Although other GPCRs such as the family of orphan receptors known as Mrg/SNSR or Mas-related G-protein coupled receptors (Mrgprs) that are expressed exclusively in the peripheral sensory neurons could also be involved in the itch sensation (47), disrupting heterodimer formation might be a viable approach for the reduction of this effect of morphine. However, the heterodimer interface must be well defined before peptides or molecules that interfere with the formation of heterodimers can be designed. As is apparent from the crystal structures of the opioid receptors, a definitive heterodimer interface might not be obvious.
Factors affecting dimer formation and stability
Insights from receptors’ crystal structures
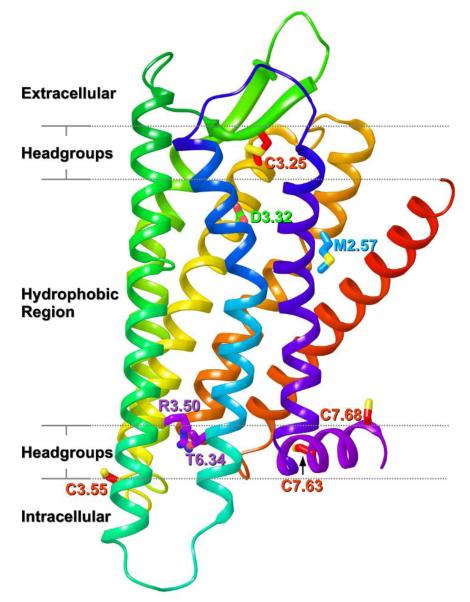
Whether ligands are activating a subset of opioid receptor splice variants or heterodimers, it is clear that receptor structures must be considered in the future design of ligands. Recently, the X-ray crystal structures of antagonist-bound inactive forms of the OPRM1, OPRD1 and OPRK1 receptors were reported (48-50). Unlike Class A GPCRs such as S1P1 (51) or Rhodopsin (52), which have their binding pockets largely shielded from the extracellular milieu, the binding pocket in all three opioid receptor structures is more open. This is largely due to the unique conformation of the EC-2 loop, which forms a β-hairpin that is pulled back from the bundle in each case despite its formation of a disulfide bridge with C3.25 (Figure 2). The openness of these binding pockets may be a contributing factor to the success of bivalent opioid ligands (53). There are also similarities between the binding pockets of OPRM1, OPRD1 and OPRK1 structures, a probable reason for the difficulties encountered in designing receptor-subtype-specific agonists. The protonated nitrogen of each antagonist in the X-ray structures interacts with D3.32 (Figure 2), a residue that is also the primary interaction site in cationic neurotransmitter GPCRs (54). On the intracellular side of all three receptors, transmembrane helix 3 (TMH3) and TMH6 are held in close proximity by an interaction between R3.50 and T6.34 (55) (Figure 2). This interaction mimics the R3.50-D/E6.30 “ionic lock” that promotes the inactive state in most Class A GPCRs. The most striking difference between the OPRM1, OPRD1 and OPRK1 structures is in the position of TMH1. In the OPRK1, TMH1 is pulled away from the bundle on the extracellular side in a manner similar to that seen in the β2-adrenergic receptor (56) (Figure 2, red helix). This conformation is promoted by the presence of the bulky residue M2.57, which prevents the top of TMH1 from moving closer to the TMH bundle (Figure 2).
Figure 2.
The OPRK1 receptor crystal structure is shown with the approximate location of lipid bilayer components delineated by dashed lines. The seven transmebrane helix (TMH) bundle is colored from red to blue (TMH1, red; TMH2, orange, TMH3, yellow; TMH4, light green; TMH5, green;TMH6, blue;TMH7, dark blue). The helical segment that begins the C terminus and is oriented parallel to the membrane is Helix 8 (purple). Extracellular loop 2 (EC-2 loop; colored green) forms a β-hairpin structure that is pulled back from the bundle despite its formation of a disulfide bridge with C3.25.
Such differences in structure could be critical in putative heterodimer formation among these opioid receptors, especially since TMH1 has been found to be part of the interface in certain dimers and oligomers (see below). A cysteine crosslinking study of the inverse agonist-bound dopamine D2 receptor identified a symmetric TMH4-TMH5 homodimer interface (57) consistent with the atomic force microscopy (AFM)-based model of the dimer interface of dark state rhodopsin (58). An additional TMH1/TMH1 interface was later found for D2 in its oligomeric state (59) and a different set of TMH4 residues was found to form the dimeric interface in activated D2 (60). The symmetric TMH4-TMH5 interface has been predicted computationally for numerous GPCRs (for a review, see (61)), including OPRD1 (62).
At first inspection, the OPRM1 receptor crystallized as intimately associated pairs, with two different interfaces: TMH5-TMH6 and TMH1–TMH2–Hx8, distinct from previous observations. The geometry of this dimer allows a close interaction between the T4-lysozyme inserted in the IC-3 loop of each monomer to promote crystal formation. The interface defined by TMH5 and TMH6 in the OPRM1 structure is much more extensive than the one defined by TMH1–TMH2–Hx8 (48). The OPRK1 receptor crystallized as parallel dimers with a single interface: TMH1–TMH2–Hx8 (49). However, the OPRD1 crystallized with only an anti-parallel arrangement of receptor molecules (50). Importantly, it is unclear if the oligomeric interfaces observed in GPCR crystal structures have physiological relevance. For instance, for crystallization, receptors are purified and detergent solubilized as monomers, which may limit their physiological relevance. Furthermore, the formation of parallel or antiparallel dimers occurs during crystallogenesis and probably reflects differences in the most energetically favorable interactions under crystallography conditions, rather than physiological conditions (50). In addition, modifications made to the GPCR structure (such as IC-3 loop insertion of T4-lysozyme to promote crystallization) may also favor certain interfaces. This may be the case for the OPRM1 receptor structure, which shows a close association of the T4-lysozyme inserts (48). However, the interfaces that are emerging from crystallographic data of unrelated GPCRs suggest the propensity of certain similar interfaces to form dimers, indicating that the observed opioid receptor interfaces may indeed be physiologically relevant. For example, the TMH5-TMH6 interface found in the OPRM1 (48) has also been found in five crystal structures of the CXCR4 receptor complexed with small molecule and cyclic peptide antagonists (63). Furthermore, the putative dimer interfaces observed with these opioid receptor crystal structures could also be interfaces for the formation of heterodimers with other GPCRs, and therefore influence the ability of ligands designed for such heterodimers to stabilize and activate the receptor.
Post-translational modifications
In addition to the influence of helix sequences in determining the most likely dimer interface and thus the stability of the receptor oligomers, post-translational modifications of the receptor also contribute to dimer stability. Palmitoylation is a post-translational modification that typically occurs in the C terminus of a GPCR. Cholesterol--palmitoyl interactions at the C-terminal (Hx8) residue, C7.68 (Figure 2), are present in the crystallographic TMH1-Hx8 dimeric interface of the β2-adrenergic receptor crystal structure (β2-AR) (56, 64). In this interaction, cholesterol is sandwiched between the receptor and the palmitoyl chain. Rat OPRM1 has two cysteines [C7.63(346) and C7.58(351)] in its C-terminus and another cysteine at the intracellular end of TMH3, C3.55(170) (Figure 2). Recent results have shown that C3.55(170) (rather than the C-terminal cysteines) is the major palmitoylation site in OPRM1 (65). Mutation of this cysteine attenuated receptor signaling and decreased the amount of cholesterol associated with the receptor signaling complex. In addition, palmitoylation was found to stabilize the receptor signaling complex and enhance morphine-induced signaling (65). In this case, the homodimer interface was proposed to be a symmetric TMH4-TMH5 interface (65) as seen in the D2 receptor (57) and rhodopsin (66). It bears mentioning that no palmitoylation sites are seen in any of the opioid receptor crystal structures. This is likely due to detergent solubilization of receptor in preparation for crystallization. Regardless of where the palmitoylation sites are located within the opioid receptors, however, differences in receptor palmitoylation could influence the ultimate stability of homo- or heterodimers, and therefore influence the signaling outcomes of ligands designed for such receptor oligomers.
Alternative approaches to reduce side effects via receptor activation
In addition to ligands that target a specific receptor, other approaches such as local administration of opioids have been used to reduce the subjective effects of these drugs (67, 68). An approach to reduce the development of tolerance by taking advantage of ligand-induced receptor endocytosis has been proposed by Whistler and co-workers. They hypothesize that ligands that can induce receptor endocytosis and recycling will induce less desensitization than those ligands that cannot, thus manipulating the Relative Activity versus Endocytosis (RAVE) ratio of the ligands (69, 70). By generating a mouse line that replaced the OPRM1 carboxyl tail sequence with that of OPRD1, Whistler and co-workers demonstrated that tolerance and addiction to morphine were reduced in these mice because morphine can cause internalization of the mutant receptor (71). Importantly, a cocktail that consisted of morphine and a small dose of methadone retained the antinociceptive response to morphine but did not promote morphine dependence (72, 73), because it promoted OPRM1 internalization. Thus, it is conceivable that such cocktails can be designed for clinical treatment of chronic pain.
Recently, another approach utilizing a mutant OPRM1 to eliminate the drug’s side effects has been reported. In this mutant receptor, in which the conserved Ser residue in TM4 (S4.54) was substituted with either Leu or Ala, classical opiate antagonists such as naloxone or naltrexone activated the receptor without altering the agonist activity (74). Naloxone and naltrexone produced antinociceptive responses in a S4.54A substitution knockin mouse without eliciting any of the chronic effects such as tolerance and dependence (75). The chronic effects were avoided because the opioid antagonists could activate the mutant receptor while inhibiting OPRD1 (76, 77). When a double-stranded adeno-associated virus type 2 (dsAAV2) was used to deliver the S4.54A OPRM1 mutant into the spinal cord of wild type or oprm1 null mice (78, 79), or other parts of pain pathway such as the ventral lateral periaqueductal gray (PAG) area (80), expression of the mutant receptor and subsequent antagonist-induced antinociceptive responses persisted for months, without development of measurable tolerance or dependence. So far, this approach involves the delivery and expression of a transgene in all cells surrounding the injection sites. The use of neural-specific promoters or even the OPRM1 promoters will limit the expression of the dsAAV2-delivered transgene to nociceptive neurons that normally express the opioid receptor. Such a gene therapy approach could be a viable method for the elimination of the side effects of opioid drugs, perhaps most applicable in terminal cancer pain.
Concluding remarks
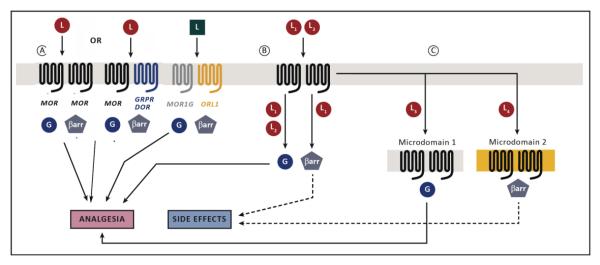
In addition to the opioid receptor crystal structures, other factors that could contribute to the receptor activities, and therefore the eventual pharmacological profiles, must also be considered during the design of drug molecules that eliminate side effects. The association of GPCRs with cellular proteins that affect their functions is well documented (81-83). Opioid receptors are no exception. Interaction of an opioid receptor with cellular proteins such as ß-arrestin, riboporin1 and GRIN1 will determine its cellular location (84). Translocation of the receptor among microdomains can influence the coupling between the receptor and signaling proteins, such as Gα or ß-arrestin (65, 85). The cellular cholesterol level could not only affect the formation of microdomains such as lipid rafts but could also facilitate the formation of the homodimers and thereby affecting the signaling and the in vivo analgesic responses (86-89). All these and other factors could contribute to the biased agonism that has been reported for all three opioid receptors (90, 91). The observed biased agonism is mainly due to the differential coupling of the receptor with cellular proteins such as G protein or ß-arrestin. But the consequences of such differential coupling can be significant; for instance, OPRM1 biased agonism can control microRNAs that regulate neuron differentiation and targeting (89), and OPRK1 biased agonism can specifically activate p38 MAP kinase in the dorsal raphe nucleus (92), which is implicated in the addiction process (86, 90, 91, 93). The differential in vivo response can be the consequence of the eventual cellular location of the activated protein kinases (94). Therefore, as summarized in Figure 3, for the future design of drug molecules or paradigms for reducing the side effects of opioid drugs to succeed, not only do the putative targets of these molecules need to be identified, (whether they are splice variants, heterodimers or receptor subtypes), but also the cellular proteins that interact with the activated receptor and the cellular locations of the activated proteins need to be considered. The ultimate goal will be the design of a drug molecule that will target a specific receptor subtype, whether it is a splice variant or a heterodimer between two opioid receptor types or between an opioid receptor and another GPCR. Thereby, the activation of a specific cellular pathway will result in the desired analgesic effect of the drug without any side effects. This has been and always will be the holy grail of opioid research.
Figure 3.
Schematic representations of future designs of opioid analgesic drugs without side effects. In (A), a ligand could be designed to interact with specific heterodimers of OPRM1 (MOR) and other GPCRs, or with a heterodimer formed between a specific MOR splice variant and other GPCR, such as ORL1. In (B) ligands can be designed to select for signaling pathways that will result in analgesia and no side effect, as in the case ligand L2 and not ligand L1. In (C), ligands can also be designed to alter the microdomain distribution of the receptor resulting in analgesia and minimal side effects, as in the case ligand L3.
Highlights.
Novel development strategies separate analgesic efficacy from opioid side effects.
Targeting receptor splice variants or receptor heterodimers could exhibit high analgesic efficacy with minimal side effects.
“Drug cocktails” to alter receptor trafficking and antagonist-activated receptor mutants are other viable approaches to minimize side effects.
Future drug designs should be guided by the resolved receptors’ crystal structures and the in vivo consequences of biased agonism.
Acknowledgement
This work is supported in parts by NIDA grants DA023905 (PYL), DA031442 (PYL), DA000564 (HHL), DA011806 (HHL) and K05 DA021358 (PHR).
Footnotes
From “Comments on the reported statistics on narcotics drugs, 2010” by International Narcotics Control Board, United Nations
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lang LJ, Pierer M, Stein C, Baerwald C. Opioids in rheumatic diseases. Ann. N.Y. Acad. Sci. 2010;1193:111–116. doi: 10.1111/j.1749-6632.2009.05343.x. [DOI] [PubMed] [Google Scholar]
- 2.Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999-2006. National Center for Health Statistics; Hyattsville (MD): 2009. NCHS data brief, no. 22. [online]. Available from URL: http://www.cdc.gov/nchs/data/databriefs/db22.htm. 2009. [PubMed] [Google Scholar]
- 3.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp, Ther. 1976;197(3):517–532. [PubMed] [Google Scholar]
- 4.Lord JAH, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides:multiple agonists and receptors. Nature. 1977;276:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 5.Dietis N, Rowbotham DJ, Lambert DG. Opioid receptor subtypes:fact or artifact? Br. J. Anaesthesia. 2011;107(1):8–18. doi: 10.1093/bja/aer115. [DOI] [PubMed] [Google Scholar]
- 6.Loh H, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res. Mol. Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 7.Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383(6603):819–23. doi: 10.1038/383819a0. [comment] [DOI] [PubMed] [Google Scholar]
- 9.Andoh T, Yageta Y, Konno M, Yamaguchi-Miyamoto T, Takahata H, Nojima H, Nemoto H, Kuraishi Y. Evidence for separate involvement of different mu-opioid receptor subtypes in itch and analgesia induced by supraspinal action of opioids. J. Pharmacol. Sci. 2008;106(4):667–670. doi: 10.1254/jphs.08004sc. [DOI] [PubMed] [Google Scholar]
- 10.Ling G, Spiegel K, Lockhart SH, Pasternak GW. Separation of opioid analgesia from respiratory depression: evidence for different receptor mechanisms. J.Pharmacol.Exp.Ther. 1985;232:149–155. [PubMed] [Google Scholar]
- 11.Zhang XaB L. Interaction and regulatory functions of μ- and d-opioid receptors in nociceptive afferent neurons. Neurosci. Bull. 2012;28(2):121–130. doi: 10.1007/s12264-012-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferre S, Navarro G, Casado V, Cortes A, Mallol J, Canela EI, et al. G protein-coupled receptor heteromers as new targets for drug development. Prog Mol Biol Transl Sci. 2010;91:41–52. doi: 10.1016/S1877-1173(10)91002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stockton SD, Jr., Devi LA. Functional relevance of mu-delta opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121(3):167–72. doi: 10.1016/j.drugalcdep.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurice P, Kamal M, Jockers R. Asymmetry of GPCR oligomers supports their functional relevance. Trends Pharmacol. Sci. 2011;32(9):514–520. doi: 10.1016/j.tips.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Zimprich A, Simon T, Hollt V. Cloning and expression of an isoform of the rat mu opioid receptor (rMOR1B) which differs in agonist induced desensitization from rMOR1. FEBS Letters. 1995;359:142–146. doi: 10.1016/0014-5793(95)00028-8. [DOI] [PubMed] [Google Scholar]
- 17.Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24(11):736–50. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 18.Abbadie C, Pan YX, Pasternak GW. Immunohistochemical study of the expression of exon11-containing mu opioid receptor variants in mouse brain. Neurosci. 2004;127(2):11–8. doi: 10.1016/j.neuroscience.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Abbadie C, Pasternak GW, Aicher SA. Presynaptic localization of the carboxyterminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord. Neuroscience. 2001;106(4):833–42. doi: 10.1016/s0306-4522(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Pan YX, Kolesnikov Y, Pasternak GW. Immunohistochemical labeling of the mu opioid receptor carboxy terminal splice variant mMOR-1B4 in the mouse central nervous system. Brain Res. 2006;1099(1):33–43. doi: 10.1016/j.brainres.2006.04.133. [DOI] [PubMed] [Google Scholar]
- 21.Pan X, Xu J, Bolan E, Moskowitz HS, Xu M, Pasternak GW. Identification of four novel exon 5 splice variants of the mouse mu-opioid receptor gene:functional consequences of C-terminal splicing. Mol. Pharmacol. 2005;68(3):866–875. doi: 10.1124/mol.105.011858. [DOI] [PubMed] [Google Scholar]
- 22.Bolan E, Pan YX, Pasternak GW. Functional analysis of MOR-1 splice variants of the mouse mu opioid receptor gene Oprm. Synapse. 2004;51(1):11–18. doi: 10.1002/syn.10277. [DOI] [PubMed] [Google Scholar]
- 23.Schuller AGP, King MA, Zhang J, Bolan E, Pan YX, Morgan DJ, Chang A, Czick ME, Unterwald EM, Pasternak GW, Pintar JE. Retention of heroin and morphine-6ß-glucuronide analgesia in a new line of mice lacking exon 1 of MOR1. Nat Neurosci. 1999;2(2):151–156. doi: 10.1038/5706. [DOI] [PubMed] [Google Scholar]
- 24.Pan X, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine actions. Proc Natl Acad Sci U S A. 2009;106(12):4917–4922. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumdar S, Grinnell S, Le Rouzic V, Burgman M, Polikar L, Ansonoff M, Pintar J, Pan Y-X, Pasternak GW. Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects. Proc. Natl. Acad. Sci. USA. 2011;108:19778–19783. doi: 10.1073/pnas.1115231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan T, Varghese G, Nguyen T, Tse R, O’Dowd B, George SR. A role for the distal carboxy tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J. Biol. Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- 28.Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin-2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist showns in vivo relevance of G protein-coupled receptor dimers. Proc. Natl. Acad. Sci. USA. 2005;102(25):9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes I, Ijzerman AP, Ye K, Maillet E, Devi LA. G protein-couple receptor heterodimerization: a role in allosteric modulation of ligand binding. Mol. Pharmacol. 2011;79:1044–1052. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilardaga J-P, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Confromational corss-talk between a2A-adrenergic and μ-opioid receptors controls cell signaling. Nature Chem. Biol. 2008;4(2):126. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 32.van Rijin R, Whistler JL, Waldhoer M. Opioid receptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol. 2010;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelhamid EES M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J.Pharmacol.Exp.Ther. 1991;258(1):299–303. [PubMed] [Google Scholar]
- 34.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 35.Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol. Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacol. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels DJ, Lenard NR, Etienne CL, Law P, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. USA. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur. J. Pharmacol. 2007;566(1-3):75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 39.He S, Zhang Z, Guan J, Liu H, Zhao B, Wang H, Li Q, Yang H, Luo J, Li Z, Wang Q, Lu Y, Bao L, Zhang X. Facilitation of -opioid receptor activity by preventing -opioid receptor-mediated codegredation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of μ- and δ-opioid receptors occurs at cell surface only and requires receptor-G protein interactions. J Biol. Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- 41.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zlhang ZN, He SQ, Zheng HC, Wu SX, Hökfelt TGM, Bao L, Zhang X. Co-expression of δ- and μ-opioid receptors in nociceptive sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 2010;107(29):13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci. Signal. 2010;20:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherrer GS, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Research. 1997;778(2):367–80. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- 45.Wang HaP VM. Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. J. Neurosci. 2001;21(9):3242–3250. doi: 10.1523/JNEUROSCI.21-09-03242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X-L L, Z-C., Sun Y-G, Ross M, Kim S, Tsai F-F, Li Q-F, Jeffry J, Kim J-Y, Loh HH, Chen Z-F. Unidirectional cross-activation of GPCR by MOR1D uncouples itch and analgesia induced by opioids. Cell. 2011;147:447–458. doi: 10.1016/j.cell.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Q, Tang ZX, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng H-J, Geng Y, Undem BJ, Kollarik M, Chen Z-F, Anderson DJ, Dong XZ. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139(7):1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu H, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC. S. Structure of the human k-opioid receptor in complex with JDTic. Nature. 2012;485(798):327–332. doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the d-opioid receptor bound to naltrindole. Nature. 2012;485(7398):400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335(6070):851–5. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 53.Pen G, Neumeyer JL. Kappa receptor bivalent ligands. Curr Top Med Chem. 2007;7(4):363–373. doi: 10.2174/156802607779941251. [DOI] [PubMed] [Google Scholar]
- 54.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci U S A. 1999;96(26):15268–73. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang P, Visiers I, Weinstein H, Liu-Chen LY. The local environment at the cytoplasmic end of TM6 of the mu opioid receptor differs from those of rhodopsin and monoamine receptors: introduction of an ionic lock between the cytoplasmic ends of helices 3 and 6 by a L6.30(275)E mutation inactivates the mu opioid receptor and reduces the constitutive activity of its T6.34(279)K mutant. Biochemistry. 2002;41(40):11972–11980. doi: 10.1021/bi026067b. [DOI] [PubMed] [Google Scholar]
- 56.Cherezov V RD, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;18(5854):1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J Biol Chem. 2003;278(7):4385–8. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 58.Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J Biol Chem. 2003;278(24):21655–20662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. Embo J. 2008;27(17):2293–304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. From The Cover: Crosstalk in G protein-coupled receptors: Changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A. 2005;102(48):17495–500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filizola M. Increasingly accurate dynamic molecular models of G-protein coupled receptor oligomers: Panacea or Pandora’s box for novel drug discovery? Life Sci. 2009;86(15-16):590–7. doi: 10.1016/j.lfs.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filizola M, Weinstein H. Structural models for dimerization of G-protein coupled receptors: The opioid receptor homodimers. Biopolymers. 2002;66(5):317–25. doi: 10.1002/bip.10311. [DOI] [PubMed] [Google Scholar]
- 63.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 Chemokine GPCR with Small-Molecule and Cyclic Peptide Antagonists. Science. 2010;330(6007):1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450(7168):383–7. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 65.Zheng H, Pearsall EA, Hurst DP, Zhang Y, Chu J, Zhou Y, Reggio PH, Loh HH, Law PY. Palmitoylation and membrane cholesterol stabilize mu-opioid receptor homodimerization and G protein coupling. BMC Cell. Biol. 2012;13:6. doi: 10.1186/1471-2121-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr. Opin. Pharmacol. 2009;9(1):3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23(4):737–746. doi: 10.1016/s0896-6273(01)80032-5. [comment] [DOI] [PubMed] [Google Scholar]
- 68.Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32(5):829–839. doi: 10.1016/s0896-6273(01)00517-7. [comment] [DOI] [PubMed] [Google Scholar]
- 69.Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr. Biol. 2008;18(2):129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berger A, Whistler JL. Morphine-induced mu opioid receptor trafficking enhances reward yet prevents compulsive drug use. EMBO Mol. Med. 2011;3(7):385–397. doi: 10.1002/emmm.201100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He L, Whistler JL. An opiate cocktail that reduces morphine tolerance and dependence. Curr. Biol. 2005;15:1028–1033. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 72.Claude PA, Wotta DR, Zhang XH, Prather PL, McGinn TM, Erickson LJ, Loh HH, Law PY. Mutation of a conserved serine in TM4 of opioid receptors confers full agonistic properties to classical antagonists. Proc. Natl. Acad. Sci. U.S.A. 1996;93(12):5715–5719. doi: 10.1073/pnas.93.12.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Claude-Geppert P, Liu J, Solberg J, Erickson-Herbrandson LJ, Loh HH, Law PY. Antagonist efficacy in MORS196L mutant is affected by the interaction between transmembrane domains of the opioid receptor. J. Pharmacol. Exp. Ther. 2005;313:216–226. doi: 10.1124/jpet.104.076505. [DOI] [PubMed] [Google Scholar]
- 74.Yang WJ, Law PY, Guo XH, Loh HH. In vivo activation of a mutant μ-opioid receptor by antagonist: Future direction for opiate pain treatment paradigm that lacks undesirable side effects. Proc. Natl. Acad. Sci. U.S.A. 2003;100(4):2117–2121. doi: 10.1073/pnas.0334906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roy S, Guo X, Kelschenbach J, Liu Y, Loh HH. In vivo activation of a mutant mu-opioid receptor by naltrexone produces a potent analgesic effect but no tolerance: role of mu-receptor activation and delta-receptor blockade in morphine tolerance. J. Neurosci. 2005;25(12):3229–3233. doi: 10.1523/JNEUROSCI.0332-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen SL, Ma HI, Han JM, Tao PL, Law PY, Loh HH. dsAAV type 2-mediated gene transfer of MORS196A-EGFP into spinal cord as a pain management paradigm. Proc Natl Acad Sci U S A. 2007;104(50):20096–101. doi: 10.1073/pnas.0703409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen S, Ma HI, Han JM, Lu RB, Tao PL, Law PY, Loh HH. Antinociceptive effects of morphine and naloxone in mu-opioid receptor knocout mice transfected with the MORS196A gene. J. Biomed. Sci. 2010;17:28. doi: 10.1186/1423-0127-17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kao J, Chen SL, Ma HI, Law PY, Tao PL, Loh HH. Intrathecal delivery of a mutant μ-opioid receptor activated by naloxone as a possible antinociceptive paradigm. J. Pharmacol. Exp. Ther. 2010;334:739–745. doi: 10.1124/jpet.109.165399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chou S, Kao JH, Tao PL, Law PY, Loh HH. Naloxone can act as an analgesic agent without measurable chronic side effects in mice with a mutant mu-opioid receptor expressed in different sites of pain pathway. Synapse. 2012;66(8):694–704. doi: 10.1002/syn.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenakin TM, L.J. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc. Natl. Acad. Sci. U.S.A. 1998;95(12):7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge X, Loh HH, Law PY. mu-Opioid receptor cell surface expression is regulated by its direct interaction with ribophorin1. Mol. Pharmacol. 2009;75:1307–1316. doi: 10.1124/mol.108.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ge X, Qiu Y, Loh HH, Law PY. GRIN1 Regulates μ-Opioid Receptor Activities by Tethering the Receptor and G Protein in the Lipid Raft. J Biol Chem. 2009;284(52):36521–36534. doi: 10.1074/jbc.M109.024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng H, Chu J, Qiu Y, Loh HH, Law PY. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci U S A. 2008;105(27):9421–6. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng H, Zou H, Liu X, Chu J, Zhou Y, Loh HH, Law PY. Cholesterol level influences opioid signaling in cell models and analgesia in mice and human. J. Lipid Res. 2012;53(6):1153–1162. doi: 10.1194/jlr.M024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng H, Loh HH, Law PY. Arrestin-dependent μ-opioid receptor-activated extracellular signal-regulated kinases (ERKs) translocate to nucleus in contrast to G protein-dependent ERK activation. Mol. Pharmacol. 2008;73(1):178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng H, Chu J, Zhang Y, Loh HH, Law PY. Modulating -Opioid Receptor Phosphorylation Switches Agonist-dependent Signaling as Reflected in PKC Activation and Dendritic Spine Stability. J. Biol. Chem. 2011;286(14):12724–12733. doi: 10.1074/jbc.M110.177089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pradhan A, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the δ-opioid receptor in vivo: two paths toward analgesic tolerance. J. Neurosci. 2010;30(49):16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Land B, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106(45):19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY. Mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol. Pharmacol. 2010;77(1):102–109. doi: 10.1124/mol.109.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng H, Zeng Y, Chu J, Kam AY, Loh HH, Law PY. Modulations of NeuroD activity contribute to the differential effects of morphine and fentanyl on dendritic spine stability. J. Neurosci. 2010;30(24):8102–8110. doi: 10.1523/JNEUROSCI.6069-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruchas M, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71(3):498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng H, Chu J, Zen,g Y, Loh HH, Law PY. Yin Yang 1 phosphorylation contributes to the differential effects of μ-opioid receptor agonists on microRNA-190 expression. J. Biol. Chem. 2010;285(29):21994–212002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature. 2012 doi: 10.1038/nature10939. [DOI] [PMC free article] [PubMed] [Google Scholar]