1. Introduction
Bacterial lipid metabolism has long had a significant impact on the understanding of the basic lipid metabolic pathways, enzyme mechanisms and transcriptional regulation. The early work in the Escherichia coli system jump-started the investigation of fatty acid and phospholipid synthesis. A recent review by Dowhan [1] recounts these early days of discovery in bacterial lipid metabolism. For decades, E. coli was considered the paradigm for bacterial metabolism; however, the advent of the genomic era revealed that genes and enzymes of lipid metabolism painstakingly investigated in E. coli are not common to all bacteria. This realization has accelerated research into the great diversity in pathways, and fatty acid and phospholipid structures that occur in nature. This review attempts to capture and organize this diversity to provide an overview of lipid metabolism in prokaryotes as it stands today.
2.1 The FASII initiation module
The function of the initiation module of FASII is to produce the primer and the building blocks to feed the elongation module (Fig. 1). The acetyl-CoA carboxylase (ACC) performs the first committed step in bacterial phospholipid synthesis to generate malonyl-coenzyme A (malonyl-CoA) through the carboxylation of acetyl-CoA [2–4]. In order to be recognized by the FASII enzymes, the malonate group from malonyl-CoA must be transferred to acyl carrier protein (ACP) by FabD [5]. The condensation of malonyl-ACP with a short-chain acyl-CoA (C2-C5) by FabH initiates the elongation cycle [6–8]. The malonyl-ACP generated by the ACC and FabD is also used by the elongation cycle to extend the growing fatty acid chain, illustrating how crucial ACC activity is to maintaining the optimum rate of membrane phospholipid synthesis. Every condensation reaction performed by FabH will result in the production of a new fatty acid to expand the membrane. Consequently, the initiation module is ideally positioned for biochemical and genetic regulation of the amount of fatty acids produced and speed at which they are manufactured.
Figure 1. The initiation module of type II fatty acid synthesis.
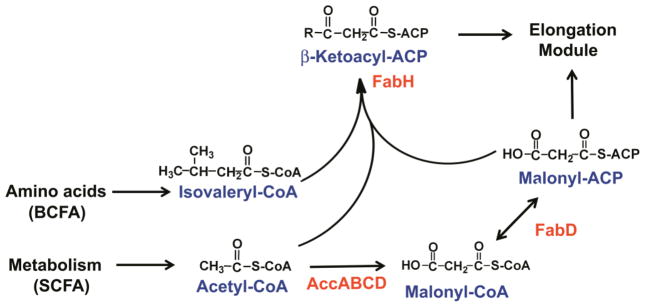
The priming reaction for the elongation cycle is performed by the by the initiating condensing enzyme, FabH, which condenses an acyl-CoA with malonyl-ACP. In bacteria that produce straight chain fatty acids (SCFA), acetyl-CoA is employed. Bacteria producing branched-chain fatty acids (BCFA) utilize an amino acid derived branched chain acyl-CoA precursor. Malonyl-CoA is formed by acetyl-CoA carboxylase, which is composed of four different protein subunits encoded by separate genes (AccABCD). Malonyl-ACP is formed by the transacylase, FadD.
2.1.1 Acetyl-CoA Carboxylase
The acetyl-CoA carboxylase enzyme is present in animals, plants, fungi and bacteria. In bacteria, the ACC is a multisubunit complex consisting of a biotin carboxylase (AccC), biotin carboxyl carrier protein (AccB, also known as BCCP) and a carboxyltransferase (AccAD). AccB and AccC exist as homodimers while AccAD is a heterotetramer [3,9,10]. In the cell, ACC is likely a multimeric complex of these four subunits; however, the complex readily dissociates following cell disruption leaving only the two ACC half reactions that can be measured. The first half reaction catalyzes the ATP-dependent carboxylation of biotin on AccB by AccC [11,12]. The carboxylation of free biotin or biotin analogs is an extremely inefficient reaction, and maximum rates required biotin to be covalently attached to a lysine residue of biotin carboxyl carrier protein (AccB) [11,13]. In the second half reaction, the carboxyl group is transferred from carboxyl-AccB to acetyl-CoA by AccAD. The two half-reactions can be assayed individually but reconstituting the complete ACC reaction is more challenging [14,15]. High concentrations of each purified subunit are required to reach a threshold concentration for the catalytically active complex to form in vitro [11,12,16]. The genetic organization of the four acc genes differs between organisms. In Escherichia coli and Staphylococcus aureus, the accAD and accBC genes are organized in operons located at different regions of the chromosome [9]. In Streptococcus pneumoniae, the acc genes are located adjacent to each other in a transcriptional unit that also contains the other FASII genes [17]. Evidence that the ACC governs the quantity of fatty acid produced by the cell was provided by a study involving the overexpression of accABCD in E. coli. Maintaining a normal lipid to protein ratio is critical for homeostasis, therefore the authors devised a means to uncouple FASII from phospholipid synthesis and excrete the excess fatty acids from the cell. The acc genes were overexpressed in combination with a soluble acyl-ACP thioesterase to uncouple FASII from membrane synthesis resulting in 5–6 fold increase in fatty acid synthesis [18]. The influence of ACC activity on the rate of fatty acid synthesis underlines the importance of stringent regulation of the ACC activity.
2.1.2 Role of FabH in determining fatty acid structure
There is remarkable diversity in the fatty acid structures produced by different bacteria. Like humans, E. coli and S. pneumoniae produce even-number straight-chain saturated and unsaturated fatty acids [19]. Many Gram-positive bacteria, such as B. subtilis and S. aureus, produce predominantly odd-numbered branched-chain fatty acids [20]. The branched/straight and even/odd characteristics are determined by the substrate specificity of FabH, the initiation condensing enzyme [21]. This enzyme catalyzes the first condensation reaction that initiates the fatty acid elongation cycle [22] (Fig. 1). E. coli FabH (EcFabH) selectively utilizes acetyl-CoA derived from intermediary metabolism, whereas FabH enzymes from B. subtilis and S. aureus preferentially utilize the bulkier branched-chain acyl-CoA primers derived from amino acids [20,21,23,24]. The branched-chain acyl-CoA substrates lead to the production of anteiso and iso fatty acids. The selectivity of E. coli FabH (EcFabH) for acetyl-CoA and S. aureus FabH (SaFabH) for branched-chain (C4-C5) acyl-CoAs is attributed to structural differences in the hydrophobic pocket adjacent to the active site that accommodates the acyl-enzyme intermediate. It is the organization of the residues in this pocket that determine which acyl-CoA primers can be accommodated by the enzyme. In the E. coli FabH structure solved by Qiu et al., the binding pocket is only large enough to fit acetyl- or propionyl-CoA [21,25,26]. Although the sequence of the residues throughout the tunnel are almost identical, in S. aureus FabH the side chains of the residues are oriented to create space for bulkier branched-chain acyl-CoAs [21]. The shape of this pocket is thought to be a major determinant of the substrate specificity of FabHs [7,19,21,27].
Confirmation for the role that FabH plays in determining the structure of fatty acids was provided by Li et al. who performed an elegant study using the FabH from Streptomyces coelicolor (ScFabH) [28]. S. coelicolor phospholipids contain predominantly branched-chain fatty acids (74%), a signature of a FabH enzyme with a preference for branched-chain acyl-CoA substrates. Li et al. deleted the endogenous fabH gene in S. coelicolor and complemented the deletion with either EcfabH or fabH from Streptomyces glaucescens (SgfabH), another organism that synthesizes mainly branched-chain fatty acids. Complementation with SgFabH had little effect on the fatty acid composition of S. coelicolor, whereas complementation with EcFabH resulted in an organism with 87.5% straight-chain fatty acids compared to 25.6% with the endogenous ScFabH enzyme. The EcFabH-complemented mutant also exhibited a major growth defect, stressing the importance of a correct fatty acid profile for bacterial membrane homeostasis.
A unique FabH initiates FASII in Mycobacteria by utilizing long-chain acyl-CoA. Mycobacterium tuberculosis uses a type one fatty acid synthase (FASI) to manufacture long-chain acyl-CoA products (C14-C26) [29]. The products are utilized by MtFabH to initiate elongation by FASII enzymes to generate the very long-chain mycolic acids (C50-C56). The ability of mtFabH to utilize such long-chain primers requires some significant structural alterations compared to the E. coli enzyme (EcFabH). MtFabH contains an extra hydrophobic channel that permits binding of longer acyl-CoA substrates. In EcFabH, this channel is blocked by a phenylalanine whereas the corresponding threonine residue in MtFabH allows an opening into a much larger hydrophobic cavity that accommodates the fatty acid [29].
The sequence similarity of different FabH enzymes varies enormously but each is characterized by having a conserved Cys-His-Asn catalytic triad [21]. Every FabH catalyzes a Claisin-type condensation reaction between an acyl-CoA substrate and malonyl-ACP using a bi-bi ping-pong mechanism [30]. The acyl-CoA binds to the enzyme and the acyl group is inserted into the hydrophobic active site tunnel of the protein [25,26,31]. The acyl chain is transferred to the active site cysteine via nucleophilic attack of the sulfhydryl on the CoA thioester, resulting in the release of CoA [31]. Malonyl-ACP then binds to the acyl-enzyme intermediate [31], and condenses with the intermediate, releasing bicarbonate and the β-ketoacyl-ACP product. The His and Asn residues of the catalytic triad are critical for the malonyl-ACP decarboxylation half reaction and are thought to aid the process by interacting with and stabilizing the developing negative charge on the malonyl-ACP carbonyl oxygen [31].
The initiation (FabH) and elongation (FabB/F) condensing enzymes are distinguished by their active site residues. However, both FabH and FabB/F catalyze identical condensation reactions, so the distinction between FabH having a His-Asn-Cys triad versus the His-His-Cys triad of FabB/F has always been unclear. The requirement for the His-Asn-Cys catalytic triad for bacterial initiation condensing enzymes was recently challenged by Yuan et al. who investigated FASII initiation in Pseudomonas aeruginosa [32]. Using bioinformatics, four genes were identified with similarity to EcFabH and each contained at least two of the three His-Asn-Cys residues of the active site. A quadruple knockout strain of P. aeruginosa was constructed with all four potential FabH genes deleted. Interestingly, this mutant strain showed no obvious growth defect suggesting none of these fabH genes were responsible for initiating FASII. The author’s identified a gene named fabY (PA5174) that was essential for P. aeruginosa viability, and restored growth in an E. coli FabH knockdown strain. This deviation from the FabH nomenclature was appropriate because FabY active site consists of a His-His-Cys triad that is characteristic of the elongation condensing enzyme (FabF/FabB). FabY differs from the FabF/FabB enzymes in preferring acyl-CoA substrates instead of the acyl-ACP substrates employed by the elongation condensing enzymes. A FabY structure may shed light on why this unique condensing enzyme has adopted the His-His-Cys configuration.
The question of whether fabH is essential for growth in E. coli was investigated by introducing the Salmonella enterica fabH gene into the E. coli chromosome [33]. The endogenous fabH gene was easily deleted from the double-fabH strain, but fabH could not be inactivated in the parent strain. This conclusion was supported by experiments in Lactococus lactis where the deletion of fabH gave rise to exogenous fatty acid auxotrophs. These data appeared to provide convincing evidence for the essentiality of FabH in E. coli, except that a different E. coli fabH null mutant was able to grow without a fatty acid supplement and has a cell volume that is 70% smaller [34]. The apparent discrepancy was traced to differences in the genotypes of the E. coli strains used in the two experiments. The strain where fabH essentiality was demonstrated contained both relA1 and spoT1 mutations that inactivate these enzymes. RelA and SpoT are involved in the regulation of the intracellular concentration of ppGpp, a small molecule that regulates a multiplicity of cellular processes [35]. However, fabH can be deleted in wild-type strains and a series of genetic experiments showed that a functional spoT gene was required in order for fabH to be dispensable in E. coli [34]. Although the accumulation of ppGpp following the inhibition of fatty acid synthesis is SpoT dependent [36] and SpoT directly interacts with ACP [37–39], the role of SpoT in FASII is a mystery and deserving for more research.
The enzyme(s) responsible for the initiation of FASII in the absence of FabH in E. coli remain to be determined. The fatty acid auxotrophy of the L. lactis fabH deletion mutant can be rescued by overproduction of the β-ketoacyl-ACP synthase II, FabF [40]. Although the catalytic mechanism of the FabF condensing enzyme is identical to FabH, FabF is unable to utilize acetyl-CoA and is instead specific for acyl-ACP primers. Importantly, as demonstrated by in vitro experiments, the FabF enzyme is able to self-prime in the absence of a suitable acyl-ACP substrate by the decarboxylation of malonyl-ACP to acetyl-ACP, which subsequently forms the acyl-enzyme intermediate. In this example, FabH is bypassed by this side-reaction of FabF [41]. The acetyl-enzyme would subsequently perform the condensation reaction with malonyl-ACP to produce the β-ketobutyryl-ACP normally generated by FabH. The efficiency of the decarboxylation reaction compared to the condensation reaction is low, explaining the need for abnormally high FabF expression to complement the FabH defect [42,43]. These studies provide insight into the phenotype in the L. lactis fabH mutant and suggest that either FabB or FabF may be compensating the lack of FabH in the E. coli fabH deletion. However, this idea remains unproven and further experimentation is required to positively identify the enzyme responsible for the initiation of FASII in the absence of FabH in E. coli.
2.2. The Elongation Module
The elongation module represents the central machinery of bacterial FASII (Fig. 2). These four enzymes work in concert to receive the product of the FabH reaction and elongate the acyl-ACP by two carbons with the completion of each turn of the cycle until a long-chain acyl-ACP is generated. The enzymes appear to be independent biochemical entities, and the cycle can be reconstituted in vitro by incubating the enzymes with necessary substrates and cofactors [44]. The FabH product enters the elongation cycle and is reduced by the NADPH dependent β-ketoacyl-ACP reductase (FabG) to form β-hydroxyacyl-ACP. The crystal structures of FabG from an assortment of organisms are known, and there is very little variation in the FabG structures between plant, Gram-positive and Gram-negative bacterial enzymes [45–48]. The similarities in FabG structure between organisms that produce distinctly different fatty acids suggests FabG does not play a role in determining fatty acid structure. The next step in the elongation cycle is dehydration of the β-hydroxyacyl-ACP to the trans-2-enoyl-ACP. In E. coli, FabA and FabZ are the enzymes that catalyze this reaction. The distinction between the two enzymes lies in the ability of FabA to perform the cis-trans isomerase reaction needed to synthesize unsaturated fatty acids (see section 2.2.3). In Gram-positive bacteria containing saturated fatty acids, only the FabZ isozyme is present, but FabZ is the only isoform in S. pneumoniae, which produces unsaturated fatty acids. Thus, FabZ must be capable of elongating these intermediates acyl-ACP also.
Figure 2. The elongation module.
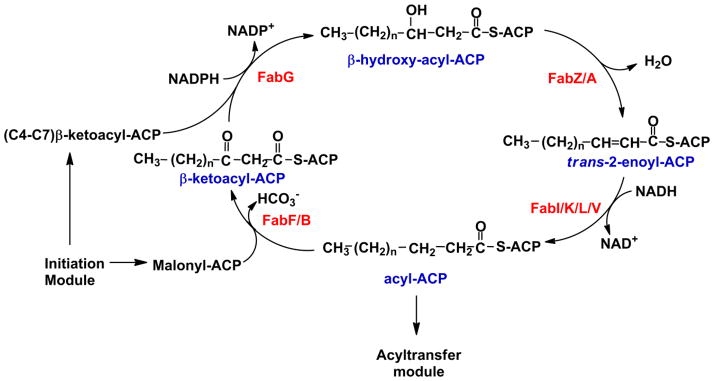
The enzymes of the elongation module work in concert to receive the β-ketoacyl-ACP and malonyl-ACP from initiation module and extend the fatty acid by two carbons with the completion of each cycle. There is a β-ketoacyl-ACP reductase (FabG) that appears to be present in all bacteria. FabZ is a universally-expressed dehydratase. FabA is always present with FabB and is also involved in unsaturated fatty acid synthesis (Fig. 3). There are multiple isoforms of enoyl-ACP reductase (FabI/K/L/V), and some organisms express more than one of these isoforms. FabF is the most-uniformly expressed elongation condensing enzyme. FabB is always found with FabA and has a special role in unsaturated fatty acid synthesis (Fig. 3). When the fatty acyl chain becomes long enough, the acyl-ACP can be utilized by the acyltransferase module (Fig. 4).
The next reaction of the elongation cycle is performed by the NAD(P)H trans-2-enoyl-ACP reductase, which in many bacteria is the FabI enzyme [49]. This reaction reduces the C2-C3 double bond on trans-2-enoyl-ACP to form acyl-ACP. FabI belongs to a subgroup within the short- chain alcohol reductase/dehydrogenase superfamily that is defined by the Tyr-Xaa6-Lys catalytic diad and a requirement for nicotinamide cofactors [50]. The lysine binds the 2′-hydroxyl group of the ribose moiety of the cofactor and the tyrosine stabilizes the enoyl-form of the thioester during catalysis. In E. coli, FabI has a preference for NADH over NADPH although the converse is true of S. aureus FabI. Bacillus subtilis possesses a FabL enoyl-ACP reductase in addition to a FabI [51]. This alternate reductase has an identical catalytic diad to FabI, but has a strong preference for NADPH over NADH. Neither FabI nor FabL are essential in B. subtilis but a double knockout cannot be obtained showing that the two enzymes function interchangeably in FASII. A structurally distinct enoyl-ACP reductase, FabV, was first discovered in V. cholerae [52] and subsequently in P. aeruginosa [53] and Burkholderia mallei [54] during studies on bacteria resistant to the FabI inhibitor, triclosan. The origin of FabV is indicated by the presence of an enzyme (BatG) in an operon in that produces a natural product FabI inhibitor [55]. The FabV homolog (BatG) confers resistance to bacteria toward this natural product, whereas bacteria that have only a FabI are sensitive to the natural product. FabV is relatively widespread in bacteria and two FabV structures have been solved [56,57]. FabV is 60% larger than FabI and also possesses the catalytic Tyr and Lys residues, albeit in a different configuration (Tyr-Xaa8-Lys). Many Streptococci use an unrelated flavoenzyme, FabK, to perform the enoyl-ACP reductase reaction [58]. This enzyme lacks the Lys and Tyr catalytic residues in FabI, but uses a His and a flavin mononucleotide (FMN) for catalysis instead [59]. It is thought that this unique reductase may have an additional function as an NAD+ re-generating system due to its NADH oxidase activity observed in the absence of a substrate, which would promote energy metabolism via glycolysis. Despite this significant structural diversity between species, each of the different enoyl-ACP reductases can complement a temperature-sensitive fabI mutant in E. coli, indicating their common function in the FASII elongation cycle. The acyl-ACP produced by the elongation module has two fates. It can be used by the acyltransferase module (Fig. 3) or it can be used by the elongation condensing enzyme, FabB or fabF, to initiate a new round of elongation (Fig. 2).
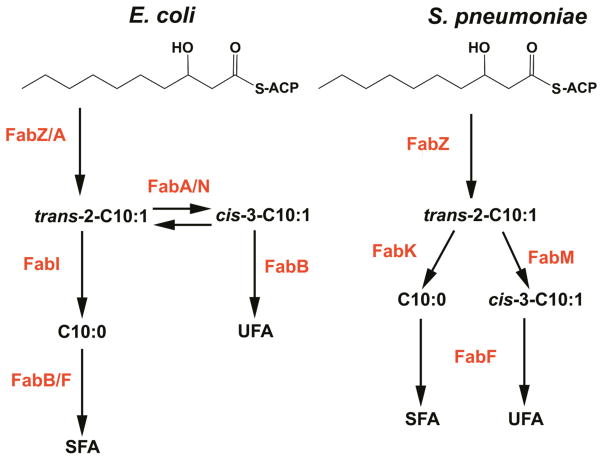
Figure 3. Branch points in unsaturated fatty acid synthesis.
Two established mechanisms exist to produce unsaturated fatty acids. The E. coli system utilizes a bifunctional dehydratase/isomerase enzyme (FabA), which acts specifically on β-C10:0-ACP. The trans-2-C10:1-ACP product of the dehydratase reaction can either be reduced by FabI to continue through the saturated fatty acid pathway or undergo the FabA-dependent isomerization reaction to generate cis-3-C10:1-ACP. FabB is required to elongate this intermediate leading to an unsaturated fatty acid. In S. pneumoniae, there is a specific isomerase (FabM) that competes for substrate with the FabK enoyl-ACP reductase. A FabF isoform elongates unsaturated fatty acids in this organism. Other mechanisms exist, but the mechanistic understanding is limited (see section 2.2.3).
Several of the intermediates generated in the elongation module are also required for other essential cellular processes. An example is lipoic acid, synthesized from octanoyl-ACP manufactured by the elongation cycle [60,61]. This sulfur containing coenzyme is present in almost all prokaryotic bacteria and is covalently attached to pyruvate dehydrogenase and glycine cleavage protein [60]. This modification is required for catalytic activity of these enzymes and depletion of lipoic acid in lipoic acid auxotrophic E. coli mutants causes growth arrest [62]. Another essential enzyme cofactor arising from FASII is biotin, which is synthesized from a pimeolyl-ACP derived from FASII [63]. The importance of biotin as a cofactor for carboxylase enzymes (including ACC) is well appreciated (section 2.1.1). In addition to enzyme cofactors, structural components not destined to the cytoplasmic membrane also require the diversion of intermediates from the elongation module. Lipid A is needed to form the hydrophobic anchor of lipopolysaccharide (LPS) in Gram-negative bacteria and usually contains at least four β-hydroxy-fatty acids derived from FASII [64]. The β-hydroxy-fatty acids are an essential structural component of the LPS and cannot be substituted for by normal fatty acids. Also, the enzymes of LPS biosynthesis are specific for ACP thioesters, thus FASII is the only source for the hydroxy-fatty acids of LPS [64], making FASII essential even in the presence of exogenous fatty acids. In Gram-positive bacteria, which lack LPS, exogenous fatty acids can be ligated to ACP and enter the elongation cycle [65]. Finally, intermediates in the elongation module are diverted to produce variety of extracellular molecules that regulate cell activity. Examples, are the quorum-sensing N-acyl-homoserine lactones that are produced by a large number of organisms [66,67]. The quinolone signals [68] and extracellular rhamnolipid surfactants [69] produced by P. aeruginosa are derived from FASII, as is the recently discovered diffusible signal factor, cis-2-decenoic acid [70].
2.2.1 The determinant role of FabI in the rate of elongation
The idea that the enoyl-ACP reductase (FabI, Fig. 2) is a rate-determining reaction in the elongation module is derived from the in vitro reconstitution of the cycle using E. coli enzymes. FabI catalyzes the final reaction of the elongation cycle and the resulting acyl-ACP product is either used by FabB/F to begin another round of elongation or it is incorporated into phospholipids by the acyltransferases (Fig. 2). The in vitro reactions catalyzed by the elongation module enzymes FabI, FabG, and FabB/F result in extensive product formation. However, the equilibrium of the β-hydroxyacyl-ACP dehydratase reactions performed by EcFabA or EcFabZ favor the formation of β-hydroxyacyl-ACP over enoyl-ACP [49]. The analysis of the acyl-ACP pool composition in FabI-depleted E. coli shows the accumulation of predominantly β-hydroxy-ACP instead of enoyl-ACP in a 9:1 ratio corroborating the in vitro results in vivo [49]. Thus, FabI pulls the elongation cycle to completion, and thus regulates of the rate at which the elongation cycle turns. Whereas this idea is consistent with all the data in E. coli, it would be wrong to think that the enoyl-ACP reductase plays the same role in all bacteria. We now know of the diversity in enoyl-ACP reductase structures among organisms, and it would not be prudent to conclude that these widely disparate entities have the same role in the pathway. Also, the FabZ enzymes of all bacteria may not have the same equilibrium position. Thus, understanding the fundamental regulation of the elongation module in E. coli may or may not bring us closer to understanding how the cycle is controlled in pathogens.
FabI also impacts the basal saturated:unsaturated fatty acid ratio in some organisms. FabA is the enzyme that catalyzes the isomerization reaction of trans-2-decenoyl-ACP to cis-3-decenoyl-ACP at the branch point in unsaturated fatty acid synthesis (section 2.2.3; Fig. 4). Thus, FabA and FabI compete for the trans-2-decenoyl-ACP. The requirement of E. coli for a minimum of 15% unsaturated fatty acid content for growth stresses the importance of maintaining a balance of FabA catalyzed cis-trans isomerization and the cycle-concluding reaction of FabI [71]. This theory is supported by studies observing growth rate attenuation in E. coli overexpressing fabI [72]. Overproduction of FabA rather surprisingly leads to an increase in saturated fatty acid synthesis due to the reversible nature of the cis-trans isomerase reaction [73]. This change can be counteracted by overproduction of FabB, which drags cis-decenoyl-ACP produced by FabA into the unsaturated fatty acid pathway [73]. Although FabI and FabA completefor the trans-2-C10-ACP intermediate, the absence of a change in unsaturated fatty acid composition in cells overexpressing fabI is consistent with FabB being the rate-limiting step in unsaturated fatty acid synthesis. The balance between the cis-trans isomerase reaction and the trans-2-enoyl-ACP reductase reaction is more important in S. pneumoniae which lacks a FabA and utilizes a monofunctional FabM enzyme for the isomerization of trans-2-decenoyl-ACP to cis-2-decenoyl-ACP instead [74]. FabM directly competes with FabK for the substrate, and the replacement of FabK with FabI leads to a decrease in unsaturated fatty acid synthesis due to the higher rates of enoyl-ACP utilization by FabI [74]. These data point to the importance of different enoyl-ACP reductases in different bacteria and illustrate the how the unsaturated fatty acid content in S. pneumoniae is regulated by the expression levels of FabK and FabM.
Figure 4. The acyltransferase module of bacterial lipid biosynthesis.
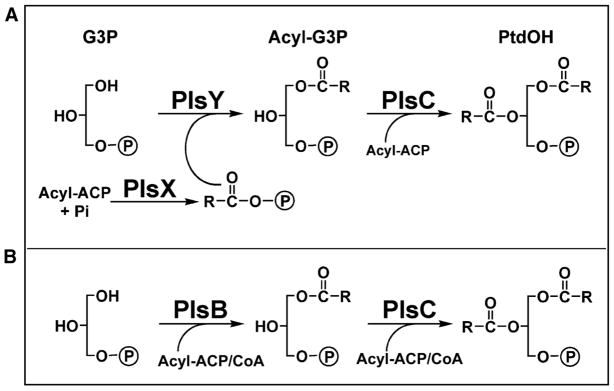
Transfer of fatty acids to glycerol-3-phosphate is accomplished by two different systems in bacteria. (A) The PlsX/Y/C system is a widely-distributed pathway and predominates in Gram-positive organissms. PlsX produces acyl-PO4 from the acyl-ACP end-products of FASII. PlsY is an acyl-PO4-specific glycerol-phosphate acyltransferase that transfers the acyl chain to the 1-position. PlsC is an acyl-ACP specific 1-acyl-glycerol-phosphate acyltransferase. (B) The PlsB/C system is primarily associated with γ-proteobacteria. PlsB transfers an acyl chain from either acyl-ACP or acyl-CoA to glycerol-3-phosphate and PlsC transfers an acyl-chain from acyl-ACP/CoA to 1-acyl-glycerol-3-phoshate to generate phosphatidic acid.
2.2.2 Condensing enzyme regulation of product structure
E. coli utilizes three condensing enzymes for fatty acid synthesis. Biochemical analysis of each of the three enzymes identified markedly differences in substrate specificities. β-Ketoacyl-ACP synthase III (KASIII) is the product of the fabH gene and is a main player during initiation of fatty acid synthesis as discussed in section 2.1.2. β-Ketoacyl-ACP synthase I (KASI), is encoded by the fabB gene and most efficiently catalyzes the condensation of short to medium chain acyl-ACPs with malonyl-ACP [75]. β-Ketoacyl-ACP synthase II (KASII) activity is the product of the fabF gene and functions in vivo to elongate palmitoleic acid (16:1Δ9) to cis-vaccinic acid (18:1Δ11), a reaction that FabB performs less efficiently [76]. All three enzymes catalyze Claisen condensation reactions between an acyl doner and malonyl-ACP to generate a β-ketoacyl-ACP elongated by two carbons. Each KAS uses a ping-pong mechanism with an acyl-enzyme intermediate, but the catalytic residues vary between FabB/FabF and FabH. FabF and FabB utilize a His-His-Cys catalytic triad whereas most FabH enzymes utilize His-Asn-Cys residues. Deletion mutants of fabB in E. coli are unsaturated fatty acid auxotrophs and rely on a supply of exogenous oleic acid for growth [77]. Disruption of the fabF gene in E. coli has no discernible effect on cell growth but almost eliminates the pool of cis-vaccinic acid containing lipids [78]. Interestingly, the fabF mutant in P. aeruginosa deficient in all three forms of motility suggesting the cis-vaccinate is important to the assembly of the motility machinery [79]. The effect of fabF on motility in E. coli has not been examined. This difference in essentiality between FabB and FabF underlines the distinct but integral roles of each condensing enzyme in unsaturated fatty acid synthesis. FabB is absolutely required to feed the cis-decenoyl-ACP product produced by FabA into the elongation system, but has poor activity with any acyl-ACP with 16 carbons or more, including palmitoleoyl-ACP. Thus, FabF is the enzyme in E. coli capable of efficiently converting palmitoleic acid to cis-vaccinic acid [80]. The FabF reaction participates in the adaptation of E. coli to changes in temperature to maintain the biophysical properties of the bilayer. As the temperature decreases, the bacteria respond by increasing the percent of unsaturated fatty acid in their membranes [75]. FabF is a naturally temperature-sensitive enzyme retaining high activity at lower temperatures to increase the rate of cis-vaccinate production in relation to saturated fatty acids [75]. Interestingly, the lack of cis-vaccinate synthesis in fabF null E. coli can be recovered through the overexpression of fabB, offsetting the poor FabB activity associated with palmitoleoyl-ACP [81]. However, this plasmid-driven cis-vaccinate biosynthesis does not substitute for the temperature-dependent increase in cis-vaccinate in FabF-positive strains [81]. The reverse experiment of overexpression of fabF in a fabB deletion host has failed to provide useful information because the overexpression of fabF is cytotoxic to E. coli [82]. Analysis of intracellular CoA and ACP thioester pools during fabF overexpression suggests a block at the malonyl-ACP transacylase (FabD) reaction may be responsible because partial growth recovery is seen when fabD and fabF are co-expressed.
The length of the fatty acids produced by any bacteria is a result of competition between the condensing enzyme and phospholipid acyltransferases for the acyl-ACP intermediates in the elongation module [83]. The upper chain-length is limited to 20–22 carbons by the substrate specificity of the condensing enzymes, and the lower limit is 12–14 carbons due to the substrate specificity of the acyltransferases. This competition concept is supported by studies blocking the acyltransferases or overexpressing fabB in E. coli and observing a substantial increase in the average fatty acyl chain length [81,84]. Overexpression of the plsB acyltransferase leads to a decrease in the average chain length. E. coli has been considered the paradigm for dissociated fatty acid synthesis for decades, although only a minority of bacteria uses an elongation module with both FabB and FabF. Most bacteria accomplish acyl-chain elongation using exclusively FabF. The molecular basis for the exclusion of longer chain lengths from the elongation condensing enzymes appears to be the size of the hydrophobic pocket adjacent to the active site that accommodates the acyl-enzyme intermediate [85], but this reasonable hypothesis has received little direct experimental support. This idea highlights the need for the coordinate regulation of expression levels of the key enzymes in the elongation and acyltransferase modules.
2.2.3 Multiple solutions for the introduction of the double bond
The production of unsaturated fatty acids is an important aspect of membrane homeostasis in bacteria [5]. The physical state of a cell membrane is manipulated by the incorporation of a mixture of fatty acids with different melting temperatures into phospholipids. A decrease in temperature increases membrane rigidity and many bacteria respond by increasing the proportion of unsaturated fatty acids incorporated into the phospholipids. When membrane fluidity increases due to rising temperature in the environment, the proportion of unsaturated fatty acids incorporated into the membrane decreases. There is considerable diversity in the mechanisms used by bacteria to generate unsaturated fatty acids. E. coli uses the bifunctional FabA enzyme to perform the dehydration of β-hydroxyacyl-ACP to trans-2-enoyl-ACP (Fig. 4), and specifically at the 10-carbon stage in the elongation module, the isomerization trans-2-decenoyl-ACP to cis-2-decenoyl-ACP [44]. The specificity of the isomerization reaction for the 10 carbon β-hydroxy-ACP is rationalized from the FabA crystal structure, which shows the active site tunnel that would perfectly fit a 10-carbon acyl-ACP [86]. But FabA cannot do the job alone. The product of the FabA isomerization reaction must be utilized by FabB to skip the reductase step and initiate the elongation of a monounsaturated fatty acid. Inactivating mutations in either the fabA or fabB genes result in unsaturated fatty acid auxotrophs. The fabA-fabB route to unstaturated fatty acids is common in the γ-proteobacteria, but most bacterial genomes do not contain these genes.
S. pneumoniae utilizes the FabM enzyme to introduce the double bond. This monofunctional trans-2, cis-3-decenoyl-ACP isomerase bears no similarity to FabA despite catalyzing the same reaction and having a strong substrate preference towards trans-2-decenoyl-ACP [74]. Interestingly, the fabM gene alone is not capable of complementing an E. coli fabA temperature-sensitive mutant. This was attributed to FabM being unable to successfully compete with FabI for the trans-2-enoyl-ACP substrate. This was remedied by expressing fabM and fabK on a plasmid based system and mitigating FabI activity using the potent FabI inhibitor triclosan [74]. This condition allowed the fabA mutant to be complemented by FabM at the non-permissive temperature. As with FabA in E. coli, FabM is essential for growth in S. pneumoniae, unless an exogenous supply of cis-vaccinic acid is available [87]. In contrast, a fabM deletion strain constructed in Streptococcus mutans was able to grow without unsaturated fatty acids, albeit with a doubling time of 159.9 minutes compared to 78.7 minutes in the wild type [88]. Both S. pneumoniae and S. mutans fabM deletions are unsaturated fatty acid auxotrophs, but the ability of S. mutans to grow without an external supply of unsaturated fatty acids identifies a difference in the requirement for unsaturated fatty acids between the two bacteria. The prototypical FabZ has a broad substrate specificity, catalyzing the dehydration of short, medium and long-chain saturated and unsaturated fatty acids [44]. The FabZ of E. coli cannot carry out the isomerization, and FabZ is the only isoform present in bacteria like S. aureus that produce only saturated fatty acids. However, this is not always the case. A FabZ-like protein in Enterococcus faecalis, called FabN, performs the same reaction as FabA and in this bacterium, FabF elongates the nascent unsaturated chain [77]. The reason why some dehydratases in the FabZ family can catalyze the isomerization reaction and others cannot is perplexing. They share the same catalytic residues. Domain swapping experiments suggest that perhaps the shape of the active site tunnel may be responsible for the exclusion of the kinked acyl chains with cis double in some enzymes [89], but this hypothesis requires further experimental validation. Recently, a gene named ufaA was demonstrated to be essential for unsaturated fatty acid synthesis in Neiserria gonorrhoeae [90]. This gene encodes a protein that is related to the FabK class of enoyl-ACP reductases, but its biochemical role in unsaturated fatty acid synthesis remains a mystery. Finally, there are many bacteria that produce unsaturated fatty acids, like Clostridium acetobutylicium, where the mechanism for unsaturated fatty acid synthesis remains unknown [91].
An alternate route for the generation of unsaturated fatty acids after the elongation cycle exists in some bacteria. The genome Bacillus subtilis does not contain a fabA gene but expresses a gene encoding a fatty acid desaturase, des, which functions to insert a double bond into phospholipid anchored acyl chains [92,93]. Unlike the anerobic isomerization of trans-2-decenoyl-ACP, bacterial fatty acid desaturases are iron-containing, oxygen-dependent enzymes. The B. subtilis des gene is induced during growth at low temperatures and functions to increase membrane fluidity by the generation of unsaturated phospholipids in a pre-existing saturated membrane [93]. Deletion of des results in a strain that has no obvious phenotype when cultured at 37°C, but is unable to grow and begins to lyse at 15°C, reinforcing the role of des in low-temperature adaptation in B. subtilis. Fatty acid compositional analysis of wild-type B. subtilis grown at 15°C degrees initially identified four different unsaturated fatty acid species, including 16:1Δ5, 16:1Δ9, 2-hydroxy-iso-17:1Δ7, and anteiso-17:1Δ7. All four species were absent upon deletion of the des gene [93]. Expression of the des gene in E. coli resulted in the production of only the 16:1Δ5 species [94]. Further mass spectroscopic investigation by Altabe et al. deduced that the double bond of unsaturated fatty acids in in B. subtilis is exclusively at the Δ5 position, designating the Des protein as a specific Δ5 phospholipid desaturase [92]. The other unsaturated species without a double bond at the Δ5 position identified by Weber et al. were attributed to errors in the algorithm used to determine the identity of products eluted from a gas-chromatography column.
Like E. coli, Pseudomonas aeruginosa produces saturated and unsaturated fatty acids by the anaerobic FabA/B pathway, but can also generate unsaturated fatty acids by two oxygen-dependent pathways [95]. The P. aeruginosa desA gene introduces the double bond into acyl chains attached to phospholipids and has a similar structure to the B. subtilis des gene described above. The second system, desBC, is a 2-gene operon responsible for the introduction of a double bond into saturated acyl-CoA in a reaction analogous to the mammalian stearoyl-CoA desaturase. Both desaturase enzymes are oxygen dependent and catalyze double bond insertion specifically at the Δ9 position of the fatty acid. Single deletion mutants of desA, desB/C or fabA are viable, although ΔfabA cells exhibit a growth defect [95]. A double knockout of fabA and desA required supplementation with either saturated (16:0/18:0) or unsaturated (18:1Δ9) fatty acids for growth whereas a triple knockout of fabA, desA, and desB could only grow in the presence of 18:1Δ9 fatty acid. The additional desaturases in P. aerugenosa give the bacteria a system to modify existing phospholipids (DesA) and to produce unsaturated fatty acids from exogenous saturated fatty acids (DesBC). Unlike the B. subtilis, Δ5 phospholipid desaturase, the expression of desA does not seem to be affected by a change in temperature but instead is expressed in response to anoxic growth conditions [95]. The transcription factors controlling desA expression and its biological function remain elusive, but it is known that expression of desBC is regulated by the transcriptional repressor DesT that will be discussed in section 4.1.2.
A less well-characterized pathway for acquiring unsaturated fatty acids is through uptake of exogenous fatty acids. S. aureus lacks a FabA enzyme and any known desaturases but is able to activate exogenous saturated and unsaturated fatty acids through ligation to ACP and subsequent utilization by the elongation and/or acyltransfer module [65]. Considering the energy intensive process of de novo fatty acid synthesis, this pathway may be an energy saving mechanism. The identity and substrate specificity of the acyl-ACP synthetase is currently unknown, therefore the biological significance of this pathway remains vague. The skin pathogen Propionibacterium acnes incorporates polyunsaturared fatty acids into phospholipids through an unusual mechanism. The bacterium imports exogenous free fatty acids and utilizes a unique polyunsaturated fatty isomerase to convert Δ9,Δ11-linoleic acid to Δ10,Δ12-linoleic acid [96]. This isomerase is highly specific for free fatty acids as opposed to thioesters. The biological reason for this reaction remains a mystery. Fatty acid analysis of P. acnes indicate the bacteria only produce branched and saturated fatty acids but as the regulation of de novo synthesis in the bacterium is unknown, this composition could simply reflect the fatty acid composition of the Brain-Heart Infusion media [97,98].
2.3 Acyltransferases and phosphatidic acid synthesis
Glycerol-3-phosphate acyltransferases are responsible for intercepting the products of the elongation cycle and transfering the acyl-chain from acyl-ACP to either the sn-1 or the sn-2 carbons of glycerol-3-phosphate (Fig. 3). These reactions produce 1,2-diacyl-sn-glycerol-3-phosphate (phosphatidic acid), which is the universal phospholipid precursor in bacteria (for a recent review see [99]). These enzymes are at the interface between FASII and membrane expansion and are thus positioned to be key regulators of both fatty acid and phospholipid synthesis. Not only does the substrate specificity of these enzymes determine if an acyl-ACP undergoes additional rounds of elongation, it also defines which position and which species of fatty acid is attached to glycerol-3-phoshphate. The initial reaction of phospholipid synthesis is catalyzed by glycerol-3-phosphate dehydrogenase. This reversible reaction produces glycerol-3-phosphate from dihydroxyacetone phosphate linking glycolysis and phospholipid synthesis. Deletion mutants of gpsA are glycerol auxotrophs and rely on a supply of exogenous glycerol-3-phosphate or GlpK, a glycerol kinase, to generate glycerol-3-phoshphate from exogenous glycerol. A block at the GpsA reaction triggers an accumulation of abnormally long-chain acyl-ACPs that are not seen in cells with a functional acyltransferase system [100–102]. This illustrates the interplay between the elongation and acyltransfer modules that determines membrane fatty acid composition.
2.3.1 The PlsB/PlsC system
The first phospholipid acyltransferases to be discovered were the PlsB/PlsC acyltransferases of E. coli [103]. PlsB ligates a fatty acid into the 1-position of glycerol-3-phosphate and PlsC the 2-position of 1-acyl-glycerol-3-phosphate (lysophosphatidic acid). These membrane bound acyltransferases utilize acyl-ACP from the elongation cycle or acyl-CoA thioesters derived from exogenous fatty acids [104]. E. coli ligates exogenous fatty acids to CoA using an acyl-CoA synthetase (FadD) [105]. Although these CoA thioesters cannot be elongated by FASII, they can be placed directly into phospholipids by PlsB or PlsC or degraded by β-oxidation to generate a carbon source for growth [106]. The attachment of FASII-derived acyl chains to ACP and exogenous fatty acids to CoA serves as a biochemical tag to prevent any endogenously synthesized fatty acids from entering the degradative β-oxidation pathway. There is distinct asymmetry in the incorporation of acyl-chains into the 1- and the 2- position of glycerol-3-phosphate. In E. coli, the 1- position is occupied by either a 16:0 or an 18:1 fatty acid whereas the 2- position predominantly contains the unsaturated fatty acids 16:1 or 18:1 [107]. This phospholipid structure arises from the substrate specificities of the two acyltransferases [108]. However, this selectivity for different fatty acids is not absolute. In an E. coli fabF fabA double mutant strain that is unable to effectively synthesize unsaturated fatty acids, 16:0 can be found in both the 1 and the 2 position, suggesting a substrate preference for PlsC towards unsaturated acyl groups rather than a complete inability to utilize saturated acyl-ACPs [109]. PlsB and PlsC homologs exist in mammals, and it was long thought that the E. coli discoveries would also extend to all bacteria. However, with the advent of whole-genome sequencing it became readily apparent that the PlsB/PlsC system is largely limited to the γ-proteobacteria [104].
2.3.2 The PlsX/PlsY/PlsC system
In 1974, Robert Bell’s group identified an E. coli mutant that was a G3P auxotroph [110]. They determined this phenotype was due to mutations in the plsB gene that resulted in a defective acyltransferase with an increased Km for G3P, a defect that is overcome by supplying exogenous G3P in the media. The missense mutation giving rise to the plsB26 allele was later identified [111]. Years later, the Bell group discovered that the G3P auxotroph strain they had developed also had a mutation in the enigmatic plsX gene, a widely-distributed gene of unknown function [112]. The second mutation was discovered after failed attempts to reproduce the G3P auxotrophy phenotype by transducing the defective plsB gene into a wild type strain. Transduction of the defective plsX gene into a wild type strain had no phenotype.
The role of PlsX in phospholipid synthesis was uncovered in 2006 [104]. PlsX converts acyl-ACP to acyl-PO4, which is subsequently utilized by the PlsY glycerol-phosphate acyltranferase. Most bacteria, including S. aureus and S. pneumoniae, use the PlsX/Y pathway for the acylation of glycerol-3-phosphate. The PlsY acyltransferase is unable to utilize acyl-ACP or acyl-CoA [104]. The essentiality of PlsX/Y and their role in coupling FASII with phospholipid synthesis was investigated in B. subtilis. Paoletti et al. [113] used a plsX, plsY and plsC deletion mutants complemented with inducible plasmid-based expression to investigate the consequences of blocking each of these reactions. Depletion of plsY apparently had no effect on the rate of fatty acid synthesis, although phospholipid synthesis was blocked and free fatty acids accumulated. The fatty acids arise from the conversion of acyl-ACP to acyl-PO4 followed by the hydrolysis of the accumulating acyl-PO4. Depletion of plsC resulted in over 200% increase in fatty acid synthesis and an accumulation of large quantities of free fatty acid and monoglyceride [113]. The free fatty acid accumulation was attributed to degradation of the monoglyceride formed by PlsY by an esterase, although the identity of this enzyme is unknown. Upon plsX depletion, the rate of fatty acid synthesis decreased precipitously, suggesting PlsX is a key regulatory point that synchronizes FASII and phospholipid synthesis. A block at the PlsX reaction would result in accumulation of acyl-ACP from the FASII elongation module. Acyl-ACP is a key feedback regulator of FASII in E. coli, and it is possible a similar regulation mechanism could be present in Bacillus. However, at this stage nothing is known about the biochemical regulation of the pathway in B. subtilis, and the biochemical analysis of FabH and ACC in B. subtilis is needed to validate this connection. The only instance of biochemical regulation of the acyltransferase enzymes that has been identified is S. pneumoniae PlsY, which is reportedly non-competitively inhibited by acyl-CoA [114]. This connection is puzzling and perhaps irrelevant because there is no known mechanism for the production of acyl-CoA in S. pneumoniae.
The reason for the retention of PlsX/Y in E. coli, which has the PlsB acyltransferase is an enigma. Yoshimura and coworkers generated targeted gene deletions of plsB, plsX and plsY (annotated as ygiH) [115]. Their studies revealed that plsB is an essential gene, whereas single deletion mutants of plsX or plsY have no detrimental effect on growth. However, construction of a plsY and plsX double deletion mutant was not successful, indicating an essential role for the PlsY and PlsX enzymes in E. coli. The expression of B. subtilis plsX can rescue the glycerol auxotroph plsB26 phenotype in an E. coli strain expressing an inactive PlsX, and a deleted plsY gene [116]. This interesting observation strongly suggests an important role for acyl-PO4 outside of lysophosphatidic acid synthesis, however, why plsX is retained in organisms with a PlsB/C pathway remains a puzzle that will require more research to unravel.
2.3.3 The diacylglycerol (DAG) kinases
Diacylglycerol kinases (Dgk) catalyze the phosphorylation of DAG to generate phosphatidic acid (Fig. 5). This reaction is critical for recycling DAG formed as a byproduct from the utilization of phospholipids in the biosynthesis of other macromolecular membrane components. Phosphatidic acid produced by Dgk can then feed back into the phospholipid biosynthetic pathway. The substrate for the major phospholipid breakdown pathways is phosphatidylglycerol (PtdGro), which donates its sn-glycerol-1-phosphate (G1P) headgroup to the either membrane-derived oligosaccharides (MDO) in Gram-negative species or lipoteichoic acid in Gram-positive bacteria. These two groups of bacteria have unique Dgks to recycle the DAG.
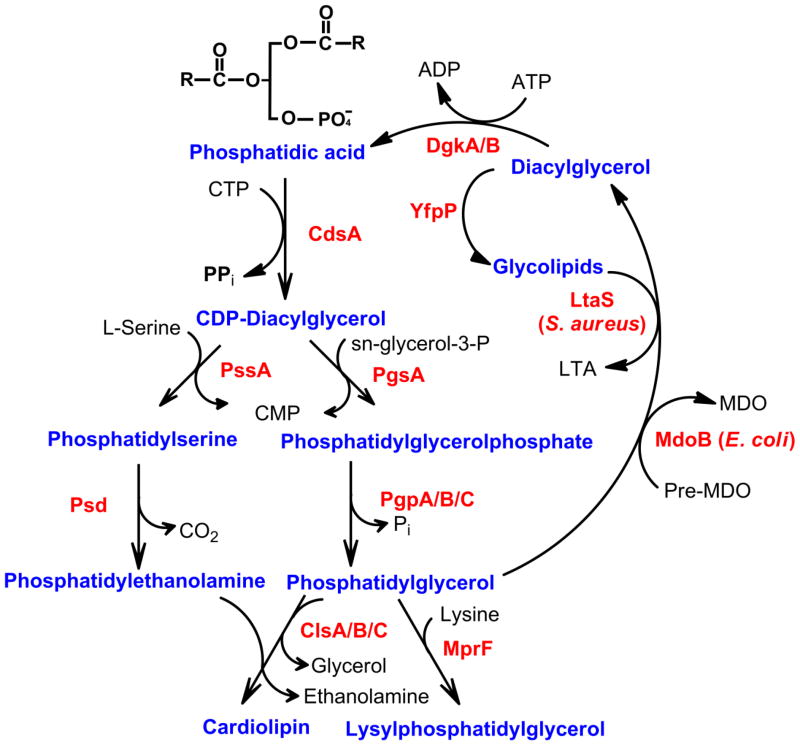
Figure 5. Phospholipid headgroup synthesis.
Phosphatidic acid is the precursor for glycerophospholipids in bacteria. Diacylglycerol is formed as a byproduct of membrane-derived oligosaccharides (MDO) synthesis (MdoB) in E. coli and lipoteichoic acid (LTA) synthesis (LtaS) in Gram-positive organisms. This intermediate is recycled to phosphatidic acid by a integral membrane kinase DgkA in Gram-negative bacteria or by a soluble, interfacial kinase, DgkB in Gram-positive bacteria. Glycolipids are produced from UDP-sugars and diacylglycerol (YfpP). Phosphatidic acid is converted to the key intermediate by CdsA, and the enzymes and pathways to the major bacterial phospholipids are illustrated. MprF is restricted to Gram-positive bacteria.
The first DAG kinase to be discovered was E. coli DgkA [117–120]. E. coli produces MDO to control the osmolarity in the periplasmic space. MDO are β-glucans containing 8–10 glucose units that are decorated with multiple G1P moieties to convert them into polyanionic polymers [121,122]. The glucose subunits of MDO are also substituted to a lesser extent with succinic acid and phosphoethanolamine residues [122]. Although MDO not essential for cell survival in normal media, a role in osmotic homeostasis in E. coli has been established [121,122]. During osmotic stress, the periplasmic levels of MDO fall to balance the osmotic pressure and maintain a constant periplasmic volume. Strains bearing mutations in the G1P transferase gene, mdoB, are unable to perform the phosphoglycerol transfer reaction from PtdGro to DAG [123]. Mutants of mdoB and mdoC, the enzyme responsible for the insertion of the succinate moiety into MDO, are hypersensitive to detergent and osmotically challenging environments [124].
As expected, inactivation of the dgkA gene in E. coli results in an accumulation of DAG [120]. The dgkA gene is not required for growth under standard laboratory conditions where MDO synthesis is not required, but is essential during growth in osmotically challenging environments [123]. In high salt conditions, the addition of arbutin to the growth media acts as an artificial acceptor of G1P, therefore accelerating the production of DAG [123]. An accumulation of such a neutral lipid in the membrane is catastrophic for the bacteria due to disruption of the bilayer [125]. Membrane proteins were unable to assemble into liposomes containing high concentrations of diaclyglycerol [126]. It was proposed that the bulky structure of DAG essentially seals the membrane in a way that the hydrophilic heads of phospholipids cannot. Thus, it is the role of DgkA to phosphorylate the accumulating DAG, allowing for new synthesis of polar lipids.
DgkA is an integral membrane protein that exists as a trimer with the active site facing the cytoplasmic side of the membrane [127]. Solving the structure of this integral membrane protein was a triumph of NMR spectroscopy and readers interested in the detailed structure and function of DgkA and its relatives are referred to the recent review by Van Horn and Sanders [128]. Because DAG is generated on the outside of the cell, it must first translocate to the inner leaflet of the cytoplasmic membrane to be acted on by DgkA. There is no known transport system for DAG and it is thought to spontaneously flip between the two sides of the bilayer. Little is known about the genetic regulation of dgkA, although it is clear that its transcription is controlled by multiple stress response regulators in E. coli [129]. Homologs of DgkA are widely distributed in bacteria, but not all of these enzymes are actually DAG kinases. It is not possible to determine from bioinformatics analysis the lipid substrates used by the DgkA homologs [128,130].
Diacylgycerol is also generated outside the cell in Gram-positive bacteria, although the G1P transferase reaction serves a different purpose. The G1P headgroup of PtdGro is used to synthesize lipoteichoic acid (LTA), a major component of the Gram-positive cell envelope. LTA is synthesized by the LtaS enzyme, which polymerizes G1P residues derived from PtdGro onto a glycolipid membrane anchor [131]. Depletion of LTA by inactivation of the ltaS gene in S. aureus results in a strain that is unable to grow at 37°C and exhibits aberrant growth at reduced temperatures [132]. Aside from the role of LTA as a major structural cell wall component, it is also thought to stabilize the cell membrane, localize the activity of cell-wall synthesis enzymes and is a major player in pathogen-host interactions during infection [133–136]. Reminiscent of the synthesis of MDO in E. coli, each subunit of G1P that is transferred to the LTA polymer results in a molecule of DAG formed as a byproduct. Therefore, the synthesis of a single LTA strand produces over 20–40 DAG molecules in the process [137]. Consequently, Gram-positive bacteria such as B. subtilis and S. aureus have developed a similar mechanism to E. coli for recycling DAG. B. subtilis was reported to have a dgkA gene but a genetic and biochemical evaluation of the enzyme identified the protein as an undecaprenol kinase [130]. The bone fide DAG kinase of B. subtilis, a soluble protein with no sequence similarity to DgkA, was discovered and named DgkB [130]. This soluble DAG kinase is highly specific for the phosphorylation of DAG over other phospholipids, and its primary sequence places it in the same protein family as the mammalian signaling DAG kinases. Unlike the integral membrane DgkA, DgkB is an interfacial enzyme that uses positively charged lysine residues on its surface to dock on the anionic surface of the cell membrane [138]. This property is demonstrated in vitro through enzymatic assays showing a robust increase in DgkB activity when DAG is incorporated into negatively-charged PtdGro vesicles, as opposed to neutrally charged bilayers [138]. Complementation studies of a dgkB mutant with an inducible plasmid expressing dgkB revealed the gene to be essential in B. subtilis unless LTA synthesis is disrupted by mutations in yflE, a gene homologous to ltaS of S. aureus. Cessation of LTA synthesis would prevent DAG formation through LTA turnover, preventing the need for a DAG recycling system [137]. The structure of DgkB from S. aureus was solved [139]. This structure illustrates how the enzyme interacts with the phospholipid bilayer, and revealed a unique structural Mg2+ site that explained the metal-dependent activity of this enzyme family.
2.4 Phospholipid headgroup diversity
An assortment of different phospholipid headgroups are synthesized by bacteria to create the optimum surface charge for the cell membrane. The charge of zwitterionic phospholipid headgroups must be balanced with those containing acidic headgroups such as phosphatidylglycerol. This balance in electrostatic charge is required for many integral membrane proteins to adopt the correct topology in the cell membrane [140,141]. This property highlights the need for stringent regulation of the synthesis of different headgroup moieties.
The key intermediate in bacterial phospholipid synthesis is CDP-DAG [142] synthesized by the cds gene product [143]. Both ribo- and deoxyribo-liponucleotides are produced by Cds [144], but the significance, if any, for the formation of these two products is unknown. Cds is an essential gene, but mutants with decreased activity have been isolated and accumulate phosphatidic acid which leads to membrane dysfunction [145–147]. A regulatory role has not been ascribed to Cds and its activity level appears to be in large excess over what is required to support phospholipid synthesis.
2.4.1 CDP-DAG-dependent reactions
In E. coli, the fate of CDP-DAG represents a branch point between synthesis of acidic phospholipids (PtdGro) and zwitterionic phospholipids (phosphatidylethanolamine). In the PtdGro pathway, PtdGro phosphate is synthesized from CDP-DAG by the displacement of cytidine monophosphate (CMP) by glycerol-phosphate catalyzed by the PgsA enzyme [148]. The PtdGro phosphate is dephosphorylated by a PtdGro phosphate phosphatase (Pgp) to form the end product, PtdGro. There are three pgp genes in the E. coli genome (pgpA, pgpB and pgpC), the last just recently discovered [149]. Perhaps not all of these are actually designed for PtdGro phosphate hydrolysis, and they may perform other functions also. The essentiality of the genes involved in PtdGro synthesis has been debated over the decades [150,151]. In S. aureus and B. subtilis, whose membranes are predominantly composed of PtdGro (50–60%), pgsA is an essential gene [152–155]. In E. coli, PtdGro is a minor component (20%) compared to phosphatidylethanolamine (PtdEtn) (70%) [150]. Weiming et al. were the first to examine the requirement of PtdGro for growth in E. coli and found that a pgsA and lpp lipoprotein deletion mutant was only able to grow when the mutation was complemented by pgsA expressed on a temperature sensitive plasmid [150]. However, one aspect of their experimental design was that they did not use an inducible promoter, but rather reached the conclusion by assaying growth arrest that occurred when the pgsA plasmid by cured by growth at 42°C. Membrane phospholipid composition is important for temperature and osmotic regulation, consequently aberration in normal membrane composition may prevent growth under strenuous conditions. This caveat was studied by Kikuchi et al. who observed that a pgsA lpp null mutant is able to grow at 37°C, but not 42°C. Growth in low osmolarity media was also compromised presumably due to the lack of MDOs [151]. The requirement for the deletion of the outer membrane lipoprotein gene in combination with the pgsA gene was investigated by Suzuki et al. [156]. The lipoprotein requires transfer of the DAG moiety from PtdGro for maturation and removal from the inner membrane. A lack of PtdGro results in accumulation of unmodified Lpp on the inner membrane which crosslinks with peptidoglycan via the carboxyl terminus of the lipoprotein. This crosslinking causes the inner membrane to be erroneously anchored to the cell wall, resulting in lysis. These studies conclude that anionic phospholipids alone are not absolutely essential for metabolic function in E. coli grown under laboratory conditions that do not stress the bacterium.
The PtdGro also serves as a precursor in the synthesis of cardiolipin, a variable component of Gram-positive and Gram-negative cell envelopes [148]. Cardiolipin derived from two PtdGro molecules is synthesized in E. coli by the ClsA protein [157]. The clsA gene is dispensable in E. coli, although trace amounts of cardiolipin are still detected in the membranes of clsA deletion mutants suggesting an alternate synthetic route is present [158]. Two additional cardiolipin synthases are found in E. coli. The clsB gene encodes a cardiolipin synthase that uses PtdGro, but whether the other substrate is PtdGro or another phospholipid is unknown [159]. Recently, the clsC gene was discovered that encodes an enzyme that uses PtdGro plus PtdEtn instead of two PdtGro molecules [160]. S. aureus possesses two genes for the synthesis of cardiolipin termed cls1 and cls2 [161]. Transcription of cls1 was induced under stress conditions such as osmotic stress whereas cls2 is the constitutive cardiolipin synthase, catalyzing its formation under standard laboratory growth conditions. Neither cls genes were found to be essential when deleted individually or in combination although the double knockout was more susceptible to osmotic stress (25% NaCl). Thus, cardiolipin appears non-essential in most organisms under laboratory growth conditions, but the multiplicity of genes and their regulation of membrane cardiolipin content suggest that this phospholipid plays an important role(s) in the survival of bacteria in the environment.
The alternative use of CDP-DAG in E. coli is to synthesize the zwitterionic phospholipid PtdEtn. PtdEtn is the major component of the E. coli cell membrane, but surprisingly mutants completely defective in PtdEtn remain viable if the growth medium is supplemented with Ca2+, Mg2+ or Sr2+, despite replacing all PtdEtn with PtdGro and cardiolipin [162]. No other cations or osmolites substitute for these cations. The phosphatidylserine (PtdSer) synthase (PssA) enzyme catalyzes the first committed step in PtdEtn synthesis, which condenses serine to the phosphatidyl moiety of CDP-DAG coupled with the release of CMP. Unlike the other enzymes involved in phospholipid headgroup synthesis, E. coli PssA is tightly associated with ribosomes instead of the cell membrane in cell fractionation studies [163,164]. However, this is likely an artifact of the propensity of PssA to bind anionic surfaces, and purified PssA exhibits substrate-dependent association with the membrane bilayer [165]. PtdSer is a transient intermediate in E. coli and constitutes less than 0.1% of the detectible lipid pool [166]. The final reaction in PtdEtn biosynthesis is performed by the PtdSer decarboxylase (Psd), which decarboxylates the serine headgroup resulting in the release of CO2. Psd uses a covalently attached pyruvate group as a cofactor that is formed by the self-cleavage of the primary transcript into two nonidentical subunits [167]. Kennedy et al. isolated a Psd temperature sensitive mutant strain of E. coli and observed an accumulation of PtdSer coupled with growth arrest [168]. However, membranes with a significant proportion of PtdSer were viable by growth of the temperature-sensitive strain at a semi-permissive temperature. In some lactic acid fermenting bacteria and nitrogen-fixing plant symbiotes, PtdSer is a major membrane lipid [169]. The specific cellular functions of PtdSer are unknown but there is a clear correlation between the presence of PtdSer in lipid membranes and the ability of the bacteria to ferment and fix nitrogen.
Two other phospholipids are less widespread in bacteria, but are nonetheless important for the physiology of the specific organisms that produce them. Phosphatidylcholine (PtdCho) is a phospholipid usually associated with eukaryotic cells, although it is estimated that 10% of bacteria produce cell membranes containing PtdCho [170]. PtdCho is present in some pathogenic bacteria, including P. aeruginosa, albeit only a few percent of the total lipid mass. P. aeruginosa uses a PtdCho synthase enzyme to condense choline with CDP-DAG. An alternate PtdCho synthesis pathway occurs in some soil-dwelling bacteria. This pathway uses choline to displace CMP similar to PssA in a reaction that is distinct from eukaryotic PtdCho biosynthesis. The diversity and regulatin of these enzymes has been covered in a recent review [171]. Agrobacterium tumefaciens synthesizes PtdCho through successive methylations of PtdEtn via methyltransferase enzymes using S-adenosyl-methionine as a methyl doner [172]. Some species of bacteria have phosphatidylinositol (PtdIns) containing membranes [173–177]. PtdIns is another lipid normally associated with mammalian cell membranes but present in various Mycobacteria and bacteria found in some more obscure environments [173–177]. It is thought the lipid rich cell wall of Mycobacteria and some Actinomycetes allows them to survive in severe envinmental conditions [175]. Actinobacteria (including Mycobacteria) are able to synthesize PtdIns from inositol and CDP-DAG using phosphatidylinositol synthase (PIS) and PtdIns can comprise up to 25% of the total membrane lipids [174]. The PIS is encoded by the pgsA gene in M. tuberculosis and is essential. It was not determined if the loss in viability in the pgsA deprived strain was due the lack of PtdIns or the lack of a metabolically deprived product of PtdIns [174].
2.4.2 DAG-dependent reactions
DAG is predominantly a byproduct of either MDO synthesis in E. coli or LTA biosynthesis in Gram-positive organisms. However, there are some lipids that require DAG as a precursor as opposed to CDP-DAG. In Gram-positive bacteria and some cyanobacteria, DAG is used to synthesize glycolipids [178]. In cyanobacteria, the glycolipids form part of the energy-generating photosystem I [179]. In Gram-positive B. subtilis and S. aureus, the glycolipids function as a scaffold for the LTA polymer to be synthesized and to physically anchor the LTA to the cell membrane. LTA is constructed from three components: (i) the glycolipid anchor that attaches the LTA to the membrane, (ii) the poly(glycerophosphate) residues derived from successive G1P transfer reactions from PtdGro and (iii) the D-alanyl moieties linked to the poly(glycerophosphate) [178]. The species of glycolipid found in B. subtilis and S. aureus is diglucosyldiacylglycerol (Glu2DAG). The enzyme that catalyzes the glucotransferase reaction (YpfP) was initially discovered in B. subtilis [180]. YpfP utilizes uridine diphosphate-glucose (UDP-glucose) as a carbohydrate donor. B. subtilis ypfP (BsYpfP) was cloned and overexpressed in E. coli, an organism that does not produce glycolipids [180]. They found that YpfP enzyme is capable of transferring up to four glucose residues to a DAG molecule, although in B. subtilis only the product of one or two glucotransferase reactions are observed. In addition to its function as the glycolipid anchor, Glu2DAG also exists as a membrane lipid, constitution approximately 8% of the molar proportion of glycolipid in S. aureus [181]. Other bacterial glycolipids appear to be synthesized by closely-related glycosyltransferases with different specificities for UDP-sugars [182].
The role of glycolipids in membrane homeostasis is highlighted by research using the Acholeplasma laidlawii model system. Alterations in membrane fatty acid composition, cholesterol content and growth temperature induce significant changes in the ratio of monoglycosyl-DAG (MGDG) to diglycosyl-DAG (DGDG) [183–186]. The MGDG:DGDG ratio increases with increasing unsaturated fatty acids or temperature suggesting that the ratio is an adaptive respone to increasing membrane disorder. This body of work is interpreted to describe a compensatory mechanism that maintains the biophysical properties of the membrane close to the lamellar to hexagonal phase transition. MGDG forms inverted nonlamellar structures as opposed to the bilayer conformation of DGDG. It has been suggested that the bacterial cell membrane is always kept at the limit of stability by the introduction of non-bilayer lipids into membrane to allow a flexible response to extracellular stimuli that perturb the membrane biophysical properties [180,187]. How this is accomplished is mechanistically unclear, but perhaps the activities of the interfacial glycosyltransferases [188] may be regulated by the physical properties of the membrane containing their substrates, but this idea needs to be put to the test.
It is difficult to examine the effect of selectively removing glycolipids from most bacteria by inactivation of the glycosyltransferases because elimination of glycolipids also results in a dramatic reduction in the amount of LTA produced [189]. Deletion of the ltaS gene is lethal in S. aureus unless cells are grown with osmoprotectants or in combination with a cyclic diadenosine monosphosphate phosphatase (gdpP) deletion [131,132,178,190]. Interestingly, a 90% reduction in LTA biosynthesis through deletion of ypfP in S. aureus SA113 is viable with minimal impact on growth in standard laboratory media or osmotically stressful conditions (2.5 M NaCl) [189]. There appears to be a significant difference in the regulation of LTA biosynthesis between S. aureus strains RN4220 and SA113 as the rate of cell-associated LTA biosynthesis remains unchanged in an RN4220 ypfP knockout, despite the lack of a Glu2DAG anchor. There is a two-fold increase in the amount of LTA secreted from RN4220 yfpP mutant, perhaps representing the lack of a stable connection to the cell membrane [189]. SA113 ypfP deletion mutant attaches the G1P polymer of LTA directly to DAG instead of Glu2DAG. Perhaps this is the normal reaction that is followed by the transfer of the G1P polymer to the Glu2DAG. Synthesis of Glu2DAG also requires a α–phosphoglucomutase (PgcA) and UTP:α –glucose-1-phosphate uridylyltransferase (GtaB) to produce the UDP-glucose for YpfP [137]. The carbohydrate moiety of the glycolipid is exclusively glucose in S. aureus and B. subtilis but this is not true throughout the bacterial kingdom. A comprehensive review by Norman Shaw describes the different carbohydrate subunits present in bacterial glycolipids that includes to galactose, mannose, rhamnose and glucaronic acid [191]. Unfortunately in many cases the composition of the diglyceride remains a mystery although bacteria containing plant-like digalactosyldiacylglycerol glycolipids have been identified [192,193]. Some Rhodococcus species and Mycobacterium tuberuculosis synthesize triacylglycerol from DAG to use as an energy store in the form of lipid droplets [194–196]. These storage lipids are normally only found in eukaryotes but have been hypothesized to aid the TB bacterium during the unique dormant phase in latent tuberculosis infections [195]. Most bacteria use polyhydroxyalkanoates as storage lipids [197], and in some cases these precursors are derived from the β-hydroxy-intermediates of the FASII elongation module [69].
2.5 Non-phosphorus lipids
In addition to glycolipids, some bacteria are able to form phosphorus-free lipids in response to phosphate-limited environments. These bacteria ligate fatty acids onto an alternative backbone to glycerol-3-phosphate. The most common substitutes are amino acids [198,199]. Although lipids synthesized using lysine, glycine, glutamine and serineglycine have been reported, most research has focused on ornthine lipids [199]. Ornithine lipids in S. meliloti, R. capsulatus, B. abortus and P. aeruginosa are synthesized by ligation of a fatty acid from acyl-ACP onto the α-amino group and the 3-hydroxyl group of ornithine resulting in an amide linked and an ester linked fatty acid. The PlsB/C acyltransferases are not capable of performing the aminoacyltransferase reations, but rather related acyltransferases, OlsB and OlsA, catalyze the stepwise transfer the fatty acids from acyl-ACP to the amino and hydroxyl group, respectively. Ornithine lipid production is an adaptation to low phosphate environments (for reviews, see [198,199]). During growth in phosphorus-limiting conditions, some α-proteobacteria such as Rhodobacter sphaeroides and S. meliloti replace the majority of their lipids with ornithine lipids plus two additional classes of phosphorus-free lipids: betaine lipids and sulfolipids [170,200–202]. In S. meliloti, the switch to non-phoshphorus lipids is transcriptionally activated by the PhoB- and PhoU-dependent phosphate sensor. Deletion mutants of either PhoB or PhoU are unable to induce non-phosphorus lipid synthesis [201]. The most abundant lipid formed under phosphorus-limitation is the betaine lipid diacylglycerol-N,N,N-trimethylhomoserine (DGTS). Sulfoquinovosyl diacylglycerol (SL) is a less abundant sulfur lipid that is also formed under phosphorus-limitation. The enzymes involved in DGTS and SL syntheses both utilize DAG as a precursor. In an effort to liberate phosphorus contained within preexisting phospholipids, S. meliloti uses a phospholipase C (PlcP) to remove the phosphocholine from PtdCho and CgmB to remove phosphoglycerol from PtdGro [200]. This turnover of phospholipids releases phosphorus to other metabolic processes that are also suffering from the phosphorus limiting growth conditions. Despite the ability of several bacteria to exclude phosphorus from membrane lipids, no organisms have been isolated that are completely devoid of glycerophospholipids. This observation begs the question whether it is possible to form biological membranes in the absence of these phospholipids.
2.6 Bacterial Sphingolipids
Sphingolipids are a class of lipids containing an aliphatic amino alcohol connected to a sphingoid backbone and a fatty acid N-linked via an amide. Sphingolipids are structural and signaling components of mammalian and yeast cells but in rare cases have been observed in bacteria. Bacterial sphingolipids are structurally distinct from eukaryotic sphingolipids. Eukaryotic sphingolipids characteristically have an unsaturated amino alcohol and an unbranched acyl-chain of 18–20 carbons. In contrast, bacterial sphingolipids are typically saturated and often branched with no more than 19 carbons [203]. Eukaryotic sphingolipids usually possess a phosphocholine headgroup, whereas most bacteria utilize an array of different headgroups not including phosphocholine [203]. Sphingolipids have been identified in a handful of bacterial genera but specifically in the Sphingomonas and Sphingobacterium classes [204–207]. Sphingomonas paucomobilis lacks LPS in their outer membrane and alternatively utilizes two glycosphingolipids [205]. Similarly to LPS, glycolipids of Sphingomonas are potent activators of natural killer T-cells during infection [208,209]. The functions of the membrane sphingolipids in bacteria must be deduced from their structure and localization because there are no genetic studies that directly demonstrate their role in membrane homeostasis. One interesting study by An et al., examined the effect of inhibiting sphingolipid biosynthesis in the sphingolipid producing bacterium Bacteroides fragelis [203]. B. fragelis forms part of the normal flora in the human small intestine but is also associated with urinary tract infections [210]. The investigators utilized myriocin, a potent, specific inhibitor of eukaryotic sphingolipid synthesis to prevent sphingolipid formation in B. fragelis. Myriocin inhibits the serine palmitoyltransferase enzyme that catalyzes the first committed step in sphingolipid biosynthesis (condensation of palmitoyl-CoA with serine to form 3-ketosphingosine) [203]. When sphingolipid synthesis was inhibited through myriocin treatment, B. fragelis is less tolerant to oxidative and heat shock stress. The authors showed that exogenous cholesterol and sphingolipids are also required to manage DNA damage stress. It is unknown if this decrease in resilience is due to the requirement of sphingolipids as a structural component, or the role sphingolipids could play in signal transduction by formation of sphingolipid microdomains in the cell envelope – a characteristic of eukaryotic sphingolipids [203].
3. Biochemical regulation of phospholipid synthesis
Biochemical regulation exerts a powerful influence in the control of membrane lipid homeostasis. Biochemical regulation is normally in the form of negative feedback, by which the product or intermediate of a metabolic pathway feedback inhibits an enzyme upstream. Not only does this allow for an extremely fast and effective response, it can overcome increases in gene expression by adjusting the net activity regardless of the overall enzyme amount. Most of the energy that it takes to construct a phospholipid is used in the biosynthesis of fatty acids. In almost all bacteria, fatty acids have no alternate fate to membrane formation. Therefore, tight biochemical regulation of FASII is an important aspect of membrane lipid homeostasis and bacterial fitness.
3.1 Regulation in the initiation module
The initiation module is an ideal point of regulation in membrane lipid biosynthesis. The two most significant enzymes in the initiation module are ACC and FabH. The ACC performs the first committed step in FASII and every turnover of FabH results in the formation of a fatty acid. In E. coli, the ACC is feedback inhibited by acyl-ACP [11]. Davis et al. synthesized acyl-ACPs of different chain lengths (C6-C20) and measured ACC activity in the presence of the different ACP species. They observed robust inhibition of ACC activity (70–75%) in the presence of 40 μM acyl-ACP, regardless of the chain length or the saturation of the acyl-chain. Although 40 μM may appear to be a high concentration for physiologically relevant inhibition, ACP is the most abundant soluble protein in E. coli. The inhibitory effect was specific for bacterial ACP, as ACP from spinach had no effect. Similarly, unacylated ACP showed no inhibition. Assays of the biotin carboxylase and carboxyltransferase reactions individually did not show any inhibition in the presence of acyl-ACP. The lack of inhibition in the ATP dependent biotin carboxylase reaction suggests that acyl-ACP does not interfere with ATP binding. Since the authors used the reverse reaction (decarboxylation of malonyl-CoA) to assay the biotin carboxylase, no information regarding the mode of inhibition with respect to acetyl-CoA can be discerned. Assay of the complete reaction showed a mixed type inhibition with respect to acetyl-CoA: a combination of competitive and non-competitive inhibition. This feedback loop was investigated in vivo, by Heath et al. who monitored the quantities of malonyl-CoA in E. coli after a block at either the elongation cycle or the acyltransfer module. The block at the elongation cycle was achieved by treatment of the cells with cerulenin, a specific FabB/FabF inhibitor, whereas the block at the acyltransfer module was formed by depriving using an E. coli plsB mutant of the glycerol it requires for growth [211]. Treatment with cerulenin resulted in the accumulation of malonyl-CoA after 10 minutes of treatment. Similarly, treatment of E. coli with the FabB/F inhibitor thiolactomycin also resulted in the sustained production of malonyl-CoA [212]. Although these data illustrate that ACC is not regulated under these conditions, the nature of the ACP thioesters that accumulate following the block in elongation remain to be precisely determined. The molecular basis for the inhibition of the ACC by acyl-ACP is unclear, but it may be that similar residues that are involved in acetyl-CoA binding are involved due to both molecules sharing a common 4′-phosphopantetheine moiety. However, the acetyl-CoA binding pocket is not large enough for ACP and an ACP binding surface on ACC has not been identified [213]. This regulatory system has been also been identified in rapeseed (Brassica napus) [214] suggesting that it is widespread. Unlike in E. coli, S. pneumoniae can ligate exogenous fatty acids directly to ACP, allowing them to feed exogenous fatty acids into either the elongation or acyltransfer modules as ACP thioesters [65]. Upon treatment with exogenous oleate for 20 minutes, the intracellular malonyl-CoA pool fell to <5% of the untreated. The effect was similar to treatment of a S. aureus culture with andrimid, an ACC inhibitor. Although the role of acyl-ACP in this physiological response has not been confirmed, it seems like a reasonable intermediate to repress ACC activity. Inhibition of the acetyl-CoA carboxylase by acyl-ACP is a logical feedback regulatory system linking the end product of the pathway with the initial reaction, and is likely to be widespread in bacteria.
Overproduction of the ACC in E. coli does not result in a significant increase in the rate of fatty acid synthesis, unless a soluble acyl-ACP thioesterase (TesA) is co-expressed to uncouple acyl-ACP utilization from phospholipid sythesis [18]. These data confirm the extremely tight regulation involving acyl-ACP in E. coli and also point to another regulatory control point in FASII outside of the ACC. This additional control point was identified as the FabH enzyme based on biochemical analysis [7]. Unlike the acyl-chain length independent effect of acyl-ACP on the ACC, the potency of the acyl-ACP increases with the length of the fatty acid (20% inhibition for 12:0-ACP and 70–80% inhibition with 20:0-ACP). This graded response helps to tune the activity of the initiation module to the elongation and acyltransfer modules, ensuring fatty acids of the desired length are synthesized. The authors determined the mode of inhibition was competitive with respect to malonyl-ACP and mixed inhibition with acetyl-CoA, indicating the acyl-ACP can bind to the apo-enzyme and the acyl-enzyme intermediate. The acyl-ACPs appear less potent to FabH compared to ACC with the authors performing in vitro inhibition assays with 100 μM of acyl-ACP to achieve similar inhibition to the ACC at 40 μM [7]. Further evidence of the regulatory effect in vivo was provided by employing an E. coli plsB mutant glycerol auxotroph. Using this strain, starving the cells of glycerol prevents utilization of acyl-ACP by the acyltransfer module, resulting in long-chain acyl-ACP accumulation. Examination of the short chain acyl-ACP pool showed depletion of the products of the FabH reaction, malonyl-ACP and acetyl-ACP (potentially arising from decarboxylation of malonyl-ACP by FabF), due to accumulation of long-chain acyl-ACP [33]. The combinatory regulation of the ACC and FabH by the product of the elongation cycle allows for some delicate synergistic feedback regulation of the initiation cycle to control the quantity of fatty acid produced in E. coli during a reduction in the rate of acyltransferase activity or consequently an increase in the rate of fatty acid elongation. The inhibition of FabH by acyl-ACP has only been characterized in E. coli but is likely to be present in other organisms also. S. aureus treated with oleic acid reduces lipid synthesis by 50%, although malonyl-CoA levels remain unchanged [65]. The lack of repression of ACC activity combined with the reduction of lipid synthesis points to FabH as the regulatory point and potentially acyl-ACP as the regulatory ligand, but further studies are needed to confirm this.
3.2 Control of fatty acid elongation
The determinant role of the elongation enzymes in the structure of fatty acids is discussed in section 2.2.2. The activity and substrate specificity of the elongation enzymes are finely balanced to produce: 1) fatty acids of the desired length; and 2) to produce the correct ratio of unsaturated:saturated fatty acids. The roles of FabZ/FabA from E. coli and FabM from S. pneumoniae in regulating the unsaturated fatty acid content are also relevant (section 2.2.3). The acyl chain length of the fatty acids generated by the cycle is a product of competition for the acyl-ACP between the G3P acyltransferases and FabB/F (or FabF in most species with a single elongation condensing enzyme). The longer chain acyl-ACPs are poor substrates for FabB and ideal substrates for the acyltransferases. A shift in the activity ratio in E. coli is accomplished by overexpressing FabB or depleting the cells of PlsB to alter the kinetic balance between the elongation and acyltranfer modules. Both perturbations result in abnormally long fatty acids [215]. The acyl-chain length specificity of FabB is explained by the size of substrate binding tunnel adjacent to the active site. Many crystal structures illustrate that the pocket accommodates acyl-chains up to 16 carbons and limit the enzyme to producing 18-carbon fatty acids [46,216–219]. The trans-2-enoyl-ACP reductase (FabI) is also inhibited by acyl-ACP. This effect is attributed to product inhibition, a property of all enzymes, but nonetheless may be very relevant to slowing the rate of the elongation module when long-chain acyl-ACP are abundant [220]. In summary, biochemical regulation at multiple steps in the pathway work in concert to provide a tight control over the amounts of fatty acid produced and their structures.
3.3 Coordination of lipid and macromolecular synthesis
The synchronization of lipid synthesis with DNA, RNA and protein synthesis must occur to maintain the correct proportion lipid to protein in the membrane. How the cell correctly balances lipid and macromolecular synthesis such that the membrane protein:lipid ratio remains constant at different growth rates is a problem in bacterial physiology that requires additional experimentation. There have been only a few studies on this topic over the past four decades. The first reports of the cross-talk between lipid and protein synthesis were published by Glaser et al., who apparently used a temperature-sensitive glycerol-phosphate acyltransferase E. coli mutant to examine the effect of diminished phospholipid synthesis on the production of DNA, RNA and protein syntheses [221]. Growth of the mutant at the non-permissive temperature resulted in an abrupt halt to DNA, RNA and protein synthesis, but it was later found that mutation in the plsA mutation, originally thought to be a acyltransferase, was actually a defective adenylate kinase [222]. Inhibition of phospholipid synthesis in mutants expressing a defective plsB gene did not cause other branches of macromolecular syntheses to slow until the cells have divided 1–2 times, despite the nucleotide pool remaining largely unchanged [223–225]. This lack of coordination was confirmed when the membranes of glycerol-starved E. coli gpsA mutants were discovered to contain significantly more protein than their glycerol-supplemented counterparts [224]. Nonetheless, protein synthesis does eventually stop under these conditions, demonstrating there is a factor which senses a reduction in growth rate or perturbation of the membrane and feeds back to other arms of metabolism. After glycerol is made available to the growth-arrested cells, lipid synthesis and growth rapidly resume, but there is a delay in the initiation of DNA, RNA and protein synthesis. This observation argues for a sensor which detects the ratio of protein to lipid in the membrane and does not restart other macromolecular syntheses until the incorrect lipid to protein ratio of the membrane has been remedied [225]. A similar pattern was observed in E. coli cells treated with the FabB inhibitor cerulenin. Despite a 90% reduction in lipid synthesis after 40 minutes of treatment, only a moderate (25%) reduction in nucleotide synthesis was observed with no change in protein synthesis [226]. Reminiscent of the E. coli gpsA mutant during glycerol starvation, after 1–2 hours complete termination of all macromolecular syntheses was observed and when the cerulenin was removed from the growth medium, lipid synthesis restarted significantly faster than protein synthesis. These studies hint at a connection between lipids and other branches of metabolism, but these studies all used strains of E. coli that also possess lesions in the synthesis of the global regulatory signalling molecule, ppGpp. Most were defective in both relA1 and spoT1 alleles that result in absence of ppGpp regulation [227]. The RelA protein catalyzes the synthesis of ppGpp from GTP and GDP whereas SpoT catalyzes its synthesis and degradation [228]. The importance of ppGpp in linking protein synthesis and lipid synthesis was investigated by one group that compared incorporation of [14C]acetate into phospholipids during starvation of leucine [229]. They compared wild-type and relA strains and found that upon leucine deprivation, the wild-type strain responded by reducing lipid synthesis by approximately 50%, whereas the relA strain did not. This effect was attributed to biochemical inhibition of the carboxyltransferase subunit by ppGpp in the wild-type strain that was absent in the relA strain. Multiple groups also demonstrated that ppGpp can inhibit the PlsB activity in vitro when palmitoyl-CoA is used as the acyldoner [230,231]. Cerulenin treatment triggers a SpoT-dependent increase in ppGpp [36], suggesting that SpoT activity is responsible for the coordination of fatty acid and protein synthesis. The discovery of a ppGpp-mediated regulatory link between amino acid availability and lipid synthesis calls to question the relevance of earlier studies to normal physiology and stresses the importance of knowing the status of the relA and spoT genes used in the experiments. More recently, Yao et al. were reminded of the connection between ppGpp and regulation of lipid synthesis when selecting for E. coli mutants that were able to grow in the presence of a defective LPS synthesis pathway [34]. Their selection conditions yielded mutations in fabH and accD, allowing the cells to grow during limited LPS biosynthesis. Based on this result, the researchers generated a targeted fabH deletion in the parent strain which they observed was viable. The debate about the essentiality of fabH was discussed in Section 2.1.2, but it was uncovered that a fabH null mutation was not viable in an E. coli recA1 spoT1 strain. The explanation for this is the lack of pathway regulation by ppGpp, which accumulates during fatty acid starvation/inhibition, but to date a conclusive mechanism has not been provided [36,232].
3.4 Phospholipid headgroup homeostasis
3.4.1 Regulation of PtdSer Synthesis
Synthesis of the PtdEtn and PtdGro in E. coli was covered in Section 2.4.1. The balance of PtdEtn to PtdGro is maintained by a series of strict biochemical regulatory controls. The branch point between zwitterionic phospholipids (PtdEtn) and anionic phospholipids (PtdGro and cardiolipin) arises after phosphatidic acid has been converted to CDP-DAG. The PtdSer synthase (Pss) and the PtdGro-phosphate synthase (PgsA) enzymes compete for the CDP-DAG. Plasmid driven overexpression (800 fold) of Pss or PgsA individually in E. coli has minimal effect on the composition of PtdEtn and PtdGro in the membrane due both enzymes being tightly controlled by independent feedback regulatory loops [233–235]. Evidence for independent regulation of Pss and PgsA comes from experiments with E. coli treated with the glycerol-1-phosphate acceptor arbutin, that stimulates the transfer of G1P from PtdGro to form arbutin-phosphoglycerol and DAG [234]. The arbutin-mediated removal of G1P from PtdGro did not significantly reduce the PtdGro content of the cell membrane. This demonstrated a huge increase (7 fold) in PgsA activity to maintain the PtdGro content of the membrane despite constant conversion of PtdGro to DAG by arbutin. No additional PgsA activity was detected in cell extracts from cells treated with arbutin, indicating this increase in in vivo activity was due to biochemical feedback regulation as opposed to increased gene expression. The synthesis of PtdEtn was not increased, proving PgsA and Pss are regulated independently.
During the purification of Pss, it was noted that it was primarily associated with the ribosomal fraction of the cell instead of the membrane fraction as expected [165]. However, if membranes supplemented with CDP-DAG are added to the ribosomal fraction, Pss switches location and associates with the membrane. Thus, CDP-DAG essentially activates Pss and promotes association of the enzyme with the membrane rather than ribosomes [165]. Pss is an interfacial enzyme which needs to associate with anionic lipids on the surface of the membrane to be catalytically active [165,236]. The requirement for anionic phospholipids for Pss activity results in a clever detection mechanism that can respond to an increase in anionic membrane phospholipids by stimulating PtdSer (and consequently PtdEtn) synthesis. This also provides a explanation for the lack of increased PtdEtn synthesis following a 800-fold overexpression of Pss because only the membrane-bound form is catalytically active. Pss from B. subtilis is an integral membrane protein with no association with the ribosomal fraction of the cell [237]. Sara et al. introduced the Pss from B. subtilis into an E. coli Pss null strain under the control of an IPTG inducible promoter [237]. In contrast to overproduction of E. coli Pss, increasing amounts of IPTG (and therefore B. subtilis Pss) caused a steady-state increase in the PtdEtn content up to a critical level, where expression of the gene became lethal [1,237]. The membrane bound B. subtilis Pss does not have the regulated activity of the E. coli enzyme and therefore must be controlled differently than in E. coli. This multilayed regulation of E. coli Pss is a combination of strict feedback regulation and a sensing mechanism for acidic phospholipids that drives the membrane association and activation of Pss.
Phosphatidylserine decarboxylase (Psd) is an intrinsic membrane protein that catalyzes the formation of PtdEtn from PtdSer. The fact that only trace amounts of PtdSer are detected in E. coli cells suggests that Psd is extremely efficient at performing the reaction. Psd is an interesting enzyme that utilizes a covalently bound pyruvate prosthetic group that is essential for catalytic activity [238]. Unlike most of the enzymes involved in membrane lipid synthesis in E. coli, Psd requires a post translational modification for activity. Li et al. reported Psd is translated as a 36 kDa proenzyme that is rapidly cleaved by reductive amination into two non-identical subunits of 7 kDa and 29 kDa [239]. This cleavage results in Ser254 being converted to the N-terminal pyruvate of the 7 kDa subunit [239]. Mutagenesis of Ser254 to a cysteine or threonine reduces the cleavage reaction whereas substitution to an alanine completely inhibits the reaction [167]. The mechanism for the cleavage is unclear but is thought to follow an autocatalytic serinolysis reaction comparable to that of the histidine decarboxylase enzyme [240]. The requirement for posttranslational processing of Psd seems to be widespread as the enzyme of Vibrio cholerae also undergoes autocatalytic cleavage into a 27.9 kDa and a 3.6 kDa subunit and the B. subtilis Psd contains a conserved cleavage segment, although the cleavage mechanism has not yet been confirmed [241–243].
3.4.2 The MprF system
Some firmicutes, including S. aureus and B. subtilis synthesize the unique lipid, lysyl- phosphatidylglycerol (Lys-PtdGro). This positively charged phospholipid is synthesized by the aminoacylation of PtdGro by the transmembrane MprF enzyme utilizing Lys-tRNA as a lysine doner (for review see ref [244]). The lysinylation of the anionic PtdGro changes the net charge of the phospholipid from -1 to +1 [245]. The enzyme was named MprF as a notation for multiple peptide resistance factor, in reference to the increased susceptibility of a S. aureus mprF null mutant towards cationic antimicrobial peptides (CAMPs) and lipopeptide antibiotics such as defensins and daptomycin [246,247]. The cationic nature of CAMPs result in high-affinity to anionic bacterial membranes. The positive net charge introduced due to the production of Lys-PtdGro perturbs this electrostatic attractions and mitigates the antimicrobial effects of the CAMPs. Although Lys-PtdGro does not have a defined structural role, it clearly has an integral role in pathogenesis in S. aureus because mprF deletion mutants have attenuated virulence [248]. Why a non-pathogenic soil-dwelling organism like B. subtilis would produce Lys-PtdGro seems less obvious, but it has been hypothesized that many soil dwelling organisms do so to mitigate the effect of antimicrobial peptides secreted by themselves and other soil bacteria [249]. MprF from S. aureus produces strictly Lys-PtdGro, whereas an MprF-homologue from P. aurugenosa produces only Ala-PtdGro [250].
The MprF enzyme from Enterococcus faecium and B. subtilis have a less strict substrate specificities and are able to utilize both Lys and Ala to modify PtdGro [251]. The MprF enzyme houses two different catalytic domains. The C-terminal domain is the Lys-PtdGro synthase domain that catalyzes the transfer of the lysine from the aminoacyl-tRNA to the hydroxyl group of terminal glycerol of PtdGro. The amino-terminal domain is the hydrophobic flippase components that catalyzes the translocation of Lys-PtdGro from the inner leaflet of the cell membrane where it is produced, to the outer leaflet where it can function to repel CAMPs. Ernst et al. [246] demonstrated that production of Lys-PtdGro alone on the inner leaflet is not sufficient to induce resistance to CAMPs. This was accomplished by expressing a truncated form of MprF that only contains the Lys-PtdGro synthase domain that actively produced Lys-PtdGro. Expression of the synthase domain in combination with the flippase domain restored resistance to CAMPs, signifying the requirement for presenting the Lys-PtdGro on the exterior of the membrane to exert its CAMP repulsing effect. The regulation of MprF activity and gene expression remains elusive. Nothing is known about the biochemical control of MprF activity in any organism.
In P. aeruginosa, Ala-PtdGro synthesis is induced by acidic growth conditions and Lys-PtdGro production in S. aureus varies throughout the stage of growth [246,250]. The mprF gene and other resistance mechanisms are upregulated in response to CAMPs by the ApsRSX three component regulator [252–255]. ApsS is the membrane bound sensor kinase that interacts with CAMPs and activates ApsR by phosphorylation, inducing transcription of mprF and genes involved in lysine biosynthesis [252,255].
3.5 Acyl-chain turnover
The turnover of PtdGro in E. coli to generate MDO and in S. aureus during LTA synthesis was discussed in Section 2.3.3, but in E. coli production of lipoproteins results in the removal of acyl chains from established phospholipids. Lipoproteins serve a plethora of different functions in the bacterial kingdom, from hemolysins that are essential for pathogenesis to peptidoglycan associated lipoproteins that have a role in cell wall maintenance [256,257]. Lipoproteins are abundant in E. coli with 1% to 3% of genes predicted to encode fatty acid or diacylglycerol modified proteins. The most thoroughly studied is the outer membrane lipoprotein Lpp [258]. There are two generalized structures of lipoproteins in bacteria: diacylated lipoproteins and triacylated lipoproteins. In E. coli, the synthesis pathway of the triacylated form is well-established [257]. The lipoprotein is translated as a preproplipoprotein containing a 20 amino acid amino-terminal signal peptide. The lipoprotein DAG transferase (Lgt) enzyme attaches a DAG molecule from PtdGro to the sulfhydryl group of a conserved cysteine. The lipoprotein signal peptidase enzyme (Lsp) specifically cleaves the prolipoprotein upstream of the lipidated Cys residue resulting in the Cys becoming the amino-terminal residue of the lipoprotein. The final stage to generate a triacylated lipoprotein by the lipoprotein N-acyl transferase (Lnt) catalyzed transfer of a fatty acid to the terminal amine residue of the cysteine. The source of the fatty acid involved in the α-aminoacylation of the lipoprotein is derived from the 1-position of PtdEtn in E. coli, creating the lysophospholipid 2-acyl-glycerophosphoethanolamine (2-acyl-GPE) in the process [259,260]. Gupta and coworkers demonstrated that the Lnt reaction isn’t absolutely specific for PtdEtn, as in the absence of PtdEtn, the fatty acid from the 1-position of PtdGro or cardiolipin can be used [261]. It is not desirable for the cell membrane to contain lysophospholipids, therefore a lysophospholipid repair or degradation pathway must be implemented to dispose of these lipids. 2-acyl-GPE can be degraded into free fatty acid and GPE by the lysophospholipase L2 enzyme (PldB). The alternative is conversion of 2-acyl-GPE to PtdEtn through the acyl-ACP synthetase:2-acyl-GPE acyltransferase enzyme (Aas) [262–265]. This membrane-bound enzyme contains two activity domains: the first catalyzes the ATP dependent formation of acyl-ACP from free fatty acid and unesterified ACP that remains tightly associated with the enzyme. The second activity transfers the fatty acid from the acyl-ACP to the 1-position of 2-acyl-GPE, in essence recycling the lysophospholipid into a bilayer forming membrane lipid. Unlike the acyl-ACP synthetase produced by V. harveyi that generates free acyl-ACP from free fatty acid and ATP, the acyl-ACP from the E. coli Aas enzyme does not dissociate from the enzyme unless non-physiological high ionic strength is applied (0.4 M LiCl) [263,266]. Other methods of removing 2-acyl-GPE include a membrane bound transacylase activity that combines two 2-acyl-GPEs to produce PtdEtn and GPE and another phospholipase that degrades 2-acyl-GPE [267,268]. In E. coli, lysophospholipids can also be generated through transfer of an acyl chain from membrane bound phospholipids to lipid A [269,270]. The recycling system is thought to be the same mechanism that is used to repair lysophospholipids generated during Lpp maturation. It is important to note that the reactions of lipoprotein acylation occur outside the cytoplasmic membrane justifying the use of phospholipids, rather than acyl-ACP, for these reactions. However, this does create a topological problem in that the phospholipid products must be transferred inside the cell to re-enter the biosynthetic pathway. DAG is thought to spontaneously cross the plasma membrane, but there is a transporter (flippase) required to move 2-acyl-GPE from the periplasm to the cell interior where it can be used by the Aas enzyme. This is accomplished that the LplT transporter that is found in the same operon as Aas [271]. In some bacteria, the proteins are fused as is found in the MprF system.
It was thought for some time that Gram-positive organisms could only generate diacylated lipoproteins due to the lack of a Lnt homologue. This view was challenged when a triacylated protein, SitC, was discovered through a mass spectrometry study in S. aureus [272,273]. This finding was not without controversy as another group almost simultaneously reported the same protein was only diacylated [274]. The discrepancy was resolved by Kurokawa et al. who determined that the acylation state of SitC was dependent on the growth conditions [275]. Growth of S. aureus in a low pH, high temperature or high salt environment or post-exponential growth phase accumulated the diacyl SitC, whereas growth under more favorable conditions resulted in accumulation of the triacylated isoform. The identity of the enzyme that creates triacylated lipoproteins in Gram-positive bacteria remains to be identified.
A additional mechanism for acyl-chain turnover in E. coli was proposed by Kol and coworkers. They examined the fate of exogenous short chain (6:0) PtdEtn and PtdSer in a Pss mutant that is unable to synthesize PtdEtn [276]. Their results showed that exogenous PtdEtn was remodeled and the short-chain fatty acids replaced with palmitate acyl chains. Exogenous PtdSer was rapidly decarboxylated to form PtdEtn, prior to remodeling. The authors think this process involved two successive deacylation and reacylation steps, but the enzymes involved remain unknown. The steady state level of phospholipid remodeling in E. coli has been reported to increase during stationary phase. Pech-Canul et al. [277]were examining the role of a fadD null mutant in E. coli and S. meliloti and observed that during stationary phase, cells lacking the FadD acyl-CoA synthetase accumulated free fatty acids. They determined that the fatty acids did not arise from an exogenous source by observing the same phenomenon after deleting FadL, the fatty acid transporter preventing utilization of fatty acids in the medium. This observation highlights the potential role of FadD in activating endogenous fatty acids released from membrane lipids and the increased rate of deacylation of membrane phospholipids during stationary phase in E. coli and S. meliloti.
4. Transcriptional regulation of bacterial lipid metabolism
4.1 Genetic regulation of lipid metabolism in Gram-negative bacteria
There is a sophisticated network of diverse transcriptional regulators of lipid metabolism in bacteria that work in concert with biochemical regulation to control the pathway (Table 1) [278]. The expression levels of different fatty acid synthesis biosynthetic genes are coordinated with growth rate, nutrient availability and environmental stimuli. In organisms possessing a fatty acid βoxidation pathway in addition to biosynthesis, the expression of the degradation machinery is balanced with fatty acid synthesis enzymes.
Table 1.
Transcriptional regulation of bacterial lipid synthesis.
| Transcription Factor | Organism | Activation | Repression | Regulator | Reference |
|---|---|---|---|---|---|
| FadR | Escherichia, Salmonella,Vibrio, Shigella, Haemophilus, Klebsiella, Yersinia | fabA, fabB, iclR | fadl, fadD, fadBA,fadE, fadF, fadIJ, plsB | Acyl-CoA | [280] 288,[304] [295] |
| FabR | Escherichia, Salmonella,Vibrio, Shigella, Haemophilus | None | fabA, fabB | Acyl-CoA and acyl-ACP | [312,372] |
| FapR | Bacillus, Staphylococcus, Clostridium, Desulfitobacterium, Carboxidothermus, Clostridium | None | fabH, fabF, ydhO, fapR, fabI, fabD, fabG, plsX | Malonyl-CoA, Malonyl-ACP | [317,325] |
| FabT | Streptococcus, Enterococcus, Lactococcus | None | fabT, fabH, fabK, fabD, fabZ, fabG, fabF, accABCD | Acyl-ACP | [17,324] |
| DesT | Pseudomonas | None | desCB, fabAB | Acyl-CoA | [309,310] |
| DesR | Bacillus | None | desA | [328] |
4.1.1 Coordination of fatty acid synthesis and degradation by FadR
The FadR transcription factor has been most thoroughly studied in E. coli (EcFadR), but homologs are present in Gram-positive and Gram-negative bacteria. E. coli FadR is a member of the GntR family of transcription factors and functions as a classical repressor of genes involved in fatty acid degradation and an activator of some genes involved in fatty acid synthesis [279,280]. The genes encoding enzymes involved in β-oxidation are spread throughout the chromosome and are responsible for the transport (fadL), activation (fadD) and degradation (fadABEFHIJ) of exogenous fatty acids [281]. β-Oxidation liberates one molecule of acetyl-CoA with each turn of the cycle, that can be further catabolized by the citric acid cycle and enzymes involved in glyoxylate bypass [282]. FadR is not the sole transcriptional regulator of genes involved in fatty acid degradation in E. coli as the ArcAB system also negative regulates the pathway whereas the global cyclic AMP receptor protein-cAMP complex activates the fad genes [281,283,284].
The binding of the FadR repressor to DNA is antagonized by the binding of long-chain acyl-CoA thioesters, resulting in a conformational change of FadR and a reduced affinity for its DNA binding site [279,285,286]. The FadL enzyme transports a fatty acid from the growth medium across the outer membrane and into the periplasmic space [287,288]. The fatty acids are moved from the periplasm to the cytoplasm through an unknown mechanism (probably spontaneous flipping) where acyl-CoAs are generated through the activation of exogenous or endogenous free fatty acids by the interfacial enzyme acyl-CoA synthetase (FadD) [105,277,289]. The acyl-CoA products of the FadD reaction can either be utilized as acyl-donors for PlsB and PlsC during phosphatidic acid biosynthesis or used by enzymes encoded by the fad regulon, that are able to disassemble the fatty acid completely to acetyl-CoA by β-oxidation. Therefore, FadR recognizes the availability of acyl-CoAs as an energy source and de-represses the genes involved in consuming the fatty acid as an energy source. An additional member of the fad regulon was recently found that encodes an acyl-CoA thioesterase, FadM [290]. The physiological role of FadM (formerly YbaW) has been postulated to be hydrolysis of 3,5-tetradecadienoyl-CoA and 9-cis,11-trans-octadecadienoyl-CoA that are intermediates in β-oxidation degradation of oleic acid and conjugated linoleic acid respectively [291,292]. The free acids are released from the cell and secreted into the growth medium. The proposed role of the thioesterase is to release the intermediates that are resistant to further degradation that would otherwise accumulate and inhibit the flow of metabolites through the pathway. Similarly to FadL and FadD, FadM is only weakly derepressed in a ΔfadR strain (2–3 fold) whereas the enzymes involved in degradation, FadH and FadBA, increase expression 5–10 fold [290,293]. The fadL and fadD genes are the only members of the fad regulon containing two FadR binding sites, despite a lesser influence of FadR on repression of fadL and fadD [293]. The less stringent regulation of FadL and FadD is logical as these enzymes are required to generate the regulatory acyl-CoA.
FadR also functions as an activator of two genes involved in unsaturated fatty acid synthesis, fabA and fabB, in addition to iclR [294–297]. The IclR protein is a repressor of the aceAB genes that encode enzymes involved in the glyoxylate shunt pathway [294]. The activation of fabA and fabB by FadR is abolished upon binding of FadR to acyl-CoA. The simultaneous activation of fatty acid degradation and repression of unsaturated fatty acid synthesis in response to exogenous fatty acids coordinates the two pathways. A possible explanation put forward by John Cronan to explain the selective deactivation of the unsaturated arm of FASII by FadR is based on the essentiality of saturated fatty acids in E. coli [295]. As described in Section 2, E. coli requires 3-hydroxymyristoyl-ACP to synthesize the lipid A component of LPS. Although exogenous fatty acids can fulfill the unsaturated fatty acid requirement of the cell, 3-hydroxymyristate is a product of saturated fatty acid biosynthesis and can only be utilized from the endogenous pathway as an ACP thioester. Biochemical studies have concluded FadR has the highest affinity for long-chain acyl-CoAs (C16-C18), and little affinity for <C10-CoA or free fatty acids [279]. The dissociation constants for palmitoyl and oleoyl-CoA are in the nanomolar range, underlining the high sensitivity of FadR to acyl-CoAs [279].
Structural data has been obtained for three forms of FadR: the free protein, FadR-DNA complex and the FadR-acyl-CoA complex [298–301,301]. The structures illustrate that FadR contains a typical DNA binding winged-helix motif at the N-terminus and a collection of α-helices as the C-terminal domain. The free protein and DNA-bound structures are almost identical whereas the acyl-CoA bound structure shows the interaction of the CoA thioester with the C-terminal domain. In order to accommodate the acyl-CoA, several residues from the DNA-binding region change conformation causing the helices in the DNA binding domain to separate. This conformational change reduces the affinity of FadR to its recognition sequence and dissociates the repressor from the DNA.
Although E. coli is considered the paradigm for bacterial FASII, the advent of genome sequencing has revealed variations in the machinery and the regulation of the pathway. FadR is not present in bacteria without a β-oxidation pathway and is not present in all bacteria which have the pathway. Not only does the affinity for acyl-CoAs vary between species, the genes within the fad regulon are also different [302]. For example, a FadR homologue was discovered in B. subtilis that also represses the genes of β-oxidation but shows no evidence of the activator function of observed in E. coli, most likely due to the lack of a FabB/FabA mediated unsaturated fatty acid synthesis pathway [303]. Although B. subtilis and E. coli FadR proteins are functionally similar, they are structurally distinct with the B. subtilis protein belonging to the TetR family while the E. coli enzyme is a GntR like repressor. An additional member of the FadR regulon was identified in Vibrio cholerae where FadR binds to the promoter region and represses transcription of plsB [304]. The potential explanation for the absence of this transcriptional regulation in E. coli arises from the different acyltransferase machinery. E. coli uses solely PlsB and PlsC to transfer fatty acids from acyl-CoA or acyl-ACP to G3P, whereas V. cholerae appears to use different acyltransferases for exogenous and endogenous fatty acids. Although E. coli possesses plsX and plsY genes, the functions remain unknown. V. cholera utilizes the PlsX/PlsY system for the acylation of glycerol-phosphate from de novo synthesized fatty acids and PlsB for exogenous fatty acids. Based on the poor affinity of B. subtilis PlsX for acyl-CoAs compared to acyl-ACPs, it is possible V. cholerae PlsX could behave the same, thereby utilizing one acyltransferase for exogenous fatty acids (PlsB) and another set for endogenously synthesized acyl chains (PlsX/PlsY) [104]. This idea is supported by data indicating a transposon insertion into plsB of V. cholerae is not lethal while plsB is essential in E. coli [115,305,306]. In contrast, PlsX is a dispensable gene in E. coli but not in V. cholera [115,305,306]. If PlsB functions purely as an exogenous fatty acid acyltransferase in V. cholerae, it seems logical that it is derepressed in response to exogenous fatty acid.
The widespread nature of the FadR enzyme indicates an important role in metabolism in several different bacteria. Deletion of fadR appears to have no effect in E. coli and S. enterica unless fatty acids are the sole carbon source, where deletion of fadR causes accelerated growth as the fatty acid degradation enzymes are overexpressed [307]. In contrast, disruption of fadR in V. vulnificus had a severe effect on cell growth in rich media and resulted in attenuated virulence in a murine subcutaneous infection model [308]. The virulence attenuation could be attributed to the lack of expression of the fabA gene in the fadD mutant, which causes a significant (13%) decrease in the unsaturated fatty acid content of the bacteria.
4.1.2 FabR/DesT control of unsaturated fatty acid synthesis
In addition to the influence of fabA and fabB in controlling unsaturated fatty acid biosynthesis, P. aeruginosa also possesses Δ9-desaturases (discussed in Section 2.2.3) that insert a double bond into the acyl-chains of existing phospholipids (DesA) or saturated acyl-CoAs (DesBC) [95]. The expression of fabAB and desBC is controlled by the DesT repressor [309,310]. DesT is a TetR family transcription factor and functions to sense the overall fatty acid composition of the acyl-CoA pools. DesT binds saturated and unsaturated acyl-CoAs with equal affinity but binding to DNA is enhanced when DesT is bound to an unsaturated acyl-CoA and perturbed when DesT is bound to a saturated acyl-CoA [309,310]. This allows P. aeruginosa to respond to the availability of exogenous saturated and unsaturated fatty acids and adjust gene expression to maintain a constant membrane fluidity. DesT only detects exogenous fatty acids in the form of long-chain acyl-CoA thioesters as acyl-ACP, short chain acyl-CoAs and free fatty acids did not alter DNA binding [309,310]. Growth of P. aeruginosa on oleate repressed expression of desC and fabAB 2- and 12-fold, respectively. In contrast, growth on stearic acid stimulated transcription of desC by approximately 8-fold [309,310]. The differential influence of DesT binding between the fabAB and desCB genes was attributed to the slightly different recognition palindromes in the promoters with the desCB recognition sequence binding DesT with the highest affinity and exerting more stringent regulation [309,310]. X-Ray crystallography of the different forms of DesT provided a molecular insight into the conformational changes that govern the binding/dissociation of DesT from its cognate DNA upon acyl-CoA binding. Miller and coworkers [311] solved crystal structures of the DesT-18:1Δ9-CoA-DNA complex and the DesT-16:0-CoA complexes. They determined that DesT adopts two conformations: a “relaxed” conformation when bound to an unsaturated acyl-CoA that facilitates DNA binding and a “tense” conformation when bound to a saturated acyl-CoA species that exhibits a lower affinity for the DNA target sequence. The L-shaped oleoyl-CoA ligand slots into the hydrophobic core of the DesT ligand binding domain and becomes an integral component of the hydrophobic domain allowing it to adopt its relaxed state. As DesT binds 16:0-CoA, the acyl chain is inserted into the protein core forcing the phenylalanine rich cluster in the hydrophobic pocket to adjust its position to accommodate the straight acyl chain locking DesT into the tense conformation. The conformational changes that occur to accommodate the saturated acyl-CoA result in a shift of the some of the helices in the DNA binding domain away from their optimal DNA binding orientations present in the relaxed form. A key residue in the phenylalanine cluster is Phe166, which is perfectly positioned where the cis-double bond of oleate sits and is thought to detect the unsaturation of the acyl-chain. Despite retaining affinity for acyl-CoA, mutagenesis of Phe166 to an alanine prevents detection of the kink in oleate and locks the protein in the tense state, preventing DNA binding. Phe166 is positioned below one of the helices in the DNA binding domain and functions to stabilize its orientation when oleoyl-CoA is bound.
There is a second transcription factor, designated FabR, which contributes to fine tuning the biophysical properties of the membrane. FabR binds to regions of the fabA and fabB promoter downstream of the FadR recognition sequence to represses transcription [280,312–314]. FabR was discovered in 2001 after McCue et al. examined a sequence upstream of the fabA and fabB genes that is conserved in E. coli and several other γ-proteobacteria [315]. Using an oligonucleotide affinity column, they isolated proteins from an E. coli crude extract that bound to the fabA and fabB promoter sequence and used proteomic techniques to identify interacting proteins. This strategy led to the identification of FabR, which was subsequently followed by genetic studies examining the transcriptional effects of deleting the fabR gene. Deletion of fabR in E. coli causes a 2–4 fold increase in fabA and fabB mRNA, confirming the role of FabR as a repressor [312]. The effect of deleting fabR was most pronounced on the expression of fabB, whereas FadR is the most prominent regulator of fabA. Deletion of FabR causes an increase in the unsaturated to saturated fatty acid ratio from approximately 1 to 2, consistent with its role in repressing transcription of fabA and fabB, the primary determinants of unsaturated fatty acid synthesis in E. coli [314]. Analysis of the an fabR insertional inactivation strain from the Keio collection did not show in increase in unsaturated fatty acid content when compared to the parent strain [278,306]. However, sequencing of the fabR gene from the parent strain used in the Keio collection identified a missense mutation in the DNA binding domain of the gene encoding the FabR that likely antagonizes the binding of FabR to its congnate sequence, presenting the phenotype of a FabR deletion mutant [278]. Zhu et al. [278] identified the regulatory ligands that increase the affinity of FabR for DNA as unsaturated acyl-CoA and acyl-ACP thioesters. FabR demonstrated high affinity for long-chain unsaturated acyl-CoA/acyl-ACP thioesters that induce binding of FabR to DNA, suppressing new unsaturated fatty acid synthesis. Both FadR and FabR can similtaniously bind to the fabB promoter sequence, demonstrating combinatorial transcriptional regulation. Binding of the FabR-18:1Δ9 CoA complex to DNA was antagonized by the presence of saturated 16:0-CoA/16:0-ACP to increase the unsaturated fatty acid content when an abundance of saturated fatty acids are available. This observation is consistent with the theory that unsaturated fatty acid ligands induce a conformational change in FabR that increases its affinity to DNA, whereas binding of saturated ligands does not induce the change in conformation. FabR essentially measures the unsaturated: saturated fatty acid ratio in the cell, as opposed to absolute amounts of either. This property is crucial as during cell growth, the concentration of intracellular acyl-ACP does not vary whereas the unsaturated content can change. This allows FabR to tune the expression of fabA and fabB according to the unsaturated fatty acid content of the ACP pool. The ability of FabR to bind both CoA and ACP thioesters indicates FabR is a sensor of both de novo and exogenously obtained fatty acids, in contrast to FadR that only senses the exogenous acyl-CoA pool.
The FabR model described by Zhu et al. [314] was questioned by Feng and Cronan [313], who observed binding of FabR to DNA in the absence of a ligand. They observed upregulation of fabA and fabB upon deletion of fabR and repression of fabA and fabB after treatment of growing cells with oleate. However, depletion of the unsaturated acyl-ACP pool through specific inhibition of FabA with 3-decynoyl-N-acetylcysteamine did not result in depression of fabAB. Under their assay conditions, Feng and Cronan showed through electrophoretic mobility shift assays that FabR Is able to bind to a fabB and fabA promoter sequence without addition of a ligand. On the surface, this finding seems to argue against the model of Zhu et al., but the equilibrium between the two conformations of FabR or DesT can be shifted by either altering the ligand or DNA concentrations in the assay [278,311]. More research is needed to clarify the details of FabR-DNA, FabR-ligand and FabR-ligand-DNA interactions, but it is clear that the properties of FabR and DesT monitor the ratio of the saturated to unsaturated acyl-CoA pools to allow P. aeruginosa and E. coli to tune its gene expression to modify fatty acid synthesis to provide the acyltransferases with an optimal selection of saturated and unsaturated substrates. The ability of these transcription factors to examine the composition of the acyl-CoA (and acyl-ACP in E. coli) pools provides an elegant mode of regulation when compared to classical repressors that respond to ligand concentration rather than ligand composition.
4.2 Genetic regulation of lipogenic genes in Gram-positive Bacteria
Firmicutes, including S. aureus, B. subtilis and S. pneumoniae, lack the FabB and FabA enzymes for synthesizing unsaturated fatty acids. S. aureus and B. subtilis produce primarily branched-chain saturated fatty acids, whereas S. pneumoniae utilizes the FabM enzyme for the cis-trans isomerization reaction to initiate unsaturated fatty acid synthesis. This difference in the fatty acid biosynthetic machinery mitigates the requirement for a Gram-negative type of transcriptional regulation identified in E. coli and P. aeruginosa to control the expression of the fabAB. In addition to the biochemical regulatory control of the key fatty acid synthesis pathway enzymes, a least two different systems for the transcriptional control of FASII gene expression exist in Gram-positive bacteria.
4.2.1 The FapR system
The first transcriptional regulator of FASII to be discovered in Gram-positive bacteria was the FapR repressor of B. subtilis. Bioinformatic homology searches suggested FapR exists in several different species of Bacilli in addition to Staphylococci and Clostridia [316]. Far from the specific gene regulators described in Section 4.1, FapR is a global regulator of phospholipid biosynthesis genes. The fap regulon of B. subtilis consists of the genes encoding the condensing enzymes, fabI, fabD, fabG, plsX and two unknown proteins (yhfC and ylpC) [316]. FapR is a classical repressor that detects malonyl-CoA, which fuels the elongation cycle [316,317]. Structural studies have revealed that FapR exists as a homodimer and contains a “hot dog” fold, which is commonly seen in thioesterases that are involved and acyl-CoA and acyl-ACP hydrolysis [318,319]. The ligand binding domain of the ligand-free FapR exists as an open cleft that surrounds the malonyl-CoA upon ligand binding. The malonyl-CoA induced structural rearrangements pull the DNA binding domains of the homodimer apart, reducing the cooperative association of the two monomers and disrupting the competent operator binding arrangement. The response of FapR to increases in malonyl-CoA levels essentially allows FapR to respond to defects in the elongation cycle by monitoring the levels of fatty acid precursor (malonyl-CoA) and increase transcription of the pathway genes accordingly [320]. Inducing a defect in the pathway by treatment of B. subtilis or S. aureus with a FabF inhibitor results in malonyl-CoA accumulation and consequently an increase in expression of genes in the fap regulon [65,316]. Transcription of FASII genes is constitutively high in cells lacking FapR and they are unaffected by pathway inhibition [316]. Unlike FadR that has a nanomolar dissociation constant for its acyl-CoA ligands, FapR is relatively insensitive to malonyl-CoA with a concentration of 25 μM required to disrupt DNA bindng [317]. Similar concentrations of malonyl-ACP also prevent DNA binding by FapR but slightly less effectively. Considering the concentration of ACP in E. coli is approximately 40 μM and assumed to be similar in B. subtilis, it seems unrealistic to assume that the majority of the intracellular ACP must be converted to malonyl-ACP to exert its physiological effect [321,322]. Similarly, the steady state levels of malonyl-CoA in E. coli were calculated to be approximately 24 μM, which is slightly lower than the calculated Kd for DNA bound FapR for malonyl-CoA (25 μM) [323]. This would in theory give the bacterium enough dynamic range to modulate expression of the fap genes. An increase in malonyl-CoA utilization through overexpression of the condensing enzymes would be expected to have the opposite effect and decrease expression of the fap regulon, as steady-state malonyl-CoA levels are decreased. However, this possibility has not been investigated. The significance of malonyl-CoA as a regulatory molecule in addition to its role as a precursor of fatty acids suggests that the malonyl-CoA pool in FapR-containing organisms must be tightly regulated by a currently unknown mechanism, potentially product inhibition or feedback regulation by acyl-ACPs as seen in E. coli. The importance of maintaining optimum expression of the genes in the fap regulon became evident after it was discovered that a B. subtilis fapR null mutant has a defective growth phenotype when grown in hypothermic conditions (15°C) [316]. Compositional analysis of the mutant strain compared to the wild type indicated longer fatty acids in the fapR strain, which would be expected to increase membrane rigidity during growth at low temperatures. The compositional defect arising from an excess of pathway enzymes could contribute to the cold intolerance of B. subtilis fapR mutants.
4.2.2 The FabT system
The fatty acid composition of membrane lipids in S. pneumoniae closely resembles the unsaturated/saturated fatty acid profile of E. coli. Despite this similarity, both organisms have evolved distinctly different mechanisms for transcriptional control. Instead of the FadR/FabR system, S. pneumoniae employs FabT as a transcriptional repressor to govern gene expression of FASII genes [17,324]. The genetic organization of the genes encoding FASII machinery in S. pneumoniae reveals they all reside in a single locus on the chromosome, including the fabT gene. The genes are divided into two operons, the first containing fabT-fabH-acpP,and the second includes fabK-fabD-fabG-fabF-accD-fabZ-accC-accD-accA with a FabT operator sequence included in the promoter region of fabT and fabK [17]. Disruption of the fabT gene distinctly altered the fatty acid composition of membrane phospholipids, sharply reducing the unsaturated:saturated fatty acid ratio from 2.6 to 0.9 and increasing the abundance of longer chain C18 species as compared to C16. The increase in acyl chain length is reminiscent of the chain lengthening effect of disrupting fapR in B. subtilis [17,316]. The ligand that enhances FabT binding to DNA is long-chain acyl-ACP. Upon binding to acyl-ACP, the affinity of FabT for the DNA binding site is significantly increased and the FabT-acyl-ACP complex docks on the DNA and represses transcription of the FAS cluster [324]. FabT binds acyl-ACPs of all chain lengths tested but only the long-chain acyl-ACPS induce the conformational change that stimulates DNA binding. cis-Vaccinate (18:1Δ11) is the most abundant fatty acid in S. pneumoniae and is the most effective of the acyl-ACP derivatives tested at inducing DNA binding [324]. The transcriptional feedback regulation involving long-chain acyl-ACPs links accumulation of the end product of the pathway with expression of the genes encoding the pathway enzymes. This regulatory circuit allows S. pneumoniae to adjust gene expression of all the FASII genes in response to a lack or an accumulation of the end product of the pathway. This effect is complemented by biochemical regulation of the initiation enzymes in response to exogenous fatty acids, presumably acyl-ACP [65]. However, the biochemical regulatory mechanism remains poorly understood.
B. subtilis lacking FapR and S. pneumoniae deficient in FabT do not produce increased quantities of lipid, despite overexpression of the pathway enzymes [17,325]. This observation strongly argues for the presence of biochemical regulatory mechanisms in both organisms that override transcriptional control to prevent excess production of phospholipids and maintain the appropriate lipid:protein ratio in the membrane.
4.3 Regulation of lipid metabolism by stress response regulators
4.3.1 The DesR thermometer
In addition to the controlling the rate of fatty acid synthesis in response to a shift in the composition of pathway intermediates, B. subtilis has developed regulatory mechanisms to detect environmental changes in temperature and adjust the expression of desaturating enzymes accordingly (for review see [326]). The ability to adjust the unsaturation of membrane phospholipids is crucial to counteract the increase in rigidity that arises when the temperature of biological membranes is reduced. Membrane lipids are fluid in the liquid crystalline state but become rigidified when the temperature drops below the melting temperature (Tm) of the membrane. The fluidity of the membrane is required for movement and activity of membrane proteins, therefore it is paramount to bacterial survival that the Tm is adjusted during growth at lower temperatures [327]. Section 2.2.3 discussed various desaturase enzymes from P. aeruginosa and the Des protein from B. subtilis, which inserts a cis-double bond into the Δ5 position of existing phospholipids [93]. Experiments demonstated that des mRNA is virtually undetectable in B. subtilis cultures grown at 37°C, but is dramatically induced upon cold shock at 20°C [328]. The temperature-dependent expression of des suggested there is a temperature responsive transcription factor regulating the expression of the desaturase in B. subtilis. The discovery of the DesRK two-component regulator identified such a system. Two component regulators typically consist of a sensor histidine kinase/phosphatase component, which phosphorylates/dephosphorylates a response regulator that exerts its effect, usually through activation of a specific target gene. The B. subtilis system consists of the membrane-associated kinase, DesK, which phosphorylates DesR and drives transcription of the des gene by recruiting RNA polymerase to the promoter [326,329–333]. It has been postulated DesK acts as a thermometer, detecting the ambient temperature by adopting different signaling states depending on the membrane fluidity and activating the response regulator, DesR by phosphorylation [331]. This regulation could occur through the phosphatase activity dominating at high temperatures and the kinase activity dominating at low temperatures. Evidence that DesK detects perturbations in the membrane fluidity are supported by experiments that show no des induction during cold shock when B. subtilis is treated with unsaturated fatty acids. The unsaturated fatty acids increase membrane fluidity when incorporated into phospholipids, reducing the need for further desaturation of existing lipids by the Des enzyme [331]. An increased proportion of branched chain anteiso fatty acids in the B. subtilis membrane also increases membrane fluidity in the absence of unsaturated fatty acids. The quantity of branched chain fatty acids produced in B. subtilis can be controlled by the availability of isoleucine in the growth medium, which is utilized by the branched-chain α-ketoacid dehydrogenase to generate the branched chain anteiso precursors utilized by FabH [334]. Starvation of B. subtilis for isoleucine at 37°C also triggers induction of des, in an attempt to increase membrane fluidity to counteract reduced branched-chain fatty acid synthesis [330]. This effect is alleviated through the addition of unsaturated fatty acids to the isoleucine free media.
The mechanism underlying the phosphorylation of DesR begins with the autophosphorylation of a histidine residue within DesK [333]. Phosphotransfer then occurs between DesK and an aspartate residue on DesR. As with the previous transcription factors discussed in this review, DesR exists in solution as a dimer. Phosphorylation by DesK promotes the association of two dimers to form a tetramer on the des promoter region where each dimer binds an adjacent, nonidentical DesR-P binding site on the des promoter DNA. The first palindrome, termed “RA”, is a high-affinity binding site and low concentrations of DesR-P bind only this site. The lower affinity site, termed “RB”, consists of only a partial repeat. Binding of DesR-P to both sites is necessary for recruitment of the RNA polymerase, as point mutations in RA or RB abolish des activation [333]. The working hypothesis for the mechanism of RNA polymerase recruitment requires binding of a dimer to the RA site initially, followed by binding to the RB site. It is the interaction of the second dimer of the tetramer at the RB site that is thought to physically interact with the RNA polymerase [332]. The requirement for binding of DesR-P to the low affinity and high affinity sites in the des promoter ensures that Des activation only occurs when a threshold amount of DesR phosphorylation occurs. The unusual dual binding site mechanism is responsible for the fine tuning of des expression and ensuring that the biophysical properties of the membrane phospholipids remain within an optimum fluidity range.
4.3.2 Alternate sigma factors
In addition to the DesRK system to sense changes in the membrane biophysical properties, B. subtilis expresses seven extracytoplasmic function (ECF) σ factors [335]. Several of the σ factors detect changes in membrane integrity and elicit a response to counteract the problem. A prime example is σw, which in contrast to DesKR, responds to an increase in membrane fluidity. σw is activated under conditions of membrane perturbation, such as high concentrations of detergent or when the lipid:protein ratio is disrupted [336–338]. σw reacts to an increase in membrane fluidity by elevating straight-chain fatty acid synthesis by the down-regulation of fabHa and increasing the average chain length by increased fabF expression [339]. There is a σw binding site within the fabHa coding sequence in the B. subtilis fabHa-fabF operon, which when occupied upregulates transcription of fabF and downregulates fabHa. Unlike most bacteria discussed in this review, the B. subtilis chromosome encodes two initiation condensing enzymes termed FabHa and FabHb, which differ in their substrate specificities [20]. FabHa has a preference for the branched chain precursors isobutyryl, isovaleryl and 2-methybutyryl-CoA, whereas FabHb shows maximum activity with the straight-chain precursor acetyl-CoA. The authors confirmed the enzymological analysis by examining the fatty acid composition of B. subtilis strains deficient in either FabHa or FabHb, which showed a decrease in either branched-chain and straight-chain fatty acids, respectively. Overexpression of σw by plasmid based expression increased transcription of fabF and decreased fabHa transcripts. The opposite changes in expression of the two condensing enzymes generates a fatty acid profile containing longer fatty acids due to the overexpression of fabF and a decreased quantity of branched-chain fatty acids produced as a consequence of fabHa transcript suppression. The decrease in branching and increase in chain length allows tighter packing of the acyl chains within the membrane and reduces the fluidity of the membrane. Deletion of σw is associated with increased susceptibility to environmental hazards such as detergents and membrane active compounds; however many of these physiological effects are likely due to the plethora of efflux pumps and detoxification enzymes that are concurrently induced as opposed to specific modification of membrane structure [340]. Whether σw is induced under other conditions which would increase membrane fluidity, such as growth under high temperature or excessive unsaturated fatty acid production remains undetermined. Therefore, the direct role of σw in membrane homeostasis may be a more general stress response as opposed to a specific reaction to changes is the biophysical properties of the membrane. Streptococcus pneumoniae uses the two component regulator WalRK (previously designated YycFG or VicRK) to increase the acyl chain length by upregulating fabF and downregulating fabH [341,342]. However, the WalRK mediated change in gene expression is modest (1.5–2x) and is not specific to fabH and fabF as all the pathway enzymes except fabH, fabM and acpP are upregulated. Regardless, activation of the WalRK two-component regulator causes an increase in 18-carbon fatty acid species compared to 16-carbon. It is not known whether WalRK is activated in response to increased membrane fluidity, but it does participate in a cell wall damage response pathway [342]. Listeria monocytogenes only expresses a single FabH isoform and lacks transcriptional control characterized in B. subtilis to regulate membrane fluidity. Instead, L. monocytogenes utilizes a FabH that displays an increased preference for anteiso precursors at low temperatures, increasing acyl chain branching and membrane fluidity. Other ECFs also regulate the membrane lipid composition in B. subtilis. σx senses membrane damage from antimicrobial peptides and responds by inducing the synthesis of the zwitterionic lipid PtdEtn, thereby decreasing the net anionic charge of the membrane [343]. Promoter regions of the pss and psd genes in B. subtilis also contain σx binding sites. The principal behind σx mediated resistance to antimicrobial peptides is similar to that of MprF present in several Gram-positive bacteria that synthesizes the positively charged Lys-PtdGro to electrostatically repel the peptides [244].
Transcriptional control by the ECF sigma factors is highly complex with many being allosterically regulated by anti-sigma factors, which in turn can be controlled by anti-anti sigma factors [344]. In addition to the autoregulation that occurs, cross regulation is also evident. An example is the σw promoter region that also contains a σa binding site. This paints a rather complex picture of the mechanisms that control each particular ECF sigma factor; however their roles in the transcription of several genes controlling phospholipid biosynthesis are evident through the genetic and physiologic studies.
5. The impact of bacterial lipid metabolism on translational medicine
5.1 Development and utility of FASII inhibitors
The emergence of multiple drug resistant bacteria has fuelled the development of drugs that target alternate macromolecular processes. The absolute growth requirement for membrane phospholipid synthesis has highlighted FASII as a potential target for development of new antimicrobial compounds. The role of the enoyl-ACP reductase, FabI, as the rate-limiting reaction of the elongation cycle has marked this enzyme as the first candidate for contemporary antimicrobial drug discovery. The anti-mycobacterial compound isoniazid is a front line treatment for latent tuberculosis, but it was almost four decades after its discovery that it was determined to be a specific inhibitor of the enoyl-ACP reductase (InhA) of M. tuberculosis [345]. Isoniazid is a prodrug and requires activation by the mycobacterial enzyme KatG for activity [346]. The dependence on KatG is basis for why isoniazid is a selective inhibitor of M. tuberculosis and has little inhibitory activity against many other pathogens. Another fatty acid synthesis inhibitor in wide use is the topical antibacterial agent, triclosan. Triclosan is an inhibitor of the enoyl-ACP reductase (FabI) enzyme in several Gram-positive and Gram-negative bacteria [347,348]. It is so effective that it has been included in an assortment of different household items and sanitizing products as a broad spectrum antimicrobial agent [349]. Despite the criticized overuse of triclosan, no triclosan resistant bacteria have been reported in the envrionment [347,350,351]. Triclosan shows excellent topical efficacy against E. coli and S. aureus, but very few studies have been performed examining the efficacy of systemic delivery, likely due to the poor pharmacological properties of the hydrophobic molecule. Triclosan has a low LD50 in rats when delivered intravenously (29 mg/kg), although when administered orally or subcutaneously it is much more tolerable (LD50 of 4000 mg/kg and 14,700 mg/kg respectively). Triclosan administered to mice subcutaneously can reduce the severity of a pathogenic E. coli infection, although very few groups have investigated further the concept of using triclosan systemically due to its extremely high serum binding capacity (>99.5%) [350,352]. With the exception of triclosan as a topical antibacterial agent, no small molecules are in clinical use that target fatty acid synthesis in the dangerous pathogen methicillin-resistant S. aureus (MRSA). The increased prevalence of MRSA in healthcare institutions and more recently in individuals with no connection to healthcare facilities, has stressed the need for the development of new therapeutic compounds to counteract the increase in resistance [353]. The viability of FASII as an effective antimicrobial target has been validated in several different animal infection studies examining the efficacy of inhibitors of FabI, FabF and ACC against S. aureus infections [352,354–359]. Many of the compounds tested were natural products, which are hindered in their therapeutic value by poor pharmacokinetics and/or complex and laborious total syntheses. Unlike cell wall active antibiotics that result in cell lysis, like β-lactams and glycopeptides, FASII inhibitors are initially bacteriostatic [360,361]. Bactericidal antibiotics were historically considered superior to bacteriostatic antibiotics for treatment of many different bacterial infections, but more recent clinical data suggest this may not always be true [361]. Based on the success of triclosan and isoniazid, there are numerous drug discovery efforts to produce inhibitors of the enoyl-ACP reductase [359,362–366] (for review see [366]). Affinium Pharmaceuticals has developed AFN-1252, a small molecule inhibitor optimized against S. aureus FabI that is currently in clinical trials [359,362,363]. The targeted approach of developing the compounds to selectively target Staphylococcal FabI has resulted in an extremely potent compound with minimum inhibitory concentrations (MICs) of less than 8 ng/ml against a collection of multidrug-resistant S. aureus strains [65,362]. The successful use of AFN-1252 in the treatment of septicemia in a mouse infection model and the imminent clinical deployment of AFN-1252 represents an exciting frontier for the future treatment of MRSA infections by utilizing inhibitors of FASII.
5.2 Is FASII a viable target for therapeutics discovery?
The development of FASII inhibitors has not been without controversy. One group questioned the viability of FASII inhibitors to treat Gram-positive bacterial infections, on the basis that in vivo fatty acids for growth can be salvaged from the host serum [367]. In Gram-negative bacteria, the de novo FASII pathway is essential for generating 2-hydroxymyristic acid necessary for the synthesis of the Lipid A component of LPS [64]. Pathogenic E. coli and P. aeruginosa activate exogenous fatty acids through ligation to CoA, however they cannot enter the elongation cycle to introduce the hydroxyl group and they are not recognized by the ACP specific machinery of Lipid A biosynthesis. Gram-positive bacterial cell walls do not contain LPS, and require only small amounts of intermediates to synthesize cofactors such as lipoic acid and biotin, both which can potentially be obtained from the growth medium. Some bacteria, including S. pneumoniae lack enzymes for synthesizing lipoic acid and consequently completely rely on scavenging lipoic acid for growth from the host/growth medium [368,369]. Brinster et al. [367] disputed the suitability of inhibiting FASII in Gram-positive bacteria through studies using the Gram-positive opportunistic pathogen Streptococcus agalactiae. They reported that it is possible to delete each of the pathway enzymes during growth in media containing fatty acids or serum. In addition, treatment with the FabF inhibitor cerulenin had no effect if S. agalactiae is grown with fatty acids. Balemans et al. [352] countered by reporting that FASII inhibitors are effective against S. aureus even in the presence of fatty acids or serum, which was criticized by Brinster et al. for not allowing S. aureus to adapt to utilizing the fatty acids [352,370]. However, Balemans et al. did confirm FASII inhibitors are ineffective at preventing growth of S. agalactiae in the presence of human serum. This vigorous debate identified a crucial lack of understanding in the dispensability of the FASII machinery in Staphylococci and Streptococci. The key to understanding the phenomenon was uncovered by Parsons et al. [65] who defined a crucial difference in the biochemical regulation of FASII by exogenous fatty acids between the two pathogens. During growth in media supplemented with oleate, S. aureus reduces the rate of fatty acid synthesis by approximately 50% as it incorporates oleate into its phospholipids. In contrast, during growth of S. pneumoniae in media containing fatty acids, complete repression of de novo fatty acid synthesis occurs as the over 95% of acyl chains in pneumococcal phospholipids were derived from the growth medium. It was revealed that exogenous fatty acids inhibit the activity of the ACC in S. pneumoniae but not S. aureus. A block at the FabI enzyme in S. aureus by AFN-1252 results in depletion of the ACP pool and an accumulation of short-chain acyl-ACP intermediates. The consequence of ACP depletion is an inability to activate exogenous fatty acids and synthesize new acyl chains. In S. pneumoniae, the repression of ACC activity prevents the fatal accumulation of acyl-ACPs in the elongation cycle and allows the unrestricted utilization of exogenous fatty acids for phospholipid biosynthesis. These data strongly suggest that FASII is an effective target in S. aureus and agree with the numerous reports demonstrating in vivo efficacy of several FASII inhibitors [352,354–359]. The reluctance of S. aureus to incorporate host fatty acids could reflect the differences in the fatty acid composition of S. aureus membrane phospholipids compared to human serum. S. aureus synthesizes primarily branched-chain fatty acids, unique to bacteria [65]. The fatty acid profile of S. pneumoniae is similar to the unsaturated/saturated fatty acid profile of human serum, implicating that it could conserve energy by salvaging fatty acids from the host, avoiding the energy intensive process of de novo synthesis [65,371]. In S. aureus, a membrane composed of solely host derived saturated and unsaturated fatty acids will likely drastically alter the biophysical properties of the membrane, resulting in potentially deleterious consequences for cell growth.
The critical differences in the regulation of FASII by exogenous fatty acids between S. aureus and S. pneumoniae highlight the dangers of extrapolating data with one species of bacteria to all others. Some basic experiments on the bench must be performed as to test the efficacy of a FASII inhibitor against a particular bacterium in the presence and absence of serum or fatty acids to answer the question. There is no dispute in the field regarding the essentiality of FASII in many Gram-negative bacteria and with the discovery of the metabolic basis of FASII inhibitor resistance in Gram-positive bacteria, there has been no additional debate interrogating the suitability of FASII as an antibiotic target in S. aureus. The importance of understanding the regulation of phospholipid synthesis in pathogens and the potential impact on human health must not be underestimated.
6. Perspectives
There is an incredible diversity in the structure, regulation and biosynthetic machinery of fatty acid synthesis in bacteria. The ability to alter the fatty acid composition depending on its environment is crucial for survival in the wide range of environmental conditions where bacteria thrive. E. coli was considered the paradigm for fatty acid synthesis for decades until key differences in the biosynthetic apparatus were discovered by genome sequencing. The more research that is performed on different bacteria, even within the Gram-positive and Gram-negative grouping, emphasizes how the regulation of each bacterium must be assessed on a species-specific basis. Fatty acid synthesis is an extremely energy intensive pathway and it is absolutely critical that the biophysical properties of the phospholipid membrane maintain a constant fluidity based on the quantity and the fatty acid composition of the phospholipids produced. Different bacteria possess distinctive biochemical and transcriptional regulatory checkpoints to control the rate of fatty acid synthesis. The initiation, elongation and acyltransferase modules of FASII all work in harmony to ensure the correct quantity and structure of fatty acids are produced. The biochemical regulation that occurs is extremely finely balanced and aberrant gene expression of the pathway enzymes in E. coli can have a significant influence on the structure of the fatty acids produced. This emphasizes the need for stringent biochemical and transcriptional control, which sense not only the accumulation intermediates, but in the case of FabR and DesT, the composition of a pathway intermediate pool. An understanding of the biochemical regulation of FASII in Gram-positive pathogens has influenced and will continue to guide the progression of therapeutic compounds targeting FASII. There are still huge gaps in our understanding of FASII in many bacteria, even E. coli. How are fatty acids ligated to ACP in Gram-positive bacteria? It is known that S. aureus ligates exogenous fatty acids to ACP, but how? What further undiscovered biochemical regulatory mechanisms are there in E. coli, S. aureus, B. subtilis, etc? There are currently no X-ray crystal structures of any of the acyltransferase enzymes, therefore much is not known about the catalytic mechanisms. As more discoveries are made which help fill these gaps each year, the more we can appreciate just how perfectly evolution has designed bacterial fatty acid synthesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dowhan W. A retrospective: Use of Escherichia coli as a vehicle to study phospholipid synthesis and function. Biochim Biophys Acta. 2012;1831:471–494. doi: 10.1016/j.bbalip.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cronan JE, Jr, Waldrop GL. Multi-subunit acetyl-CoA carboxylases. Prog Lipid Res. 2002;41:407–435. doi: 10.1016/s0163-7827(02)00007-3. [DOI] [PubMed] [Google Scholar]
- 3.Choi-Rhee E, Cronan JE. The biotin carboxylase-biotin carboxyl carrier protein complex of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 2003;278:30806–30812. doi: 10.1074/jbc.M302507200. [DOI] [PubMed] [Google Scholar]
- 4.Li S-J, Cronan JE., Jr The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-CoA carboxylase. J Biol Chem. 1992;267:16841–16847. [PubMed] [Google Scholar]
- 5.Zhang Y-M, Rock CO. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 6.Tsay J-T, Oh W, Larson TJ, Jackowski S, Rock CO. Isolation and characterization of the β-ketoacyl-acyl carrier protein synthase III gene (fabH) from Escherichia coli K-12. J Biol Chem. 1992;267:6807–6814. [PubMed] [Google Scholar]
- 7.Heath RJ, Rock CO. Inhibition of β-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:10996–11000. doi: 10.1074/jbc.271.18.10996. [DOI] [PubMed] [Google Scholar]
- 8.Alhamadsheh MM, Musayev F, Komissarov AA, Sachdeva S, Wright HT, Scarsdale N, Florova G, Reynolds KA. Alkyl-CoA disulfides as inhibitors and mechanistic probes for FabH enzymes. Chem Biol. 2007;14:513–524. doi: 10.1016/j.chembiol.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 9.James ES, Cronan JE. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J Biol Chem. 2004;279:2520–2527. doi: 10.1074/jbc.M311584200. [DOI] [PubMed] [Google Scholar]
- 10.Smith AC, Cronan JE. Dimerization of the bacterial biotin carboxylase subunit is required for acetyl coenzyme A carboxylase activity in vivo. J Bacteriol. 2012;194:72–78. doi: 10.1128/JB.06309-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis MS, Cronan JE., Jr Inhibition of Escherichia coli acetyl coenzyme A carboxylase by acyl-acyl carrier protein. J Bacteriol. 2001;183:1499–1503. doi: 10.1128/JB.183.4.1499-1503.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gucchait RB, Polakis E, Dimroth P, Stoll E, Moss J, Lane MD. Acetyl coenzyme A carboxylase system of Escherichia coli. Purification and proterties of the biotin carboxylase, carboxyltransferase, and carboxyl carrier protein components. J Biol Chem. 1974;249:6633–6645. [PubMed] [Google Scholar]
- 13.Fall RR, Nervi AM, Alberts AW, Vagelos PR. Acetyl CoA carboxylase: isolation and characterization of native biotin carboxyl carrier protein. Proc Natl Acad Sci U S A. 1971;68:1512–1515. doi: 10.1073/pnas.68.7.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Fortin PD, Walsh CT. Andrimid producers encode an acetyl-CoA carboxyltransferase subunit resistant to the action of the antibiotic. Proc Natl Acad Sci U S A. 2008;105:13321–13326. doi: 10.1073/pnas.0806873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janiyani K, Bordelon T, Waldrop GL, Cronan JE., Jr Function of Escherichia coli biotin carboxylase requires catalytic activity of both subunits of the homodimer. J Biol Chem. 2001;276:29864–29870. doi: 10.1074/jbc.M104102200. [DOI] [PubMed] [Google Scholar]
- 16.Fall RR. Stabilization of an acetyl-CoA carboxylase comples from Pseudomonas citronellolis. Biochim Biophys Acta. 1976;450:475–480. doi: 10.1016/0005-2760(76)90022-9. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y-J, Rock CO. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol Microbiol. 2006;59:551–566. doi: 10.1111/j.1365-2958.2005.04951.x. [DOI] [PubMed] [Google Scholar]
- 18.Davis MS, Solbiati J, Cronan JE., Jr Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem. 2000;275:28593–28598. doi: 10.1074/jbc.M004756200. [DOI] [PubMed] [Google Scholar]
- 19.Khandekar SS, Gentry DR, Van Aller GS, Warren P, Xiang H, Silverman C, Doyle ML, Konstantinidis AK, Brandt M, Daines RA, Lonsdale JT. Identification, substrate specificity, and inhibition of the Streptococcus pneumoniae β-ketoacyl-acyl carrier protein synthase III (FabH) J Biol Chem. 2001;276:30024–30030. doi: 10.1074/jbc.M101769200. [DOI] [PubMed] [Google Scholar]
- 20.Choi K-H, Heath RJ, Rock CO. β-Ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J Bacteriol. 2000;182:365–370. doi: 10.1128/jb.182.2.365-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu X, Choudhry AE, Janson CA, Grooms M, Daines RA, Lonsdale JT, Khandekar SS. Crystal structure and substrate specificity of the β-ketoacyl-acyl carrier protein synthase III (FabH) from Staphylococcus aureus. Prot Sci. 2005;14:2087–2094. doi: 10.1110/ps.051501605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackowski S, Murphy CM, Cronan JE, Jr, Rock CO. Acetoacetyl-acyl carrier protein synthase: a target for the antibiotic thiolactomycin. J Biol Chem. 1989;264:7624–7629. [PubMed] [Google Scholar]
- 23.Reynolds CM, Kalb SR, Cotter RJ, Raetz CR. A phosphoethanolamine transferase specific for the outer 3-deoxy-D-manno-octulosonic acid residue of Escherichia coli lipopolysaccharide. Identification of the eptB gene and Ca2+ hypersensitivity of an eptB deletion mutant. J Biol Chem. 2005;280:21202–21211. doi: 10.1074/jbc.M500964200. [DOI] [PubMed] [Google Scholar]
- 24.He X, Reynolds KA. Purification, characterization, and identification of novel inhibitors of the β-ketoacyl-acyl carrier protein synthase III (FabH) from Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:1310–1318. doi: 10.1128/AAC.46.5.1310-1318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu X, Janson CA, Smith WW, Head M, Lonsdale J, Konstantinidis AK. Refined structures of β-ketoacyl-acyl carrier protein synthase III. J Mol Biol. 2001;307:341–356. doi: 10.1006/jmbi.2000.4457. [DOI] [PubMed] [Google Scholar]
- 26.Qiu X, Janson CA, Konstantinidis AK, Nwagwu S, Silverman C, Smith WW, Khandekar S, Lonsdale J, Abdel-Meguid SS. Crystal structure of β-ketoacyl-acyl carrier protein synthase III. A key condensing enzyme in bacterial fatty acid biosynthesis. J Biol Chem. 1999;274:36465–36471. doi: 10.1074/jbc.274.51.36465. [DOI] [PubMed] [Google Scholar]
- 27.He X, Reynolds KA. Purification, characterization, and identification of novel inhibitors of the β-ketoacyl-acyl carrier protein synthase III (FabH) from Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:1310–1318. doi: 10.1128/AAC.46.5.1310-1318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Florova G, Reynolds KA. Alteration of the fatty acid profile of Streptomyces coelicolor by replacement of the initiation enzyme 3-ketoacyl acyl carrier protein synthase III (FabH) J Bacteriol. 2005;187:3795–3799. doi: 10.1128/JB.187.11.3795-3799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarsdale JN, Kazanina G, He X, Reynolds KA, Wright HT. Crystal structure of the Mycobacterium tuberculosis β-ketoacyl-acyl carrier protein synthase III. J Biol Chem. 2001;276:20516–20522. doi: 10.1074/jbc.M010762200. [DOI] [PubMed] [Google Scholar]
- 30.Kruh NA, Borgaro JG, Ruzsicska BP, Xu H, Tonge PJ. A novel interaction linking the FAS-II and phthiocerol dimycocerosate (PDIM) biosynthetic pathways. J Biol Chem. 2008;283:31719–31725. doi: 10.1074/jbc.M802169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies C, Heath RJ, White SW, Rock CO. The 1.8 Å cystal structure and active site architecture of β-ketoacyl-[acyl carrier protein] synthase III (FabH) from Escherichia coli. Structure. 2000;8:185–195. doi: 10.1016/s0969-2126(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 32.Yuan Y, Schdeva M, Leeds JA, Meredith TC. Fatty acid biosynthesis in Pseudomonas aeruginosa is initiated by FabY: A new class of β-ketoacyl-acyl carrier protein synthases. J Bacteriol. 2012;194:5171–5184. doi: 10.1128/JB.00792-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CY, Cronan JE. β-Ketoacyl-acyl carrier protein synthase III (FabH) is essential for bacterial fatty acid synthesis. J Biol Chem. 2003;278:51494–51503. doi: 10.1074/jbc.M308638200. [DOI] [PubMed] [Google Scholar]
- 34.Yao Z, Davis RM, Kishony R, Kahne D, Ruiz N. Regulation of cell size in response to nutrient availability by fatty acid biosynthesis in Escherichia coli. Proc Natl Acad Sci U S A. 2012;109:E2561–E2568. doi: 10.1073/pnas.1209742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson GC, Tenson T, Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS ONE. 2011;6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seyfzadeh M, Keener J, Nomura M. spoT-dependent accumulation of guanosine tetraphosphate in response to fatty acid starvation in Escherichia coli. Proc Natl Acad Sci U S A. 1993;90:11004–11008. doi: 10.1073/pnas.90.23.11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gully D, Bouveret E. A protein network for phospholipid synthesis uncovered by a variant of the tandem affinity purification method in Escherichia coli. Proteomics. 2005;6:282–293. doi: 10.1002/pmic.200500115. [DOI] [PubMed] [Google Scholar]
- 38.Battesti A, Bouveret E. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol Microbiol. 2006;62:1048–1063. doi: 10.1111/j.1365-2958.2006.05442.x. [DOI] [PubMed] [Google Scholar]
- 39.Battesti A, Bouveret E. Bacteria possessing two RelA/SpoT-Like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J Bacteriol. 2009;191:616–624. doi: 10.1128/JB.01195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan-Kiss RM, Cronan JE. The Lactococcus lactis FabF fatty acid synthetic enzyme can functionally replace both the FabB and FabF proteins of Escherichia coli and the FabH protein of Lactococcus lactis. Arch Microbiol. 2008;190:427–437. doi: 10.1007/s00203-008-0390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberts AW, Bell RM, Vagelos PR. Acyl carrier protein. XV. Studies of β-ketoacyl-acyl carrier protein synthetase. J Biol Chem. 1972;247:3190–3198. [PubMed] [Google Scholar]
- 42.Alberts A, Vagelos PR. Acyl-CoA Carboxylases. In: Boyer PD, editor. The Enzymes. Academic Press, Inc; New York: 1972. pp. 37–82. [Google Scholar]
- 43.Arnvig MK, McGuire JN, Wettstein-Knowles P. Acyl carrier protein (ACP) inhibition and other differences between β-ketoacyl synthase (KAS) I and II. Biochem Soc Trans. 2000;28:607–610. doi: 10.1042/0300-5127:0280607. [DOI] [PubMed] [Google Scholar]
- 44.Heath RJ, Rock CO. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J Biol Chem. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- 45.Fisher M, Kroon JT, Martindale W, Stuitje AR, Slabas AR, Rafferty JB. The X-ray structure of Brassica napus β-ketoacyl carrier protein reductase and its implications for substrate binding and catalysis. Structure. 2000;8:339–347. doi: 10.1016/s0969-2126(00)00115-5. [DOI] [PubMed] [Google Scholar]
- 46.Price AC, Zhang Y-M, Rock CO, White SW. The structure of β-ketoacyl-[acyl carrier protein] reductase from Escherichia coli: negative cooperativity and its structural basis. Biochemistry. 2001;40:12772–12781. doi: 10.1021/bi010737g. [DOI] [PubMed] [Google Scholar]
- 47.Cohen-Gonsaud M, Ducasse S, Hoh F, Zerbib D, Labesse G, Quemard A. Crystal structure of MabA from Mycobacterium tuberculosis, a reductase involved in long-chain fatty acid biosynthesis. J Mol Biol. 2002;320:249–261. doi: 10.1016/S0022-2836(02)00463-1. [DOI] [PubMed] [Google Scholar]
- 48.Simons RW, Hughes KT, Nunn WD. Regulation of fatty acid degradation in Escherichia coli: dominance studies with strains merodiploid in gene fadR. J Bacteriol. 1980;143:726–730. doi: 10.1128/jb.143.2.726-730.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 50.Baker ME. Enoyl-acyl-carrier-protein reductase and Mycobacterium tuberculosis InhA do not conserve the Tyr-Xaa-Xaa-Xaa-Lys motif in mammalian 11β- and 17β-hydroxysteroid dehydrogenases and Drosophila alcohol dehydrogenase. Biochem J. 1995;309:1029–1030. doi: 10.1042/bj3091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath RJ, Su N, Murphy CK, Rock CO. The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis. J Biol Chem. 2000;275:40128–40133. doi: 10.1074/jbc.M005611200. [DOI] [PubMed] [Google Scholar]
- 52.Massengo-Tiasse RP, Cronan JE. Vibrio cholerae fabV defines a new class of enoyl acyl-carrier-protein reductase. J Biol Chem. 2008;283:1308–1316. doi: 10.1074/jbc.M708171200. [DOI] [PubMed] [Google Scholar]
- 53.Zhu L, Lin J, Ma J, Cronan JE, Wang H. Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrobiol Agents Chem. 2010;54:689–698. doi: 10.1128/AAC.01152-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu H, Tonge PJ. Mechanism and inhibition of the FabV enoyl-ACP reductase from Burkholderia mallei. Biochemistry. 2010;49:1281–1289. doi: 10.1021/bi902001a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattheus W, Masschelein J, Gao LJ, Herdewijn P, Landuyt B, Volckaert G, Lavigne R. The kalimantacin/batumin biosynthesis operon encodes a self-resistance isoform of the FabI bacterial target. Chem Biol. 2010;17:1067–1071. doi: 10.1016/j.chembiol.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Hirschbeck MW, Kuper J, Lu H, Liu N, Neckles C, Shah S, Wagner S, Sotriffer CA, Tonge PJ, Kisker C. Structure of the Yersinia pestis FabV enoyl-ACP reductase and its interaction with two 2-pyridone inhibitors. Structure. 2012;20:89–100. doi: 10.1016/j.str.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Zhang X, Bi L, He J, Jiang T. Determination of the crystal structure and active residues of FabV, the enoyl-ACP reductase from Xanthomonas oryzae. PLoS ONE. 2011;6:e26743. doi: 10.1371/journal.pone.0026743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heath RJ, Rock CO. A triclosan-resistant bacterial enzyme. Nature (London) 2000;406:145–146. doi: 10.1038/35018162. [DOI] [PubMed] [Google Scholar]
- 59.Saito J, Yamada M, Watanabe T, Iida M, Kitagawa H, Takahata S, Ozawa T, Takeuchi Y, Ohsawa F. Crystal structure of enoyl-acyl carrier protein reductase (FabK) from Streptococcus pneumoniae reveals the binding mode of an inhibitor. Protein Sci. 2008 doi: 10.1110/ps.073288808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jordan SW, Cronan JE., Jr A new metabolic link; the acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J Biol Chem. 1997;272:17903–17906. doi: 10.1074/jbc.272.29.17903. [DOI] [PubMed] [Google Scholar]
- 61.Zhao X, Miller JR, Cronan JE. The reaction of LipB, the octanoyl-[acyl carrier protein]:protein N-octanoyltransferase of lipoic acid synthesis, proceeds through an acyl-enzyme intermediate. Biochemistry. 2005;44:16737–16746. doi: 10.1021/bi051865y. [DOI] [PubMed] [Google Scholar]
- 62.Reed KE, Cronan JE., Jr Lipoic acid metabolism in Escherichia coli: Sequencing and functional characterization of the lipA and lipB genes. J Bacteriol. 1993;175:1325–1336. doi: 10.1128/jb.175.5.1325-1336.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin S, Hanson RE, Cronan JE. Biotin synthesis begins by hijacking the fatty acid synthetic pathway. Nat Chem Biol. 2010;6:682–688. doi: 10.1038/nchembio.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parsons JB, Frank MW, Subramanian C, Saenkham P, Rock CO. Metabolic basis for the differential susceptibility of Gram-positive pathogens to fatty acid synthesis inhibitors. Proc Natl Acad Sci U S A. 2011;108:15378–15383. doi: 10.1073/pnas.1109208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.More MI, Finger LD, Stryker JL, Fuqua C, Eberhard A, Winans SC. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 67.Val DL, Cronan JE., Jr In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer synthases. J Bacteriol. 1998;180:2644–2651. doi: 10.1128/jb.180.10.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bredenbruch F, Nimtz M, Wray V, Morr M, Muller R, Haussler S. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J Bacteriol. 2005;187:3630–3635. doi: 10.1128/JB.187.11.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu K, Rock CO. RhlA converts β-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the β-hydroxydecanoyl-β-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. J Bacteriol. 2008;190:3147–3154. doi: 10.1128/JB.00080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol Microbiol. 2012;83:840–855. doi: 10.1111/j.1365-2958.2012.07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cronan JE, Jr, Gelmann EP. An estimate of the minimum amount of unsaturated fatty acid required for growth of Escherichia coli. J Biol Chem. 1973;248:1188–1195. [PubMed] [Google Scholar]
- 72.Goh S, Good L. Plasmid selection in Escherichia coli using an endogenous essential gene marker. BMC Biotechnol. 2008;8:61–69. doi: 10.1186/1472-6750-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clark DP, de Mendoza D, Polacco ML, Cronan JE., Jr β-Hydroxydecanoyl thioester dehydrase does not catalyze a rate-limiting step in Escherichia coli unsaturated fatty acid synthesis. Biochemistry. 1983;22:5897–5902. doi: 10.1021/bi00294a032. [DOI] [PubMed] [Google Scholar]
- 74.Marrakchi H, Choi K-H, Rock CO. A new mechanism for anaerobic unsaturated fatty acid formation in Streptococcus pneumoniae. J Biol Chem. 2002;277:44809–44816. doi: 10.1074/jbc.M208920200. [DOI] [PubMed] [Google Scholar]
- 75.Jackowski S, Zhang Y-M, Price AC, White SW, Rock CO. A missense mutation in the fabB (β-ketoacyl-acyl carrier protein synthase I) gene confers thiolactomycin resistance to Escherichia coli. Antimicrob Agents Chemother. 2002;46:1246–1252. doi: 10.1128/AAC.46.5.1246-1252.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garwin JL, Klages AL, Cronan JE., Jr β-Ketoacyl-acyl carrier protein synthase II of Escherichia coli. Evidence for function in the thermal regulation of fatty acid synthesis. J Biol Chem. 1980;255:3263–3265. [PubMed] [Google Scholar]
- 77.Wang H, Cronan JE. Functional replacement of the FabA and FabB proteins of Escherichia coli fatty acid synthesis by Enterococcus faecalis FabZ and FabF homologues. J Biol Chem. 2004;279:34489–34495. doi: 10.1074/jbc.M403874200. [DOI] [PubMed] [Google Scholar]
- 78.Ulrich AK, de Mendoza D, Garwin JL, Cronan JE., Jr Genetic and biochemical analyses of Escherichia coli mutants altered in the temperature-dependent regulation of membrane lipid composition. J Bacteriol. 1983;154:221–230. doi: 10.1128/jb.154.1.221-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Overhage J, Lewenza S, Marr AK, Hancock REW. Identification of genes Involved in swarming motility using a Pseudomonas aeruginosa PAO1 Mini-Tn5-lux Mutant Library. J Bacteriol. 2007;189:2164–2169. doi: 10.1128/JB.01623-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Edwards P, Nelsen JS, Metz JG, Dehesh K. Cloning of the fabF gene in an expression vector and in vitro characterization of recombinant fabF and fabB encoded enzymes from Escherichia coli. FEBS Lett. 1997;402:62–66. doi: 10.1016/s0014-5793(96)01437-8. [DOI] [PubMed] [Google Scholar]
- 81.de Mendoza D, Klages Ulrich A, Cronan JE., Jr Thermal regulation of membrane fluidity in Escherichia coli. Effects of overproduction of β-ketoacyl-acyl carrier protein synthase I. J Biol Chem. 1983;258:2098–2101. [PubMed] [Google Scholar]
- 82.Subrahmanyam S, Cronan JE., Jr Overproduction of a functional fatty acid biosynthetic enzyme blocks fatty acid synthesis in Escherichia coli. J Biol Chem. 1998;180:4596–4602. doi: 10.1128/jb.180.17.4596-4602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Y-M, Rock CO. Acyltransferases in bacterial glycerophospholipid synthesis. J Lipid Res. 2008;49:1867–1874. doi: 10.1194/jlr.R800005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cronan JE, Jr, Weisberg LJ, Allen RG. Regulation of membrane lipid synthesis in Escherichia coli. Accumulation of free fatty acids of abnormal length during inhibition of phospholipid synthesis. J Biol Chem. 1975;250:5835–5840. [PubMed] [Google Scholar]
- 85.White SW, Zheng J, Zhang Y-M, Rock CO. The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem. 2005;74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- 86.Leesong M, Henderson BS, Gillig JR, Schwab JM, Smith JL. Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure. 1996;4:253–264. doi: 10.1016/s0969-2126(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 87.Altabe S, Lopez P, de Mendoza D. Isolation and characterization of unsaturated fatty acid auxotrophs of Streptococcus pneumoniae and Streptococcus mutans. J Bacteriol. 2007;189:8139–8144. doi: 10.1128/JB.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fozo EM, Quivey RG. The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J Bacteriol. 2004;186:4152–4158. doi: 10.1128/JB.186.13.4152-4158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu Y-J, White SW, Rock CO. Domain swapping between Enterococcus faecalis FabN and FabZ proteins localizes the structural determinants for isomerase activity. J Biol Chem. 2005;280:30342–30348. doi: 10.1074/jbc.M504637200. [DOI] [PubMed] [Google Scholar]
- 90.Isabella VM, Clark VL. Identification of a conserved protein involved in anaerobic unsaturated fatty acid synthesis in Neiserria gonorrhoeae: implications for facultative and obligate anaerobes that lack FabA. Mol Microbiol. 2011;82:489–501. doi: 10.1111/j.1365-2958.2011.07826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu L, Cheng J, Luo B, Feng S, Lin J, Wang S, Cronan JE, Wang H. Functions of the Clostridium acetobutylicium FabF and FabZ Proteins in unsaturated fatty acid biosynthesis. BMC Microbiol. 2009;9:119. doi: 10.1186/1471-2180-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Altabe SG, Aguilar P, Caballero GM, de Mendoza D. The Bacillus subtilis acyl lipid desaturase is a Δ5 desaturase. J Bacteriol. 2003;185:3228–3231. doi: 10.1128/JB.185.10.3228-3231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weber MHW, Klein W, Muller L, Niess UM, Marahiel MA. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol Microbiol. 2001;39:1321–1329. doi: 10.1111/j.1365-2958.2001.02322.x. [DOI] [PubMed] [Google Scholar]
- 94.Aguilar PS, Cronan JE, Jr, de Mendoza D. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J Bacteriol. 1998;180:2194–2200. doi: 10.1128/jb.180.8.2194-2200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu K, Choi K-H, Schweizer HP, Rock CO, Zhang Y-M. Two aerobic pathways for the formation of unsaturated fatty acids in Pseudomonas aeruginosa. Mol Microbiol. 2006;60:260–273. doi: 10.1111/j.1365-2958.2006.05088.x. [DOI] [PubMed] [Google Scholar]
- 96.Liavonchanka A, Hornung E, Feussner I, Rudolph MG. Structure and mechanism of the Propionibacterium acnes polyunsaturated fatty acid isomerase. Proc Natl Acad Sci U S A. 2006;103:2576–2581. doi: 10.1073/pnas.0510144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ray LF, Kellum RE. Corynebacterium acnes from human skin. Identification by morphologic, cultural, biochemical, serological, and chromatographic methods. Arch Dermatol. 1970;101:36–40. doi: 10.1001/archderm.101.1.36. [DOI] [PubMed] [Google Scholar]
- 98.Moss CW, Dowell VR, Jr, Lewis VJ, Schekter MA. Cultural characteristics and fatty acid composition of Corynebacterium acnes. J Bacteriol. 1967;94:1300–1305. doi: 10.1128/jb.94.5.1300-1305.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao J, Rock CO. Phosphatidic acid synthesis in bacteria. Biochim Biophys Acta. 2013;1831:495–502. doi: 10.1016/j.bbalip.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang P, Cronan JE., Jr Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol. 1994;176:2814–2821. doi: 10.1128/jb.176.10.2814-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ray PH, White DC. Effect of glycerol deprivation on the phospholipid metabolism of a glycerol auxotroph of Staphylococcus aureus. J Bacteriol. 1972;109:668–677. doi: 10.1128/jb.109.2.668-677.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ray PH, Lillich TT, White DC. Consequences of glycerol deprivation on the synthesis of membrane components in a glycerol auxotroph of Staphylococcus aureus. J Bacteriol. 1972;112:413–420. doi: 10.1128/jb.112.1.413-420.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bell RM. Mutants of Escherichia coli defective in membrane phospholipid synthesis: properties of wild type and Km defective sn-glycerol-3-phosphate acyltransfersae activities. J Biol Chem. 1975;250:7147–7152. [PubMed] [Google Scholar]
- 104.Lu Y-J, Zhang Y-M, Grimes KD, Qi J, Lee RE, Rock CO. Acyl-phosphates initiate membrane phospholipid synthesis in gram-positive pathogens. Molec Cell. 2006;23:765–772. doi: 10.1016/j.molcel.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 105.Greenway DLA, Silbert DF. Altered acyltransferase activity in Escherichia coli associated with mutations in acyl coenzyme A synthetase. J Biol Chem. 1983;258:13034–13042. [PubMed] [Google Scholar]
- 106.Polacco ML, Cronan JE., Jr Mechanism of the apparent regulation of Escherichia coli unsaturated fatty acid synthesis by exogenous oleic acid. J Biol Chem. 1977;252:5488–5490. [PubMed] [Google Scholar]
- 107.Goelz SE, Cronan JE., Jr The positional distribution of fatty acids in Escherichia coli phospholipids is not regulated by sn-glycerol 3-phosphate levels. J Bacteriol. 1980;144:462–464. doi: 10.1128/jb.144.1.462-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rock CO, Goelz SE, Cronan JE., Jr Phospholipid synthesis in Escherichia coli. Characteristics of fatty acid transfer from acyl-acyl carrier protein to sn-glycerol-3-phosphate. J Biol Chem. 1981;256:736–742. [PubMed] [Google Scholar]
- 109.Jackson MB, Cronan JE., Jr An estimate of the minimum amount of fluid lipid required for the growth of Escherichia coli. Biochim Biophys Acta. 1978;512:472–479. doi: 10.1016/0005-2736(78)90157-8. [DOI] [PubMed] [Google Scholar]
- 110.Cronan JE, Jr, Bell RM. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn-glycerol 3-phosphate acyltransferase Km mutants. J Bacteriol. 1974;120:227–233. doi: 10.1128/jb.120.1.227-233.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heath RJ, Rock CO. A missense mutation accounts for the defect in the glycerol-3-phosphate acyltransferase expressed in the plsB26 mutant. J Bacteriol. 1999;181:1944–1946. doi: 10.1128/jb.181.6.1944-1946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Larson TJ, Ludtke DN, Bell RM. sn-Glycerol-3-phosphate auxotrophy of plsB strains of Escherichia coli: Evidence that a second mutation, plsX, is required. J Bacteriol. 1984;160:711–717. doi: 10.1128/jb.160.2.711-717.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paoletti L, Lu Y-J, Schujman GE, de Mendoza D, Rock CO. Coupling of fatty acid and phospholipid synthesis in Bacillus subtilis. J Bacteriol. 2007;189:5816–5824. doi: 10.1128/JB.00602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Y-J, Zhang F, Grimes KD, Lee RE, Rock CO. Topology and active site of PlsY: the bacterial acylphosphate:glycerol-3-phosphate acyltransferase. J Biol Chem. 2007;282:11339–11346. doi: 10.1074/jbc.M700374200. [DOI] [PubMed] [Google Scholar]
- 115.Yoshimura M, Oshima T, Ogasawara N. Involvement of the YneS/YgiH and PlsX proteins in phospholipid biosynthesis in both Bacillus subtilis and Escherichia coli. BMC Microbiol. 2007;7:69. doi: 10.1186/1471-2180-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hara Y, Seki M, Matsuoka S, Hara H, Yamashita A, Matsumoto K. Involvement of PlsX and the acyl-phosphate dependent sn-glycerol-3-phosphate acyltransferase PlsY in the initial stage of glycerolipid synthesis in Bacillus subtilis. Genes Genet Syst. 2008;83:433–442. doi: 10.1266/ggs.83.433. [DOI] [PubMed] [Google Scholar]
- 117.Schulman H, Kennedy EP. Relation of turnover of membrane phospholipids to synthesis of membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1977;252:4250–4255. [PubMed] [Google Scholar]
- 118.Raetz CRH, Newman KF. Diglyceride kinase mutants of Escherichia coli: inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J Bacteriol. 1979;137:860–868. doi: 10.1128/jb.137.2.860-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goldberg DE, Rumley MK, Kennedy EP. Biosynthesis of membrane-derived oligosaccharides: a periplasmic phosphoglyceroltransferase. Proc Natl Acad Sci U S A. 1981;78:5513–5517. doi: 10.1073/pnas.78.9.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raetz CRH, Newman KF. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J Biol Chem. 1978;253:3882–3887. [PubMed] [Google Scholar]
- 121.Miller KJ, Kennedy EP, Reinhold VN. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986;231:48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- 122.Kennedy EP. Osmotic regulation and biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982;79:1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackson BJ, Bohin JP, Kennedy EP. Biosynthesis of membrane-derived oligosaccharides: characterization of mdoB mutants defective in phosphoglycerol transferase I activity. J Bacteriol. 1984;160:976–981. doi: 10.1128/jb.160.3.976-981.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rajagopal S, Eis N, Bhattacharya M, Nickerson KW. Membrane-derived oligosaccharides (MDOs) are essential for sodium dodecyl sulfate resistance in Escherichia coli. FEMS Microbiol Lett. 2003;223:25–31. doi: 10.1016/S0378-1097(03)00323-9. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi H, Watanabe M, Quinn PJ, Kato S, Murayama S, Ohki K, Hatta I. Effects of diacylglycerol on the structure and phase behaviour of non-bilayer forming phospholipid. Biophys Chem. 1999;77:37–48. doi: 10.1016/s0301-4622(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 126.Kawashima Y, Miyazaki E, Muller M, Tokuda H, Nishiyama K. Diacylglycerol specifically blocks spontaneous integration of membrane proteins and allows detection of a factor-assisted integration. J Biol Chem. 2008;283:24489–24496. doi: 10.1074/jbc.M801812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van Horn WD, Sanders CR. Prokaryotic diacylglycerol kinase and undecaprenol kinase. Annu Rev Biophys. 2011;41:81–101. doi: 10.1146/annurev-biophys-050511-102330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Horn WD, Kim HJ, Ellis CD, Hadziselimovic A, Sulistijo ES, Karra MD, Tian C, Sönnichsen FD, Sanders CR. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science. 2009;324:1726–1729. doi: 10.1126/science.1171716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wahl A, My L, Dumoulin R, Sturgis JN, Bouveret E. Antagonistic regulation of dgkA and plsB genes of phospholipid synthesis by multiple stress responses in Escherichia coli. Mol Microbiol. 2011;80:1260–1275. doi: 10.1111/j.1365-2958.2011.07641.x. [DOI] [PubMed] [Google Scholar]
- 130.Jerga A, Lu Y-J, Schujman GE, de Mendoza D, Rock CO. Identification of a soluble diacylglycerol kinase required for lipoteichoic acid production in Bacillus subtilis. J Biol Chem. 2007;282:21738–21745. doi: 10.1074/jbc.M703536200. [DOI] [PubMed] [Google Scholar]
- 131.Grundling A, Schneewind O. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104:8478–8483. doi: 10.1073/pnas.0701821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oku Y, Kurokawa K, Matsuo M, Yamada S, Lee BL, Sekimizu K. Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J Bacteriol. 2009;191:141–151. doi: 10.1128/JB.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller GA, Urban J, Jackson RW. Effects of a streptococcal lipoteichoic acid on host responses in mice. Infect Immun. 1976;13:1408–1417. doi: 10.1128/iai.13.5.1408-1417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gutberlet T, Frank J, Bradaczek H, Fischer W. Effect of lipoteichoic acid on thermotropic membrane properties. J Bacteriol. 1997;179:2879–2883. doi: 10.1128/jb.179.9.2879-2883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yamada S, Sugai M, Komatsuzawa H, Nakashima S, Oshida T, Matsumoto A, Suginaka H. An autolysin ring associated with cell separation of Staphylococcus aureus. J Bacteriol. 1996;178:1565–1571. doi: 10.1128/jb.178.6.1565-1571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seo HS, Michalek SM, Nahm MH. Lipoteichoic acid is important in innate immune responses to gram-positive bacteria. Infect Immun. 2008;76:206–213. doi: 10.1128/IAI.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grundling A, Schneewind O. Genes required for glycolipid synthesis and lipoteichoic acid anchoring in Staphylococcus aureus. J Bacteriol. 2007;189:2521–2530. doi: 10.1128/JB.01683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jerga A, Miller DJ, White SW, Rock CO. Molecular determinants for interfacial binding and conformational change in a soluble diacylglycerol kinase. J Biol Chem. 2009;284:7246–7254. doi: 10.1074/jbc.M805962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miller DJ, Jerga A, Rock CO, White SW. Analysis of the Staphylococcus aureus DgkB structure reveals a common catalytic mechanism for the soluble diacylglycerol kinases. Structure. 2008;16:1036–1046. doi: 10.1016/j.str.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang W, Campbell HA, King SC, Dowhan W. Phospholipids as determinants of membrane protein topology. Phosphatidylethanolamine is required for the proper topological organization of the γ-aminobutyric acid permease (GabP) of Escherichia coli. J Biol Chem. 2005;280:26032–26038. doi: 10.1074/jbc.M504929200. [DOI] [PubMed] [Google Scholar]
- 141.Xie J, Bogdanov M, Heacock P, Dowhan W. Phosphatidylethanolamine and monoglucosyldiacylglycerol are interchangeable in supporting topogenesis and function of the polytopic membrane protein lactose permease. J Biol Chem. 2006;281:19172–19178. doi: 10.1074/jbc.M602565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanfer J, Kennedy EP. Metabolism and function of bacterial lipids II. Biosynthesis of phospholipids in Escherichia coli. J Biol Chem. 1964;239:1720–1726. [PubMed] [Google Scholar]
- 143.Kishida S, Shirataki H, Sasaki T, Kato M, Kaibuchi K, Takai Y. Rab3A GTPase-activating protein-inhibiting activity of Rabphilin- 3A, a putative Rab3A target protein. J Biol Chem. 1993;268:22259–22261. [PubMed] [Google Scholar]
- 144.Sparrow CP, Raetz CR. Purfication and properties of the membrane-bound CDP-diglyceride synthetase from Escherichia coli. J Biol Chem. 1985;260:12084–12091. [PubMed] [Google Scholar]
- 145.Ganong BR, Leonard JM, Raetz CRH. Phosphatidic acid accumulation in the membranes of Escherichia coli mutants defective in CDP-diglyceride synthetase. J Biol Chem. 1980;255:1623–1629. [PubMed] [Google Scholar]
- 146.Ganong BR, Raetz CRH. Massive accumulation of phosphatidic acid in conditionally lethal CDP-diglyceride synthetase mutants and cytidine auxotrophs of Escherichia coli. J Biol Chem. 1982;257:389–394. [PubMed] [Google Scholar]
- 147.Ganong BR, Raetz CRH. pH-sensitive CDP-diglyceride synthetase mutants of Escherichia coli: phenotypic suppression by mutations at a second site. J Bacteriol. 1983;153:731–738. doi: 10.1128/jb.153.2.731-738.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miyazaki C, Kuroda M, Ohta A, Shibuya I. Genetic manipulation of membrane phospholipid composition in Escherichia coli: pgsA mutants defective in phosphatidylglycerol synthesis. Proc Natl Acad Sci U S A. 1985;82:7530–7534. doi: 10.1073/pnas.82.22.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu YH, Guan Z, Zhao J, Raetz CR. Three phosphatidylglycerol-phosphate phosphatases in the inner membrane of Escherichia coli. J Biol Chem. 2011;286:5506–5518. doi: 10.1074/jbc.M110.199265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia W, Dowhan W. Phosphatidylinositol cannot substitute for phosphatidylglycerol in supporting cell growth of Escherichia coli. J Bacteriol. 1995;177:2926–2928. doi: 10.1128/jb.177.10.2926-2928.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kikuchi S, Shibuya I, Matsumoto K. Viability of an Escherichia coli pgsA null mutant lacking detectable phosphatidylglycerol and cardiolipin. J Bacteriol. 2000;182:371–376. doi: 10.1128/jb.182.2.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martin PK, Li T, Sun D, Biek DP, Schmid MB. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J Bacteriol. 1999;181:3666–3673. doi: 10.1128/jb.181.12.3666-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hashimoto M, Takahashi H, Hara Y, Hara H, Asai K, Sadaie Y, Matsumoto K. Induction of extracytoplasmic function sigma factors in Bacillus subtilis cells with membranes of reduced phosphatidylglycerol content. Genes Genet Syst. 2009;84:191–198. doi: 10.1266/ggs.84.191. [DOI] [PubMed] [Google Scholar]
- 154.Den Kamp JA, Redai I, van Deenen LL. Phospholipid composition of Bacillus subtilis. J Bacteriol. 1969;99:298–303. doi: 10.1128/jb.99.1.298-303.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Short SA, White DC. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971;108:219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suzuki M, Hara H, Matsumoto K. Envelope disorder of Escherichia coli cells lacking phosphatidylglycerol. J Bacteriol. 2002;184:5418–5425. doi: 10.1128/JB.184.19.5418-5425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nishijima S, Asami Y, Uetake N, Yamagoe S, Ohta A, Shibuya I. Disruption of the Escherichia coli cls gene responsible for cardiolipin synthesis. J Bacteriol. 1988;170:775–780. doi: 10.1128/jb.170.2.775-780.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pluschke G, Hirota Y, Overath P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 1978;253:5048–5055. [PubMed] [Google Scholar]
- 159.Guo D, Tropp BE. A second Escherichia coli protein with CL synthase activity. Biochim Biophys Acta. 2000;1483:263–274. doi: 10.1016/s1388-1981(99)00193-6. [DOI] [PubMed] [Google Scholar]
- 160.Tan BK, Bogdanov M, Zhao J, Dowhan W, Raetz CR, Guan Z. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc Natl Acad Sci U S A. 2012;109:16504–16509. doi: 10.1073/pnas.1212797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tsai M, Ohniwa RL, Kato Y, Takeshita SL, Ohta T, Saito S, Hayashi H, Morikawa K. Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiol. 2011;11:13. doi: 10.1186/1471-2180-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.DeChavigny A, Heacock PN, Dowhan W. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem. 1991;266:5323–5332. [PubMed] [Google Scholar]
- 163.Dowhan W. Phosphatidylserine synthase from Escherichia coli. Methods Enzymol. 1992;209:287–298. doi: 10.1016/0076-6879(92)09036-3. [DOI] [PubMed] [Google Scholar]
- 164.Larson TJ, Dowhan W. Ribosomal-associated phosphatidylserine synthetase from Escherichia coli: purification by substrate-specific elution from phosphocellulose using cytidine 5’-diphospho-1,2-diacyl-sn-glycerol. Biochemistry. 1976;15:5212–5218. doi: 10.1021/bi00669a003. [DOI] [PubMed] [Google Scholar]
- 165.Louie K, Chen YC, Dowhan W. Substrate-induced membrane association of phosphatidylserine synthase from Escherichia coli. J Bacteriol. 1986;165:805–812. doi: 10.1128/jb.165.3.805-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ames GF. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968;95:833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li QX, Dowhan W. Studies on the mechanism of formation of the pyruvate prosthetic group of phosphatidylserine decarboxylase from Escherichia coli. J Biol Chem. 1990;265:4111–4115. [PubMed] [Google Scholar]
- 168.Hawrot E, Kennedy EP. Phospholipid composition and membrane function in phosphatidylserine decarboxylase mutants of Escherichia coli. J Biol Chem. 1978;253:8213–8220. [PubMed] [Google Scholar]
- 169.van Golde LM, Akkermans-Kruyswijk J, Franklin-Klein W, Lankhorst A, Prins RA. Accumulation of phosphatidylserine in strictly anaerobic lactate fermenting bacteria. FEBS Lett. 1975;53:57–60. doi: 10.1016/0014-5793(75)80681-8. [DOI] [PubMed] [Google Scholar]
- 170.Sohlenkamp C, Lopez-Lara IM, Geiger O. Biosynthesis of phosphatidylcholine in bacteria. Prog Lipid Res. 2003;42:115–162. doi: 10.1016/s0163-7827(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 171.Geiger O, Lopez-Lara IM, Sohlenkamp C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim Biophys Acta. 2013;1831:503–513. doi: 10.1016/j.bbalip.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 172.Sherr SI, Law JH. Phosphatidylcholine synthesis in Agrobacterium tumefaciens. II. Uptake and utilization of choline. J Biol Chem. 1965;240:3760–3765. [PubMed] [Google Scholar]
- 173.Nimaichand S, Sanasam S, Zheng LQ, Zhu WY, Yang LL, Tang SK, Ningthoujam DS, Li WJ. Rhodococcus canchipurensis sp. nov., a novel actinomycete isolated from a limestone deposit site in Manipur, India. Int J Syst Evol Microbiol. 2012 doi: 10.1099/ijs.0.036087-0. In press. [DOI] [PubMed] [Google Scholar]
- 174.Jackson M, Crick DC, Brennan PJ. Phosphatidylinositol is an essential phospholipid of mycobacteria. J Biol Chem. 2000;275:30092–30099. doi: 10.1074/jbc.M004658200. [DOI] [PubMed] [Google Scholar]
- 175.Morita YS, Fukuda T, Sena CB, Yamaryo-Botte Y, McConville MJ, Kinoshita T. Inositol lipid metabolism in mycobacteria: biosynthesis and regulatory mechanisms. Biochim Biophys Acta. 2011;1810:630–641. doi: 10.1016/j.bbagen.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 176.Bian GK, Feng ZZ, Qin S, Xing K, Wang Z, Cao CL, Liu CH, Dai CC, Jiang JH. Kineococcus endophytica sp. nov., a novel endophytic actinomycete isolated from a coastal halophyte in Jiangsu, China. Antonie Van Leeuwenhoek. 2012;102:621–628. doi: 10.1007/s10482-012-9757-4. [DOI] [PubMed] [Google Scholar]
- 177.Kozloff LM, Turner MA, Arellano F, Lute M. Phosphatidylinositol, a phospholipid of ice-nucleating bacteria. J Bacteriol. 1991;173:2053–2060. doi: 10.1128/jb.173.6.2053-2060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kiriukhin MY, Debabov DV, Shinabarger DL, Neuhaus FC. Biosynthesis of the glycolipid anchor in lipoteichoic acid of Staphylococcus aureus RN4220: role of YpfP, the diglucosyldiacylglycerol synthase. J Bacteriol. 2001;183:3506–3514. doi: 10.1128/JB.183.11.3506-3514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature (London) 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- 180.Jorasch P, Wolter FP, Zahringer U, Heinz E. A UDP glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol Microbiol. 1998;29:419–430. doi: 10.1046/j.1365-2958.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 181.Koch HU, Haas R, Fischer W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur J Biochem. 1984;138:357–363. doi: 10.1111/j.1432-1033.1984.tb07923.x. [DOI] [PubMed] [Google Scholar]
- 182.Reichmann NT, Grundling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wieslander A, Rilfors L, Johansson LB, Lindblom G. Reversed cubic phase with membrane glucolipids from Acholeplasma laidlawii. 1H, 2H, and diffusion nuclear magnetic resonance measurements. Biochemistry. 1981;20:730–735. doi: 10.1021/bi00507a010. [DOI] [PubMed] [Google Scholar]
- 184.Christiansson A, Wieslander A. Membrane lipid metabolism in Acholeplasma laidlawii A EF 22. Influence of cholesterol and temperature shift-down on incorporation of fatty acids and synthesis of membrane lipid species. Eur J Biochem. 1978;85:65–76. doi: 10.1111/j.1432-1033.1978.tb12212.x. [DOI] [PubMed] [Google Scholar]
- 185.Wieslander A, Rilfors L. Qualitative and quantitative variations of membrane lipid species in Acholeplasma laidlawii A. Biochim Biophys Acta. 1977;466:336–346. doi: 10.1016/0005-2736(77)90229-2. [DOI] [PubMed] [Google Scholar]
- 186.Niemi AR, Rilfors L, Lindblom G. Influence of monoglucosyldiacylglycerol and monoacylmonoglucosyldiacylglycerol on the lipid bilayer of the membrane from Acholeplasma laidlawii strain A-EF22. Biochim Biophys Acta. 1995;1239:186–194. doi: 10.1016/0005-2736(95)00132-m. [DOI] [PubMed] [Google Scholar]
- 187.Morein S, Andersson A-S, Rilfors L, Lindblom G. Wild-type Escherichia coli cells regulate the membrane lipid composition in a "window" between gel and non-lamellar structures. J Biol Chem. 1996;271:6801–6809. doi: 10.1074/jbc.271.12.6801. [DOI] [PubMed] [Google Scholar]
- 188.Lind J, Ramo T, Klement ML, Barany-Wallje E, Epand RM, Epand RF, Maler L, Wieslander A. High cationic charge and bilayer interface-binding helices in a regulatory lipid glycosyltransferase. Biochemistry. 2007;46:5664–5677. doi: 10.1021/bi700042x. [DOI] [PubMed] [Google Scholar]
- 189.Fedtke I, Mader D, Kohler T, Moll H, Nicholson G, Biswas R, Henseler K, Gotz F, Zahringer U, Peschel A. A Staphylococcus aureus ypfP mutant with strongly reduced lipoteichoic acid (LTA) content: LTA governs bacterial surface properties and autolysin activity. Mol Microbiol. 2007;65:1078–1091. doi: 10.1111/j.1365-2958.2007.05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shaw N. Bacterial glycolipids. Bacteriol Rev. 1970;34:365–377. doi: 10.1128/br.34.4.365-377.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Awai K, Watanabe H, Benning C, Nishida I. Digalactosyldiacylglycerol is required for better photosynthetic growth of Synechocystis sp. PCC6803 under phosphate limitation. Plant Cell Physiol. 2007;48:1517–1523. doi: 10.1093/pcp/pcm134. [DOI] [PubMed] [Google Scholar]
- 193.Tang Y, Hollingsworth RI. Digalactosyl diacylglycerols, plant glycolipids rarely found in bacteria, are major membrane components of bacteroid forms of Bradyrhizobium japonicum. Glycobiology. 1997;7:935–942. doi: 10.1093/glycob/7.7.935. [DOI] [PubMed] [Google Scholar]
- 194.Elamin AA, Stehr M, Spallek R, Rohde M, Singh M. The Mycobacterium tuberculosis Ag85A is a novel diacylglycerol acyltransferase involved in lipid body formation. Mol Microbiol. 2011;81:1577–1592. doi: 10.1111/j.1365-2958.2011.07792.x. [DOI] [PubMed] [Google Scholar]
- 195.Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol. 2004;186:5017–5030. doi: 10.1128/JB.186.15.5017-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kalscheuer R, Waltermann M, Alvarez M, Steinbuchel A. Preparative isolation of lipid inclusions from Rhodococcus opacus and Rhodococcus ruber and identification of granule-associated proteins. Arch Microbiol. 2001;177:20–28. doi: 10.1007/s00203-001-0355-5. [DOI] [PubMed] [Google Scholar]
- 197.Madison LL, Huisman GW. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999;63:21–53. doi: 10.1128/mmbr.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Geiger O, Gonzalez-Silva N, Lopez-Lara IM, Sohlenkamp C. Amino acid-containing membrane lipids in bacteria. Prog Lipid Res. 2010;49:46–60. doi: 10.1016/j.plipres.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 199.Vences-Guzman MA, Geiger O, Sohlenkamp C. Ornithine lipids and their structural modifications: from A to E and beyond. FEMS Microbiol Lett. 2012;335:1–10. doi: 10.1111/j.1574-6968.2012.02623.x. [DOI] [PubMed] [Google Scholar]
- 200.Zavaleta-Pastor M, Sohlenkamp C, Gao JL, Guan Z, Zaheer R, Finan TM, Raetz CR, Lopez-Lara IM, Geiger O. Sinorhizobium meliloti phospholipase C required for lipid remodeling during phosphorus limitation. Proc Natl Acad Sci U S A. 2010;107:302–307. doi: 10.1073/pnas.0912930107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Geiger O, Rohrs V, Weissenmayer B, Finan TM, Thomas-Oates JE. The regulator gene phoB mediates phosphate stress-controlled synthesis of the membrane lipid diacylglyceryl-N,N,N-trimethylhomoserine in Rhizobium (Sinorhizobium) meliloti. Mol Microbiol. 1999;32:63–73. doi: 10.1046/j.1365-2958.1999.01325.x. [DOI] [PubMed] [Google Scholar]
- 202.Riekhof WR, Andre C, Benning C. Two enzymes, BtaA and BtaB, are sufficient for betaine lipid biosynthesis in bacteria. Arch Biochem Biophys. 2005;441:96–105. doi: 10.1016/j.abb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 203.An D, Na C, Bielawski J, Hannun YA, Kasper DL. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc Natl Acad Sci U S A. 2011;108:4666–4671. doi: 10.1073/pnas.1001501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.LaBach JP, White DC. Identification of ceramide phosphorylethanolamine and ceramide phosphorylglycerol in the lipids of an anaerobic bacterium. J Lipid Res. 1969;10:528–534. [PubMed] [Google Scholar]
- 205.Olsen I, Jantzen E. Sphingolipids in Bacteria and Fungi. Anaerobe. 2012;7:103–112. [Google Scholar]
- 206.Nichols FC, Riep B, Mun J, Morton MD, Bojarski MT, Dewhirst FE, Smith MB. Structures and biological activity of phosphorylated dihydroceramides of Porphyromonas gingivalis. J Lipid Res. 2004;45:2317–2330. doi: 10.1194/jlr.M400278-JLR200. [DOI] [PubMed] [Google Scholar]
- 207.Miyagawa E, Azuma R, Suto T, Yano I. Occurrence of free ceramides in Bacteroides fragilis NCTC 9343. J Biochem. 1979;86:311–320. doi: 10.1093/oxfordjournals.jbchem.a132528. [DOI] [PubMed] [Google Scholar]
- 208.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature (London) 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 209.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature (London) 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 210.Bagley D, Kliger A, Weiss RM. Anaerobic urinary infections: Bacteroides fragilis bacteremia from the urinary tract. J Urol. 1980;124:160–161. doi: 10.1016/s0022-5347(17)55344-6. [DOI] [PubMed] [Google Scholar]
- 211.Heath RJ, Rock CO. Regulation of malonyl-CoA metabolism by acyl-acyl carrier protein and β-ketoacyl-acyl carrier protein synthases in Escherichia coli. J Biol Chem. 1995;270:15531–15538. doi: 10.1074/jbc.270.26.15531. [DOI] [PubMed] [Google Scholar]
- 212.Furukawa H, Tsay J-T, Jackowski S, Takamura Y, Rock CO. Thiolactomycin resistance in Escherichia coli is associated with the multidrug resistance efflux pump encoded by emrAB. J Bacteriol. 1993;175:3723–3729. doi: 10.1128/jb.175.12.3723-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bilder P, Lightle S, Bainbridge G, Ohren J, Finzel B, Sun F, Holley S, Al-Kassim L, Spessard C, Melnick M, Newcomer M, Waldrop GL. The structure of the carboxyltransferase component of acetyl-coA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochemistry. 2006;45:1712–1722. doi: 10.1021/bi0520479. [DOI] [PubMed] [Google Scholar]
- 214.Andre C, Haslam RP, Shanklin J. Feedback regulation of plastidic acetyl-CoA carboxylase by 18:1-acyl carrier protein in Brassica napus. Proc Natl Acad Sci U S A. 2012;109:10107–10112. doi: 10.1073/pnas.1204604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cronan JE, Weisberg LJ, Allen RG. Regulation of membrane lipid synthesis in Escherichia coli. Accumulation of free fatty acids of abnormal length during inhibition of phospholipid synthesis. J Biol Chem. 1975;250:5835–5840. [PubMed] [Google Scholar]
- 216.Huang W, Jia J, Edwards P, Dehesh K, Schneider G, Lindqvist Y. Crystal structure of β-ketoacyl-acyl carrier protein synthase II from E. coli reveals the molecular architecture of condensing enzymes. EMBO J. 1998;17:1183–1191. doi: 10.1093/emboj/17.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Moche M, Schneider G, Edwards P, Dehesh K, Lindqvist Y. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. J Biol Chem. 1999;274:6031–6034. doi: 10.1074/jbc.274.10.6031. [DOI] [PubMed] [Google Scholar]
- 218.Price AC, Choi KH, Heath RJ, Li Z, Rock CO, White SW. Inhibition of β-ketoacyl-[acyl carrier protein] synthases by thiolactomycin and cerulenin: structure and mechanism. J Biol Chem. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- 219.Olsen JG, Kadziola A, Wettstein-Knowles P, Siggaard-Andersen M, Larsen S. Structures of β-ketoacyl-acyl carrier protein synthase I complexed with fatty acids elucidate its catalytic machinery. Structure. 2001;9:233–243. doi: 10.1016/s0969-2126(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 220.Heath RJ, Rock CO. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:1833–1836. doi: 10.1074/jbc.271.4.1833. [DOI] [PubMed] [Google Scholar]
- 221.Glaser M, Bayer WH, Bell RM, Vagelos PR. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973;70:385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Glaser M, Nulty W, Vagelos PR. Role of adenylate kinase in the regulation of macromolecular biosynthesis in a putative mutant of Escherichia coli defective in membrane phospholipid biosynthesis. J Bacteriol. 1975;123:128–136. doi: 10.1128/jb.123.1.128-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bell RM. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974;117:1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McIntyre TM, Bell RM. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effect of cessation of net phospholipid synthesis on cytoplasmic and outer membranes. J Biol Chem. 1975;250:9053–9059. [PubMed] [Google Scholar]
- 225.McIntyre TM, Chamberlain BK, Webster RE, Bell RM. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effects of cessation and reinitiation of phospholipid synthesis on macromolecular synthesis and phospholipid turnover. J Biol Chem. 1977;252:4487–4493. [PubMed] [Google Scholar]
- 226.Goldberg I, Walker JR, Bloch K. Inhibition of lipid synthesis in Escherichia coli cells by the antibiotic cerulenin. Antimicrob Agents Chemother. 1973;3:549–554. doi: 10.1128/aac.3.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fiil NP, Willumsen BM, Friesen JD, von Meyenburg K. Interaction of alleles of the relA, relC and spoT genes in Escherichia coli: analysis of the interconversion of GTP, ppGpp and pppGpp. Mol Gen Genet. 1977;150:87–101. doi: 10.1007/BF02425329. [DOI] [PubMed] [Google Scholar]
- 228.Srivatsan A, Wang JD. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol. 2008;11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 229.Polakis SE, Guchhait RB, Lane MD. Stringent control of fatty acid synthesis in Escherichia coli. Possible regulation of acetyl coenzyme A carboxylase by ppGpp. J Biol Chem. 1973;248:7957–7966. [PubMed] [Google Scholar]
- 230.Ray TK, Cronan JE., Jr Acylation of sn-glycerol-3-phosphate in Escherichia coli. Study of reaction with native palmitoyl-acyl carrier protein. J Biol Chem. 1975;250:8422–8427. [PubMed] [Google Scholar]
- 231.Lueking DR, Goldfine H. The involvement of guanosine 5’-diphosphate-3’-diphosphate in the regulation of phospholipid biosynthesis in Escherichia coli. Lack of ppGpp inhibition of acyltransfer from acyl-ACP to sn-glycerol-3-phosphate. J Biol Chem. 1975;250:4911–4917. [PubMed] [Google Scholar]
- 232.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 233.Ohta A, Waggoner K, Louie K, Dowhan W. Cloning of genes involved in membrane lipid synthesis. Effects of amplification of phosphatidylserine synthase in Escherichia coli. J Biol Chem. 1981;256:2219–2225. [PubMed] [Google Scholar]
- 234.Jackson BJ, Gennity JM, Kennedy EP. Regulation of the balanced synthesis of membrane phospholipids. Experimental test of models for regulation in Escherichia coli. J Biol Chem. 1986;261:13464–13468. [PubMed] [Google Scholar]
- 235.Ohta A, Waggoner K, Radominska-Pyrek A, Dowhan W. Cloning of genes involved in membrane lipid synthesis: effects of amplification of phosphatidylglycerophosphate synthase in Escherichia coli. J Bacteriol. 1981;147:552–562. doi: 10.1128/jb.147.2.552-562.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Therisod H, Weissborn aC, Kennedy EP. An essential function for acyl carrier protein in the biosynthesis of membrane-derived oligosaccharides of Escherichia coli. Proc Natl Acad Sci U S A. 1986;83:7236–7240. doi: 10.1073/pnas.83.19.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Saha SK, Nishijima S, Matsuzaki H, Shibuya I, Matsumoto K. A regulatory mechanism for the balanced synthesis of membrane phospholipid species in Escherichia coli. Biosci Biotechnol Biochem. 1996;60:111–116. doi: 10.1271/bbb.60.111. [DOI] [PubMed] [Google Scholar]
- 238.Satre M, Kennedy EP. Identification of bound pyruvate essential for the activity of phosphatidylserine decarboxylase of Escherichia coli. J Biol Chem. 1976;253:479–483. [PubMed] [Google Scholar]
- 239.Li QX, Downhan W. Structural characterization of the Escherichia coli phosphatidylserine decarboxylase. J Biol Chem. 1988;263:11516–11522. [PubMed] [Google Scholar]
- 240.Recsei PA, Snell EE. Pyruvoyl enzymes. Annu Rev Biochem. 1984;53:357–387. doi: 10.1146/annurev.bi.53.070184.002041. [DOI] [PubMed] [Google Scholar]
- 241.Thanawastien A, Montor WR, Labaer J, Mekalanos JJ, Yoon SS. Vibrio cholerae proteome-wide screen for immunostimulatory proteins identifies phosphatidylserine decarboxylase as a novel Toll-like receptor 4 agonist. PLoS Pathog. 2009;5:e1000556. doi: 10.1371/journal.ppat.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Matsumoto K, Okada M, Horikoshi Y, Matsuzaki H, Kishi T, Itaya M, Shibuya I. Cloning, sequencing, and disruption of the Bacillus subtilis psd gene coding for phosphatidylserine decarboxylase. J Bacteriol. 1998;180:100–106. doi: 10.1128/jb.180.1.100-106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dowhan W. Phosphatidylserine decarboxylases: pyruvoyl-dependent enzymes from bacteria to mammals. Methods Enzymol. 1997;280:81–88. doi: 10.1016/s0076-6879(97)80104-8. [DOI] [PubMed] [Google Scholar]
- 244.Ernst CM, Peschel A. Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids. Mol Microbiol. 2011;80:290–299. doi: 10.1111/j.1365-2958.2011.07576.x. [DOI] [PubMed] [Google Scholar]
- 245.Andra J, Goldmann T, Ernst CM, Peschel A, Gutsmann T. Multiple peptide resistance factor (MprF)-mediated Resistance of Staphylococcus aureus against antimicrobial peptides coincides with a modulated peptide interaction with artificial membranes comprising lysyl-phosphatidylglycerol. J Biol Chem. 2011;286:18692–18700. doi: 10.1074/jbc.M111.226886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ernst CM, Staubitz P, Mishra NN, Yang SJ, Hornig G, Kalbacher H, Bayer AS, Kraus D, Peschel A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009;5:e1000660. doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Rubio A, Conrad M, Haselbeck RJ, Kedar GC, Brown-Driver V, Finn J, Silverman JA. Regulation of mprF by antisense RNA restores daptomycin susceptibility to daptomycin-resistant isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:364–367. doi: 10.1128/AAC.00429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Peschel A, Jack RW, Otto M, Collins LV, Staubitz P, Nicholson G, Kalbacher H, Nieuwenhuizen WF, Jung G, Tarkowski A, van Kessel KP, Van Strijp JA. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the novel virulence factor MprF is based on modification of membrane lipids with l-lysine. J Exp Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Staubitz P, Peschel A. MprF-mediated lysinylation of phospholipids in Bacillus subtilis--protection against bacteriocins in terrestrial habitats? Microbiology. 2002;148:3331–3332. doi: 10.1099/00221287-148-11-3331. [DOI] [PubMed] [Google Scholar]
- 250.Klein S, Lorenzo C, Hoffmann S, Walther JM, Storbeck S, Piekarski T, Tindall BJ, Wray V, Nimtz M, Moser J. Adaptation of Pseudomonas aeruginosa to various conditions includes tRNA-dependent formation of alanyl-phosphatidylglycerol. Mol Microbiol. 2009;71:551–565. doi: 10.1111/j.1365-2958.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 251.Roy H, Ibba M. Broad range amino acid specificity of RNA-dependent lipid remodeling by multiple peptide resistance factors. J Biol Chem. 2009;284:29677–29683. doi: 10.1074/jbc.M109.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci U S A. 2007;104:9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mehta S, Cuirolo AX, Plata KB, Riosa S, Silverman JA, Rubio A, Rosato RR, Rosato AE. VraSR two-component regulatory system contributes to mprF -mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:92–102. doi: 10.1128/AAC.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Otto M. Bacterial sensing of antimicrobial peptides. Contrib Microbiol. 2009;16:136–149. doi: 10.1159/000219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 256.Rodriguez-Herva JJ, Ramos-Gonzalez MI, Ramos JL. The Pseudomonas putida peptidoglycan-associated outer membrane lipoprotein is involved in maintenance of the integrity of the cell cell envelope. J Bacteriol. 1996;178:1699–1706. doi: 10.1128/jb.178.6.1699-1706.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kovacs-Simon A, Titball RW, Michell SL. Lipoproteins of bacterial pathogens. Infect Immun. 2011;79:548–561. doi: 10.1128/IAI.00682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Babu MM, Priya ML, Selvan AT, Madera M, Gough J, Aravind L, Sankaran K. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J Bacteriol. 2006;188:2761–2773. doi: 10.1128/JB.188.8.2761-2773.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rock CO. Turnover of fatty acids in the 1-position of phosphatidylethanolamine in Escherichia coli. J Biol Chem. 1984;259:6188–6194. [PubMed] [Google Scholar]
- 260.Jackowski S, Rock CO. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli. J Biol Chem. 1986;261:11328–11333. [PubMed] [Google Scholar]
- 261.Gupta SD, Dowhan W, Wu HC. Phosphatidylethanolamine is not essential for the N-acylation of apolipoprotein in Escherichia coli. J Biol Chem. 1991;266:9983–9986. [PubMed] [Google Scholar]
- 262.Jackowski S, Jackson PD, Rock CO. Sequence and function of the aas gene in Escherichia coli. J Biol Chem. 1994;269:2921–2928. [PubMed] [Google Scholar]
- 263.Cooper CL, Hsu L, Jackowski S, Rock CO. 2-Acylglycerolphosphoethanolamine acyltransferase/acyl-acyl carrier protein synthetase is a membrane-associated acyl carrier protein binding protein. J Biol Chem. 1989;264:7384–7389. [PubMed] [Google Scholar]
- 264.Jackowski S, Hsu L, Rock CO. 2-Acylglycerophosphoethanolamine acyltransferase/acyl-[acyl-carrier-protein] synthetase from Escherichia coli. Methods Enzymol. 1992;209:111–117. doi: 10.1016/0076-6879(92)09015-u. [DOI] [PubMed] [Google Scholar]
- 265.Hsu L, Jackowski S, Rock CO. Isolation and characterization of Escherichia coli K-12 mutants lacking both 2-acyl-glycerophosphoethanolamine acyltransferase and acyl-acyl carrier protein synthetase activity. J Biol Chem. 1991;266:13783–13788. [PubMed] [Google Scholar]
- 266.Jiang Y, Chan CH, Cronan JE. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain acyl-CoA synthetase family. Biochemistry. 2006;45:10008–10019. doi: 10.1021/bi060842w. [DOI] [PubMed] [Google Scholar]
- 267.Homma H, Nojima S. Transacylation between diacylphospholipids and 2-acyl lysophospholipids catalyzed by Escherichia coli extract. J Biochem (Tokyo) 1982;91:1093–1101. doi: 10.1093/oxfordjournals.jbchem.a133791. [DOI] [PubMed] [Google Scholar]
- 268.Doi O, Nojima S. Lysophospholipase of Escherichia coli. J Biol Chem. 1975;250:5208–5214. [PubMed] [Google Scholar]
- 269.Brozek KA, Bulawa CE, Raetz CR. Biosynthesis of lipid A precursors in Escherichia coli. A membrane-bound enzyme that transfers a palmitoyl residue from a glycerophospholipid to lipid X. J Biol Chem. 1987;262:5170–5179. [PubMed] [Google Scholar]
- 270.Bishop RE, Gibbons HS, Guina T, Trent MS, Miller SI, Raetz CR. Transfer of palmitate from phospholipids to lipid A in outer membranes of gram-negative bacteria. EMBO J. 2000;19:5071–5080. doi: 10.1093/emboj/cdd507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Harvat EM, Zhang Y-M, Tran CV, Zhang Z, Frank MW, Rock CO, Saier MH. Lysophospholipid flipping across the Escherichia coli inner membrane catalyzed by a transporter (LplT) belonging to the major facilitator superfamily. J Biol Chem. 2005;280:12028–12034. doi: 10.1074/jbc.M414368200. [DOI] [PubMed] [Google Scholar]
- 272.Asanuma M, Kurokawa K, Ichikawa R, Ryu KH, Chae JH, Dohmae N, Lee BL, Nakayama H. Structural evidence of α-aminoacylated lipoproteins of Staphylococcus aureus. FEBS J. 2011;278:716–728. doi: 10.1111/j.1742-4658.2010.07990.x. [DOI] [PubMed] [Google Scholar]
- 273.Kurokawa K, Lee H, Roh KB, Asanuma M, Kim YS, Nakayama H, Shiratsuchi A, Choi Y, Takeuchi O, Kang HJ, Dohmae N, Nakanishi Y, Akira S, Sekimizu K, Lee BL. The triacylated ATP binding cluster transporter substrate-binding lipoprotein of Staphylococcus aureus functions as a native ligand for Toll-like receptor 2. J Biol Chem. 2009;284:8406–8411. doi: 10.1074/jbc.M809618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Tawaratsumida K, Furuyashiki M, Katsumoto M, Fujimoto Y, Fukase K, Suda Y, Hashimoto M. Characterization of N-terminal structure of TLR2-activating lipoprotein in Staphylococcus aureus. J Biol Chem. 2009;284:9147–9152. doi: 10.1074/jbc.M900429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Kurokawa K, Kim MS, Ichikawa R, Ryu KH, Dohmae N, Nakayama H, Lee BL. Environment-mediated accumulation of diacyl lipoproteins over their triacyl counterparts in Staphylococcus aureus. J Bacteriol. 2012;194:3299–3306. doi: 10.1128/JB.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kol MA, Kuster DW, Boumann HA, de Cock H, Heck AJ, de Kruijff B, de Kroon AI. Uptake and remodeling of exogenous phosphatidylethanolamine in E. coli. Biochim Biophys Acta. 2004;1636:205–212. doi: 10.1016/j.bbalip.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 277.Pech-Canul A, Nogales J, Miranda-Molina A, Alvarez L, Geiger O, Soto MJ, Lopez-Lara IM. FadD is required for utilization of endogenous fatty acids released from membrane lipids. J Bacteriol. 2011;193:6295–6304. doi: 10.1128/JB.05450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang Y-M, Rock CO. Transcriptional regulation in bacterial membrane lipid synthesis. J Lipid Res. 2009;50:S115–S119. doi: 10.1194/jlr.R800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.DiRusso CC, Heimert TL, Metzger AK. Characterization of FadR, a global transcriptional regulator of fatty acid metabolism in Escherichia coli. Interaction with the fadB promoter is prevented by long chain fatty acyl coenzyme A. J Biol Chem. 1992;267:8685–8691. [PubMed] [Google Scholar]
- 280.Feng Y, Cronan JE. Escherichia coli unsaturated fatty acid synthesis: Complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J Biol Chem. 2009;284:29526–29535. doi: 10.1074/jbc.M109.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Feng Y, Cronan JE. Overlapping repressor binding sites result in additive regulation of Escherichia coli FadH by FadR and ArcA. J Bacteriol. 2010;192:4289–4299. doi: 10.1128/JB.00516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.DiRusso CC, Nyström T. The fats of Escherichia coli during infancy and old age: regulation by global regulators, alarmones and lipid intermediates. Mol Microbiol. 1998;27:1–8. doi: 10.1046/j.1365-2958.1998.00645.x. [DOI] [PubMed] [Google Scholar]
- 283.Cho BK, Knight EM, Palsson BO. Transcriptional regulation of the fad regulon genes of Escherichia coli by ArcA. Microbiology. 2006;152:2207–2219. doi: 10.1099/mic.0.28912-0. [DOI] [PubMed] [Google Scholar]
- 284.Pauli G, Ehring R, Overath P. Fatty acid degradation in Escherichia coli: requirement of cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein for enzyme synthesis. J Bacteriol. 1974;117:1178–1183. doi: 10.1128/jb.117.3.1178-1183.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Cronan JE., Jr In vivo evidence that acyl coenzyme A regulates DNA binding by the Escherichia coli FadR global transcription factor. J Bacteriol. 1997;179:1819–1823. doi: 10.1128/jb.179.5.1819-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.DiRusso CC, Tsvetnitsky V, H⊘jrup P, Knudsen J. Fatty acyl-CoA binding domain of the transcription factor FadR - Characterization by deletion, affinity labeling, and isothermal titration calorimetry. J Biol Chem. 1998;273:33652–33659. doi: 10.1074/jbc.273.50.33652. [DOI] [PubMed] [Google Scholar]
- 287.van den BB, Black PN, Clemons WM, Jr, Rapoport TA. Crystal structure of the long-chain fatty acid transporter FadL. Science. 2004;304:1506–1509. doi: 10.1126/science.1097524. [DOI] [PubMed] [Google Scholar]
- 288.Higashitani A, Nishimura Y, Hara H, Aiba H, Mizuno T, Horiuchi K. Osmoregulation of the fatty acid receptor gene fadL in Escherichia coli. Mol Gen Genet. 1993;240:339–347. doi: 10.1007/BF00280384. [DOI] [PubMed] [Google Scholar]
- 289.Rock CO, Jackowski S. Pathways for the incorporation of exogenous fatty acids into phosphatidylethanolamine in Escherichia coli. J Biol Chem. 1985;260:12720–12724. [PubMed] [Google Scholar]
- 290.Feng Y, Cronan JE. A New Member of Escherichia coli fad Regulon: Transcriptional Regulation of fadM (ybaW) J Bacteriol. 2009;191:6320–6328. doi: 10.1128/JB.00835-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Nie L, Ren Y, Janakiraman A, Smith S, Schulz H. A novel paradigm of fatty acid βoxidation exemplified by the thioesterase-dependent partial degradation of conjugated linoleic acid that fully supports growth of Escherichia coli. Biochemistry. 2008;47:9618–9626. doi: 10.1021/bi801074e. [DOI] [PubMed] [Google Scholar]
- 292.Nie L, Ren Y, Schulz H. Identification and characterization of Escherichia coli thioesterase III that functions in fatty acid β-oxidation. Biochemistry. 2008;47:7744–7751. doi: 10.1021/bi800595f. [DOI] [PubMed] [Google Scholar]
- 293.Feng Y, Cronan JE. Crosstalk of Escherichia coli FadR with global regulators in expression of fatty acid transport genes. PLoS ONE. 2012;7:e46275. doi: 10.1371/journal.pone.0046275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gui L, Sunnarborg A, LaPorte DC. Regulated expression of a repressor protein: FadR activates iclR. J Bacteriol. 1996;178:4704–4709. doi: 10.1128/jb.178.15.4704-4709.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Campbell JW, Cronan JE., Jr Escherichia coli FadR positively regulates transcription of the fabB fatty acid biosynthetic gene. J Bacteriol. 2001;183:5982–5990. doi: 10.1128/JB.183.20.5982-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Henry MF, Cronan JE., Jr Escherichia coli transcription factor that both activates fatty acid synthesis and represses fatty acid degradation. J Mol Biol. 1991;222:843–849. doi: 10.1016/0022-2836(91)90574-p. [DOI] [PubMed] [Google Scholar]
- 297.DiRusso CC, Heimert TL, Metzger AK. Characterization of FadR, a global transcriptional regulator of fatty acid metabolism in Escherichia coli. Interaction with the fadB promoter is prevented by long chain fatty acyl coenzyme As. J Biol Chem. 1992;267:8685–8691. [PubMed] [Google Scholar]
- 298.van Aalten DM, DiRusso CC, Knudsen J. The structural basis of acyl coenzyme A-dependent regulation of the transcription factor FadR. EMBO J. 2001;20:2041–2050. doi: 10.1093/emboj/20.8.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.van Aalten DM, DiRusso CC, Knudsen J, Wierenga RK. Crystal structure of FadR, a fatty acid-responsive transcription factor with a novel acyl coenzyme A-binding fold. EMBO J. 2000;19:5167–5177. doi: 10.1093/emboj/19.19.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.van Aalten DM, Knudsen J, DiRusso CC, Kokko T, Wierenga RK. Crystallization and X-ray diffraction studies of the fatty-acid responsive transcription factor FadR from Escherichia coli. Acta Crystallogr D Biol Crystallogr. 2000;56:469–471. doi: 10.1107/s0907444900000937. [DOI] [PubMed] [Google Scholar]
- 301.Xu Y, Heath RJ, Li Z, Rock CO, White SW. The FadR-DNA complex: Transcriptional control of fatty acid metabolism in Escherichia coli. J Biol Chem. 2001;276:17373–17379. doi: 10.1074/jbc.M100195200. [DOI] [PubMed] [Google Scholar]
- 302.Iram SH, Cronan JE. Unexpected functional diversity among FadR fatty acid transcriptional regulatory proteins. J Biol Chem. 2005;280:32148–32156. doi: 10.1074/jbc.M504054200. [DOI] [PubMed] [Google Scholar]
- 303.Matsuoka H, Hirooka K, Fujita Y. Organization and function of the YsiA regulon of Bacillus subtilis involved in fatty acid degradation. J Biol Chem. 2007;282:5180–5194. doi: 10.1074/jbc.M606831200. [DOI] [PubMed] [Google Scholar]
- 304.Feng Y, Cronan JE. The Vibrio cholerae fatty acid regulatory protein, FadR, represses transcription of plsB, the gene encoding the first enzyme of membrane phospholipid biosynthesis. Mol Microbiol. 2011;81:1020–1033. doi: 10.1111/j.1365-2958.2011.07748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Cameron DE, Urbach JM, Mekalanos JJ. A defined transposon mutant library and its use in identifying motility genes in Vibrio cholerae. Proc Natl Acad Sci U S A. 2008;105:8736–8741. doi: 10.1073/pnas.0803281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Iram SH, Cronan JE. The β-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J Bacteriol. 2006;188:599–608. doi: 10.1128/JB.188.2.599-608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Brown RN, Gulig PA. Regulation of fatty acid metabolism by FadR is essential for Vibrio vulnificus to cause infection of mice. J Bacteriol. 2008;190:7633–7644. doi: 10.1128/JB.01016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Subramanian C, Zhang Y-M, Rock CO. DesT coordinates the expression of anaerobic and aerobic pathways for unsaturated fatty acid biosynthesis in Pseudomonas aeruginosa. J Bacteriol. 2010;192:280–285. doi: 10.1128/JB.00404-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Zhang Y-M, Zhu K, Frank MW, Rock CO. A Pseudomonas aeruginosa transcription factor that senses fatty acid structure. Mol Microbiol. 2007;66:622–632. doi: 10.1111/j.1365-2958.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- 311.Miller DJ, Zhang Y-M, Subramainian C, Rock CO, White SW. Transcriptional regulation of membrane lipid homeostasis. Nat Struct Mol Biol. 2010;17:971–975. doi: 10.1038/nsmb.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang Y-M, Marrakchi H, Rock CO. The FabR (YijC) transcription factor regulates unsaturated fatty acid biosynthesis in Escherichia coli. J Biol Chem. 2002;277:15558–15565. doi: 10.1074/jbc.M201399200. [DOI] [PubMed] [Google Scholar]
- 313.Feng Y, Cronan JE. Complex binding of the FabR repressor of bacterial unsaturated fatty acid biosynthesis to its cognate promoters. Mol Microbiol. 2011;80:195–218. doi: 10.1111/j.1365-2958.2011.07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhu K, Zhang YM, Rock CO. Transcriptional regulation of membrane lipid homeostasis in Escherichia coli. J Biol Chem. 2009;284:34880–34888. doi: 10.1074/jbc.M109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.McCue L, Thompson W, Carmack C, Ryan MP, Liu JS, Derbyshire V, Lawrence CE. Phylogenetic footprinting of transcription factor binding sites in proteobacterial genomes. Nucleic Acids Res. 2001;29:774–782. doi: 10.1093/nar/29.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Andrews JC, Short SA. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986;165:434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Martinez MA, Zaballa ME, Schaeffer F, Bellinzoni M, Albanesi D, Schujman GE, Vila AJ, Alzari PM, de Mendoza D. A novel role of malonyl-ACP in lipid homeostasis. Biochemistry. 2010;49:3161–3167. doi: 10.1021/bi100136n. [DOI] [PubMed] [Google Scholar]
- 318.Schujman GE, Guerin M, Buschiazzo A, Schaeffer F, Llarrull LI, Reh G, Vila AJ, Alzari PM, de Mendoza D. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. EMBO J. 2006;25:4074–4083. doi: 10.1038/sj.emboj.7601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Albanesi D, Reh G, Guerin ME, Schaeffer F, Debarbouille M, Buschiazzo A, Schujman GE, de MD, Alzari PM. Structural basis for feed-rorward transcriptional regulation of membrane lipid homeostasis in Staphylococcus aureus. PLoS Pathog. 2013;9:e1003108. doi: 10.1371/journal.ppat.1003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Schujman GE, Altabe S, de Mendoza D. A malonyl-CoA-dependent switch in the bacterial response to a dysfunction of lipid metabolism. Mol Microbiol. 2008;68:987–996. doi: 10.1111/j.1365-2958.2008.06202.x. [DOI] [PubMed] [Google Scholar]
- 321.Rock CO, Jackowski S. Regulation of phospholipid synthesis in Escherichia coli. Composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982;257:10759–10765. [PubMed] [Google Scholar]
- 322.Jackowski S, Rock CO. Ratio of active to inactive forms of acyl carrier protein in Escherichia coli. J Biol Chem. 1983;258:15186–15191. [PubMed] [Google Scholar]
- 323.Jackowski S, Rock CO. Consequences of reduced intracellular coenzyme A content in Escherichia coli. J Bacteriol. 1986;166:866–871. doi: 10.1128/jb.166.3.866-871.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Jerga A, Rock CO. Acyl-acyl carrier protein regulates transcription of fatty acid biosynthetic genes via the FabT repressor in Streptococcus pneumoniae. J Biol Chem. 2009;284:15364–15368. doi: 10.1074/jbc.C109.002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Schujman GE, Paoletti L, Grossman AD, de Mendoza D. FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis. Dev Cell. 2003;4:663–672. doi: 10.1016/s1534-5807(03)00123-0. [DOI] [PubMed] [Google Scholar]
- 326.Mansilla MC, de Mendoza D. The Bacillus subtilis desaturase: a model to understand phospholipid modification and temperature sensing. Arch Microbiol. 2005;183:229–235. doi: 10.1007/s00203-005-0759-8. [DOI] [PubMed] [Google Scholar]
- 327.Seltmann G, Holst O. The Bacterial Cell Wall. Springer; 2002. The Outer Membrane Gram-Negative Bacteria; pp. 18–22. [Google Scholar]
- 328.Aguilar PS, Lopez P, de Mendoza D. Transcriptional control of the low-temperature-inducible des gene, encoding the Δ5 desaturase of Bacillus subtilis. J Bacteriol. 1999;181:7028–7033. doi: 10.1128/jb.181.22.7028-7033.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cybulski LE, del Solar G, Craig PO, Espinosa M, de Mendoza D. Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J Biol Chem. 2004;279:39340–39347. doi: 10.1074/jbc.M405150200. [DOI] [PubMed] [Google Scholar]
- 330.Cybulski LE, Albanesi D, Mansilla MC, Altabe S, Aguilar PS, de Mendoza D. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol Microbiol. 2002;45:1379–1388. doi: 10.1046/j.1365-2958.2002.03103.x. [DOI] [PubMed] [Google Scholar]
- 331.Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 2001;20:1681–1691. doi: 10.1093/emboj/20.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Najle SR, Inda ME, de Mendoza D, Cybulski LE. Oligomerization of Bacillus subtilis DesR is required for fine tuning regulation of membrane fluidity. Biochim Biophys Acta. 2009;1790:1238–1243. doi: 10.1016/j.bbagen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 333.Albanesi D, Mansilla MC, de Mendoza D. The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol. 2004;186:2655–2663. doi: 10.1128/JB.186.9.2655-2663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, Stuart MK, Wilkinson BJ. Insertional inactivation of branched-chain α-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl Environ Microbiol. 2008;74:5882–5890. doi: 10.1128/AEM.00882-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Luo Y, Asai K, Sadaie Y, Helmann JD. Transcriptomic and phenotypic characterization of a Bacillus subtilis strain without extracytoplasmic function sigma factors. J Bacteriol. 2010;192:5736–5745. doi: 10.1128/JB.00826-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zweers JC, Nicolas P, Wiegert T, van Dijl JM, Denham EL. Definition of the σW regulon of Bacillus subtilis in the absence of stress. PLoS ONE. 2012;7:e48471. doi: 10.1371/journal.pone.0048471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zweers JC, Wiegert T, van Dijl JM. Stress-responsive systems set specific limits to the overproduction of membrane proteins in Bacillus subtilis. Appl Environ Microbiol. 2009;75:7356–7364. doi: 10.1128/AEM.01560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Huang X, Gaballa A, Cao M, Helmann JD. Identification of target promoters for the Bacillus subtilis extracytoplasmic function sigma factor, σW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 339.Kingston AW, Subramanian C, Rock CO, Helmann JD. A σW-dependent stress response in Bacillus subtilis that reduces membrane fluidity. Mol Microbiol. 2011;81:69–79. doi: 10.1111/j.1365-2958.2011.07679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Eiamphungporn W, Helmann JD. The Bacillus subtilis σW regulon and its contribution to cell envelope stress responses. Mol Microbiol. 2008;67:830–848. doi: 10.1111/j.1365-2958.2007.06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ng WL, Tsui HC, Winkler ME. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J Bacteriol. 2005;187:7444–7459. doi: 10.1128/JB.187.21.7444-7459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Gutu AD, Wayne KJ, Sham LT, Winkler ME. Kinetic characterization of the WalRKSpn (VicRK) two-component system of Streptococcus pneumoniae: dependence of WalKSpn (VicK) phosphatase activity on its PAS domain. J Bacteriol. 2010;192:2346–2358. doi: 10.1128/JB.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Cao M, Helmann JD. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J Bacteriol. 2004;186:1136–1146. doi: 10.1128/JB.186.4.1136-1146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ho TD, Ellermeier CD. Extra cytoplasmic function sigma factor activation. Curr Opin Microbiol. 2012;15:182–188. doi: 10.1016/j.mib.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Baldock C, Rafferty JB, Sedeinikova SE, Baker PJ, Stuitje AR, Slabas AR, Hawkes TR, Rice DW. A mechanism of drug action revealed by structural studies of enoyl reductase. Science. 1996;274:2107–2110. doi: 10.1126/science.274.5295.2107. [DOI] [PubMed] [Google Scholar]
- 346.Lei B, Wei CJ, Tu SC. Action mechanism of antitubercular isoniazid. Activation by Mycobacterium tuberculosis KatG, isolation, and characterization of InhA inhibitor. J Biol Chem. 2000;275:2520–2526. doi: 10.1074/jbc.275.4.2520. [DOI] [PubMed] [Google Scholar]
- 347.Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB. Molecular basis of triclosan activity. Nature (London) 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- 348.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem. 1999;274:11110–11114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 349.Dann AB, Hontela A. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol. 2011;31:285–311. doi: 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- 350.Sharma S, Ramya TN, Surolia A, Surolia N. Triclosan as a systemic antibacterial agent in a mouse model of acute bacterial challenge. Antimicrob Agents Chemother. 2003;47:3859–3866. doi: 10.1128/AAC.47.12.3859-3866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Yazdankhah SP, Scheie AA, Hoiby EA, Lunestad BT, Heir E, Fotland TO, Naterstad K, Kruse H. Triclosan and antimicrobial resistance in bacteria: an overview. Microb Drug Resist. 2006;12:83–90. doi: 10.1089/mdr.2006.12.83. [DOI] [PubMed] [Google Scholar]
- 352.Balemans W, Lounis N, Gilissen R, Guillemont J, Simmen K, Andries K, Koul A. Essentiality of FASII pathway for Staphylococcus aureus. Nature (London) 2010;463:E3. doi: 10.1038/nature08667. [DOI] [PubMed] [Google Scholar]
- 353.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Freiberg C, Pohlmann J, Nell PG, Endermann R, Schuhmacher J, Newton B, Otteneder M, Lampe T, Habich D, Ziegelbauer K. Novel bacterial acetyl-coenzyme A carboxylase inhibitors with antibiotic efficacy in vivo. Antimicrob Agents Chemother. 2006;50:2707–2712. doi: 10.1128/AAC.00012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature (London) 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 356.Payne DJ, Miller WH, Berry V, Brosky J, Burgess WJ, Chen E, DeWolf JW, Jr, Fosberry AP, Greenwood R, Head MS, Heerding DA, Janson CA, Jaworski DD, Keller PM, Manley PJ, Moore TD, Newlander KA, Pearson S, Polizzi BJ, Qiu X, Rittenhouse SF, Slater-Radosti C, Salyers KL, Seefeld MA, Smyth MG, Takata DT, Uzinskas IN, Vaidya K, Wallis NG, Winram SB, Yuan CC, Huffman WF. Discovery of a novel and potent class of FabI-directed antibacterial agents. Antimicrob Agents Chemother. 2002;46:3118–3124. doi: 10.1128/AAC.46.10.3118-3124.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Miller WH, Seefeld MA, Newlander KA, Uzinskas IN, Burgess WJ, Heerding DA, Yuan CC, Head MS, Payne DJ, Rittenhouse SF, Moore TD, Pearson SC, Berry V, DeWolf WE, Jr, Keller PM, Polizzi BJ, Qiu X, Janson CA, Huffman WF. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI) J Med Chem. 2002;45:3246–3256. doi: 10.1021/jm020050+. [DOI] [PubMed] [Google Scholar]
- 358.Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, Gonzalez I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Discovery of platencin, a dual FabF and FabH inhibitor with in vivo antibiotic properties. Proc Natl Acad Sci U S A. 2007;104:7612–7616. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kaplan N, Albert M, Awrey D, Bardouniotis E, Berman J, Clarke T, Dorsey M, Hafkin B, Ramnauth J, Romanov V, Schmid MB, Thalakada R, Yethon J, Pauls HW. Mode of action, in vitro activity, and in vivo efficacy of AFN-1252, a selective antistaphylococcal FabI inhibitor. Antimicrob Agents Chemother. 2012;56:5865–5874. doi: 10.1128/AAC.01411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Escalada MG, Harwood JL, Maillard JY, Ochs D. Triclosan inhibition of fatty acid synthesis and its effect on growth of Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother. 2005;55:879–882. doi: 10.1093/jac/dki123. [DOI] [PubMed] [Google Scholar]
- 361.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 362.Karlowsky JA, Kaplan N, Hafkin B, Hoban DJ, Zhanel GG. AFN-1252, a FabI inhibitor, demonstrates a Staphylococcal-specific spectrum of activity. Antimicrob Agents Chemother. 2009;53:3544–3548. doi: 10.1128/AAC.00400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Karlowsky JA, Laing NM, Baudry T, Kaplan N, Vaughan D, Hoban DJ, Zhanel GG. In vitro activity of API-1252, a novel FabI inhibitor, against clinical isolates of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2007;51:1580–1581. doi: 10.1128/AAC.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Escaich S, Prouvensier L, Saccomani M, Durant L, Oxoby M, Gerusz V, Moreau F, Vongsouthi V, Maher K, Morrissey I, Soulama-Mouze C. The MUT056399 inhibitor of FabI is a new antistaphylococcal compound. Antimicrob Agents Chemother. 2011;55:4692–4697. doi: 10.1128/AAC.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Park HS, Yoon YM, Jung SJ, Yun IN, Kim CM, Kim JM, Kwak JH. CG400462, a new bacterial enoyl-acyl carrier protein reductase (FabI) inhibitor. Int J Antimicrob Agents. 2007;30:446–451. doi: 10.1016/j.ijantimicag.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 366.Lu H, Tonge PJ. Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res. 2008;41:11–20. doi: 10.1021/ar700156e. [DOI] [PubMed] [Google Scholar]
- 367.Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature (London) 2009;458:83–86. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 368.Martin N, Christensen QH, Mansilla MC, Cronan JE, de Mendoza D. A novel two-gene requirement for the octanoyltransfer reaction of Bacillus subtilis lipoic acid biosynthesis. Mol Microbiol. 2011;80:335–349. doi: 10.1111/j.1365-2958.2011.07597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 370.Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. Brinster et al reply. Nature (London) 2010;463:E4. doi: 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 371.Shimomura Y, Sugiyama S, Takamura T, Kondo T, Ozawa T. Quantitative determination of the fatty acid composition of human serum lipids by high-performance liquid chromatography. J Chromatogr. 1986;383:9–17. doi: 10.1016/s0378-4347(00)83435-0. [DOI] [PubMed] [Google Scholar]
- 372.Zhu K, Zhang YM, Rock CO. Transcriptional regulation of membrane lipid homeostasis in Escherichia coli. J Biol Chem. 2009;284:34880–34888. doi: 10.1074/jbc.M109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]