Abstract
OBJECTIVES
Factors influencing xerostomia during intensity-modulated radiation therapy (IMRT) were assessed.
METHODS
A 6-week study of 32 head and neck cancer (HNC) patients was performed. Subjects completed the Xerostomia Inventory (XI) and provided stimulated saliva (SS) at baseline, week two and at end of IMRT. Influence of SS flow rate (SSFR), calcium and mucin 5b (MUC5b) concentrations and radiation dose on xerostomia was determined.
RESULTS
HNC subjects experienced mean SSFR decline of 36% by visit two (N=27; p=0.012) and 57% by visit three (N=20; p=0.0004), Concentrations of calcium and MUC5b increased, but not significantly during IMRT (p>0.05). Xerostomia correlated most with decreasing salivary flow rate as determined by Spearman correlations (p<0.04) and linear mixed models (p<0.0001).
CONCLUSIONS
Although IMRT is sparing to the parotid glands, it has an early effect on SSFR and the constituents in saliva in a manner that is associated with the perception of xerostomia.
Keywords: burning mouth, burning tongue, calcium, gland sparing, head and neck cancer, hyposalivation, intensity modulated radiation therapy, IMRT, irradiation, mucin, MUC5B, saliva, xerostomia
More than 50,000 patients have head and neck cancer (HNC) treated with radiation therapy (RT) each year1, and significant oral discomfort accompanies this therapy and post-therapy. A major factor contributing to decreased oral comfort is decreased salivation.2–6 Hyposalivation is caused by radiation damage to the salivary glands, and typically arises when doses exceed 1,500 to 2,000 cGy.6–8 Xerostomia and the sensation of oral dryness is caused in part by hyposalivation,9, 10 and has been documented to occur when unstimulated saliva production is reduced by at least 40%.11, 12 Changes in saliva composition also appear contributory, as many patients with low salivary flow rates do not report xerostomia.13
Saliva composition changes as a result of HN irradiation.3, 14 Radiation induces a decrease in amylase activity, bicarbonate, and pH.14–16 Significant increases have been observed in the osmolality, viscosity, lactoferrin, protein, sodium and chloride concentrations after irradiation;3, 15, 17–19 although some studies report sodium and protein content being lower in saliva after RT.14–16 Interestingly, there are limited reports regarding altered calcium concentration in saliva after irradiation, and secretion of saliva is calcium dependent.20 Salivary calcium concentration has decreased in rats exposed to irradiation21, 22 but have been observed at higher concentration in nine HNC patients one to two years after recovery from RT.12, 16 Calcium concentrations also have been shown to be higher in patients who have oral dryness, and to correlate with the severity of oral dryness.23 However, there is insufficient knowledge of calcium concentrations in saliva of HNC patients during RT, the period when oral dryness often first appears.
There are eleven human mucins associated with the secretory functions of the gastrointestinal and respiratory tracts.24 Mucin 5b (MUC5b) and MUC7 are two of the main mucins secreted by the salivary glands.25 MUC7 is a watery, low molecular weight mucin found in saliva while MUC5b is the sticky, high molecular weight glycosylated protein that adheres to mucosa. MUC5b forms multimers and a viscous coat that protects the mucosal surface from harm and theoretically contributes to oral cavity hydration, lubrication and moistening, and thus may play a role in the perception of xerostomia.26 To date, we were able to find only one study that examined MUC5b concentrations in HNC patients, and that study analyzed samples procured 12 months after radiation therapy.27 The absence of studies that have evaluated the role of MUC5b in xerostomia during RT is an impetus for this study.
Intensity-modulated RT (IMRT) spares the parotid glands from doses accumulating in other oral tissues28 and reduces the incidence of hyposalivation.29–31 Nevertheless, IMRT is associated with, and predictive of, xerostomia and the development of sticky saliva.32, 33,34, 35 At present, our understanding of the concurrent changes in salivary flow and composition that occur during IMRT is incomplete. The hypothesis of the present study was that stimulated saliva flow rate (SSFR) and concentrations of calcium and MUC5b may be disturbed by the physiological effects caused by dosage-sparing IMRT. The inter-relationship between these factors were investigated during IMRT, because the perception of xerostomia often initiates during the course of HN radiation therapy.36
METHODS
Patients
Patients were eligible for study if they underwent IMRT delivered by linear acceleration or tomotherapy as treatment for HNC at the University of Kentucky Chandler Medical Center between July 20, 2009 and December 31, 2011. The diagnosis of HNC was based on established standard clinical and pathologic criteria. Exclusion criteria included age less than 18 years, pregnant or nursing, not fluent in English, history of previous RT, history of Sjögren’s syndrome, unable or unwilling to provide informed consent or samples, treatment with chemotherapeutic drugs or anti-organ rejection drugs within the last year, or presence of a febrile illness or active infection at the time of enrollment. Once screened, 37 consecutive patients who were interested in participation were enrolled and demographic and medical history information was obtained. In addition, a cohort of eleven healthy subjects (i.e., their medical history and physical examination was negative for HNC and any current or recent symptoms) was recruited to provide baseline comparative saliva samples for establishment of MUC5b standard curves and to validate that sample processing and storage did not produce unexpected abnormalities. The controls were a convenience sample and did not have any systemic disease and did not take any medications. The study was approved by the University’s Institutional Review Board and written informed consent was obtained from all patients before their procedure.
Treatment
For all patients, a planning CT-scan was performed in the treatment position using 2.5 mm or smaller slice thickness. A custom Aquaplast mask was used for immobilization. The gross tumor volume (GTV) was contoured on the CT image dataset to include all areas of gross disease. This included the primary tumor and any pathologically enlarged lymph nodes ≥ 1.0 cm detected on imaging modalities. A 1.0 cm expansion of the GTV was utilized to create the clinical treatment volume (CTV) which included areas with potential microscopic spread. An additional 5 mm expansion was used to generate the planning treatment volume (PTV). Target volumes were designated according to areas of gross or high risk disease (PTV 1), and areas of subclinical disease (PTV 2). We included the ipsilateral and/or involved lymph node levels in the high risk PTV 1. The contralateral neck was included in the high risk volume in clinical situations such as a bulky primary tumor crossing midline, level II adenopathy >3 cm, and disease involving the medial one third of the soft palate. In other situations the contralateral neck was treated electively and designated as PTV 2.
All patients were treated using Step-and-Shoot Intensity Modulated Radiation Therapy (IMRT), and treatment planning was performed on Xio Planning Treatment System (version 4.5). All patients were treated using a simultaneous integrated boost technique. PTV 1 was treated to a cumulative dose of 70 Gy in 35 fractions (2Gy/frction); and PTV 2 was treated to 56 Gy in 35 fractions (1.6Gy/fx). Treatments were delivered using a 6 MV linear accelerator. Organs at risk (OAR) were contoured on the CT image dataset based on standard anatomical boundaries. Specific to this manuscript, the bilateral parotid and submandibular glands were contoured separately. The sublingual space was contoured to account for an estimated dose to these glands, respectively. All OARs were delineated by a single team dedicated to head and neck cancers. IMRT plans were created to deliver the aforementioned doses, but also with goals of sparing the bilateral parotid glands to 50% of the cumulative volumes to receiving <26 Gy. In situations where this could not be met, attempts were made to spare the glands to 50% < 30 Gy, and in certain situations, obtaining tumor control precluded the ability to spare these glands.
Clinical assessments and specimens
The 14-question Xerostomia Inventory (XI) as described by Thomson37 (Fig. 1) was administered to all cases immediately prior to saliva collection at each visit. Stimulated whole expectorated saliva samples were collected from each subject at baseline, visit two (i.e., two weeks into RT), and visit three (following completion of RT). Stimulated saliva (SS) was collected because it has been reported to more closely correlate with the severity of dry mouth-related symptoms than unstimulated flow rate38, and it was predicted that unstimulated whole saliva would be difficult to obtain as the number of radiation visits increased. SS was collected according to the method of Navazesh and Kumar.39 Patients were instructed not to eat, drink (except water), or smoke for at least one hour prior to sample collection. Once they arrived for their appointment, patients were instructed to sit motionless and lean their head over a paper cup. They were then asked to swallow and void the mouth of saliva before inserting paraffin into their mouth. Subjects were then instructed to chew the paraffin (approximately 70 strokes per minute). The researcher instructed the patient to spit every minute. The first two-minute sample was discarded and a sterile test tube was used to collect the subsequent sample for eight minutes. All samples were immediately placed on ice, transported to the laboratory on ice within an hour of collection, centrifuged, separated into aliquots and stored at −80°C until analyzed which occurred within six months. SS was collected from the healthy controls in the same manner at a single visit, as no significant changes were expected in saliva composition over time. Volume was measured in a graduated pipette within 10 minutes of collection.
Figure 1.
Fourteen question xerostomia inventory (XI).
Immunoassays
All samples (n=81 HNC and 11 control) were analyzed in duplicate within six months of storage. Unpublished data from our lab indicate that concentrations of biomarkers are consistently maintained when stored for six months at −80°C. Calcium was analyzed as a putative marker of sensory perception23 and for its role in saliva secretion20 using the Quantichrom calcium ELISA assay from BioAssay Systems (Hayward, CA) according to the manufacturer’s directions. MUC5b was analyzed as a putative marker of oral wetness26 according to the ELISA method described by Almståhl et al.40 Anti-MUC5b goat polyclonal IgG (Santa Cruz, California), rabbit anti-goat IgG-alkaline phosphatase and phosphatase substrate (p5994-25tab) from Sigma-Aldrich Corp (St. Louis, MO) were used in the assay. All assays were performed in duplicate by a certified medical technologist in the Center for Oral Health Research core laboratory at the University of Kentucky. Standards were included on all runs and all results are reported within the linearity of the assays.
Statistical analysis
Interval level variables are presented as means, and standard deviations (SD) and categorical variables as percentages. Radiation doses were calculated as volume-weighted averages of each major salivary gland. For analysis of xerostomia, the text responses for the XI were converted to Likert scale scores from 1 (never) to 5 (very often), with higher scores indicating greater perception of dryness and a maximum score being 70. Correlations were computed using Spearman’s correlation coefficient. Since measurements are taken over time a series of linear mixed models (LMM)s were constructed to determine if mean calcium concentrations, salivary flow levels, and MUC5b concentrations varied significantly over time. A backward selection algorithm was used in the LMMs to identify covariates correlated with various XI scores. In all LMMs, each patient was considered as a separate experimental unit sharing the same random effect under a compound symmetry assumption for the three visits. Total scores were considered from the total of questions 1 through 14 in the XI. Possible covariates in the analyses were calcium, MUC5b, volume and dose to the major salivary glands. These latter analyses were repeated using the data from each visit separately (general linear model) and then for outcome XI score ≥ 42 (generalized linear model for a dichotomous outcome). Analyses were also performed after correcting calcium and MUC5b concentrations for salivary flow rate. Statistical significance was set at p < 0.05 throughout. A power analysis was performed and determined that a sample size of 30 was needed to detect a Pearson correlation of 0.5 with 80% power. Statistical analyses were performed using the PC SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Of 37 patients enrolled, 32 patients with HNC who received IMRT provided baseline samples. Five subjects failed to provide baseline samples because either they enrolled in the study and subsequently refused to participate or after enrollment they were unable to tolerate chewing on the paraffin to collect the initial sample. The study group was 85.3% male and 94.1% Caucasian, with a mean age 61.3 years (range 40–78 years). Squamous cell carcinoma was the predominant diagnosis (91.2%) and the majority of cases (88.2%) involved the tongue, oropharynx/tonsil and/or larynx. Twenty-seven had stage IV disease (79.4%), 11.8% were stage III, 5.9% were stage II and one patient was stage I. Mean cumulative radiation doses to salivary glands were 1604 centigray (cGy) at visit two and 5425 cGy at visit three; at completion the range was 3839 to 6107 cGy (10th and 90th percentiles). Mean cumulative doses to the contralateral glands were significantly less than the ipsilateral glands, except the sublingual at visit 2 (Table 1).
Table 1.
Mean radiation doses (cGy) to major salivary glands.
| Parotid | Submandibular | Sublingual | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ipsi | Contra | Both | Ipsi | Contra | Both | Ipsi | Contra | Both | |
| Visit 2 | 1385 | 1150 | 1270 | 2190 | 2012 | 1802 | 1888* | 1833* | 1872 |
| Visit 3 | 4392 | 3475 | 3816 | 6784 | 6260 | 6511 | 5531 | 5338 | 5424 |
Ipsi = ipsilateral, Contra = contralateral.
Contra was significantly less than Ipsi (p < 0.05) for all major glands except the sublingual at visit 2.
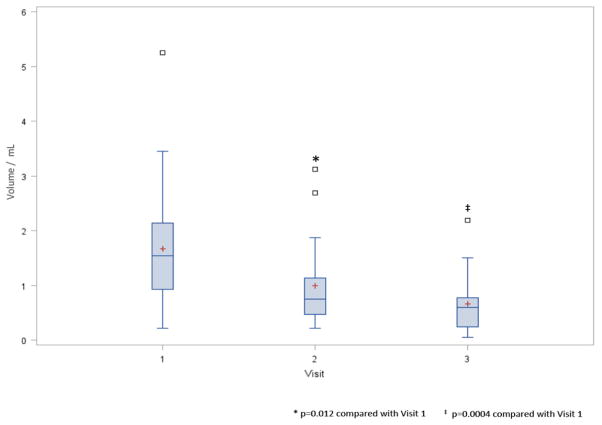
SS volume was determined from 32 baseline, 27 visit two, and 20 visit three specimens. The decreasing sample size reflects patient dropout and the inability to produce expectorated saliva, which in six cases was related to the placement of a percutaneous endoscopic gastrostomy (PEG) feeding tube. SSFR significantly declined 36.5% by visit two (p = 0.012) and 57.1% by visit 3 (p = 0.0004) and was observed in 74.1% of patients (Fig. 2). Of note, stimulated salivary flow did not diminish in all patients. Eight patients maintained ≥70% of their baseline volume, without apparent difference in the radiation method and dose between these patients and those who experienced significant decreases in SSFR. SSFR demonstrated an inverse correlation with overall radiation dose (r =−0.5, p=0.016) and total dose to the sublingual gland (−0.51, p=0.01). In contrast, the correlation between saliva volume and radiation dose to the parotid (r=−0.37, p=0.08) and submandibular glands (r =−0.32, p=0.16) trended toward significance.
Figure 2.
Boxplots of salivary flow rate by visit. SSFR is calculated by taking the volume shown divided by eight minutes (i.e., collection time). Red plus is the mean. Open squares are outliers. * p=0.012 compared with Visit 1. ‡ p=0.0004 compared with Visit 1.
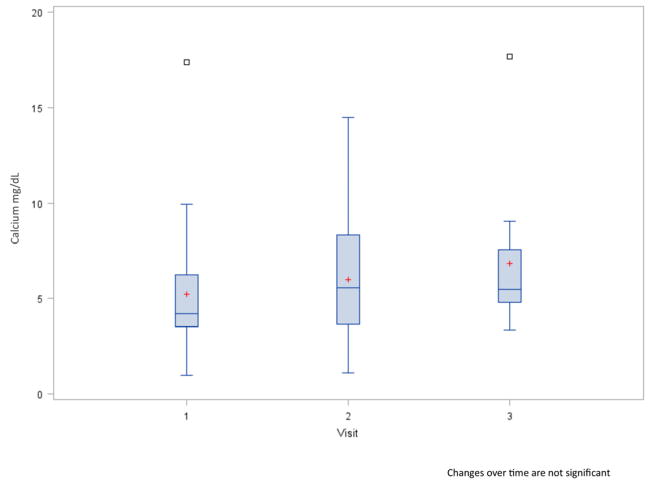
The mean calcium concentration was 5.2 mg/dL at baseline, and the majority ranged between 3.5 and 6.2 mg/dL (i.e., lower and upper quartiles) (Fig. 3). By visit two, the mean concentration rose 14.2% and by visit three it increased 32.2% compared to baseline (p>0.05). A significant correlation was observed between calcium concentration and radiation dose to the major salivary glands. The correlation (r=0.59, p=0.003) was evident by visit two, and increased by visit three (r=0.839, p=0.0006). The correlation occurred between calcium concentration and the individual gland dosage (parotid gland, r=0.76, p=0.004; sublingual gland r=0.71, p=0.009; and submandibular gland r=0.71, p=0.02). The change in calcium concentration was also highly correlated with changes in radiation doses between visit one and two (r ≥ 0.52, p ≤ 0.02), but less so between visit two and three.
Figure 3.
Boxplots of saliva calcium concentration by visit. Red plus is the mean. Open squares are outliers.
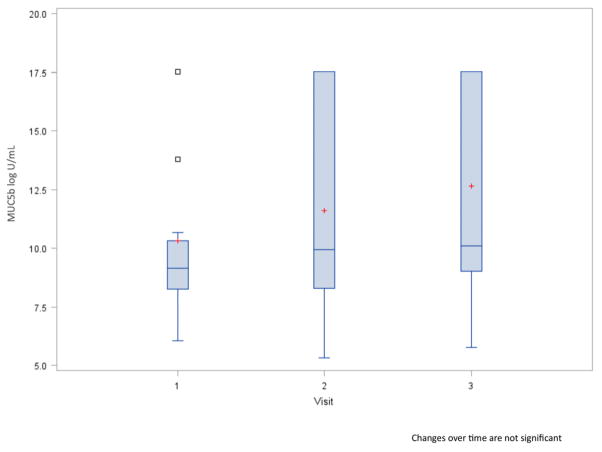
The mean MUC5b concentration was 10.1 units/mL at baseline, and ranged mostly between 8.2 and 10.3 units/mL. Figure 4 demonstrates that MUC5b concentrations increased over time, but not as dramatically as and with more variation than calcium. By visit two, MUC5b increased 11.9% and by visit three concentrations increased by 20.8% compared with baseline. MUC5b correlated with radiation dose to the parotid gland only (r=0.64, p=0.02). Overall, the correlation between radiation doses and MUC5b was weak, and the change in MUC5b did not correlate with changes in radiation doses.
Figure 4.
Boxplots of saliva MUC5b concentration by visit. Red plus is the mean. Open squares are outliers.
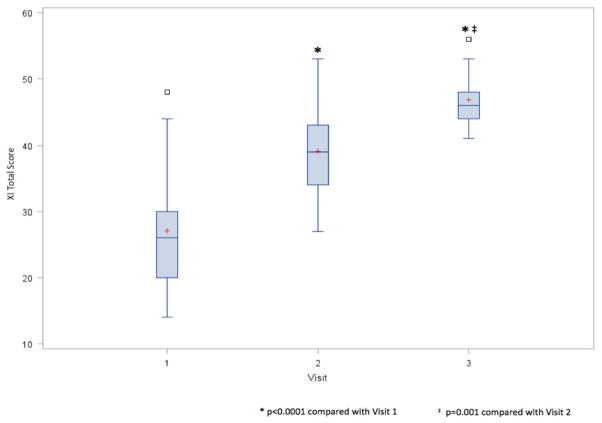
XI scores increased significantly as patients received increasing IMRT (Fig. 5). The effect was significant between all visits (p≤0.001). The relationships between the perception of xerostomia, salivary flow, and salivary calcium and MUC5b concentrations were examined. The total XI score was used as a measure of the severity of xerostomia. Here correlations with XI scores were observed with SSFR at visit 1 (−0.37, p=0.045) and visit 2 (r=−0.44, p=0.04). Salivary concentrations of calcium and MUC5b were unrelated to the perception of xerostomia. Increasing XI scores over time were most notable for SSFR (r=−0.40, p=0.057), not for calcium or MUC5b. Of note, SSFR correlated with 12 XI items (all but questions 1 and 5; p≤0.04), calcium correlated with five XI items (questions 9, 10, 11, 13, and 14; p≤0.03), whereas MUC5b concentrations did not correlation with any XI question.
Figure 5.
Boxplots of total XI scores by visit. Maximum score possible = 70. Red plus is the mean. Open squares are outliers. * p<0.001 compared with Visit 1. ‡ p=0.001 compared with Visit 2.
Next we applied three strategies to determine significant predictors of xerostomia as determined by XI scores in this cohort. In strategy one, the use of general linear models by visit yielded SSFR as the predictor. Correcting for salivary flow rate in this analysis resulted in calcium concentration also becoming a significant predictor of xerostomia, however the relationship with calcium was evident only at visit 1, not visit 2 or 3 after IMRT was administered (data not shown). In strategy two, analysis using LMMs over all visits produced visit SSFR and cumulative mean dose to the ipsilateral submandibular gland as predictors. In strategy three, a threshold of total XI score of 42 was used as an indicator of xerostomia (generalized linear model). In that analysis SSFR at the third visit was a significant predictor of xerostomia. Thus, these statistical approaches demonstrated ‘SSFR’ is a better predictor of xerostomia than salivary concentrations of calcium and MUC5b in the context of IMRT.
DISCUSSION
This study’s findings provide an understanding of the effects of IMRT on salivary flow and specific salivary constituents reported to be involved in xerostomia. Our findings show that 1) despite IMRT SSFR declines significantly after 2 weeks of therapy, 2) although flow rates significantly decline in the majority of patients, not all patients who undergo HN IMRT develop hyposalivation, 3) radiation doses are highly correlated with salivary calcium concentrations, 4) MUC5b concentrations are rather independent of radiation dose, and 5) xerostomia and tongue burning sensations demonstrate a stronger relationship with SSFR compared with salivary concentrations of calcium and MUC5b.
Intensity modulated radiation therapy (IMRT) was used first clinically in the mid to late 1990s.41 The goal of IMRT is to deliver radiation more precisely to the tumor while sparing adjacent normal tissues.42 To date, IMRT has shown promise in effectively treating HNC while limiting the dose to one or both parotid glands.29–35, 43, 44 In this study, all patients received IMRT either by linear acceleration or tomotherapy. Both forms of therapy are sparing and reduce the radiation dose to one or both of the parotid glands and improve salivary flow over conventional RT.45, 46 We observed that 74% of patients (20/27) experienced loss of salivary function ≥30% of their baseline and this effect was rapid with 50% declines appearing when doses exceeded 2000 cGy as found by others.6, 8, 29, 47 The findings are consistent with those of Jham et al. who found 63% of 207 patients undergoing RT suffered from salivary dysfunction and associated symptoms.48 An interesting observation is that both studies demonstrated that a minority of patients do not experience significant hyposalivation. Although the exact cause as to why a minority is protected is not yet known, Nishimura et al. reported that the effect may be partially explained by the size of the glands. His computed tomography analysis showed that patients with larger parotid glands were more protected.49
The results of several statistical analyses in this study demonstrated that xerostomia was more related to SSFR than salivary concentrations of calcium or MUC5b. This occurred in the correlational analyses of the individual visits and total visits, as well as in the linear models and after accounting for salivary flow rates. Consistent with those findings was the observation that SSFR influenced 12 of the 14 items in the XI, whereas calcium influenced only five items and MUC5b influenced none. Also, insight is provided into the potential relationship between xerostomia and the sensation of oral “burning” in that the only covariate that demonstrated a relationship with XI questions 7 and 8 that inquired of a “burning sensation” in the gums or tongue was SSFR. Not surprisingly, burning mouth symptoms have been linked to xerostomia in several other studies50–53 yet more needs to be done in addressing the hyposalivation and xerostomia that exists in many of those patients.
We observed increasing concentrations in calcium in the saliva of HNC patients during IMRT and a significant correlation between calcium and radiation dose. The findings are similar to those of Almståhl and Wikström16 and Funegård et al.54 who noted that calcium concentration in stimulated parotid saliva were higher one year after completion of radiation therapy compared to baseline values. These findings suggest that the altered function in calcium secretion is maintained for many months after RT, and could be contributory to mucosal discomfort as reported by Agha-Hosseini.23 Also, the accompaniment of hyposalivation with changes in salivary calcium concentrations could contribute to changes in oral health including altered mineralization of tooth surfaces, neutralization of acids55, as well as microbial species that occur in these susceptible patients.56
MUC5b binds to mucosa and helps to hydrate these surfaces.24 The level of hydration provided by MUC5b appears to be influenced by the volume of saliva in the mouth57 and the amount produced by the glandular sources of MUC5b (i.e., minor salivary glands, sublingual glands and submandibular glands). In this study, increasing doses were contoured to these glandular sources resulting in differential dosages and salivary volume decline, however MUC5b concentrations increased slightly during the four to six weeks of radiotherapy. Although the small sample size may have prevented us to observe a significant relationship involving MUC5b and xerostomia, the interaction between salivary flow and MUC5b could contribute to reduced hydration in RT patients. For example, select mucins may experience reduced water content due to post-translational modification (e.g., sulfo/glycosylation) which has been observed in other patients experiencing a dry mouth (i.e., Sjögren syndrome).58 These physiological changes may also contribute to the development of ‘sticky saliva’.
Within the limitations of the small sample size and limited duration of this study, we can conclude that HNC patients during IMRT demonstrated decreased SSFR within two weeks of initiating therapy, and that decreasing SSFR is a major contributor to xerostomia and oral burning sensations. Our data seem to exclude salivary concentrations of MUC5b as being important in contributing to xerostomia, whereas salivary calcium concentration is associated with select aspects of changes in the XI. Longitudinal studies that extend the examination period of RT patients who develop and maintain hyposalivation will likely provide us further insight into the factors that predict xerostomia and complaints of oral burning. Also, these findings do not show whether decreased SSFR induced by IMRT persist long term and do not argue against the benefit of IMRT in the treatment of HNC patients.
Clinical Relevance.
Xerostomia developed in the majority of patients undergoing IMRT, and the perception of dry mouth and oral burning sensations correlated with diminished salivary flow to a greater extent than the change in concentration of select salivary constituents.
Acknowledgments
Sources of Support: This study was supported by grants from the National Institute of Health U01 DE017793 and M01-RR02602, the University of Kentucky General Clinical Research Core and partially supported by the Clinical Translational Science Award: UL1RR033173.
We thank Laura Reichel, RN; Jackie Sims, RN; Karen Meekins, RN in the University Department of Radiation Medicine for their assistance in patient and sample management.
Footnotes
Conflict of interest: The authors report no conflicts of interest related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Surveillance Epidemiology and End Results NCI Cancer Stat Fact Sheets. [Accessed July 20 2012]. [Google Scholar]
- 2.Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71:1363–69. doi: 10.1177/00220345920710070301. [DOI] [PubMed] [Google Scholar]
- 3.Valdez IH, Atkinson JC, Ship JA, Fox PC. Major salivary gland function in patients with radiation-induced xerostomia: flow rates and sialochemistry. Int J Radiat Oncol Biol Phys. 1993;25(1):41–7. doi: 10.1016/0360-3016(93)90143-j. [DOI] [PubMed] [Google Scholar]
- 4.Shannon IL, Starcke EN, Wescott WB. Effect of radiotherapy on whole saliva flow. J Dent Res. 1977;56(6):693. doi: 10.1177/00220345770560062201. [DOI] [PubMed] [Google Scholar]
- 5.Mossman KL, Shatzman AR, Chencharick JD. Effects of radiotherapy on human parotid saliva. Radiat Res. 1981;88(2):403–12. [PubMed] [Google Scholar]
- 6.Wescott WB, Mira JG, Starcke EN, Shannon IL, Thornby JI. Alterations in whole saliva flow rate induced by fractionated radiotherapy. AJR Am J Roentgenol. 1978;130(1):145–9. doi: 10.2214/ajr.130.1.145. [DOI] [PubMed] [Google Scholar]
- 7.Fall-Dickson JM, Berger AM. Oral manifestations and complications of cancer therapy. In: Berger AM, Shuster JL, Von Roenn JH, editors. Principles and Practice of Palliative Care and Supportive Oncology. 3. Philadelphia: Lippincott Williams & WIlkins; 2007. pp. 205–20. [Google Scholar]
- 8.Eneroth CM, Henrikson CO, Jakobsson PA. Pre-irradiation qualities of a parotid gland predicting the grade of functional disturbance by radiotherapy. Acta Otolaryngol. 1972;74(6):436–44. doi: 10.3109/00016487209128474. [DOI] [PubMed] [Google Scholar]
- 9.Osailan S, Pramanik R, Shirodaria S, Challacombe SJ, Proctor GB. Investigating the relationship between hyposalivation and mucosal wetness. Oral Dis. 2011;17(1):109–14. doi: 10.1111/j.1601-0825.2010.01715.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Lee SW, Chung SC, Kim YK, Kho HS. Analysis of residual saliva and minor salivary gland secretions in patients with dry mouth. Arch Oral Biol. 2002;47(9):637–41. doi: 10.1016/s0003-9969(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 11.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66(Spec No):648–53. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 12.Wolff MS, Kleinberg I. The effect of ammonium glycopyrrolate (Robinul)-induced xerostomia on oral mucosal wetness and flow of gingival crevicular fluid in humans. Arch Oral Biol. 1999;44(2):97–102. doi: 10.1016/s0003-9969(98)00113-7. [DOI] [PubMed] [Google Scholar]
- 13.Bergdahl M, Bergdahl J. Low unstimulated salivary flow and subjective oral dryness: association with medication, anxiety, depression, and stress. J Dent Res. 2000;79(9):1652–8. doi: 10.1177/00220345000790090301. [DOI] [PubMed] [Google Scholar]
- 14.Chitra S, Shyamala Devi CS. Effects of radiation and alpha-tocopherol on saliva flow rate, amylase activity, total protein and electrolyte levels in oral cavity cancer. Indian J Dent Res. 2008;19(3):213–8. doi: 10.4103/0970-9290.42953. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Aryeh H, Gutman D, Szargel R, Laufer D. Effects of irradiation on saliva in cancer patients. Int J Oral Surg. 1975;4(5):205–10. doi: 10.1016/s0300-9785(75)80027-5. [DOI] [PubMed] [Google Scholar]
- 16.Almstahl A, Wikstrom M. Electrolytes in stimulated whole saliva in individuals with hyposalivation of different origins. Arch Oral Biol. 2003;48(5):337–44. doi: 10.1016/s0003-9969(02)00200-5. [DOI] [PubMed] [Google Scholar]
- 17.Eliasson L, Almstahl A, Lingstrom P, Wikstrom M, Carlen A. Minor gland saliva flow rate and proteins in subjects with hyposalivation due to Sjogren’s syndrome and radiation therapy. Arch Oral Biol. 2005;50(3):293–9. doi: 10.1016/j.archoralbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Zhao D, Gong B, et al. Decreased saliva secretion and down-regulation of AQP5 in submandibular gland in irradiated rats. Radiat Res. 2006;165(6):678–87. doi: 10.1667/RR3569.1. [DOI] [PubMed] [Google Scholar]
- 19.Brown LR, Dreizen S, Rider LJ, Johnston DA. The effect of radiation-induced xerostomia on saliva and serum lysozyme and immunoglobulin levels. Oral Surg Oral Med Oral Pathol. 1976;41(1):83–92. doi: 10.1016/0030-4220(76)90255-3. [DOI] [PubMed] [Google Scholar]
- 20.Baum BJ, Ambudkar IS. Regulation of calcium handling by rat parotid acinar cells. Mol Cell Biochem. 1988;82(1–2):67–73. doi: 10.1007/BF00242518. [DOI] [PubMed] [Google Scholar]
- 21.Funegard U, Johansson I, Franzen L, Ericson T. Acute radiation effects on saliva composition in rats with different vitamin A levels in serum. Acta Oncol. 1991;30(8):975–80. doi: 10.3109/02841869109088252. [DOI] [PubMed] [Google Scholar]
- 22.Vissink A, Konings AW, Ligeon EE, s-Gravenmade EJ. Irradiation-induced changes in secretion and composition of rat saliva. J Biol Buccale. 1990;18(1):3–8. [PubMed] [Google Scholar]
- 23.Agha-Hosseini F, Mirzaii-Dizgah I, Mansourian A, Zabihi-Akhtechi G. Serum and stimulated whole saliva parathyroid hormone in menopausal women with oral dry feeling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(6):806–10. doi: 10.1016/j.tripleo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Amerongen AV, Bolscher JG, Veerman EC. Salivary mucins: protective functions in relation to their diversity. Glycobiology. 1995;5(8):733–40. doi: 10.1093/glycob/5.8.733. [DOI] [PubMed] [Google Scholar]
- 25.Offner GD, Troxler RF. Heterogeneity of high-molecular-weight human salivary mucins. Adv Dent Res. 2000;14:69–75. doi: 10.1177/08959374000140011101. [DOI] [PubMed] [Google Scholar]
- 26.Tabak LA. In defense of the oral cavity: structure, biosynthesis, and function of salivary mucins. Annu Rev Physiol. 1995;57:547–64. doi: 10.1146/annurev.ph.57.030195.002555. [DOI] [PubMed] [Google Scholar]
- 27.Dijkema T, Terhaard CH, Roesink JM, et al. MUC5B levels in submandibular gland saliva of patients treated with radiotherapy for head-and-neck cancer: a pilot study. Radiat Oncol. 2012;7(1):91. doi: 10.1186/1748-717X-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49(4):907–16. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 29.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ship JA, Eisbruch A, D’Hondt E, Jones RE. Parotid sparing study in head and neck cancer patients receiving bilateral radiation therapy: one-year results. J Dent Res. 1997;76(3):807–13. doi: 10.1177/00220345970760031401. [DOI] [PubMed] [Google Scholar]
- 31.Jones RE, Takeuchi T, Eisbruch A, et al. Ipsilateral parotid sparing study in head and neck cancer patients who receive radiation therapy: results after 1 year. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(6):642–8. doi: 10.1016/s1079-2104(96)80068-0. [DOI] [PubMed] [Google Scholar]
- 32.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 33.Beetz I, Schilstra C, van der Schaaf A, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: The role of dosimetric and clinical factors. Radiother Oncol. 2012;105(1):101–6. doi: 10.1016/j.radonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Beer KT, Zehnder D, Lussi A, Greiner RH. Sparing of contralateral major salivary glands has a significant effect on oral health in patients treated with radical radiotherapy of head and neck tumors. Strahlenther Onkol. 2002;178(12):722–6. doi: 10.1007/s00066-002-0961-4. [DOI] [PubMed] [Google Scholar]
- 35.Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007–14. doi: 10.1016/j.ijrobp.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson WM, Williams SM. Further testing of the xerostomia inventory. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):46–50. doi: 10.1016/s1079-2104(00)80013-x. [DOI] [PubMed] [Google Scholar]
- 37.Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999;16(1):12–7. [PubMed] [Google Scholar]
- 38.Suh KI, Lee JY, Chung JW, Kim YK, Kho HS. Relationship between salivary flow rate and clinical symptoms and behaviours in patients with dry mouth. J Oral Rehabil. 2007;34(10):739–44. doi: 10.1111/j.1365-2842.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 39.Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008;139 (Suppl):35S–40S. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 40.Almstahl A, Wikstrom M, Groenink J. Lactoferrin, amylase and mucin MUC5B and their relation to the oral microflora in hyposalivation of different origins. Oral Microbiol Immunol. 2001;16(6):345–52. doi: 10.1034/j.1399-302x.2001.160605.x. [DOI] [PubMed] [Google Scholar]
- 41.Eisbruch A, Ship JA, Martel MK, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: techniques and early results. Int J Radiat Oncol Biol Phys. 1996;36(2):469–80. doi: 10.1016/s0360-3016(96)00264-7. [DOI] [PubMed] [Google Scholar]
- 42.Teh BS, Woo SY, Butler EB. Intensity modulated radiation therapy (IMRT): a new promising technology in radiation oncology. Oncologist. 1999;4(6):433–42. [PubMed] [Google Scholar]
- 43.Bhide SA, Nutting CM. Advances in radiotherapy for head and neck cancer. Oral Oncol. 2010;46(6):439–41. doi: 10.1016/j.oraloncology.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 44.O’Sullivan B, Rumble RB, Warde P. Intensity-modulated Radiotherapy in the Treatment of Head and Neck Cancer. Clin Oncol (R Coll Radiol) 2012 doi: 10.1016/j.clon.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Scrimger R, Kanji A, Parliament M, et al. Correlation between saliva production and quality of life measurements in head and neck cancer patients treated with intensity-modulated radiotherapy. Am J Clin Oncol. 2007;30(3):271–7. doi: 10.1097/01.coc.0000258081.70643.3d. [DOI] [PubMed] [Google Scholar]
- 46.Parliament MB, Scrimger RA, Anderson SG, et al. Preservation of oral health-related quality of life and salivary flow rates after inverse-planned intensity- modulated radiotherapy (IMRT) for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58(3):663–73. doi: 10.1016/S0360-3016(03)01571-2. [DOI] [PubMed] [Google Scholar]
- 47.Dose AM. The symptom experience of mucositis, stomatitis, and xerostomia. Semin Oncol Nurs. 1995;11(4):248–55. doi: 10.1016/s0749-2081(05)80005-1. [DOI] [PubMed] [Google Scholar]
- 48.Jham BC, Reis PM, Miranda EL, et al. Oral health status of 207 head and neck cancer patients before, during and after radiotherapy. Clin Oral Investig. 2008;12(1):19–24. doi: 10.1007/s00784-007-0149-5. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura Y, Nakamatsu K, Shibata T, et al. Importance of the initial volume of parotid glands in xerostomia for patients with head and neck cancers treated with IMRT. Jpn J Clin Oncol. 2005;35(7):375–9. doi: 10.1093/jjco/hyi108. [DOI] [PubMed] [Google Scholar]
- 50.Maresky LS, van der Bijl P, Gird I. Burning mouth syndrome. Evaluation of multiple variables among 85 patients. Oral Surg Oral Med Oral Pathol. 1993;75(3):303–7. doi: 10.1016/0030-4220(93)90141-p. [DOI] [PubMed] [Google Scholar]
- 51.Bergdahl M, Bergdahl J. Burning mouth syndrome: prevalence and associated factors. J Oral Pathol Med. 1999;28(8):350–4. doi: 10.1111/j.1600-0714.1999.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 52.Hakeberg M, Berggren U, Hagglin C, Ahlqwist M. Reported burning mouth symptoms among middle-aged and elderly women. Eur J Oral Sci. 1997;105(6):539–43. doi: 10.1111/j.1600-0722.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 53.Soares MS, Chimenos-Kustner E, Subira-Pifarre C, Rodriguez de Rivera-Campillo ME, Lopez-Lopez J. Association of burning mouth syndrome with xerostomia and medicines. Med Oral Patol Oral Cir Bucal. 2005;10(4):301–8. [PubMed] [Google Scholar]
- 54.Funegard U, Franzen L, Ericson T, Henriksson R. Parotid saliva composition during and after irradiation of head and neck cancer. Eur J Cancer B Oral Oncol. 1994;30B(4):230–3. doi: 10.1016/0964-1955(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 55.Van Nieuw Amerongen A, Bolscher JG, Veerman EC. Salivary proteins: protective and diagnostic value in cariology? Caries Res. 2004;38(3):247–53. doi: 10.1159/000077762. [DOI] [PubMed] [Google Scholar]
- 56.Almstahl A, Wikstrom M, Fagerberg-Mohlin B. Microflora in oral ecosystems in subjects with radiation-induced hyposalivation. Oral Dis. 2008;14(6):541–9. doi: 10.1111/j.1601-0825.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 57.Pramanik R, Osailan SM, Challacombe SJ, Urquhart D, Proctor GB. Protein and mucin retention on oral mucosal surfaces in dry mouth patients. Eur J Oral Sci. 2010;118(3):245–53. doi: 10.1111/j.1600-0722.2010.00728.x. [DOI] [PubMed] [Google Scholar]
- 58.Alliende C, Kwon YJ, Brito M, et al. Reduced sulfation of muc5b is linked to xerostomia in patients with Sjogren syndrome. Ann Rheum Dis. 2008;67(10):1480–7. doi: 10.1136/ard.2007.078246. [DOI] [PubMed] [Google Scholar]