Abstract
Background & Aims
Cancer cells often lose contact inhibition to undergo anchorage-independent proliferation and become resistant to apoptosis by inactivating the Hippo signaling pathway, resulting in activation of the transcriptional co-activator yes-associated protein (YAP). However, the oncogenic mechanisms of YAP are unclear.
Methods
Using cross-species analysis of expression data, the Notch ligand Jagged-1 (Jag-1) was identified as downstream target of YAP in hepatocytes and hepatocellular carcinoma (HCC) cells. We analyzed the functions of YAP in HCC cells via overexpression and RNA silencing experiments. We used transgenic mice that overexpressed a constitutively activated form of YAP (YAPS127A), and measured protein levels in HCC and colorectal and pancreatic tumor samples from patients.
Results
Human HCC cell lines and mouse hepatocytes that overexpress YAPS127A upregulated Jag-1, leading to activation of the Notch pathway and increased proliferation. Induction of Jag-1, activation of Notch, and cell proliferation required binding of YAP to its transcriptional partner TEAD4; TEAD4 binding required Mst1/2, but not WNT-β-catenin signaling. Levels of YAP correlated with Jag-1 expression and Notch signaling in human tumor samples and shorter survival times of patients with HCC or colorectal cancer.
Conclusion
The transcriptional regulator YAP upregulates Jag-1 to activate Notch signaling in HCC cells and mouse hepatocytes. YAP-dependent activity of Jag-1 and Notch correlate in human HCC and colorectal tumor samples with patient survival times, suggesting the use of YAP and Notch inhibitors as therapeutics for gastrointestinal cancer.
Keywords: transcriptional regulators, liver cancer, colorectal cancer, pancreatic cancer
Introduction
The Hippo-signaling pathway is a key regulator of organ size control and links cell density with cell proliferation, apoptosis, and stem cell/progenitor cell expansion.1–2 The Hippo signaling cascade, consisting of protein kinases and regulatory proteins including merlin/NF2, Mst1/2 (the mammalian Hippo homolog), salvador/WW45, and Lats1/2, antagonizes the transcriptional coactivator yes-associated protein (YAP) through its phosphorylation and binding to 14-3-3 protein resulting in cytoplasmic sequestration.3 Inactivation of the Hippo pathway leads to the nuclear translocation of YAP and subsequent interaction with several transcription factors including TEAD and SMAD family members, Runx2, or p73.2
YAP overexpression has been reported for different human tumor entities including colon, prostate, ovarian, lung cancer,4 and hepatocellular carcinoma (HCC).5–6 Several lines of evidence from mouse models suggest that dysregulation of the Hippo/YAP cascade may be critically involved in the development of different gastrointestinal tumors. Ex vivo manipulation of murine liver progenitor cells and subsequent transplantation promoted hepatocarcinogenesis partly through activation of YAP.7 In addition, overexpression of YAP or inactivation of its upstream regulators (e.g., Mst1/2) results in hepatomegaly and the eventual development of multiple liver tumors or severe dysplasia located along the intestinal epithelium and development of colonic adenomas.5,8–11
Expression profiling studies of different murine tumor models (YAP transgenics and Mst1/2-deficient animals) aimed at elucidating YAP-mediated downstream mechanisms in intestinal tumors revealed partially overlapping target gene signatures including regulators of proliferation and apoptosis (e.g., glypican-3, sox4) as well as secreted proteins (e.g., CTGF, osteopontin),5,10 indicating that the Hippo pathway not only regulates tumor cell autonomous processes but also may affect its microenvironment. Recent data demonstrated that the membrane-associated receptor tyrosine kinase AXL regulates tumor cell proliferation and migration in a YAP-dependent manner.6 Despite these examples, the relevant downstream effectors of Hippo/YAP signaling involved in carcinogenesis remain poorly delineated.
Here, we aimed to define effector mechanisms mediating YAP-dependent activity in hepatocarcinogenesis. We show that YAP-dependent Jag-1 triggers activation of Notch-signaling in vitro and in vivo, and that sequential activation of YAP and Notch signaling mediates YAP-dependent effects on tumor progression in HCC as well as in colorectal and pancreatic cancer.
Materials and Methods
For a detailed description of methods and antibodies used in this study see Supplemental Material and Supplemental Table S1.
Results
YAP Overexpression Supports HCC Cell Viability and Migration
Comprehensive immunohistochemical analysis of human specimens revealed weak/absent hepatocellular staining for nuclear or cytoplasmic YAP in normal or cirrhotic livers, dysplastic nodules, and hepatocellular adenomas (Suppl. Figure S1A). In contrast, nuclear YAP expression significantly increased in HCC, exhibiting moderate to high expression in up to 67% of all cases (Suppl. Figure S1B, S1C). Nuclear YAP expression correlated with proliferation (Ki67 staining; r=0.3; p<0.001), but was not associated with gender, age, TNM classification, or tumor etiology.
To examine the functional relevance of YAP in hepatocarcinogenesis, its expression was knocked down by siRNAs in HCC cell lines showing high-levels of YAP (Suppl. Figure S2A, S2B). Efficient inhibition of YAP reduced cell viability compared to scramble siRNA-transfected or untransfected cells (HuH-7; Suppl. Figure S2C). Conversely, vector-based expression of a wild type (YAPwt) or a constitutively active YAP isoform (YAPS127A) in HCC cells with low-level expression of YAP increased tumor cell viability compared to the respective controls (PLC/PRF/5; Suppl. Figure S2D). Cells expressing YAPS127A showed a more pronounced increase in viability than cells overexpressing YAPwt, demonstrating that enforced nuclear YAP accumulation more efficiently supports viability than YAP which remains subjected to its physiological nucleo-cytoplasmic shuttling.
Because a YAP/TAZ signature has been identified in a group of breast cancer patients with higher probability of metastasis,12 the mobility and invasive capacities of HuH-7 cells after YAP knockdown were analyzed. Migration and invasion of HCC cells following YAP silencing was significantly reduced (Suppl. Figure S2E, S2F), which was supported by 2D-scratch assays showing a diminished capacity for transversal migration (Suppl. Figure S2G). These results provide further evidence that overexpression of YAP promotes HCC cell growth and dissemination.
The Notch Ligand Jagged-1 is a Functional YAP Target
To define the effector mechanisms mediating protumorigenic properties of YAP, expression profiles of human HuH-7 cells after YAP knockdown were compared with transcriptomic data of murine hepatocytes with inducible YAP expression (Figure 1A).5 YAP-dependent expression of genes inversely regulated (including CTGF, CXCR4, TGFB2, and Jagged-1 (Jag-1)) was confirmed using real-time PCR in human HCC cell lines (Figure 1A). Due to the important role of the Jag/Notch pathway in carcinogenesis, we further analyzed the possible functional crosstalk between YAP and Notch cascades in liver cancer.
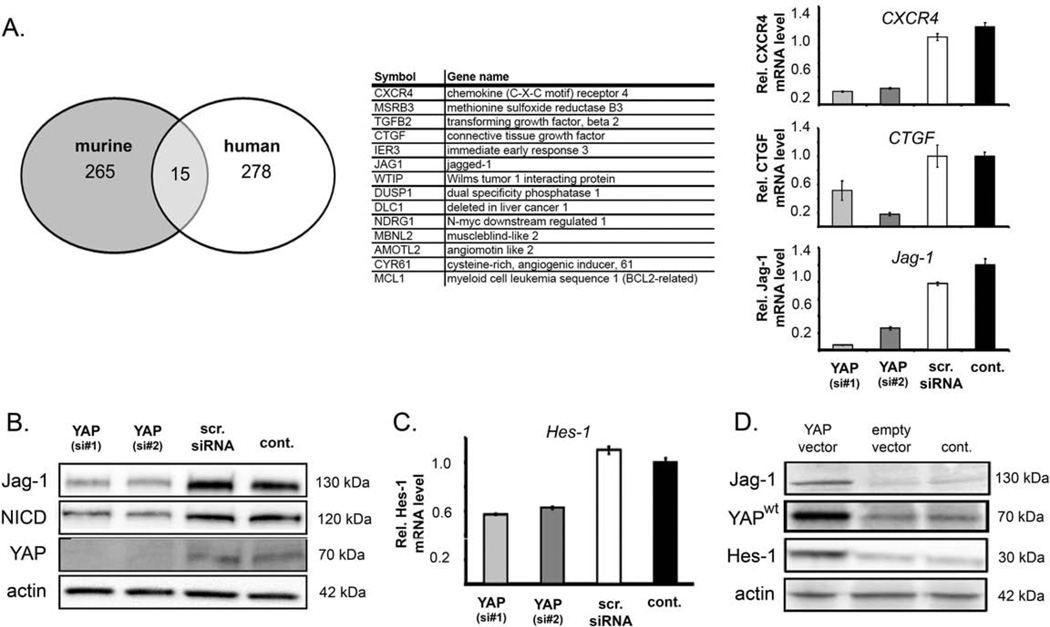
Figure 1. YAP-dependent expression of Jag-1 in HCC cells.
(A) Comparative intersection analysis of elevated transcripts in murine hepatocytes expressing YAP and HuH-7 cells after YAP silencing (transfection of si#1) revealed 15 inversely regulated candidate genes.5 Selected candidates were validated in HuH-7 by real-time PCR using an additional YAP-specific siRNA (si#2). (B) Western blot analysis of YAP, Jag-1, and NICD, 24 h after siRNA-mediated knockdown of YAP in HuH-7 cells. (C) Relative mRNA levels of the Notch pathway target gene Hes-1 in HuH-7 cells 24 h after transfection of YAP-specific siRNAs. Untreated cells (cont.) were used for calibration. (D) Western blot analysis of PLC/PRF/5 protein extracts after vector-based overexpression of YAPwt. Amounts of Jag-1, YAPwt, and Hes-1 were detected 24 h after transfection. Untreated (cont.) and empty vector (mock)-transfected cells served as controls. For (B) and (D) actin was used as loading control.
Firstly, the effects of YAP reduction on Notch axis components and pathway activity were defined. YAP knockdown in HuH-7 cells not only reduced Jag-1 protein levels but also diminished Notch intracellular domain (NICD; Figure 1B) and expression of the Notch target gene Hes-1 (Figure 1C), indicating diminished Notch pathway activity. Accordingly, in cells with low YAP protein concentrations, overexpression of YAPwt induced Jag-1 and Hes-1 expression (Figure 1D).
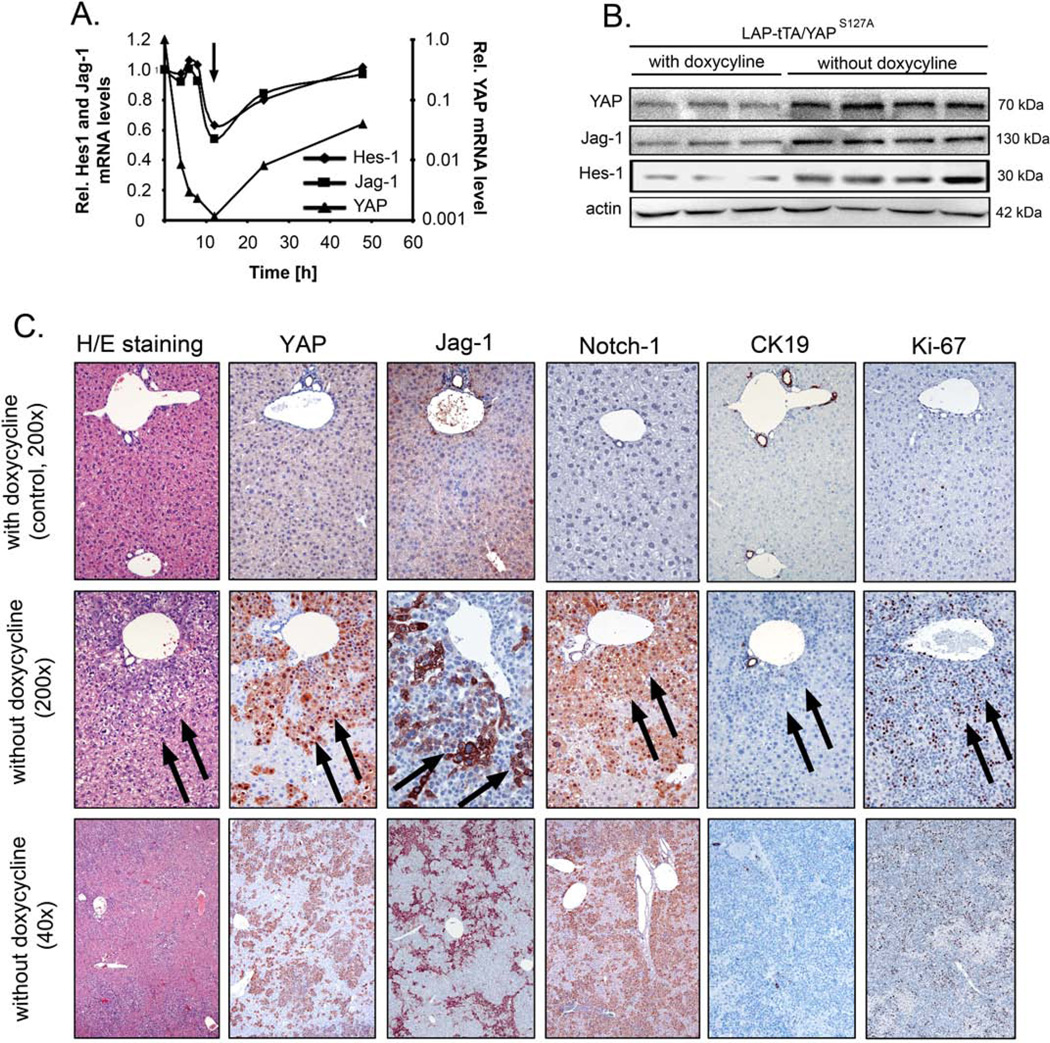
Then, we tested whether YAP-dependent regulation of the Jag-1/Notch pathway also occurred in non-malignantly transformed liver cells. For this purpose, primary murine hepatocytes were isolated from 10 week old transgenic animals expressing YAPS127A under negative control of the liver-specific LAP doxycycline transactivator domain.8 Short-term administration of doxycycline and subsequent downregulation of YAPS127A were followed by reduced expression of Jag-1 and Hes-1 in cultured hepatocytes (Figure 2A). Doxycycline depletion led to increasing YAP, Jag-1, and Hes-1 mRNA concentrations. Livers of LAP-tTA/YAPS127A animals showed elevated YAP, Jag-1, and Hes-1 protein levels after doxycycline deprivation (Figure 2B). Eight weeks after doxycycline withdrawal, LAP-tTA/YAPS127A mice showed massive hepatomegaly accompanied by an increased number of densely packed, small hepatocytes, exhibiting an elevated nuclear/cytoplasm ratio and an increased number of mitotic figures (Figure 2C). Immunohistochemical analysis of LAP-tTA/YAPS127A livers revealed a remarkable YAP positivity, which co-localized with Jag-1 and Notch1 staining (Suppl. Figure S3A). Also, LAP-tTA/YAPS127A livers exhibited a significantly higher proliferation index, as assessed by Ki-67 staining, when compared with control livers (16.8 ± 5.8 vs. 0.5 ± 0.4, respectively; p<0.001). Staining for nuclear YAP, Jag-1, Notch-1, and Ki-67 was almost exclusively detected in the CK19-negative, smallsized hepatocytes expanding from periportal regions.
Figure 2. YAP overexpression induces Jag-1/Notch in murine hepatocytes.
(A) Transcript levels of YAPS127A, Jag-1, and Hes-1 in primary hepatocytes of LAP-tTA/YAPS127 animals with and without doxycycline treatment 24 h after isolation. For each analyzed gene, the ratio between dox+/dox− is depicted. Untreated hepatocytes were used for calibration. Arrow indicates time-point of doxycycline depletion. (B) Western blot analysis of liver extracts from LAP-tTA/YAPS127A animals fed with (controls, n=3) and without doxycycline (n=4) for 4 weeks. YAP, Jag-1, and Hes-1 protein amounts were detected. Actin was used as loading control. (C) H/E overview staining and representative immunohistochemical analysis for YAP, Jag-1, Notch1, CK19, and Ki-67 in livers from LAP-tTA/YAPS127A mice fed with (control) and without doxycycline for 8 weeks. Arrows indicate densely packed, proliferating hepatocytes. Original magnification: x200 in control livers; ×200 and ×40 in LAP-tTA/YAPS127A mice.
The role of Notch signaling in tumorigenesis is controversial.13–16 Thus, we first tested whether Jag-1 knockdown produced a phenocopy of the effects observed after YAP inhibition in HCC cells. Indeed, siRNA-mediated inhibition of Jag-1 diminished cell viability (Suppl. Figure S3B) and migration (Suppl. Figure S3C). The effects on cell viability were significant but less pronounced than the effects of YAP silencing. Similarly, blocking Notch signaling using a γ-secretase inhibitor diminished HuH-7 viability in a concentration-dependent manner (Suppl. Figure S3D).
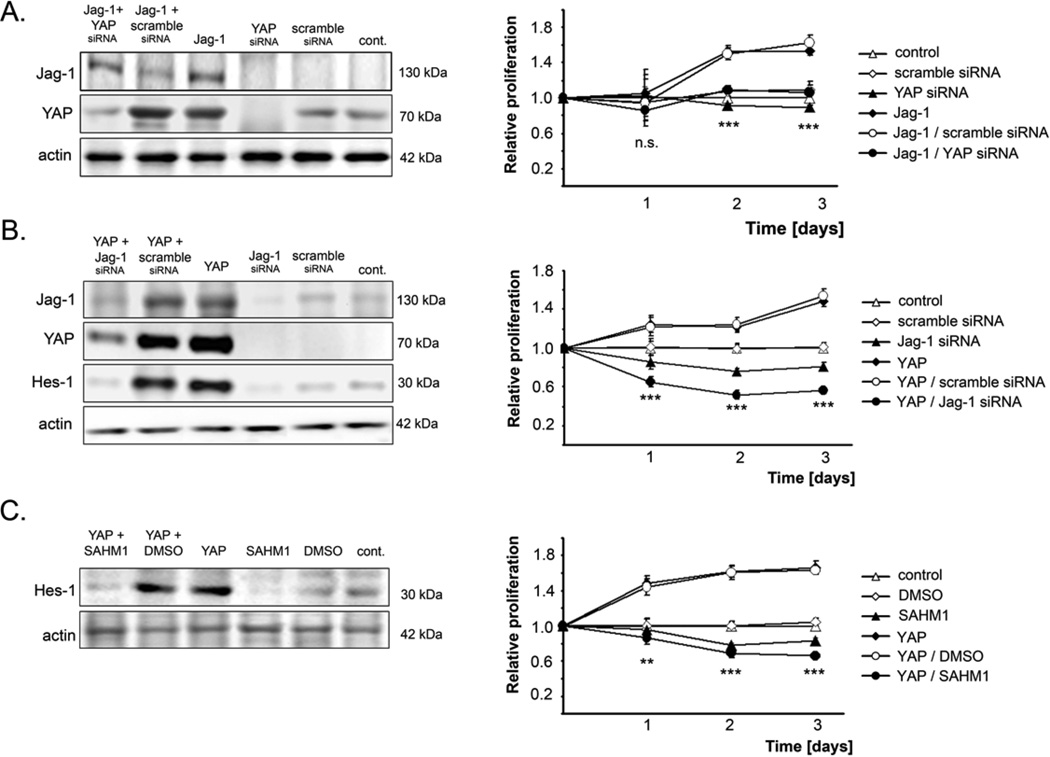
Importantly, inhibition of YAP in PLC/PRF/5 cells expressing Jag-1 abolished the Jag-1-dependent increase of tumor cell proliferation (Figure 3A). Conversely, suppression of Jag-1 in PLC/PRF/5 cells stably overexpressing YAPS127A reduced the expression of Hes-1 and abolished YAPS127A-induced cell proliferation and antiapoptosis, leading to growth restraint (Figure 3B, Suppl. Figure S3E). Noticeably, growth suppression in HCC cells expressing YAPS127A was significantly more pronounced than in untransfected cells following Jag-1 silencing. Likewise, administration of the Notch transcription factor inhibitor SAHM1 completely blocked Hes-1 expression and pro-mitogenic effects of YAPS127A (Figure 3C).
Figure 3. Jag-1 mediates tumor-supporting propertied of YAP in HCC cells.
(A) Jag-1 and YAP expression and cell proliferation of PLC/PRF/5 cells after overexpression of Jag-1 and concomitant inhibition of YAP by gene-specific siRNA. Statistics compares Jag-1/scramble siRNA with Jag-1/YAP siRNA. (B) Jag-1, YAP, Hes-1 protein levels and proliferation of PLC/PRF/5 cells after overexpression YAPS127A and simultaneous Jag-1 silencing. Statistical testing compares Jag-1 siRNA and YAPS127A/Jag-1 siRNA. (C) Hes-1 expression and cell proliferation of PLC/PRF/5 cells after expression of YAP and concomitant administration of the Notch transcriptional complex inhibitor SAHM1. Statistics compared SAHM1 with YAP/SAHM1. For (A) and (B) untreated (control) and scramble siRNA-transfected cells served as controls. For (C) untreated and DMSO-treated cells were used as controls. For (A), (B), and (C), cell proliferation was measured 24, 48, and 72 h after transfection. Actin was used as loading control. Values represent means ± SD. Statistical test: Mann-Whitney U. n.s. - not significant, **p<0.01, ***p<0.001.
Altogether, these results demonstrate that overexpression of YAP induces functionally relevant Jag-1 expression and Notch pathway activity in hepatocytes and HCC cells.
TEAD4 Mediates YAP-Dependent Induction of Jag-1
YAP gains its target gene-specificity through a panel of transcription factors, such as SMADs and TEAD family members.17 To identify the transcription factors responsible for YAP-induced Jag-1 expression, Jag-1 transcript levels were examined after siRNA-mediated inhibition of 14 known YAP binding partners in the human tumor cell line HuH-7. Other than siRNAs targeting Yap and Jag-1, only the TEAD4-specific siRNA led to a comparable reduction in Jag-1 transcript and protein levels (Figure 4A, Suppl. Figure S4A). Interestingly, reduction of YAP levels also diminished TEAD4 levels, indicating that YAP positively regulates TEAD4.
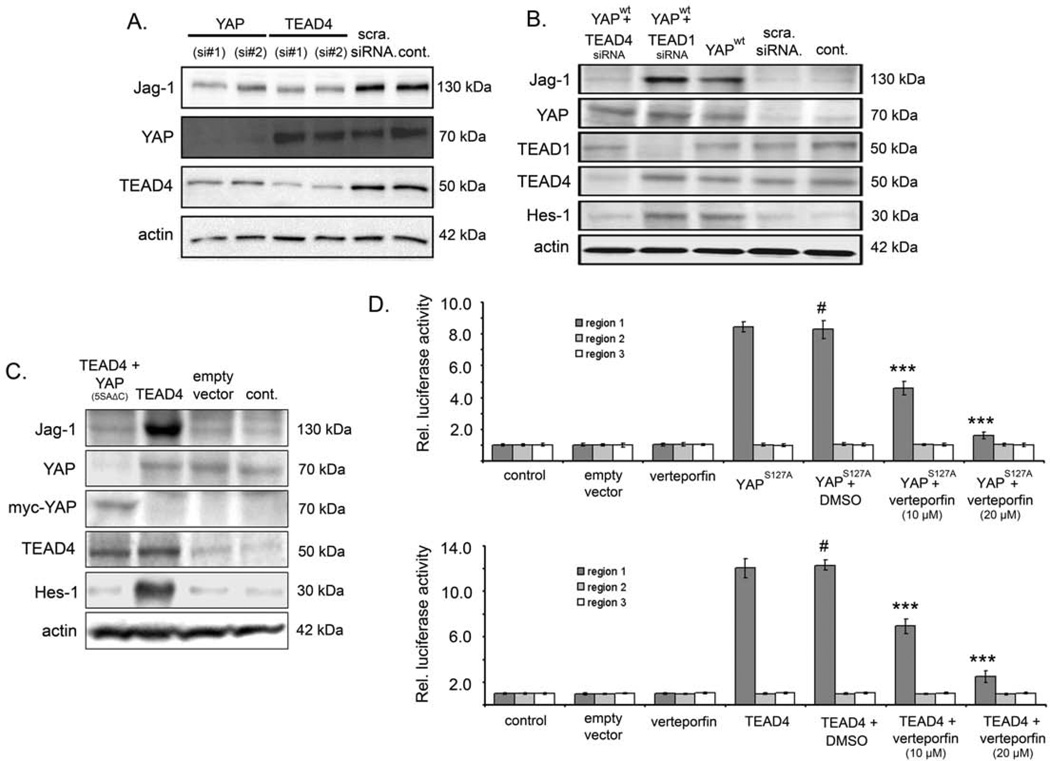
Figure 4. YAP regulates Jag-1 in a TEAD4-dependent manner.
(A) Western blot analysis of Jag-1, YAP, and TEAD4 in HuH-7 cells after siRNA-mediated inhibition of YAP and TEAD4 using two independent siRNAs. (B) Protein analysis of Jag-1, YAP, TEAD1/4, and Hes-1 in PLC/PRF/5 cell lysates after overexpression of YAPwt and concomitant silencing of TEAD1 or TEAD4 by RNAi. (C) Western blot of Jag-1, YAP, myc-tagged YAP, TEAD4, and Hes-1 in PLC/PRF/5 cell lysates after overexpression of TEAD4 and simultaneous expression of dominant negative YAP5SAΔC. Note that YAP is myc-tagged in order to discriminate between exogenous and endogenous protein. (D) Luciferase reporter gene assay testing YAP and TEAD4 responsiveness on three regions of the human Jag-1 gene (region 1–3). Expression of YAP or TEAD4 increased luciferase activity of the region 1 construct, which is partly abrogated after administration of a chemical compound disrupting YAP/TEAD interaction (verteporfin). Values represent means ± SD. Statistical test: Mann-Whitney U. # - reference for statistical comparison, ***p<0.001. For (A), (B), and (C) actin was used as loading control.
Overexpression of YAP and concomitant inhibition of TEAD1 or TEAD4 revealed that silencing of TEAD4 but not of TEAD1 abolished YAP-induced expression of Jag-1 and Hes-1 (Figure 4B). In addition, the inhibition of PLC/PRF/5 cell viability after YAP overexpression was much more pronounced following silencing of TEAD4 than TEAD1, suggesting that the specific interaction between YAP and TEAD4 mostly supported tumor cell proliferation (Suppl. Figure S4B). To further substantiate the TEAD4-dependance, the effect of dominant negative YAP5SAΔC on TEAD4 activity was analyzed.3 In HuH-7 cells overexpressing TEAD4, co-expression of mutant YAP5SAΔC abolished the induction of Jag-1 and Hes-1 genes (Figure 4C). Expression of YAP5SAΔC also abrogated TEAD4-induced HCC cell proliferation (Suppl. Fig. S4C) and increased apoptosis (Suppl. Figure S4D).
Since a previous study demonstrated that YAP/TEAD complexes can directly bind the Jag-1 promoter in breast cancer cells,18 a luciferase reporter assay was employed in HCC cells. For this purpose, three fragments of the Jag-1 promoter were cloned, namely region 1 (−2906 to −1794), region 2 (−1713 to +126) and region 3 (+168 to +1908; scheme depicted in Suppl. Figure S4E). Co-transfection of either YAPS127A or TEAD4 plasmid with each of the Jag-1 promoter regions in PLC/PRF/5 cells showed that the relative luciferase activity of Jag-1 region 1 increased when compared to control plasmid, while no such rise was detected for Jag-1 promoter region 2 and 3 (Figure 4D). Importantly, the increased luciferase activity of Jag-1 region 1 was inhibited in a dose-dependent manner when either YAPS127A or TEAD4 plasmid transfection was paralleled by treatment with verteporfin, a drug, which disrupts the YAP-TEAD binding (Figure 4D).19
Furthermore, the interaction between YAP and TEAD4 was demonstrated by immunoprecipitation of tagged-YAPS127A and detection of TEAD4 (Suppl. Figure S5A). In contrast, no pull down of TEAD4 was observed after transfection of the mutant YAPS94A/S127A isoform lacking TEAD4 binding capacity (Suppl. Figure S5B).20 Equally, expression of biologically active YAPS127A supported HCC cell viability while YAPS94A/S127A did not affect viability or apoptosis (Suppl. Figure S2D, S5C). Moreover, in protein extracts isolated from LAP-tTA/YAPS127A mouse livers, interaction of YAP and TEAD4 was detected exclusively in samples with YAPS127A overexpression (Suppl. Figure S5D).
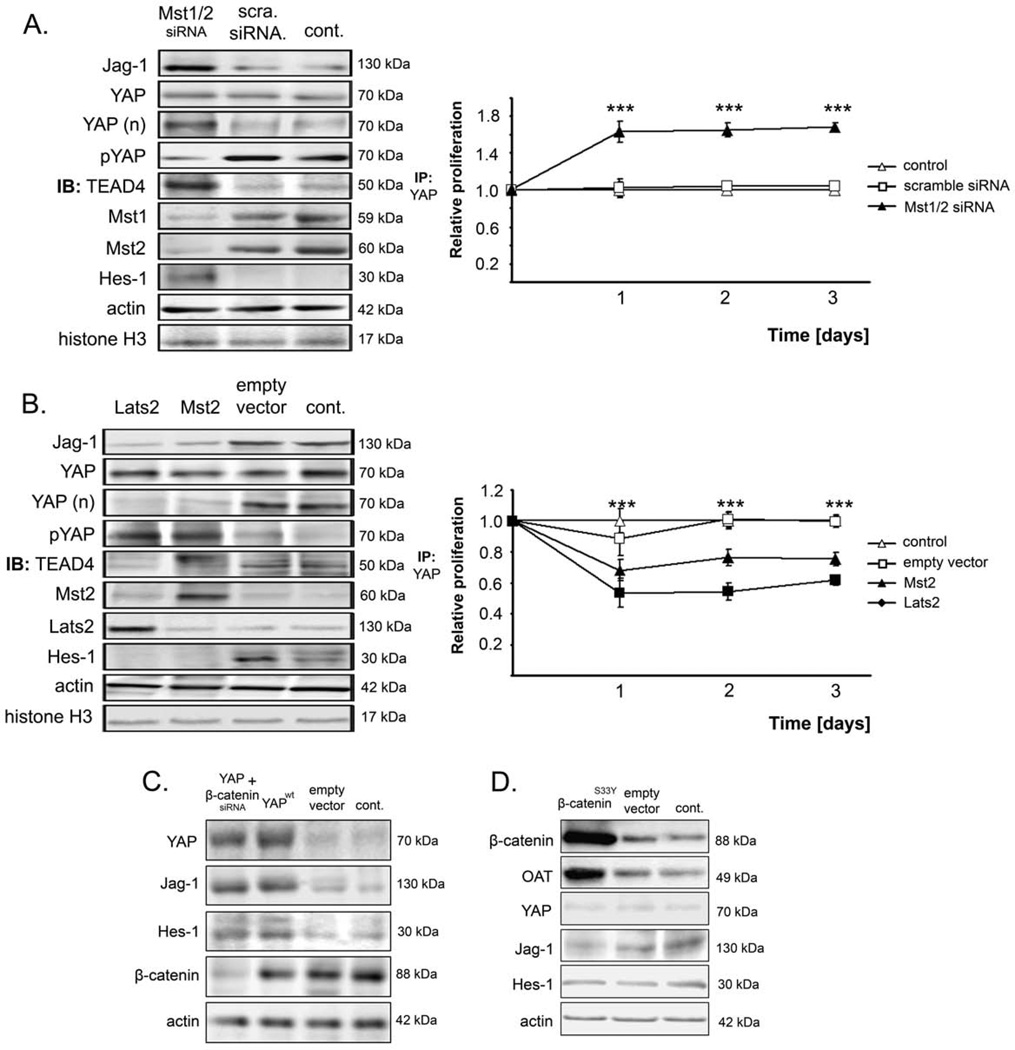
Mst and Lats but not β-catenin Regulate YAP-Dependent Notch Activation
It has been demonstrated that deficiency of the Hippo pathway members, Mst1/2, results in liver overgrowth and cancer,10 probably due to negative regulation of YAP bioactivity.11 We therefore tested whether Mst1/2 and Lats2 control YAP-dependent activation of Jag-1/Notch signaling. As hypothesized, Mst1/2 knockdown in SNU-449 cells augmented the levels of nuclear YAP and significantly increased HCC cell viability, while decreasing apoptosis (Figure 5A. Suppl. Figure S5E). In addition, overexpression of Mst2 or Lats2 induced YAP inactivation/phosphorylation, and decreased Jag-1 and Hes-1 levels, resulting in a decline in HCC cell viability and a rise in apoptosis (Figure 5B, Suppl. Figure S5F).
Figure 5. YAP regulates Jag-1 in a β-catenin-independent and MST/Latsdependent manner.
(A) Analyses of Jag-1, total YAP, nuclear YAP, phospho-YAP, TEAD4 (after IP with YAP antibody), Mst1, Mst2, and Hes-1 and corresponding relative proliferation of SNU-449 cells after combined inhibition of Mst1 and Mst2. SNU-449 cells were chosen because they show high-level expression of Mst1 and Mst2. Statistical analysis compares scramble siRNA and Mst1/2 siRNA. (B) Protein analyses of Jag-1, total YAP, nuclear YAP (after fractionation), phosphorylated YAP, TEAD4 (after IP with YAP antibody), Mst2, Lats2, and Hes-1 and corresponding cell proliferation of HuH-1 cells (showing low-levels of Mst2 and Lats2) after expression of Lats2 or Mst2. Statistical testing compares empty vector and Mst2 or Lats2, respectively. (C) Western blot analysis of YAP, Jag-1, Hes-1, and β-catenin in PLC/PRF/5 cells after overexpression of YAPwt and concomitant inhibition of β-catenin. (D) Analysis of β-catenin, OT, YAP, Jag-1, and Hes-1 in PLC/PRF/5 after overexpression of the constitutively active β-cateninS33Y mutant. For all Western blots, untreated (control), scramble siRNA-transfected, or empty vector-transfected cells were used as controls. Actin served as loading control. Values represent means ± SD. For statistical comparison, the Mann-Whitney U test was used. ***p<0.001.
Since YAP enhances the transcriptional activity of β-catenin in colon cancer cells21 and because Jag-1 is a direct target gene of the β-catenin/TCF complex,22 we tested if regulation of Jag-1 is WNT/β-catenin-dependent in HCC cells. Inhibition of β-catenin by siRNA in PLC/PRF/5 cells overexpressing YAP did not affect Jag-1 or Hes-1 expression (Figure 5C). In addition, expression of the constitutively active mutant β-cateninS33Y did not change YAP, Jag-1, or Hes-1 levels, while a prominent induction of the known β-catenin target gene ornithine aminotransferase (OT) was detectable (Figure 5D). Identical results were obtained for other tumor cell types such as PDAC cells (not shown). Lastly, there was no correlation between YAP/Jag-1 and activated β-catenin expression in HCC, pancreatic carcinoma, and colorectal carcinoma specimens (not shown).
These data demonstrate that YAP stimulates Jag-1 expression, thus inducing the Notch pathway in a Mst1/2- and TEAD4-dependent but β-catenin-independent manner.
YAP-dependent Oncogenic Activation of Notch in Human Gastrointestinal Malignancies
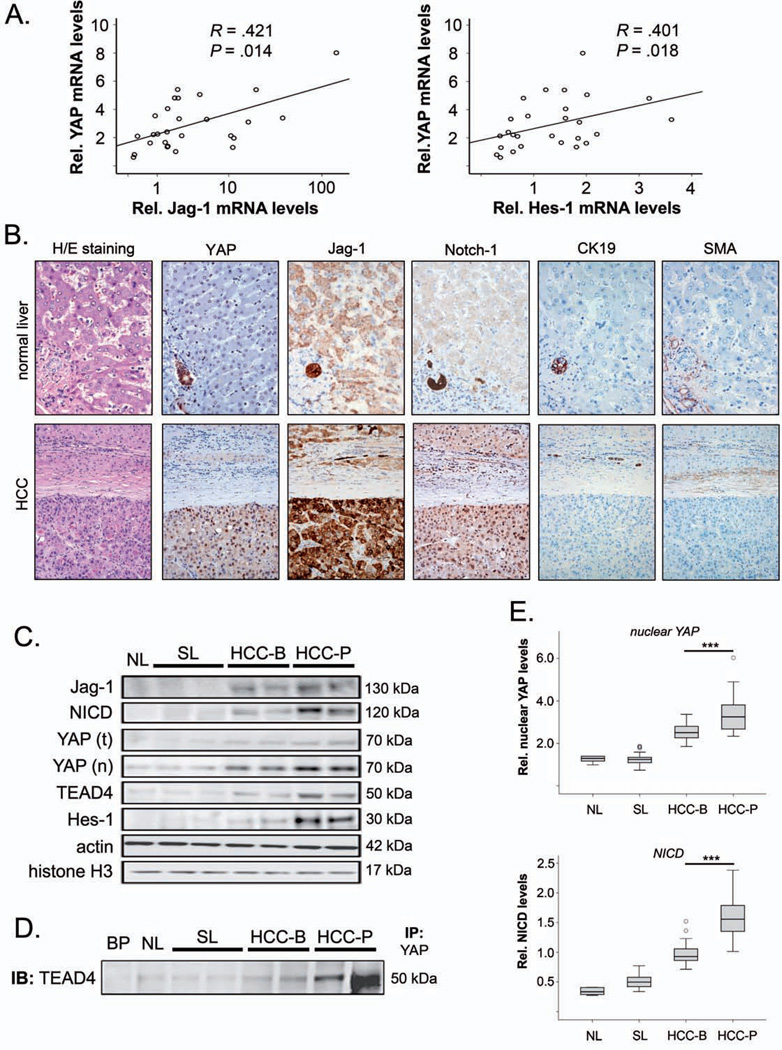
To confirm the prognostic relevance of the regulatory connection between Hippo- and Notch signaling in human carcinogenesis, the mRNA levels of YAP, Jag-1, and Hes-1 were analyzed in total RNA extracts isolated from fresh frozen human HCC samples. Positive correlations between YAP and Jag-1 transcript levels (r=0.42) and between YAP and Hes-1 transcript levels (r=0.4) were detected (Figure 6A).
Figure 6. Activation of YAP-induced Jag-1/Notch signaling correlates with poor prognosis of HCC patients.
(A) Association between YAP and Jag-1 as well as between YAP and Hes-1 transcript levels in 27 primary human HCCs. The Spearman rank coefficient was used as a statistical measure of association. (B) Representative H/E staining and immunohistochemical analysis for YAP, Jag-1, Notch-1, CK19, and α-smooth muscle actin (SMA) in normal human liver and HCC (n=70). Original magnification: ×200. (C) Representative Western blot of cell lysates from human normal livers (NL; n=6), surrounding liver tissue (SL; n=48), HCC with better prognosis (HCC-B; n= 22), and HCC with poor prognosis (HCC-P; n=26). Protein analyses of Jag-1, NICD, total YAP, nuclear YAP (after fractionation), TEAD4, and Hes-1 were detected. (D) For analyzing the interaction between YAP and TEAD4 in primary HCC samples, YAP was immunoprecipitated (IP) and TEAD4 was detected by immunoblotting (IB). BP: negative control for IP. (E) Exemplary boxplot and statistical analyses of nuclear YAP and NICD in all NLs, SLs, HCC-B, and HCC-P after densitometric protein quantification. Actin and histone H3 were used for normalization of total protein (Jag-1, NICD, TEAD4, and Hes-1) and nuclear protein fractions (YAP), respectively. Open circles indicate outliers. Statistical test: Mann-Whitney U. ***p<0.001.
Next, we assessed the staining pattern of YAP, Jag-1, and Notch1 in a collection of HCC (n=70) and corresponding non-tumorous surrounding livers (Figure 6B). Strong YAP, Jag-1, and Notch1 immunoreactivity was detected in 67%, 51%, and 49% HCC, respectively. Of note, 68% of HCCs showing elevated YAP immunoreactivity concomitantly exhibited upregulation of Jag-1 and Notch1.
To test whether YAP, Jag-1, and Hes-1 protein levels correlated with the clinical outcome of HCC patients, normal liver tissues, tumor-surrounding liver tissue, and HCCs with good and poor prognosis were examined by Western blotting. Total and nuclear YAP protein levels were lowest in normal liver tissue, higher in non-tumorous surrounding liver tissue, and highest in HCC. In parallel, Jag-1, NICD, and Hes-1 protein levels as well as binding of YAP and TEAD4 were significantly increased in most analyzed HCC samples than in the respective surrounding liver tissues (Figure 5C, 5D; p<0.001 for all proteins). Most importantly, elevated nuclear YAP, NICD, Jag-1, and Hes-1 levels were significantly increased in patients with poor prognosis (p<0.001 for all factors; Figure 5E) and higher alpha-fetoprotein (AFP) serum levels (p<0.05 for YAP, NICD, and Jag-1) compared to samples isolated from patients with better prognosis. In contrast, no such differences between patients with better and poor prognosis were found in the surrounding liver tissue (p>0.05). Interestingly, total amounts of TEAD4 protein levels also increased in patients with poor prognosis (p<0.001, Figure 5C), which may contribute to the detected high-level interaction of YAP and TEAD4 in HCC tissues (Figure 5D).
We next asked if the functional link between aberrant YAP expression and subsequent activation of Jag-1-dependent Notch signaling observed in HCCs applies to other cancer entities. In cell lines derived from solid human malignancies, such as NSCLC, colon adenocarcinoma, breast cancer, and pancreatic ductal adenocarcinoma (PDAC), YAP knockdown reduced Jag-1 levels (Suppl. Figure S6). To confirm the general relevance of YAP/Jag-1 crosstalk in other human malignancies, additional gastrointestinal tumor entities without (PDAC) and with (colon cancer; CRC) known YAP overexpression were further analyzed.
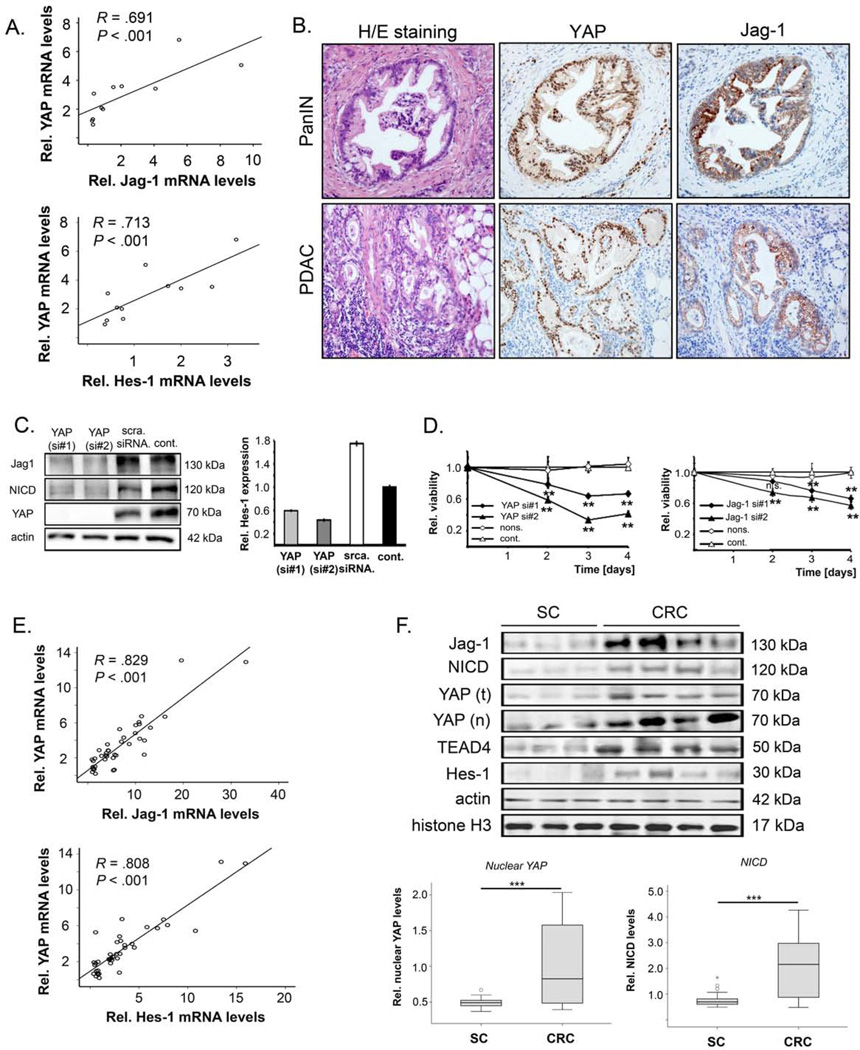
In PDAC specimens, elevated YAP transcript levels significantly correlated with Jag-1 (r=0.691) and Hes-1 (r=0.713) mRNA abundance (Figure 7A, Suppl. Figure S7A), and a significant correlation between YAP and Jag-1 staining was observed in PDAC TMAs (r=0.442, p<0.001; Figure 7B, Suppl. Figure S7B). In PDAC cell lines with high-level expression of YAP (Panc-1, Suppl. Figure S7C), siRNA-mediated knockdown of YAP reduced Jag-1, NICD, and Hes-1 levels (Figure 7C) and significantly reduced tumor cell viability, migration, and invasion (Figure 7D, Suppl. Figure S7D).
Figure 7. Concomitant activation of YAP/Jag-1 in pancreatic and colon cancer specimens.
(A) Association between YAP and Jag-1 and between YAP and Hes-1 transcript levels in 11 human PDACs. (B) Representative H/E staining and immunohistochemical analysis for YAP and Jag-1 in pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC) specimens. Original magnification: ×200. (C) Western blot analysis of YAP, Jag-1, and NICD in Panc-1 cells after siRNA-mediated silencing of YAP. Untreated (cont.) and scramble siRNA-transfected cells served as controls. Hes-1 transcript levels were determined by real-time PCR. (D) Viability kinetics of Panc-1 cells after inhibition of YAP measured by MTT-assay 48, 72, and 96 h after siRNA transfection for YAP (left) and Jag-1 (right). Untreated (cont.) and scramble-transfected cells served as controls. Values represent means ± SD. Statistical test: Mann-Whitney U; n.s. - not significant, **p<0.01. (E) Association between YAP and Jag-1 as well as between YAP and Hes-1 transcript levels in 40 human CRC samples. (F) Representative Western blots from human CRC tissue samples (n=40) and respective surrounding colon tissue (SC; n=40). Jag-1, NICD, total YAP, nuclear YAP (after fractionation), TEAD4, and Hes-1 were detected. Actin and histone H3 were used as loading controls for total protein and nuclear protein fractions, respectively. For (A) and (E) the Spearman rank coefficient was used as a statistical measure of association.
In CRC specimens, YAP transcripts significantly correlated with Jag-1 (r=0.829) and Hes-1 (r=0.808) transcript levels (Figure 7E). Total YAP protein levels, nuclear YAP, TEAD4, Jag-1, NICD, and Hes-1 were higher in CRC samples than in corresponding non-tumorous tissue samples (p<0.001 for all proteins; Figure 7F, Suppl. Figure S7E). Again, interaction of YAP and TEAD4 was higher in CRC samples (p<0.001), and the amount of YAP/TEAD4 complexes correlated with poor patient prognosis (p<0.001; Suppl. Figure S7F).
These data demonstrate that concomitant nuclear enrichment of YAP and activation of TEAD4-dependent Jag-1/Notch signaling represents a general tumor-supporting mechanism in different gastrointestinal malignancies.
Discussion
Accumulating evidence supports the importance of the tumor-suppressive Hippo pathway and its mediator YAP in cancer.1,3–7,12,23 Here, we demonstrate that overexpression of YAP exerts its tumor-supporting properties partly through TEAD4-dependent Jag-1 expression and subsequent activation of the Notch pathway in gastrointestinal cancers. Our in vitro data provide evidence that YAP not only regulates proliferation and apoptosis but also affects cell migration in tumor cell lines of different origin, indicating that elevated YAP levels contribute to tumor growth and dissemination after malignant transformation. Therefore, YAP enrichment may not only support initiation but also progression of solid cancers. This conclusion is substantiated by recent data demonstrating that YAP regulates HCC cell proliferation and migration partly through upregulation of the receptor tyrosine kinase AXL.6
The mechanisms whereby YAP exerts its oncogenic properties have not been defined in detail. Known YAP target genes include CTGF, FGF-1, and amphiregulin3,5,10,23 suggesting the existence of secondary effector mechanisms integrating additional signaling pathways in an auto- or paracrine manner. Here, we unraveled an effector mechanism mediated by the Notch receptor ligand Jag-1, which is positively regulated by YAP both in non-tumorous cells and transformed cells. YAP-mediated induction of Jag-1 inversely links Hippo and Notch signaling and to a significant extent exerts tumor-supporting properties of YAP. The importance of the Notch pathway in YAP-driven growth is further underscored by the capability of YAP/TEAD4 complex to transcriptionally upregulate Notch1 in HCC cells (Calvisi et al., unpublished data). Previous data suggested the importance of YAP/Notch connection in different model systems. For instance, in Drosophila, expression of the YAP orthologue Yorkie increased the levels of the Notch ligand Serrate, the mammalian orthologue of Jag-1 and Jag-2.24 In mammals, Mst1/2-deficient intestinal cells showed increased nuclear YAP, elevated levels of NICD and Jag-1, and activation of typical Notch target genes.21 Furthermore, a YAP-induced intestinal dysplastic phenotype was partly reversed by blocking Notch activation using a γ-secretase inhibitor.8
Mounting evidence indicates that Hippo/Yap signaling is integrated in a broader network of pathways by direct crosstalk or sequential activation of target genes. This was demonstrated for TGFβ/SMAD-,25 WNT/β-catenin,21 and EGFR signaling.26 The Hippo/YAP axis may also regulate the activity of additional pathways, including Sonic hedgehog (Shh)27 as well as FGF, BDNF, and CTGF signaling.3,28 As has been demonstrated for other cellular pathways, intensive cross talk argues for a context-dependent behavior of Hippo/YAP under physiological and pathophysiological conditions.
Co-activation of the WNT and the Notch pathways has recently been demonstrated in Mst1/2-deficient animals expressing high-levels of YAP.21 Since Jag-1 is a direct target gene of the β-catenin/TCF complex,22 it was speculated that YAP regulates Jag-1 in a β-catenin-dependent manner.21 Our results exclude this possibility for HCC and PDAC cells used in this study, since neither inactivation nor activation of β-catenin affected either YAP levels or Notch signaling. In addition, no correlation between nuclear β-catenin, YAP, or Jag-1 was detectable in human HCC and CRC samples. We therefore conclude that, in these entities, YAP regulates Jag-1-mediated activation of protumorigenic Notch signaling in a β-catenin-independent manner.
Our results confirm previous reports that dysregulation of the Hippo pathway, as evidenced by YAP overexpression or inactivation of pathway constituents (e.g., Mst1), occurs in 50 to 70% of HCCs.3,5,11 Also, functional studies have revealed oncogenic properties for nuclear YAP.6 However, the role of the Notch pathway in carcinogenesis remains controversial. In HCC, some studies have shown anti-tumorigenic effects of Notch1 overexpression, and have demonstrated Notch1-dependent sensitization of HCC cells for TRAIL-induced apoptosis.14–15 Moreover, a recent publication revealed that blocking Notch signaling in mice lacking RB1 genes increased tumor formation, and that a Notch signature predicts better prognosis of HCC patients.29 In contrast, our data argue for a protumorigenic character of activated Notch signaling in HCC cells. Inhibition of Jag-1 or blockade of the Notch pathway produces a phenocopy of the tumor-suppressive effects of Yap knockdown, and reduction of Jag-1 expression rescues YAP-induced proliferation of HCC cells. Moreover, our data reveal that high-level protein expression of Jag-1, NICD, and Hes-1 significantly correlate with poorer prognosis of HCC patients. This is supported by published results showing that high-level expression of Notch1 is associated with increased metastatic capacity of tumor cells in HCC patients, and that inhibition of Notch1 drastically reduced tumor cell dissemination in an orthotopic liver implantation model.16 In addition, very recent data demonstrate that TNFα-mediated activation of Notch1 signaling is critical for inflammation-mediated tumorigenesis in the liver,30 and that expression of NICD alone or in combination with AKT is sufficient to induce liver tumor formation.31–32
The reasons for the observed Notch pathway bifunctionality are probably due to varying activation thresholds and crosstalk with other pathways. It is worth noting that tumor-suppressor as well as tumor-promoting properties of Notch have also been described for PDAC cells, supporting the bifunctional potential of this pathway.33 Specifically, the mode of oncogenic Notch activity seems to be highly context-dependent, as shown by a recent publication demonstrating that myofibroblast-derived Jag-1 expression promotes hepatic progenitor cells (HPCs) to differentiate into cholangiocytes in chronic liver disease.34 Equally, both HCC and cholangiocellular carcinoma (CCC) develop in mice after overexpression of NICD and NICD/AKT, respectively, demonstrating the potential of this signaling pathway to drive tumorigenesis of specific liver cell types depending on cross-talk and environmental conditions.31–32 Also, LAP-tTA/YAPS127A transgenic mice show upregulation of Jag-1/Notch signaling without a switch toward the ductular/biliary phenotype, as indicated by morphological analysis of the lesions as well as by the lack of upregulation of biliary markers such as CK19. Thus, YAP-dependent activation of the Jag-1/Notch pathway in these mice is probably relevant for the formation of HCC.5,8,32,35
YAP regulates target gene transcription in tumor and non-malignantly transformed cells through interaction with different transcription factors including TEAD family members.18 Our results demonstrate that YAP-dependent expression of Jag-1 strictly depends on the presence of TEAD4, while other known binding partners/transcription factors of YAP - including TEAD1, TEAD2, and TEAD3 - did not significantly affect Jag-1 expression. Interestingly, TEAD4 expression increases in HCC and CRC samples compared to the respective non-tumorous tissue samples, and especially in the group of HCCs, significantly elevated TEAD4 amounts were detectable in samples from patients with poor prognosis. Because a clear reduction of TEAD4 after YAP inhibition was detectable (Figure 4A), it is tempting to speculate that YAP itself positively regulates TEAD4 levels in tumor cells, which would further augment its transcriptional activity.
This rigorous necessity for a YAP/TEAD4 interaction to induce Jag-1/Notch signaling offers new therapeutic options. Although transcription factors are believed to be non druggable due to large and stable protein/DNA surface interactions, crystal structure analysis of the YAP/TEAD4 complex demonstrated that the proteins are connected mainly via two short helices, one in the C-terminal domain of TEAD4 and one in the N-terminal region of YAP.36 Disruption of this interaction through specific small compounds such as verteporfin has shown encouraging results in YAP-driven hepatocarcinogenesis,19 and may provide an approach for the treatment of cancers with elevated YAP levels.
Supplementary Material
Acknowledgement
We thank M. Bissinger, U. Müller, and S. Messnard for technical assistance. We thank T.R. Brummelkamp (Whitehead Institute for Biomedical Research, Cambridge) and F.D. Camargo (Stem Cell Program, Children's Hospital, Boston) for providing the Col1A1-YAPS127A mice.
Grant Support
This study was supported by grants from the DFG (SFB/TRR77 Liver Cancer - From Molecular Pathogenesis to targeted Therapies to K.B. and P.S.; Ev168/2-1 and Do622/2-1 to M.E. and F.D., respectively), the Helmholtz Alliance Immunotherapy of Cancer (to P.S. and D.F.T.), the Virtual Liver Network (to P.S. and K.B.), the Monika Kutzner Stiftung (to D.F.T.), and NIH R01CA136606 (to X.C.). This project was supported by the tissue bank of the National Center for Tumor Disease (NCT) Heidelberg.
Abbreviations
- CRC
colorectal cancer
- HCC
hepatocellular carcinoma
- Jag1
Jagged-1
- NICD
Notch intracellular domain
- PDAC
pancreatic ductal adenocarcinoma
- TEAD
TEA domain family member
- YAP
yes-associated protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest No conflicts of financial interest to declare.
Transcript Profiling
Microarray information was deposited at the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=jxepvsumwosqkve&acc=GSE35004).
Author Contributions
DF Tschaharganeh - acquisition of data; X Chen - acquisition of data; P Latzko - acquisition of data; M Malz - acquisition of data; MM Gaida - acquisition of data; K Felix - acquisition of tissue samples; S Ladu - acquisition of data; S Singer - acquisition of data; F Pinna - acquisition of data; N Gretz - data interpretation; C Sticht - acquisition of data; ML Tomasi - acquisition of data; S Delogu - acquisition of data; M Evert - acquisition of data; B Fan - acquisition of data; S Ribback - acquisition of tissue samples; L Jiang - acquisition of data; S Brozzetti - acquisition of tissue samples; F Bergmann - collecting data and statistical interpretation; F Dombrowski - study supervision; P Schirmacher - study supervision; DF Calvisi - study supervision, drafting manuscript; K Breuhahn - study supervision, drafting manuscript
References
- 1.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu MZ, Yao TJ, Lee NP, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 13.Cantarini MC, de la Monte SM, Pang M, et al. Aspartyl-asparagyl beta hydroxylase over-expression in human hepatoma is linked to activation of insulin-like growth factor and notch signaling mechanisms. Hepatology. 2006;44:446–457. doi: 10.1002/hep.21272. [DOI] [PubMed] [Google Scholar]
- 14.Qi R, An H, Yu Y, et al. Notch1 signaling inhibits growth of human hepatocellular carcinoma through induction of cell cycle arrest and apoptosis. Cancer Res. 2003;63:8323–8329. [PubMed] [Google Scholar]
- 15.Wang C, Qi R, Li N, et al. Notch1 signaling sensitizes tumor necrosis factorrelated apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expression. J Biol Chem. 2009;284:16183–16190. doi: 10.1074/jbc.M109.002105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Wang XQ, Zhang W, Lui EL, et al. Notch1-Snail1-E-cadherin pathway in metastatic hepatocellular carcinoma. Int J Cancer. 2011 doi: 10.1002/ijc.27336. [DOI] [PubMed] [Google Scholar]
- 17.Bertini E, Oka T, Sudol M, et al. YAP: at the crossroad between transformation and tumor suppression. Cell Cycle. 2009;8:49–57. doi: 10.4161/cc.8.1.7259. [DOI] [PubMed] [Google Scholar]
- 18.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B, Kim J, Ye X, et al. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Zhang Y, Wu H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–E1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodilla V, Villanueva A, Obrador-Hevia A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Ji JY, Yu M, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho E, Feng Y, Rauskolb C, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 25.Varelas X, Samavarchi-Tehrani P, Narimatsu M, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Yi C, Kissil JL. Merlin in organ size control and tumorigenesis: Hippo versus EGFR? Genes Dev. 2010;24:1673–1679. doi: 10.1101/gad.1964810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez LA, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urtasun R, Latasa MU, Demartis MI, et al. Connective tissue growth factor autocriny in human hepatocellular carcinoma: Oncogenic role and regulation by epidermal growth factor receptor/yes-associated protein-mediated activation. Hepatology. 2011;54:2149–2158. doi: 10.1002/hep.24587. [DOI] [PubMed] [Google Scholar]
- 29.Viatour P, Ehmer U, Saddic LA, et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J Exp Med. 2011;208:1963–1976. doi: 10.1084/jem.20110198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu M, Lee DF, Chen CT, et al. IKKalpha Activation of NOTCH Links Tumorigenesis via FOXA2 Suppression. Mol Cell. 2012;45:171–184. doi: 10.1016/j.molcel.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villanueva A, Alsinet C, Yanger K, et al. Notch Signaling is Activated in Human Hepatocellular Carcinoma and Induces Tumor Formation in Mice. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 34.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dill MT, Tornillo L, Fritzius T, et al. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology. 2012 doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Chan SW, Zhang X, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.