Abstract
Nanoscale objects, whether of biologic origin or synthetically created, are being developed into devices for a variety of bionanotechnology diagnostic and pharmaceutical applications. However, the potential immunotoxicity of these nanomaterials and mechanisms by which they may induce adverse reactions have not received sufficient attention. Nanomaterials, depending on their characteristics and compositions, can interact with the immune system in several ways and either enhance or suppress immune system function. Cytokines perform pleiotropic functions to mediate and regulate the immune response and are generally recognized as biomarkers of immunotoxicity. While the specificity and validity of certain cytokines as markers of adverse immune response has been established for chemicals, small and macromolecular drugs, research on their applicability for predicting and monitoring the immunotoxicity of engineered nanomaterials is still ongoing. The goal of this review is to provide guidelines as to important cytokines that can be utilized for evaluating the immunotoxicity of nanomaterials and to highlight the role of those cytokines in mediating adverse reactions, which is of particular importance for the clinical development of nanopharmaceuticals and other nanotechnology-based products. Importantly, the rational design of nanomaterials of low immunotoxicity will be discussed, focusing on synthetic nanodevices, with emphasis on both the nanoparticle-forming materials and the embedded cargoes.
1. Introduction
Nanomedicines are emerging as potential therapeutics and diagnostics for a wide variety of diseases, and have also found uses in vaccine development, engineering, and materials science applications.1–4 As a “depot” for various cargoes, they have been successfully used for delivery of hydrophobic and hydrophilic small molecule drugs (e.g. anticancer drugs) and biomacromolecules, such as recombinant proteins, enzymes, hormones, peptides, and monoclonal antibodies. In addition, they have been used to deliver nucleic acids of various sizes and structures.5–8 The macro- and ultra-structures of these nanosized materials can be tailored to accommodate particular therapeutics and to protect them against hydrolytic or enzymatic degradation, provide the appropriate environment for solubility and for gated drug release. In addition, they can be equipped with “smart” components (antibodies, peptides, proteins, sugars, aptamers, etc.) to aid in their delivery to target organs, tissues and subcellular compartments.9 Several nanomedicine products are already on the market and in different phases of clinical trials, and many more are still under rigorous investigation, optimization and screening to select the most promising candidates for therapeutic and diagnostic applications.
Nanoparticles can interact with various components of the immune system and either enhance or inhibit its function.10–13 Modulation of the immune function by nanomaterials can be useful or detrimental, depending on the intended use.11 Nanoparticles can be designed to be immunomodulatory to serve specific functions (e.g. vaccine adjuvants, anti-inflammatory, immunosuppressive drugs). Concerns are raised, however, when an engineered nanomaterial not intended for interaction with the immune system alters its function. It has been established that certain nanomaterials can be immunotoxic, although no standard immunotoxicity assay has been described thus far that is specific to their nano size.10–15 It is generally agreed that the same set of immunological studies routinely used to assess immunotoxicity of chemicals, medical devices and drugs can be applied to engineered nanomaterials.13
Cytokines are proteins produced by various types of cells including immune cells in response to activation. They play a pivotal role in homeostasis by both modulating and regulating immune response. Cytokine functions are pleiotropic, in that they perform multiple actions and often overlap, acting synergistically or antagonizing each other. This is why cytokine interactions are often referred to as a network. Cytokine release can be characterized by fever, hypotension, nausea, headache, chills, vomiting, and muscle pain, and is the cause of infusion reactions commonly associated with antibody-based biotherapeutics16 which, in some cases, may be life-threatening.17 Hence, it became a common practice in the pharmaceutical industry to monitor cytokines in preclinical studies to understand, prevent and control undesirable cytokine responses to biotherapeutics.
Since nanoparticles can interact with proteins, and proteins, including antibodies, are often used to target nanoparticles to specific cells and tissues, understanding the use of cytokines as biomarkers of undesirable immunostimulation associated with engineered nanomaterials is emerging as an essential component of nanoparticle safety testing. Evaluation of the immunotoxicity of nanomaterials by measuring the levels of cytokines, in particular the proinflammatory cytokines can be useful tools in evaluating nanoparticle immunotoxicity. High levels of cytokines upon treatment with nanoparticles are usually associated with toxicity, adverse reactions and low therapeutic efficacy, as will be discussed later. Hence, cytokines might be utilized to partially predict the nanoparticle immunotoxicity.
Here, we review the possible interactions between the various components of nanomaterials and the immune system with respect to induction of cytokines. We highlight studies demonstrating the utility of cytokines as biomarkers of both desirable immunostimulation and undesirable immunotoxicity of engineered nanomaterials. In addition, the rational design of nanomaterials with low immunogenicity and high therapeutic efficacy is discussed, with special attention to chemical modifications to both drugs and the nanoparticle-forming constituents utilized for their delivery.
2. The immune system
2.1 Structure of the immune system
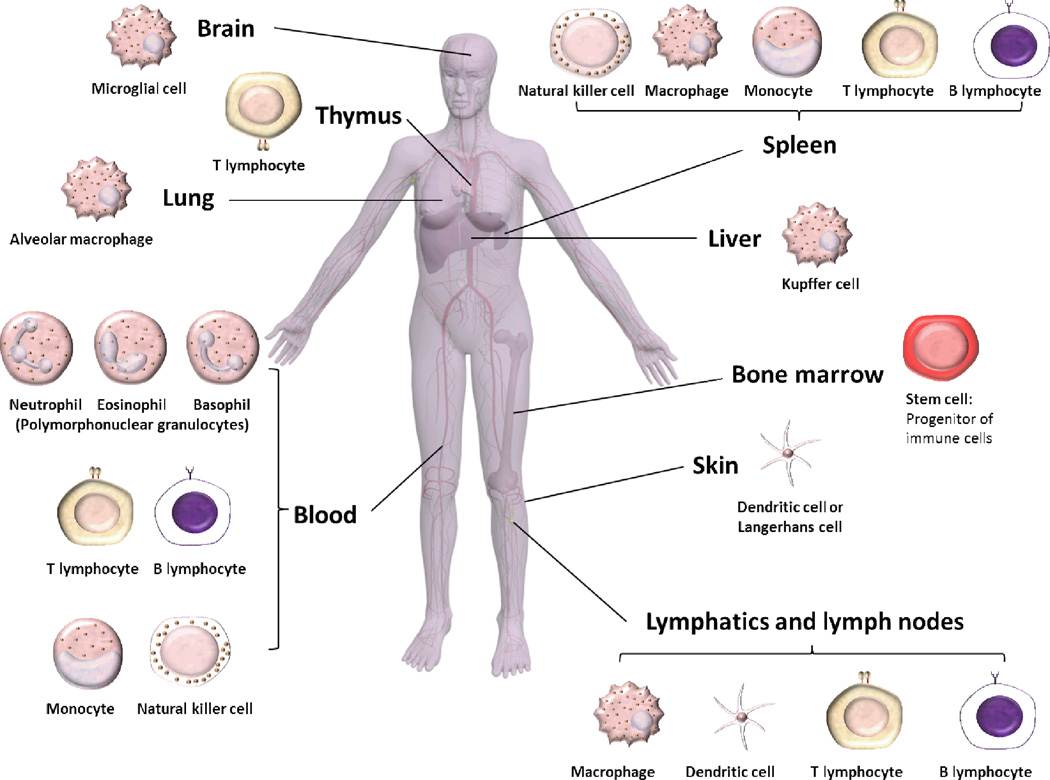
The immune system has a complex architecture comprised of various organs and cell types that interact and communicate via chemical conductors to orchestrate an immune response towards a particular event (Box 1 and Figure 1). The parts of the immune system are connected via the blood and lymphatic circulatory systems. Bone marrow, thymus, spleen, lymph nodes and mucosa-associated lymphoid tissues are the main organs of the immune system, which are involved in the manufacturing, maturation, differentiation, proliferation and storage of immune cells. The blood is composed of red blood cells and white blood cells, which are suspended in the blood together with other molecules, such as, various complement proteins and immunoglobulins. White blood cells (leukocytes), which play the major role in the immune system, are made up mainly of polymorphonuclear granulocytes (PMN), in addition to monocytes, natural killer cells, B and T lymphocytes. PMN are composed mainly of neutrophils (phagocytic cells), along with eosinophils and basophils. T and B lymphocytes, natural killer cells and PMN are the main cells of the immune system. In addition, dendritic cells and macrophages are essential parts of the immune system that act as scavengers or antigen-presenting cells (APCs). Mononuclear phagocytic system (MPS) is the name given to the part of the immune system that consists of phagocytic cells, such as blood monocytes and macrophages accumulated in lymph nodes, liver, spleen and other tissues (Box 1).
Box 1 | The immune system.
Organs of the immune system: Bone marrow, thymus, spleen, lymph nodes and mucosa-associated lymphoid tissues. During hematopoiesis, bone marrow-derived stem cells differentiate into either mature cells or into precursors of cells that migrate out of the bone marrow to continue their maturation elsewhere, for example, maturation of T lymphocytes occurs in the thymus. The spleen acts as an immunologic filter of the blood, whereas the nodes filter the tissue fluids (i.e. lymph). The mucosa-associated lymphoid tissues are aggregates of lymphoid tissues near the mucosal surfaces.
Cells of the immune system: Leukocytes (white blood cells) consist of: (a) Polymorphonuclear granulocytes (PMN): Neutrophils, eosinophils and basophils; (b) Monocytes: can be differentiated into macrophages; (c) Natural killer cells; (d) B lymphocytes: produce antibodies and (e) T lymphocytes: T helper cells, suppressor T cells and cytotoxic T cells. Lymphocytes and mononuclear phagocytes play a central role in the immune response.37
Immune response: Antigen presenting cells (APCs) are cells that capable of processing an antigen and presenting part of it onto the MHC II where it can interact with the appropriate immune cell receptors. Dendritic cells, macrophages and B cells are the main APCs for T cells, whereas follicular dendritic cells are the main APCs for B cells. APCs and B or T cells communicate together, either directly or via cytokines, to initiate an immune response. Antigen processing and presentation signal these cells to proliferate and secrete antibodies (B cells), cytokines (CD4+), or become activated to kill cells expressing the antigen presented by the APC (CD8+). The antibodies secreted by B cells can directly bind to that antigen, which accelerate the clearance by the PMN or macrophages. The antibodies may also initiate the complement destruction cascade by attracting the serum protein to bind to the immobilized antibodies that are bound to the antigen, and thereby aiding in the phagocytosis process and elimination of the immune complex.
Mononuclear phagocytic system (MPS): The original term used to refer to the phagocytic system is reticulo-endothelial system (RES). It refers to the mononuclear cells of mysenchymal origin that reside in the reticular organs including the liver, spleen and lymph nodes and other organs, and possess the collective property of rapidly engulfing colloids and particulate materials. Since not all endothelial and reticular cells are phagocytic, the term RES was recently replaced with MPS, however in the literature, RES and MPS are often used interchangeably. MPS is distributed throughout the body and are mainly responsible for the phagocytosis, clearance and initiation of the immune response due to the introduction of the nanomaterials, foreign particles or antigens in the body, and it also engulfs and clears aged cells in the blood and tissues. Phagocytic cells include PMN, blood monocytes and tissue macrophages (Kupffer cells in the liver, alveolar macrophages in the lung, splenic macrophages, peritoneal cells in the peritoneal fluids, microglial cells in the central nervous tissues, histiocytes of the connective tissues, and dendritic cells). These cells have a number of surface receptors that allow them to bind carbohydrates, complement protein and immunoglobulins. If the MHC II is expressed on the surface of the phagocytes, it enables them to function as APCs. Opsonins (“prepare food for”) are antibodies, complement proteins and other serum components that upon binding to foreign particles make them easier targets for phagocytes.
Complement system: It is a part of the immune system comprised of a biochemical proteolytic cascade that aids (complement) the antibodies and phagocytic cells to eliminate pathogens from the body.
Figure 1.
Human body showing the various organs of the immune system and the distribution of the various immune cells into the immune organs and other organs that are rich in macrophages.
Innate and adaptive immunity refer to in-born and acquired immune defense lines, respectively. Innate immunity is rapid and provides a first line defense, while an adaptive response is more involved and takes more time. Phagocytic cells, NK cells and secondary messenger molecules (e.g. cytokines, eicosanoids, prostaglandins) produced in response to a pathogen challenge, as well as the complement system, are the major players in an innate immune response. The two main types of adaptive immune responses are cell-mediated and humoral- or antibody-mediated immunity, and they are mediated mainly by T and B lymphocytes. The interaction between innate and adaptive immunity is regulated through mediator molecules such as complement proteins and cytokines.
Phagocytic cells (e.g. monocytes, neutrophils, dendritic cells, B cells, platelets and macrophages) can be found in the blood, skin, mucous membranes and in various organs, such as, the liver, spleen, lymph nodes, lung and brain (Box 1). Some of them (e.g. monocytes and neutrophils) roam through the body and act as scavengers to attack and engulf foreign particles, remove pathogens, old or dead cells, and synthesize complement proteins and cytokines that are essential for synchronization of the various parts of the immune system. These cells may attack and engulf particles that are tagged by opsonins (adsorbed molecules that enhance or accelerate clearance by the immune system) or non-opsonized particles.
APCs engulf and digest foreign antigens and present fragments of the antigens on their surface-bound receptors, major histocompatibility complexes (MHC), to other cells of the immune system such as T cells and B cells. There are two types of MHC: MHC I that are found in most of the body tissues, and MHC II that are only present in the APCs. The MHC II receptors wrap the antigen and present it to T cell receptors (TCR), resulting in activation of T cells. T lymphocytes confer cell-mediated immunity and cooperate with B cells, enabling them to secrete antibodies for specific antigens. T lymphocytes include T helper (TH) cells, suppressor T cells and cytotoxic T cells, with the categorization depending on the expressed surface protein. TH cells express CD3 and CD4, whereas the suppressor and cytotoxic T cells express CD3 and CD8 receptors. The T cells also express other receptors. For example, TCR, which can identify a broad range of specific antigens presented on MHC molecules. The CD4+ cells recognize antigens presented on the MHC II by the APCs, whereas CD8+ cells recognize antigens on the MHC I. CD4+ cells are TH0 cells that differentiate into either TH1 or TH2 cells. TH1 cells participate in cell-mediated immunity and regulate the steps of inflammation “T inflammatory cells”, while TH2 cells induce proliferation of mast cells and eosinophils and favor the differentiation of B cells to produce IgG and IgE, thereby promoting humoral immunity.18,19 TH1 cells secrete large quantities of interferon (IFN)-γ, interleukin (IL)-2, IL-3, granulocyte macrophage colony-stimulating factor (GM-CSF) and small quantities of tumor necrosis factor (TNF). TH2 cells secrete large quantities of IL-3, IL-4, IL-5, IL-6, IL-10, and small quantities of GM-CSF and TNF. The cytokines produced by specific TH cells (e.g. IFN-γ from TH1 cells and IL-10 from TH2 cells) usually inhibit the action of other type of T cells and thereby potentiate a particular pattern of immune response. IFN-γ and IL-4 are the main markers of TH1 and TH2 cells, respectively. It is accepted that the main function of TH1 cells is to fight viruses and intracellular pathogens, while that of TH2 cells is controlling humoral response and extracellular pathogens. More recently, other subsets of T cells were discovered which are distinct from TH1 and TH2 cells. They are TH9 cells, which produce IL-9 and are involved in fighting parasites, TH17 cells, which produce IL-17 and are involved in autoimmune response, regulatory T cells (Tregs), characterized by the presence of FoxP3 and antigen-experienced follicular helper T cells, identified by expression of CXCR5.20,21 The TCR and B cell-bound antibodies recognize antigens and initiate an immune response by recruiting other immune cells, cytokines and complement proteins.
2.2 Proinflammatory cytokines
The TH1/TH2 hypothesis, proposed more than 20 years ago, suggested that naïve CD4+ T cells can differentiate into distinct subsets performing various functions to protect the host from certain pathogens.22 The initial studies which provided the background for the TH1/TH2 hypothesis were performed in mice and were later adopted to human cells. The balance between the TH1 and TH2 cytokines was thought to be clinically significant. Some studies have suggested that it should be evaluated23, because this balance is also of particular importance to prevent the occurrence of several diseases (e.g., arthritis, diabetes, asthma, cancer).23–28 However, not all immune responses can be described through TH1/TH2 theory. For example, some substances (e.g. omega-3 fatty acids) have considerable effects on inflammatory and autoimmune conditions without significant shift in TH1/TH2 balance, while other substances (e.g. melanine, probiotics and zinc) affect TH1/TH2 balance but do not cause inflammatory and autoimmune diseases.26 The limitations of this theory were further clarified by the discovery of other types of T effector cells (TH9, TH17, Tregs). However, in spite of this, the type of immunostimulation and its outcome to the host is still often judged by the TH1 and TH2 type cytokines, and most commercially available assays are aimed at the TH1/TH2 panel.
Many cytokines including IL-1, IL-6 and TNF-α activate functions of inflammatory cells during acute inflammatory responses. These cytokines increase the vascular permeability and thus cause swelling and redness associated with inflammation. IL-1 and IL-6 are responsible for fever reactions, TNF-α stimulates endothelial cells and is responsible for hypotension, IL-8 is a chemokine which plays a key role in the activation of neutrophils and their recruitment to the site of inflammation. Many cytokines act together to initiate and regulate the inflammation process. For example, IFN-γ plays a significant role in the inflammatory process, as it can attract macrophages to sites where antigens are present.29 Although TH cells are the major source of cytokines that regulate the immune response, other cells, such as keratinocytes, can produce cytokines that serve as mediators of inflammatory and immunologic reactions in skin exposed to irritants.30–32 These cytokines can also affect the proliferation and differentiation of keratinocytes and control the production of other cytokines.33 These proinflammatory cytokines have been well studied and characterized as participants in the basic inflammatory process and mediators of cellular infiltration.30–32,34–36
3. Nanomaterials
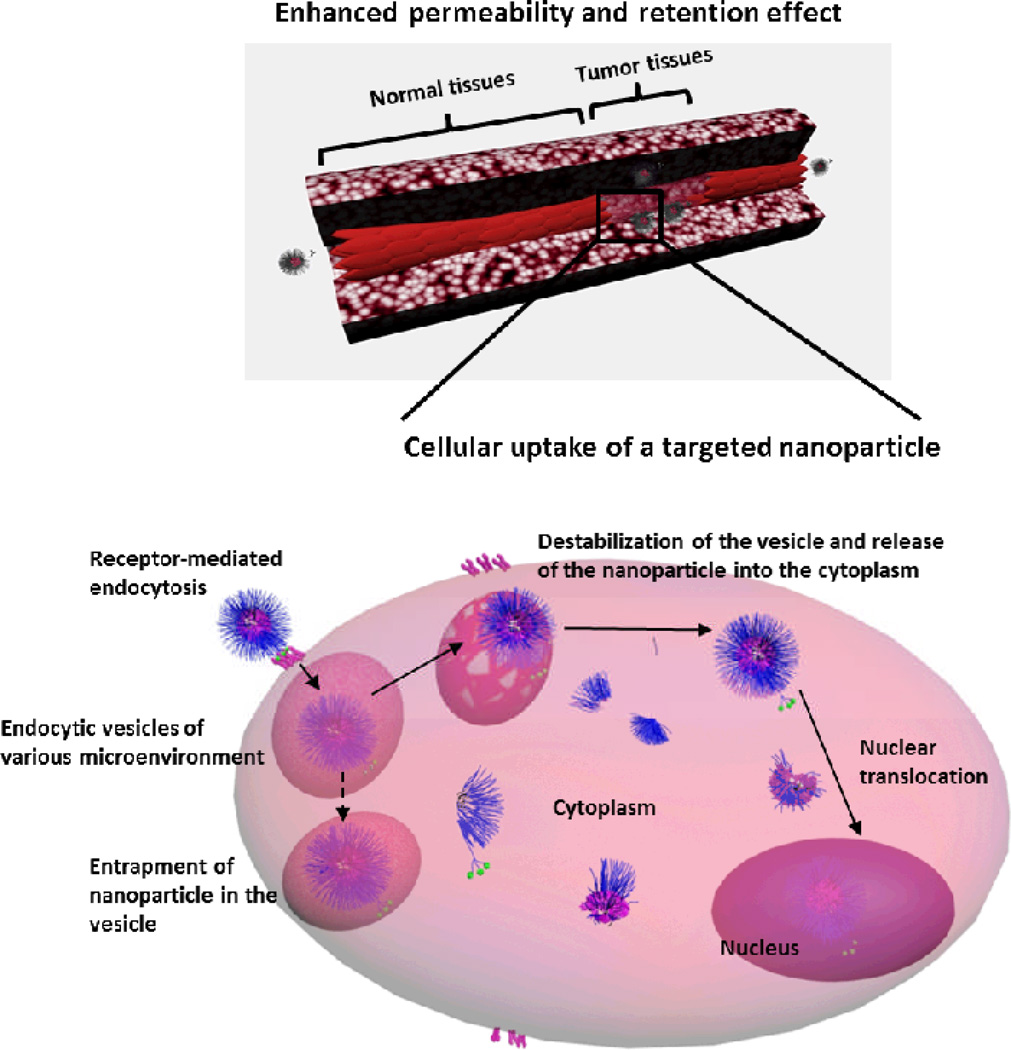
The structure and composition of nanoparticles can be tailored to carry drugs of various sizes and solubilities (Box 2 and Figure 2).4 Nanoparticles can also be engineered to carry charged macromolecules (e.g. amino acids, nucleic acids or enzymes). Attachment or encapsulation of these drugs into nanoparticles can improve their stability, protect them from systemic exposure and recognition by the immune system and control their release. For certain types of engineered nanomaterials, coating with hydrophilic polymers may impart further protection against the external environment and increase blood circulation times. The coating is a non-ionic hydrophilic flexible shell which prevents the adsorption of opsonins, thereby limiting uptake by the MPS and prolonging the circulation half-life of the encapsulated drug. Prolonged circulation times allow for passive targeting of the nanoparticles into tumors via the enhanced permeation and retention (EPR) effect (Figure 3). The EPR effect is explained by the leaky vasculature and impaired lymphatic drainage at tumor sites, which results in the deposition of colloidal particles in tumor peripheries. Once deposited in tissues, the hydrophilic corona facilitates transport of the nanomedicine through the extracellular matrix. Nanoparticles have also been decorated with targeting ligands to allow specific cellular uptake or direct the delivery system to a diseased site, and to circumvent various physiological barriers (Figure 3).2 They can also be equipped with pH- or thermo-responsive (i.e. smart) components to allow release of the drug only in diseased tissues, thereby reducing the toxicity to healthy tissues.4,38
Box 2 | Nanomaterials in biomedicine.
Nanomedicine: “The design of diagnostics and/or therapeutics on the nanoscale, which provides opportunities of coincident transport and delivery of the active species with mediation of their navigation within the biological systems for the treatment, prevention and diagnosis of diseases”.4
Types:
- Organic nanoparticles:
- Polymeric micelles (e.g. NK012, NK105, SP1049C, NC-6004, Genexol): Self-assembly of amphiphilic copolymer chains in aqueous milieu presenting a core/shell architecture with a hydrophobic core and hydrophilic corona. Depending on the structure of the core forming polymer and the forces driving the assembly, they may be classified also as polyion complex micelles or polymer-metal complex micelles.49–51
- Protein-based and polymeric nanoparticles (e.g. Abraxane®, BIND-014): Colloidal particles with a rigid core that are either made from a polymeric or lipidic matrix in which a drug is dissolved or dispersed or from drug nanocrystals stabilized by a polymer.41,53,54 Polymer brushes, unimolecular (e.g. dendrimers) and hyperbranched structures utilized for drug delivery may also fall into this category.55,56
- Lipoplexes and polyplexes (e.g. ALN-VSP): Complexes between nucleic acids (DNA/RNA) and cationic lipids or polymers, and when PEGylated are categorized as PEGylated lipoplexes and polyion complex micelles, respectively.9,60–62 Stable nucleic acid lipid particles (SNALP) may also fall into this category.63,64
- Inorganic nanoparticles:
- Metal and metal-oxide nanoparticles:
- Gold nanoparticles (e.g. Aurimune®): Nanoparticles that display interesting optical and electrical properties.65
- Other nanoparticles: Other kinds of nanoparticles do also exist, such as, titanium oxide, platinum- and diamond-based nanoparticles, etc.
- Quantum dots: Inorganic semiconductor nanoparticles that are widely used as fluorophores for biological imaging.67
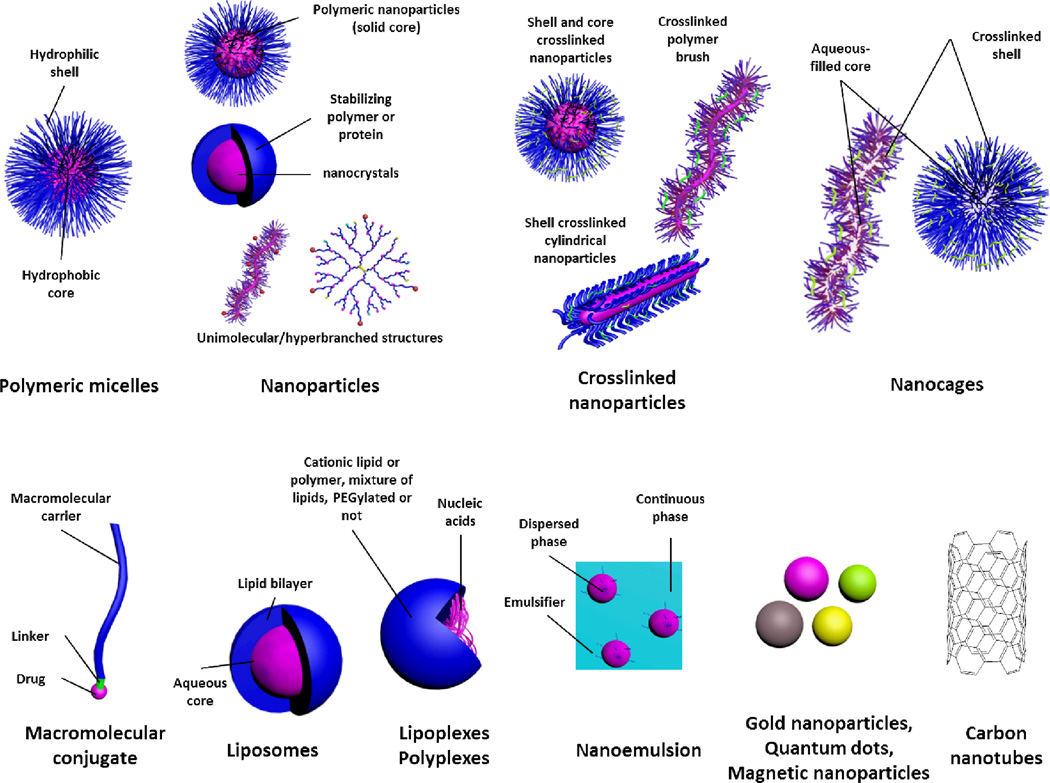
Figure 2.
Common nanoparticulates utilized for delivery of a wide range of therapeutics. Brief explanation of each category is indicated in Box 2.
Figure 3.
Passive and active targeting features of multifunctional nanomaterials. In passive targeting, nanoparticles accumulate into pathological sites with leaky vasculature (e.g. tumor) due to the enhanced permeability and retention effect. In active targeting, the targeting ligands on the surface of nanoparticles enhance cellular uptake by binding to specific receptors overexpressed on the diseased cells, and can also be achieved via facilitating the escape from endosomes and/or enhancing nuclear translocation. Multifunctional nanoparticles have additional functionalities to deliver more than one cargo (e.g. more than one type of therapeutic and/or diagnostic), or combine more than one targeting mechanism (i.e. passive and active targeting). Reproduced by permission of the Royal Society of Chemistry.4
Nanoparticles of different structures and compositions have been developed for biomedical delivery applications, such as, polymeric, lipidic, metallic and graphite-based nanoparticles (Box 2 and Figure 2).1,4,39–42 Much focus has been given to the design of nanoparticles with tailored properties that can accommodate drugs of various sizes, structures and physicochemical properties, and to allow them to circulate for long times in the blood and to circumvent the various physiological barriers encountered on the way to the target sites, and to overcome cellular and subcellular barriers.4 In addition, careful design of the various components of these nanosized particles to reduce toxicity and to enhance biocompatibility of nanoparticles has been shown to significantly enhance the safety of these formulations. For instance, the use of neutral hydrophilic layers on the surface of various nanostructures (e.g. nanocrystals, polymeric or lipidic drug complexes) has been shown to reduce the toxicity of these formulations by limiting the interactions of core components and biomacromolecules.
The benefits of using nanotechnology platforms for drug delivery are often challenged by concerns regarding the safety of these materials. Nanoparticle interactions with components of the immune system represent one category of safety concerns. The potential immunomodulatory effects of nanoparticles need to be understood in order to estimate whether or not nanomaterials are capable of inducing adverse reactions and complications. Information regarding nanoparticle immunotoxicity is also needed to ensure that interactions with the immune system will not affect the therapeutic efficacy of these nanotherapeutics.43,44 Interactions between the various types of nanoparticles (organic and inorganic) and various components of the immune system, including plasma proteins have been described elsewhere.10–15 However, at the time when these reports were prepared, the role of cytokines in nanoparticle-mediated immune responses had not been investigated in depth. This review presents a more recent and comprehensive overview of the currently available literature on this subject, with a focus on engineered nanomaterials.
4. Cytokines as biomarkers of immunomodulatory properties of nanomaterials
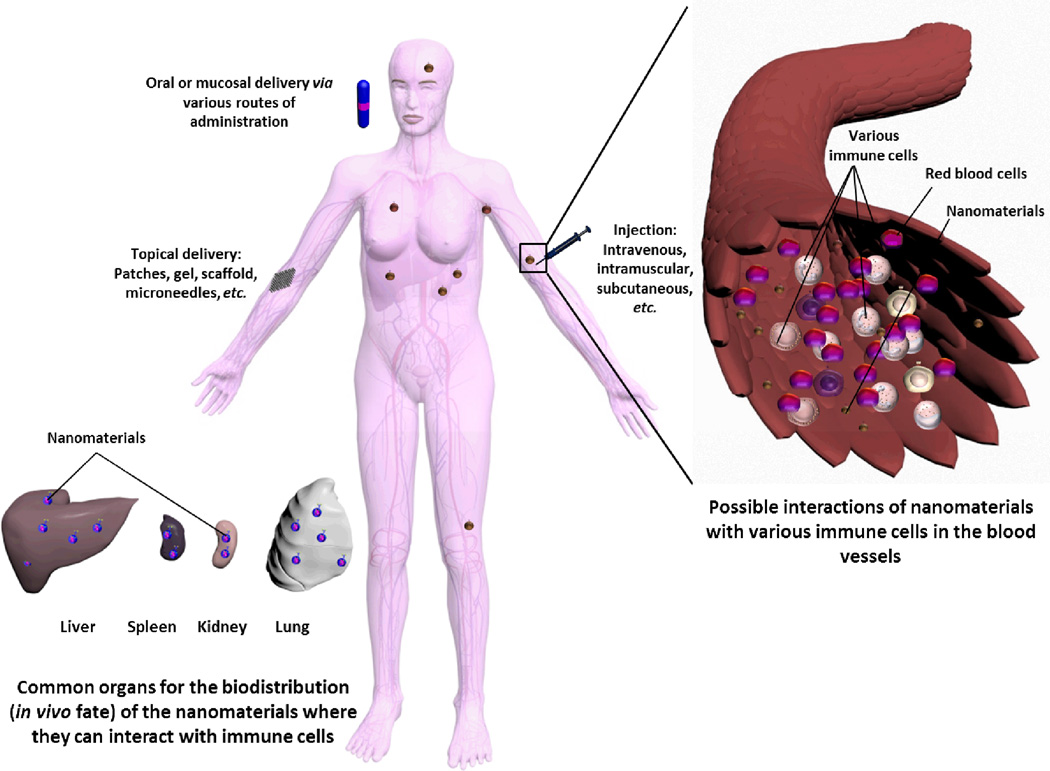
The ability of nanoparticles to induce an immune response (immunogenicity) is a product of the nanoparticles’ physicochemical properties (size, charge, hydrophobicity, etc.). It is also influenced by other factors such as the surface targeting moieties and therapeutic payload, the animal model, and the route of administration. Nanoparticles can reach the systemic circulation via different sites, where the route of administration plays a pivotal role in dictating the fate of the nanomaterials and their immunotoxicity (Figure 4). The immune system may recognize many of the components of nanoparticles (e.g. shell, core, surface-decorating moieties, and cargoes) as foreign, and initiates an immune response through a complex process.
Figure 4.
The possible interactions of nanoparticles with the various components of the immune system after entering the body via various routes of administration (oral, mucosal, systemic or topical).
There are several markers and measures, which can be utilized to study and predict the immune response of biomaterials, such as, lymphocyte proliferation, cell surface markers, morphological and histopathological examination. Soluble mediators, such as, antibodies (e.g. IgG, IgE, IgM), complement proteins (e.g. C3a, C5a), and cytokines (e.g. TH1 and TH2 cytokines) have also been exploited to evaluate the immunogenicity of various therapeutics. This review focuses on utilizing cytokines as biomarkers for nanoparticles immunotoxicity. However, the selection of the functional assay and biomarkers depends on several factors, such as, the structure and composition of the medical device, route of administration and therapeutic application. Combination of several markers might also be useful to understand the underlying mechanisms of immunotoxicity induced by nanomaterials.
4.1 Proinflammatory cytokines as biomarkers of nanoparticle immunotoxicity
The proinflammatory cytokines are often measured to predict immunomodulatory effects of nanomaterials and the possibility of inflammation-mediated toxicity. They may also alter therapeutic outcomes of the active pharmaceutical ingredients delivered through nanomaterials. The administration of nanoparticles may polarize the balance between the TH1and TH2 cytokines towards one specific pathway.14,70–72 For instance, administration of poly-hydroxylated metallofullerenol polarized the cytokine balance towards TH1 cytokines via decreasing the production of TH2 cytokines (IL-4, IL-5 and IL-6), and increasing the production of TH1 cytokines (IL-2, IFN-γ and TNF-α) in the serum of the treated mice.71 In another study, systemic administration of cationic protamine DNA lipoplexes to mice resulted in production of large amounts of proinflammatory cytokines (TNF-α, IL1-β, IL-12 and IFN-γ).44 These cytokines were associated both with toxicities in animals and inhibition of transgene expression (short duration of gene expression and refractoriness to repeated dosing at frequent intervals). The inhibition of gene expression was tested by preinjection of the vehicle and was correlated with the levels of cytokines production. In addition, intraperitoneal injection of dexamethasone suppressed cytokine production and led to higher levels of transgene expression. As commonly recognized, the unmethylated CpG motifs, especially when combined with cationic vehicles are responsible for immunomodulatory effects. It was reported recently that polyethyleneimine (PEI) (linear in vivo-jetPEI), a commonly utilized cationic polymer for nucleic acid delivery, is a selective TLR5 agonist and can elicit the production of TLR5-inducible cytokines in a dose-dependent manner, whereas no secretion of these cytokines were found in the Tlr5−/− mice.36 Intraperitoneal administration of PEI to mice induced significant and selective upregulation of the chemokine KC (the mouse functional homolog of human IL-832,35) 2-h after injection.36 In another study, increased levels of IL-6 and G-CSF were detected after treatment with PEGylated PEI (branched PEI, molecular weight = 25 kDa).34 However, further studies are still required to understand the detailed mechanisms of these immunomodulatory patterns and the particular specificity to PEI. The release of proinflammatory cytokines has also been observed with inorganic nanoparticles of various types and morphologies (titanium dioxide (TiO2), nanodiamond and nanoplatinum) in addition to other manifestations of immunotoxicity, such as dendritic cell maturation and activation and proliferation of naïve T cells.73,74
Nanoparticle-induced triggering of the NLRP3 inflammasome results in secretion of IL-1β, which is a key pro-inflammatory cytokine.75–78 Inflammasomes are multiprotein complexes that act as a major mediator for inflammatory responses. Among the various inflammasomes, NLRP3 inflammasomes are being the most studied and their activation has been linked to exposure of the biological system to nanomaterials of various compositions. For instance, double-walled carbon nanotubes enhanced the release of the pro-inflammatory cytokine IL-1β from human monocytes via NLRP3 inflammasome activation pathway.78 Production of IL-1β has also been observed upon incubation of human monocytes with silver nanoparticles, and the cytokine release was enhanced upon reducing the size of the particles.76 It was found that the silver nanoparticles induced inflammasome formation which triggered the release of the IL-1β.
4.2 Route of administration
Intravenous route
In general, parenteral administration is associated with stronger immune response than oral administration, may be due to the lower bioavailability from the oral route, in contrast to the readily available immune cells to interact with the nanoparticles administered via the parenteral routes. Poly(D,L-lactide-co-glycolide) (PLGA) particles loaded with pertussis toxoid and filamentous haemagglutinin were able to induce potent T cell and antibody response to higher extent when administered parenterally, as compared to the oral immunization, and hence lower dose and less frequent administration were required.79 Nanoparticles introduced into the body via an intravenous (i.v.) route are exposed to a complex environment of blood cells and proteins immediately upon injection. Most immediately adsorb blood proteins on their surfaces. The adsorption of plasma proteins to nanoparticle surfaces, composition of the protein corona and interactions with blood cells determine biodistribution and therapeutic efficacy of the nanoparticles, and may also contribute to immunotoxicity.80,81 Opsonization is considered to be one of the major barriers to nanoparticle stability and delivery in vivo. The most common components of the nanoparticle protein corona are immunoglobulins, complement proteins, albumin, apolipoprotein and fibrinogen.10 Attachment of immunoglobulins and complement to the surface of nanoparticles tags them for attack by the MPS.81 Blood cells exposed to certain nanoparticles may produce cytokines, but the mechanisms of cytokine induction are poorly understood. Unlike traditional antigens (lipoproteins, polysaccharides, DNA, RNA etc.), which induce cytokines after triggering TLRs, it is unknown whether unfunctionalized nanoparticles are recognized by the immune cells through a specific TLR, a combination of TLRs, or by other receptors. It is also unclear whether unfunctionalized nanoparticles are recognized by receptors on the cell surface or upon internalization.
Subcutaneous and dermal routes
Most nanoparticles do not cross intact skin barriers, but can be engineered for dermal or transdermal delivery. Skin is an immunologically active site rich in immune cells such as the epidermal Langerhans’ cells and dermal dendritic cells. This is why delivery of nanoparticles via skin can initiate an inflammatory response. Several studies have demonstrated that small (<50nm) nanoparticles administered subcutaneously distribute through lymphatic drainage into draining lymph nodes where they stimulate antigen-presenting cells and lymphocytes.82,83 Lymphatic drainage is thought to promote an adaptive immune response through several mechanisms involving local complement activation and stimulation of T-cells.82,83 This property has been used in the development of nanoparticle-based vaccines, however, there is no study which demonstrates a similar mechanism for nanomaterials which are not intended for subcutaneous delivery. Subcutaneous administration of PLGA nanoparticles loaded with antigens (tyrosinase-related protein 2 and 7-acyl lipid A) in mice activated dendritic cells and transmitted the immune response to the draining lymph nodes and spleen, as demonstrated by the secretion of IFN-γ at lymph nodes and spleen.84 The levels of the proinflammatory cytokines at the tumor tissues of the treated mice (bearing melanoma B16 tumors) were higher than the control group (mice immunized with empty nanoparticles). The route of administration not only influences the extent of immune response, but also the type of response. Intraperitoneal and intramuscular administration of pertussis toxoid and filamentous haemagglutinin in PLGA microparticles resulted in TH1 response, whereas parenteral and oral immunization of the antigens in solution or loaded into PLGA nanoparticles resulted in TH2 or mixed TH1/TH2 responses.79 The size, surface area, availability of antigens in various formulations and route of administration might explain this difference in responses. In this study, immune response following subcutaneous immunization was inferior to the other parenteral routes of administration (i.e. intraperitoneal and intramuscular).
Nasal and oral routes
Delivery of nanoparticles via nasal and oral routes brings them in contact with the mucosa-associated lymphoid tissues and again may cause interaction with the residing macrophages or lymphocytes with possibilities of subsequent transportation of cells or the immune products to the circulation and other body tissues. Singh et al.85 demonstrated that the intranasal administration of PLGA-b-poly(ε-caprolactone) nanoparticles-loaded with diphtheria toxoid induced higher production of the IL-6 and IFN-γ, as compared to the intramuscular administration of the same nanoparticles. However, this difference cannot be generalized to other types of nanomaterials as it depends on the characteristics of the nanoparticles and the nature of the antigenic payloads.79,86,87 In addition, the site of administration and the type of the APCs that first present the antigen and the cytokine environment at the activation site can all contribute to the type and extent of immune response and to the profile of the induced cytokines.79,87–90
Intraperitoneal
Some researchers consider this route for nanoparticle-mediated drug delivery into tumors and it has been utilized for the treatment of other diseases. However, peritonitis (inflammation in the peritoneum) due to stimulation and recruitment of immune cells as well as systemic distribution of the particles and related off-target toxicities are common concerns. Several studies have demonstrated that certain nanomaterials can distribute systemically, cause inflammation and cytokine responses upon intraperitoneal administration. For example, Liu et al.91 has recently reported that mesoporous hollow silica nanoparticles administered via the intraperitoneal route cause induction of proinflammatory cytokines (IL-1β, TNF-α), liver damage and activation of Kupffer cells. Another study demonstrated that nanosized TiO2 nanoparticles caused oxidative stress, neutrophil activation and inflammation in lungs 4 hours after intraperitoneal administration into mice. Analysis of the bronchoalveolar fluid from these animals has also revealed high levels of inflammatory cytokines TNF-α, IL-1β, and macrophage inflammatory protein (MIP)-2, the functional homolog of the human chemokine IL-8. Elevated gene expression of TNF and IL-1β was also found in lung tissues of animals exposed to TiO2 particles via intraperitoneal route.92 Other examples of off-target toxicity associated with induction of inflammatory cytokine responses by nanomaterials administered via the intraperitoneal route include brain inflammation and activation of microglial cells by co-administration of nano-sized TiO2 particles and low levels of endotoxin93 and allergic sensitization and lung inflammation caused by co-administration of TiO2 nanoparticles and ovalbumin.94
Distribution of nanoparticles from the peritoneum to other compartments and across the blood brain barrier is interesting. The mechanisms of such traffic are poorly understood, but this strategy is being explored for therapeutic applications. For example, intraperitoneal administration of a C60 fullerene derivative into mice 9 hours after challenge with gram negative bacteria reduced inflammatory cytokine levels and protected mice from bacterial meningitis.95 Similarly, intraperitoneal administration of dendrimer antisense oligonucleotide conjugates reduced cytokine secretion associated with infection of Japanese encephalitis.96 Another application is vaccine delivery. For example, antigen-loaded solid lipid nanoparticles were efficient in eliciting TH1 type immune responses and cytokines in mice upon intraperitoneal administration 97
4.3 Endocytic pathways
The endocytic pathway, surface decorating moieties on the nanoparticles and engagement of specific cellular receptors in the endocytosis process dictate the immune response. Phagocytosis is a receptor-mediated endocytosis restricted to professional phagocytes (e.g. macrophages, dendritic cells, platelets). There are four main phagocytic routes, mannose-receptor (MR)-, complement receptor (CR)-, Fcγ receptor (FcγR) and scavenger receptor (SR)-mediated phagocytosis. Mannose, complement proteins, and immunoglobulins are opsonins targeting the MR, CR and FcγR routes, respectively.98 SRs are thought to react with negatively charged surfaces.98 Phagocytic cells can also utilize other routes to engulf and eliminate foreign materials. Some of these pathways are receptor-independent and include various forms of pinocytosis: macropinocytosis, clathrin-dependent, caveolin-dependent and clathrin/caveolin-independent pinocytosis. In addition to phagocytic receptors, immune cells utilize many other surface and transmembrane proteins to sense and endocytose foreign materials. One type of such receptors are Toll-like receptors (TLRs). TLRs are pattern recognition receptors which can either be associated with the membrane surfaces (e.g. TLRs 1, 2, 5, 4 and 6) or localized into the endosomes (e.g. TLRs 3, 7, 8 and 9). Each of these receptors can act alone or in cooperation with other members of this protein family to effectively recognize so-called Pathogen Associated Molecular Patterns (PAMP). Examples of common PAMPs are bacterial lipoproteins, lipopolysaccharides, unmethylated cytosine-phosphate-guanine (CpG) motifs, and single- and double stranded RNA.99,100 Activation of TLRs by their respective PAMPs results in production of various cytokines, initiates inflammation and coordinates cellular and humoral immunity.100,101 There are also other components of the immune system that regulate the immune response, such as cytoplasmic immunoreceptors (e.g. retinoid inducible gene-1 protein, NOD-like receptors (NLR), and RIG-like receptors).102 Activation of these receptors initiates receptor-linked intracellular signaling pathways (e.g. nuclear factor-kappaB (NF-κB), interferon responsive factors (IRF), activating protein-1 (AP-1), interleukin-1 receptor-associated kinase, etc.).101,103
Various surface functionalization strategies have been undertaken to create nanoparticles with “pathogen-like” surface properties. For example, it has been demonstrated that nanoparticles can be engineered to target specific receptors (e.g. MR-mediated phagocytosis) and induce cytokine responses by introducing mannosylated chitosan residues onto their surface.104 In another study, di-mannose and galactose, employed to modify the surface of polyanhydride nanoparticles, resulted in an increase in mannose receptor expression on the surface of alveolar macrophages, which was accompanied by an increased uptake of the particles (presumably through MR-mediated phagocytosis). Binding to the receptors initiated downstream signaling pathway that end up with the production of proinflammatory cytokines (IL-1β, IL-6 and TNF-α), mainly through the activation of NF-κB. The production of the cytokines was higher for the targeted nanoparticles than for the non-targeted ones.105 Likewise, “grafting” the particle surface with C-type lectins was shown to increase their uptake into bone marrow derived dendritic cells and induce inflammatory cytokine secretion.106 Another strategy involves conjugation or encapsulation of specific PAMPs to nanoparticles. For example, it has been demonstrated that TLR ligands such as poly-IC, R848 and lipid A are effectively delivered into immune cells and act as adjuvants through the induction of cytokine secretion.8,84,107 All these strategies are employed to generate deliberate cytokine responses to improve vaccine efficacy. However, nanoparticles unintended to be immunostimulatory (not designed to be vaccine adjuvants), but inducing cytokine responses, do not carry mannose or other ligands which would target the pro-inflammatory pathways. The mechanism of cytokine induction by this category of nanoparticles is less clear. For example, one recent study has shown that polyacrylic acid-conjugated gold nanoparticles interact with the integrin receptor Mac-1 and induce secretion of inflammatory cytokines through a mechanism dependent on the binding and unfolding of fibrinogen.108 Interestingly, not all particles capable of binding plasma fibrinogen shared this property.108 It was proposed that activation of the Mac-1 receptors increases NF-κB activity by increasing the degradation of IκB, which allow the translocation of NF-κB into the nucleus and upregulate the expression of the proinflammatory genes. The fibrinogen-bound PAA-gold nanoparticles induced the release of IL-8 and TNF-α, although fibrinogen and PAA-gold nanoparticles could not induce the release of these cytokines. Inhibition of the NF-κB pathway reduced the release of these cytokines.
4.4 The role of the nanocarriers: Physicochemical characteristics
The extent of opsonization, destabilization and clearance depends mainly on the nanoparticle characteristics.4 The composition of the nanoparticle, the size, charge, morphology, and most importantly, surface chemistry, dictate the toxicity and immune response induced by nanoparticles. The ability of nanoparticles to move into specific regions (e.g. lymphoid tissues) and to activate APCs greatly affects their immunomodulatory effects. Hence, it can be seen how the selective uptake and biodistribution of nanoparticles can greatly affect their immunogenicity. The stronger the interaction between the nanoparticle and cells, the higher the expected immune response. Hence, it is expected that cationic carriers administered via certain routes (e.g. subcutaneous or intradermal) will induce higher immunotoxicity and serve as better adjuvants (i.e. materials not antigenic per se but capable of enhancing the immune response to the antigen). Cationic liposomes were shown to induce the expression of TH1 cytokines, most notably TNF-α, IFN-β and IL-12, which is associated with tumor static effects.109 Cytokine induction was reported for other (non-cationic) nanomaterials as well. For instance, exposure of dendritic cells to zinc oxide nanoparticles with negative zeta potentials (−31 to −36 mV) upregulated the expression of CD80 and CD86 and stimulated the release of proinflammatory cytokines (IL-6 and TNF-α), although there was no observable cytotoxic effects.110 CD80 and CD86 are proteins that can be found on activated B cells, monocytes, and dendritic cells and macrophages that work as co-stimulators for T cells activation (i.e. acting as ligands for proteins on T cell surface), and they are also well-known markers for dendritic cells activation and maturation.
Size
It has been reviewed previously in detail how the size of nanoparticles can determine their cellular uptake, intracellular trafficking pathways, biodistribution and in vivo fate.4,111 It has been generally found that the strength and type of immune response depends also on the size of the nanoparticles.112,113 Although it is still controversial, it has been reported several times that the nanometer sized particles are more toxic than micrometer sized particles, probably due to the increased surface to volume ratio.112,114–119 Hussain et al. have found that even small differences in the size of the particles can cause significant changes in the inflammatory responses.112 The immunotoxicity of carbon black and TiO2 particles with average sizes of (13, 21 and 95 nm) and (15 and 25–75 nm), respectively, were tested at concentrations that are not cytotoxic to cells. The smallest nanoparticles of both types resulted in the highest induction of the GM-CSF cytokine.112 The same study demonstrated also a dose- and cellular uptake-dependent induction of the inflammatory cytokine.
It has been demonstrated that the size of a nanomaterial affects both the type and the strength of the immune response to an antigen. For example, in an interesting study examining the effect of particle size on immune response, nanometer and micrometer (220, 500 and 1200 nm) RNA/protamine particles were utilized.120 It was concluded that the smaller particles elicited viral-like responses by triggering the release of IFN-α, whereas the larger particles developed bacterial-like immune responses and induced the release of TNF-α, whereas neither of the nanoparticle components (RNA or protamine) could increase the level of any of the tested cytokines (IFN-α and TNF-α) in human peripheral blood mononuclear cells. It was suggested that the immune system distinguishes the size of the particles associated with the antigen (i.e. single stranded RNA) to trigger antiviral and antibacterial/antifungal immune responses for the nano- and micro-sized particles, respectively. In other studies, it was observed that only small nanoparticles (about 50 nm) conjugated to a model antigen could induce TH1 response, whereas antigen conjugates to larger particles tend to promote a TH2 response.121–123
Shape and hydrophobicity
The effect of shape of zinc oxide nanoparticles (spherical vs. sheet) of approximately similar specific surface area was studies. Although the higher cellular association of the spherical particles, there were no significant differences in the generation of the reactive oxygen species and in stimulating the secretion of proinflammatory cytokines (IL-6 and TNF-α).110 The secretion of TNF-α was higher in RAW 264.7 mouse macrophages treated with spherical zinc oxide nanoparticles, as compared to the sheet zinc oxide particles. In contrast, the opposite pattern was observed in mouse primary dendritic cells, where the sheet-like nanoparticles induced higher release of TNF-α than the spherical ones. Hence, the cell type is an important factor in dictating the immune response to nanoparticulates. The hydrophobicity is among the factors that affect the immunotoxicity of nanoparticles. For instance, immune response to poly(ε-caprolactone) nanoparticles following both intramuscular and intranasal administration was increased, as compared to PLGA, due to the lower hydrophobicity of the latter.85 The same trend (greater immune response for nanoparticles with higher hydrophobicity) was observed with other particles of varying degrees of hydrophobicities.124
Composition and Surface modifications
The effects of surface modification on the inflammatory effects of silica nanoparticles (30–1000 nm) have been studied by Morishige et al. both in vitro and in vivo.125 The smaller particles (30 and 70 nm) induced higher production of TNF-α than did larger particles in vitro, and stronger inflammatory responses upon intraperitoneal administration. The mechanism involved in the immune toxicity was likely through the production of reactive oxygen species and the activation of mitogen activated protein kinases (MAPKs). Surface modification of the particles with carboxyl groups reduced the activation of MAPKs and subsequently the inflammatory responses both in vitro and in vivo. Partial modification (15%) of cationic shell crosslinked knedel-like nanoparticles with histamine (instead of primary amines) was found to significantly reduce the toxicity and immunotoxicity of the nanoparticles, probably due to the lower charge density (lower primary amine content) which was confirmed by the lower zeta-potential value and lower cellular binding/uptake.126 The immunotoxicities of the 0%- and 15%-Histamine modified particles were studied by measuring the levels of 23 cytokines upon treatment of RAW 264.7 mouse macrophages with the nanoparticles for 24 h. Generally, lower secretion of the cytokines was observed from cells treated with the histamine-modified nanoparticles. A similar trend was observed for most of the tested cytokines, although the differences between the levels of the secreted cytokines were significant for 12 cytokines, IL-3, IL-6, IL-9, IL-10, IL-12(p40), IL-13, Eotaxin, RANTES, monocyte chemotactic protein (MCP)-1, MIP-1β, KC and TNF-α.
4.5 The contribution of the payloads
The cargo, not only the nanoparticle carrier composition, is of great importance. Several examples will be given in this section to emphasize the effect of the payload on the immunotoxicity of nanoparticles, and will include nucleic acids and taxanes.
The administration of lipoplexes of pDNA or antisense oligonucleotides is usually associated with toxicity. Cationic lipoplexes (composed of DOTAP, cholesterol, protamine and plasmid DNA (pDNA)) stimulated the expression of CD80/CD86 on dendritic cells, and induced the release of TNF-α.109 It was concluded that both DNA and the cationic lipid (DOTAP) are required for full immunostimulation. The immunostimulatory sequences in the plasmid or smaller DNA structures (i.e. bacterial CpG motifs) can activate the immune system via the endosomal TLR9.127,128 The immune stimulation triggers the proliferation of the B cells and natural killer cells and the release of inflammatory cytokines (e.g. IFN-γ, IFN-α/β, IL-6, IL-12, GM-CSF and TNF-α), which may lead to serious local and systemic inflammatory reactions.44,129 It has been reported in clinical and preclinical studies that the administration of pDNA lipoplexes results in mild “flu-like” inflammatory syndrome in humans and increased levels of inflammatory cells and cytokines within the lungs of mice.43,130,131 It is commonly observed that the DNA lipoplexes induce the secretion of inflammatory cytokines by TH1 cells in a CpG-dependent manner132,133 and this immunostimulation is usually associated with reduction of gene expression in a dose-dependent manner.43,134 Although this opens up the possibility of taking advantage of immunostimulatory effects of CpG motifs for vaccination, it might complicate the situation and may result in uncontrollable adverse reactions. In addition, they will be burdensome to the use of these vehicles as platforms for other biomedical delivery applications where immunostimulation is not desirable.
The systemic administration of RNA is often associated with immune stimulation both in vitro and in vivo, predominantly due to the interaction with TLRs 3, 7 and/or 8, and sometimes due to recognition by other immunoreceptors which recognize RNA and subsequently release pro-inflammatory cytokines.135–137 When formulated with lipids, RNA can trigger both the lipid- and RNA-sensing TLRs and cytoplasmic immunoreceptors.101,137–140 As with DNA, the use of cationic lipids for siRNA delivery may result in inflammation and anaphylactic reactions.137,141,142 It has been reported that the systemic administration of lipid siRNA complexes triggers the release of proinflammatory cytokines and enhances the level of serum transaminases in mice at high doses.139,142–144 Examples of the cytokines that are usually induced upon systemic administration of siRNA include IL-1α, IL1-β, IL-6, IL-10, IL-12, keratinocyte-derived cytokine, TNF-α, IFN-α, IFN-γ and MCP-1.101,140,142,144
Immune responses to chemotherapeutic agents are also well-established, and were reported to contribute significantly to their anticancer activity.145 For instance, taxanes are mitotic inhibitors that include paclitaxel and docetaxel. Their anticancer activity is achieved through binding to tubulins, and inhibition of cell mitosis at the G2/M phase through stabilization of the microtubules, which triggers apoptosis.146 However, the immunostimulation induced by taxanes contributes significantly to their anticancer activity.147 It has been reported several times that the biological and immunostimulatory effects of taxanes are similar to those of lipopolysaccharides (bacterial components that elicit strong immune response).147,148 Taxanes activate macrophages to initiate cytotoxicity against tumor cells. Activated macrophages also secrete cytokines (e.g. IL-1β, IL-8, IL-12, GM-CSF and TNF-α), which in turn stimulate the cytotoxic lymphocytes and natural killer cells against cancer cells (tumoricidal activity).147,149
5. Mechanisms of nanoparticle immunotoxicity
The exact mechanism of nanoparticle immunotoxicity and correlation with the endocytic pathways have not been clarified yet, due to the biological variability and dependence of the results on the exact composition of nanomaterials, cell type, cell cycle, animal model, disease status, etc. In addition, most of the studies utilize the various markers to predict the possible immunomodulatory effects of nanomaterials, rather than investigating the exact mechanisms beyond the immunotoxic effects and/or the differences in immune responses to nanoparticles of different size, shape, surface chemistry and composition. Sometimes, the induction of both proinflammatory cytokines (e.g. IL-6 and TNF-α) and anti-inflammatory cytokines (e.g. IL-10) due to unregulated innate immune response (i.e. cytokine storm) makes it harder to understand the underlying mechanisms of immunotoxicity.150,151 Dissection of the mechanism(s) of the proinflammatory response is often complicated by the presence of endotoxin in the nanoparticle formulations. Such contamination is common,152 undesirable and often overlooked. Removal of endotoxin from nanoparticles has been shown to eliminate cytokine response and fever reactions.153,154 Of interest is the increasing data demonstrating that some nanoparticles per se do not induce cytokine response, but significantly enhance a cytokine response initiated by low concentrations of endotoxin. In some cases the mechanism involves the NLR3 inflammasome.155,156 For example, fibrous, TiO2 nanoparticles did not induce cytokines, but when these particles were combined with low, unreactive amounts of endotoxin they resulted in a strong induction of cytokines of the IL-1 family (IL-1β, IL18, IL33) through a cathepsin B-mediated mechanism.156 Cytokine storms and exaggeration of inflammatory reactions to endotoxin were also reported for several environmental nanoparticles.157–160 Another common culprit in undesired cytokine induction by engineered nanomaterials are chemical impurities (e.g. synthesis by-products and metal catalysts). For example, early studies on carbon nanotubes demonstrated that these particles can induce proinflammatory cytokine responses161, but when these same particles were purified from iron contaminants, they were later shown to be non-immunostimulatory and did not induce cytokines.162,163 Although all these complications in studying the mechanisms of immunotoxicity, mechanisms that have been mostly involved in the induction of cytokines release due to treatment of cells or animals with nanomaterials will be briefly discussed in this section.
Recognition of PAMPs derived from various pathogens by TLRs and/or cytoplasmic immunoreceptors (e.g. retinoid inducible gene-1) in the various immune cells stimulates the innate immune response and initiates signaling pathways that activate the transcription factor NF-κB and other pathways (e.g. p38/AP1, PI3K and interferon regulatory factor 3/5/7), which lead to production of proinflammatory cytokines.101,109,164–166 Activation of NF-κB can occur via various mediators, such as, proinflammatory cytokines, TLRs, reactive oxygen species and others.103,164,165 The NF-κB controls the expression of several proinflammatory cytokines and upregulation of costimulatory molecules on dendritic cells, which are required for activation of T cells. In addition, it plays a pivotal role in coordinating innate and adaptive immunity.164–166 This transcription factor presents in the cytoplasmic inactive form that bound to inhibitory proteins called IkBs. Activation of NF-κB upon cell stimulation, which can occur through multiple pathways, phosphorylates and degrades the IκBs, where the free NF-κB translocates from the cytoplasm to the nucleus to control the transcription of several cytokines. The detailed mechanisms of the NF-κB activation and subsequent induction of secreting inflammatory cytokines have been reviewed elsewhere.164–166 Activation of TLRs can occur through the NF-κB and MAPKs and stimulates the production of proinflammatory cytokines, maturation of dendritic cells and expression of costimulatory molecules (e.g. CD80 and CD86) on their surfaces.36,74,109,110 The activated dendritic cells can then migrate to the local lymphoid tissues for antigen presentation. It was reported in one study that cationic liposomes (DOTAP) stimulated the expression of CD80 and CD86 on dendritic cells without inducing the release of TNF-α, due to NF-κB independent pathway.109 Another study suggested the presence of multiple pathways for induction of toxicities and release of cytokines. In this study, pretreatment with inhibitors of multiple pathways (e.g. PI3K, mTOR, p38/AP1 and NF-κB) partially inhibited the release of cytokines induced by siRNA-lipid nanoparticles.101
Recognition of immunostimulatory lipids by TLR2 and TLR4 on the cell surface of macrophages and other cells initiates proinflammatory transcriptional programs, including induction of several cytokines.142,167 In one study, several cytokines (IL-1α, IL-1β, IL-6, KC, IL-10, IFN-γ and TNF-α) were released in the plasma of mice treated with lipid nanoparticles and inflammatory reactions were mainly attributed to the lipid components.142 The mice treated with the same lipid (cationic lipid, CLinDMA), but formulated in emulsion, caused a similar cytokine release response in mice. Pre-treatment with intraperitoneal injection of dexamethasone (glucocorticoid receptor agonist) inhibited the cytokine release and inflammation in multiple tissues in a dose-dependent manner. Usually, activation of this cytoplasmic nuclear hormone receptor inhibits the transcriptional activity of NF-κB and can inhibit multiple pathways of inflammatory reactions.165,168,169
In several other studies, it was reported that both lipids and nucleic acids are required for immunostimulation, which can be explained by several hypotheses.109 One of the possible reasons is that cationic complexes enhance cellular uptake of DNA/RNA. For instance, induction of cytokines and cellular influx in the lung airway were observed following intratracheal administration of an N-[1-(2–3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride/cholesterol/plasmid positively charged complexes in mice.132 The cytokine release was enhanced by complexing the plasmid to the cationic lipid. Another hypothesis is that the synergistic effect is due to separate responses to the nucleic acid and lipid, which results in two different sets of cytokines. In addition, the released cytokines due to treatment with one component may induce the secretion of other cytokines. A potential mechanism could also be the protection afforded by the electrostatic complexation against enzymatic degradation, which sustain the immunostimulatory effect of nucleic acids. Furthermore, the cationic carriers facilitate delivery of DNA/RNA to local lymphoid tissues, where they can activate APCs.
Nanoparticles may also generate large quantities of reactive oxygen species that can trigger the release of proinflammatory cytokines through the activation of NF-κB.110,125 The production of reactive oxygen species might be higher for nanoparticles than microparticles due to the enhanced surface area and surface reactivity.125 Carbon black, TiO2 nanoparticles induced the release of cytokines in bronchial epithelial cell line via oxidative stress.112,170 The inflammatory effect and release of cytokines were inhibited by catalase, an enzyme that protect against oxidative stress by catalyzing the decomposition of hydrogen peroxide into oxygen and water. Crystalline silica nanoparticles generated reactive oxygen species that triggered TNF-receptors, which activated the cellular NF-κB transcription factor and resulted in inflammatory response.34
6. Relationship between cytokines and adverse reactions
Modification of the immune system functions can lead to various consequences ranging from mild adverse reactions to serious and fatal immune complications. Lymphocyte proliferation in response to simulation by an antigen or mitogen is a direct reflection of cellular immunity, and is usually associated with the production of antibodies or cytokines.23 In vitro and in vivo studies are confirming that nanomaterials can interact with the immune system and stimulate the production of proinflammatory cytokines, which are capable of recruiting inflammatory cells including basophils, macrophages, dendritic cells, T cells, neutrophils and eosinophils.10,11,29,37,71,171 The immune response against the nanoparticles or their payloads may be beneficial or detrimental depending on the intended use of the given nanoformulation, its mode of action and the route of administration.11 For example, inflammation and granuloma formation have been observed in tissues exposed to unfunctionalized multiwalled carbon nanotubes.171,172
Cytokines play an important role in orchestrating and controlling the inflammatory process and their continual presence or excessive production can result in serious adverse reactions that can be life-threatening. They are also involved in important processes, such as fetal recognition, placental development and regulation of gene expression during organogenesis.173 They are directly or indirectly involved in pathogenesis of several immune-mediated disorders and their levels are usually altered in patients with cancers, cardiovascular diseases, inflammatory diseases (e.g. rheumatoid arthritis) and infectious diseases (e.g. hepatitis).24,26,174–177 For instance, it was found that the levels of several cytokines (IL-1β, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, TNF, IFN-β, GM-CSF, G-CSF, MCP-1, MIP-1α, Eotaxin) were significantly higher in individuals before disease onset of rheumatoid arthritis than in control subjects and further increased after the disease.24
Cytokines can mediate both local and systemic inflammatory responses, and they can also be distributed throughout the circulation to various sites of activity. Resulting immune system derangement can lead to increased incidence of autoimmune, allergic and even neoplastic diseases. Some cytokines, such as IL-1, IL-2, IL-6, TNF, transforming growth factor (TGF)-β and IFNs are also involved in modulating the expression of several P450 isoforms (i.e. modification of hepatic metabolism).173 Some of these circulating cytokines can enter some organs through fenestrated capillaries where they induce the production of prostaglandins (PG), such as PGE2, a centrally controlled mediator of fever.173 The role of cytokines in the immunotoxicity of small and macromolecules has been established and molecular mechanisms are well understood. Studies connecting cytokines to specific toxicities associated with the use of engineered nanomaterials have been reported, but the molecular mechanisms have yet to be defined. For example, an interesting study was performed to confirm the role of cytokines in inducing toxicities. Systemic administration of siRNA-encapsulated in lipid nanoparticles induced the release of several cytokines such as TNF-α, IL-6, IFN-γ and MCP-1.101 These cytokines were correlated with toxicity. Mild alleviation of the toxic responses was observed when the toxicity of the nanoparticles was re-evaluated in mouse lines deficient in one of these cytokines. Hence, it can be concluded that knocking down one or some of these cytokines is not enough to prevent the toxicity associated with the administration of the siRNA-loaded nanoparticles. Another recent study linked induction of IL-8 to dermal toxicity induced by iron oxide nanoparticles178 and to pulmonary toxicity associated with the in vivo use of various metal oxide nanomaterials.179 Similarly, induction of acute inflammatory cytokines (IL-1, TNF-α, IL-6, IL-8, etc.) was linked to nephrotoxicity associated with the in vivo use of TiO2 nanoparticles.180 Since the analysis of cytokine levels in blood is more accessible than analysis of specific pulmonary, dermal and nephrotoxicity markers, ex vivo analysis of cytokines may contribute to identification and understanding of the mechanisms of these toxicities.
7. Evaluation of nanoparticle immunotoxicity
There are a variety of methods for evaluating the immunotoxicity of nanomaterials both in vitro and in vivo, with each technique subject to specific limitations.13 According to the practical strategies suggested during the National Cancer Institute’s Nanoparticle Immunotoxicity Workshop, it is essential to test for endotoxin contamination before studying the immunotoxicity of nanomaterials in vitro and/or in vivo.13 Recently, a book became available which outlines various approaches that can be pursued for testing the safety of nanomaterials including sterility (endotoxin and microbial contamination) and immunological assays (hemolytic and thrombogenic properties, complement activation, uptake by macrophages and cellular chemotaxis).181 The various assays available to test the toxicity of nanomaterials have also been reviewed elsewhere.182–185
Most cytokines have a broad spectrum of biological activity on a wide variety of cells and are involved in hematopoiesis and recruitment of cells for host defense. Quantifying the levels of specific cytokines in cells, animals or patients treated with nanoparticles is of particular importance because variation in the levels may provide an insight to the mechanisms of immunomodulation. Understanding the underlying mechanisms that induce the secretion of cytokines and the associated adverse reactions is essential in the design of safe nanoparticulates. In addition, cytokines act as mediators of various adverse reactions and provide insight into the prognosis of several diseases. They are also commonly used as therapeutics, for instance, for cancer immunotherapy.186,187 The use of dynamic assays that include the assessment of the immune response to nanoparticles via simultaneous measurements of various cytokines can offer significant advantages, although it may be difficult to predict a clinically relevant response. These levels are often compared to the levels in untreated subjects and can also be compared to the normal physiological range for the cytokine of interest, provided such data is available.188
Multiplexing principles and complications
Principles
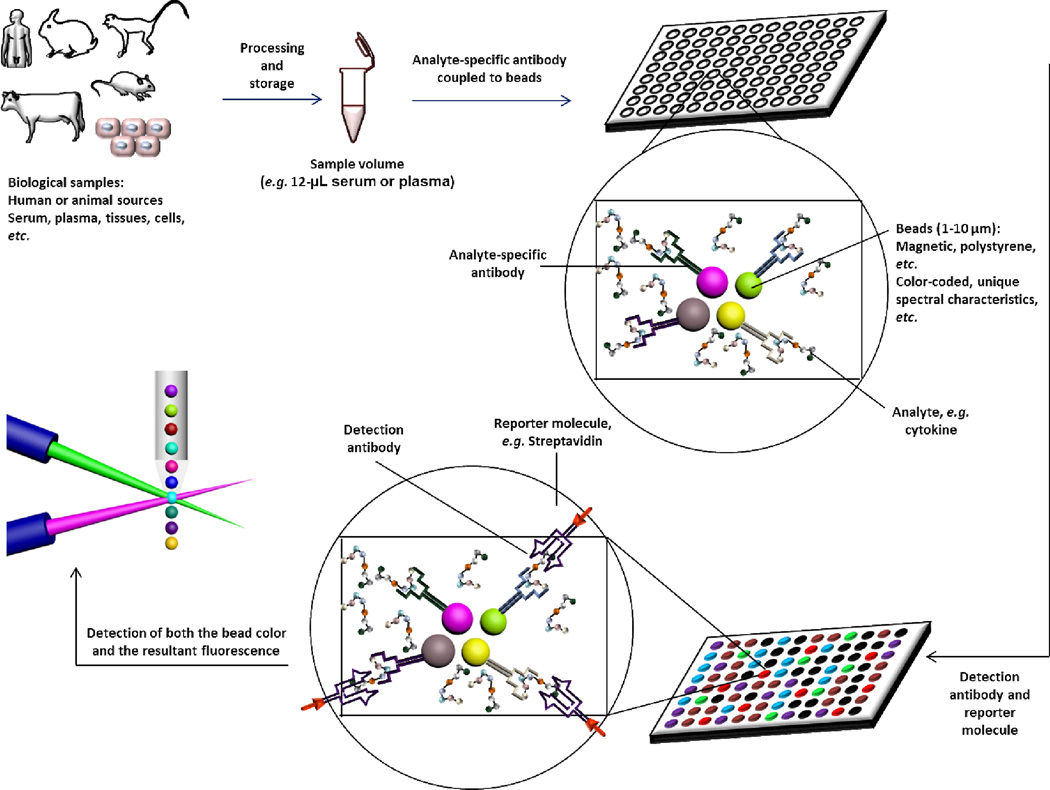
Even in the absence of an acute cellular or in vivo toxicity, elevated cytokines may induce an immune response and result in long-term undesirable immunotoxicity by affecting immune cell function. Hence, it is important to measure cytokines while establishing the safety profiles of engineered nanomaterials. There are various techniques for quantitative measurements of cytokines, proteins, and various inflammatory mediators, with varying degrees of specificity and accuracy. One of the most common and earliest approaches is the enzyme-linked immunosorbent assay (ELISA). More recently, multiplex arrays gained popularity due to the reduced cost per single cytokine and often reduced sample volume.174,189 There are several available technologies that utilize capture antibodies immobilized on microspheres, the most common being the cytometric bead assay, coupled particle light scattering and multi-analyte profiling (xMAP) technology.190,191 This review will not detail the comprehensive procedures or types of these immunoassays, but will highlight the key features of the multiplex arrays as a new strategy for evaluating a large number of biomolecules (up to 100) in a few microliters (e.g. as low as 12 µL of serum or plasma samples) in a single sample with high sensitivity and accuracy.
The multiplex assay is a modified ELISA assay that has been adapted to measure multiple cytokines in the same sample simultaneously by utilizing several techniques, most commonly flow cytometric technology. It is less time-consuming and labor intensive, and requires lower sample volume and provides higher throughput analysis than traditional ELISA assays. The assay is based on incubation of samples from various sources (plasma, serum, tissue culture supernatant, cell or tissue lysates, lavage samples, or other matrices) that contain cytokines or other inflammatory mediators with a mixture of beads (Figure 5). Each bead is coated with specific detection antibodies for every cytokine. The incubation is followed by a series of washes to remove the unbound cytokines. Biotinylated-antibodies are then added to bind to the cytokine-detection antibody complex (on a secondary site of the cytokine), which is conjugated onto the bead to form a sandwich-like complex. The detection products are then formed by the addition of a reporter molecule (e.g. streptavidin-phycoerythrin conjugate) that has a high binding affinity to the biotin (in the biotinylated antibody) and serves at the same time as a reporter or fluorescent indicator (correlated to cytokine concentration). The microscopic beads themselves are then recognized based on their chromogenic or florescence pattern (having unique color codes or spectral properties that correlate with the cytokine type) during the analysis (Figure 5). Although methods of cell stimulation are more or less optimized and harmonized, evaluation of cytokine levels is still subjective and usually conducted based on convenience, affordability and instrument availability in each individual laboratory. This emphasizes the need for greater harmonization and standardization of cytokine evaluation platforms.
Figure 5.
Schematic representation of the steps of multiplex assay for the simultaneous detection and measurements of cytokines in biological samples of various sources. Detailed procedures are not included in this schematic diagram and the detection methods (e.g. color code vs. fluorescence, or the use of various reporters, or the flow cytometric detection) vary depending on the specific instrument and kit utilized for the assay.
Complications
The common limitations of the multiplex arrays include signal “leach-over” between various analytes and the necessity of testing the same sample at multiple dilutions to “concentrate” some low abundance cytokines (e.g. IL4, IL-5) and dilute higher abundance cytokines (e.g. IL-8, TNF-α, IL1) to the assay range. Information on the expected serum levels of various cytokines is required for the proper dilution of samples. For instance, among 48 different cytokines analyzed in human serum, the mean values for most of the cytokines were <100 pg/mL.188 However, some cytokines (e.g. IL-1β, G-CSF, and β-nerve growth factor (NGF)) had levels lower than the detection limit of the instruments (e.g. ~ 1.5 pg/mL for Bio-plex®, Bio-Rad Laboratories, Inc., Hercules, CA), whereas platelet-derived growth factor (PDGF)-BB, regulated upon activation normal T-cell expressed and presumably secreted (RANTES) and stem cell growth factor (SCGF)-β concentrations were too high and required further dilutions.188 Another hypothesis is that the antibody pair and the detection reagents need to be tuned to detect the cytokines that are either below or above the detection limits (i.e. these might be technique-related limitations). The multiplex arrays-based analyses are also extremely sensitive to the handling procedures and good laboratory practices (e.g. pipetting, consistency, use of standard operating procedures, validation, calibration, etc.).
Cytokines have short half-lives, which must be taken into consideration during analysis. Several cytokines have plasma half-lives ranging from minutes to a few hours (e.g. around 6–7 minutes for TNF-α.192,193). Hence, cytokines that can be detected in plasma over a long period of time suggests an intensive production. The timing is of particular importance for evaluating the levels of released cytokines. For instance, the release of 27 cytokines from corneal bilayers treated with nanoparticles was measured 1 hour and 24 hours after exposure to cobalt-chromium nanoparticles. Among the 27 cytokines, IL6, GM-CSF, growth regulated oncogene (GRO), MCP-1 and IL8 were significantly induced, but this was only observed at the 24 hour time point.194 A general trend throughout the literature is that the levels of cytokines change significantly during the time course of the treatment with nanomaterials, both in vitro and in vivo. Hence, it is critical to compare samples that were collected at the same time, and stored, transported and analyzed under identical conditions. In addition to the effect of timing, the possibility of interactions between nanoparticles and the secreted cytokines should also be considered. Adsorption of cytokines (e.g. GM-CSF, IL-6 and TNF-α) on various types of inorganic nanoparticles (e.g. carbon black and TiO2 nanoparticles) has been observed in vitro, which might result in artefacts and misinterpretation of the data.112,170,195,196 Other problems that could further complicate the assay and negatively affect the accuracy of the collected data include the presence of certain proteins in the analyzed samples, particularly the plentiful endogenous proteins in the blood, or the iron produced from blood hemolysis, which may interfere with the formation of cytokine-antibody complexes.
8. Controlling the immunotoxicity of nanomaterials
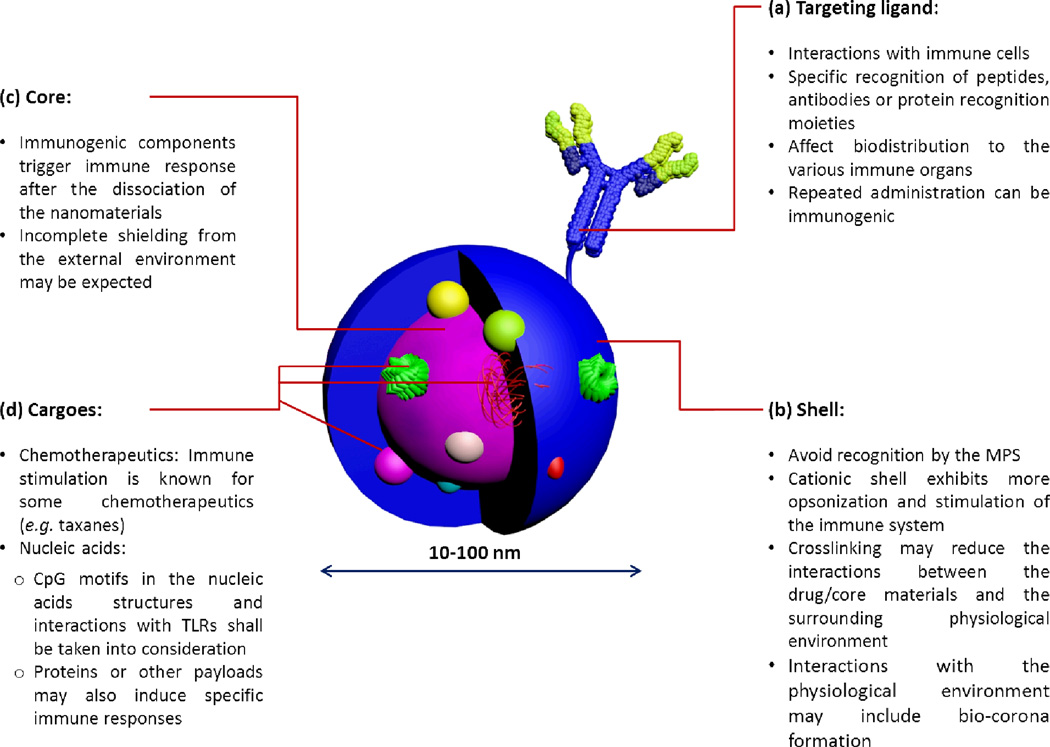
Proper design of nanomaterials has been always a subject of interest to improve their in vitro and in vivo characteristics, mainly to impart greater stability to their cargoes and to prolong blood circulation times to allow the accumulation at target sites, as well as greater safety. However, little impetus has been directed towards designing nanomaterials of low immunogenicity. When designing nanoparticles of low toxicity and immunogenicity, every component in the nanomaterial should be considered and systematic studies should be carried out to evaluate the effect of structural modifications on immunotoxicities. It is equally important to consider both the nanoparticle-forming material and the drug/payload (Figure 6).
Figure 6.
The general composition of a multifunctional nanoparticle for biomedical delivery applications is illustrated with highlighting some important considerations for the design of nanoparticles of low immunogenicity. The size of nanomaterials usually ranges from 10–100 nm.
8.1 Nanoparticle-related factors
The first parameter to be considered in the design of nanoparticles is the shell thickness, density and type, and accessibility of any molecules used for surface decoration (e.g. targeting ligands, contrast agents). Functionalization of PLGA nanoparticles with thiol groups was tested to decrease interactions with opsonins and phagocytic cells, and found to reduce protein adsorption, complement activation, and platelet activation of PLGA nanoparticles.197 Cationic nanoparticles are believed to be more toxic, rapidly-cleared and induce higher inflammatory reactions than their anionic or neutral counterparts. Encapsulation of therapeutics inside the nanocarrier is expected to reduce the immune response induced by the drug. Considering the approximate size of plasma proteins and constituents (~1–10 nm), it is desirable to keep the spacing between PEG chains as small as possible to minimize the interactions between the plasma components and the core material.198–200 Crosslinking the corona by biodegradable crosslinkers is also important to retain the spacing and avoid the dissociation and segregation of the PEG chains. Shell decoration with different moieties is necessary for the preparation of multifunctional nanocarriers. However, it is a prerequisite to keep the functionalization ratio as low as possible. In addition, the use of moieties of low immunogenicity (e.g. galactose instead of antibody for targeting) is recommended. Comprehensive studies to identify the critical parameters (e.g. PEG length and density198–200) that influence the toxicity and immunotoxicity of nanoparticles are required. In vitro studies to determine which blood components are involved in the destabilization/opsonization of nanoparticles is equally important for the rational design of nanoparticles. Polymer biodegradability and biocompatibility are essential for patient safety.
Careful selection of the ingredients of nanomaterials is critical in predicting the immune response upon in vivo administration. Indeed, Huang and coworker have been systemically investigating the effect of the nanoparticle design on the immunotoxicity of nanoparticles designed for siRNA delivery. Targeted nanoparticles-based on anisamide (ligand for sigma receptors overexpressed in some cancer cell lines)-PEGylated liposomes (DOTAP and cholesterol)-protamine-hyaluronic acid complexes (diameter of 115 nm and zeta-potential of 25 mV) were developed for the systemic delivery of siRNA.201 These nanoparticles showed low immunotoxicity in a dose range of 0.15–1.2 mg siRNA/kg in mice. On the contrary, another formulation that has the same composition but uses pDNA or calf thymus DNA instead of the hyaluronic acid had a narrower therapeutic index (e.g. a dose range of 0.15–0.45 mg/kg for the calf thymus DNA).201,202 The differences between the two formulations were the type of the condensing materials that employed to enhance the condensation of siRNA by cationic liposomes (DNA instead of hyaluronic acid). Intravenous injection of the siRNA formulated in both formulations in mice was tested and proinflammatory cytokines (IL-6 and IL-12) levels in serum of treated mice were measured 2-h after injection. The hyaluronic acid-based formulation induced lower immunotoxicity than the calf thymus or the pDNA-based nanoparticles, although both formulations showed similar characteristics and gene silencing activity. The lower immunotoxicity might be due to the removal of the foreign DNA and the CpG immunostimulatory sequences and also because hyaluronic acid is a biogenic compound that widely distributed in the human body fluids. Continuing on their work, nanoparticles built with a degradable calcium phosphate core were utilized instead of the non-degradable ones for the systemic delivery of siRNA and resulted in higher silencing activity in vitro and similar in vivo gene silencing. Importantly, they induced lower immunogenicity in mice as compared to the DNA/protamine-based nanoparticles (lower induction of IL-6 and IL-12 at higher doses).203
8.2 Drug-related factors
Careful design of the drug itself can modulate the immunotoxicity of nanoparticles. For instance, while the injection of pDNA usually induces strong immune responses, designing plasmid structures that do not contain unmethylated CpG motifs could reduce the immunological reactions and enhance the transgene expression efficiency.43 Surprisingly, even a single CpG motif in the DNA structure could elicit an inflammatory response. Methylation of pDNA has also been confirmed to significantly reduce the levels of inflammatory cytokines.44 Similarly, chemical modifications and sequence selection of other small nucleic acids (e.g. siRNA) can greatly modify/amend their immunotoxicity.204 It has been shown in one study that the immunostimulatory response of siRNA could be completely abolished, with no induction of cytokines (e.g. IFN-α and TNF), by designing chemically modified siRNA contains 2'-O-methyl modified nucleosides (<20%) into one of the siRNA strands without affecting their gene-silencing activity, both in vitro and in vivo, whereas the unmodified siRNA induced the induction of cytokines and toxicity in the treated mice.140
9. Conclusions
With the rapid and extensive research now underway into the design of novel nanomedicines, it is critical that attention be directed to their potential immunotoxicity. Evaluation of the immunotoxicity of nanomaterials, for example, by measuring the levels of cytokines or other immune-indicators, is of particular importance for their clinical safety and for maximizing therapeutic benefits. Measuring the levels of proinflammatory cytokines and other inflammatory mediators and monitoring the balance of TH1/TH2 cytokines can be useful tools in evaluating nanoparticle immunotoxicity. Moreover, screening of a single reporter is inadequate; combinations of cytokine levels should be measured, due to the cytokine network influence on the immune system. Monitoring the levels of cytokines, in particular the proinflammatory ones, following administration of nanomaterials, appears to be an important tool for partially screening their immunomodulatory effects. High levels of cytokines, as compared to the untreated controls, are considered as biomarkers of nanoparticle immunotoxicity. The latter is usually associated with toxicity, adverse reactions and lower therapeutic efficacy. Sometimes, treatment with anti-inflammatory drugs might be useful in reducing the inflammatory effects associated with the administration of nanoparticles. Designing nanocarriers with biodegradable cores and coatings that are capable of shielding the core ingredients during circulation can minimize the immune response. Equally important is the design of the drug itself, for example via chemical modifications of nucleic acid (DNA/RNA) backbones to mask or remove immunostimulatory sequences. Further understanding of the underlying mechanisms of nanomaterial-mediated toxicities and inflammatory reactions, and the role of the various chemical mediators and ligands will secure the development of biocompatible and non-immunogenic nanomaterials for a variety of biomedical delivery applications.
Acknowledgments
We gratefully acknowledge financial support from the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C) and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01-DK082546). The Welch Foundation is gratefully acknowledged for support through the W. T. Doherty-Welch Chair in Chemistry, Grant No. A-0001. Dr. Marina A. Dobrovolskaia and Dr. Scott E. McNeil are acknowledged with immense gratitude, for their significant and profound contributions to the scientific contents of this manuscript and the valuable discussions.
Biographies

Mahmoud Elsabahy is Co-Assistant Director of the Laboratory for Synthetic-Biologic Interactions at the Department of Chemistry, Texas A&M University, a Lecturer at the Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Egypt and Director of the Assiut International Center of Nanomedicine. He received a B.S. in Pharmaceutical Sciences from Assiut University, and then he finished his M.Sc. and Ph.D. degrees from the Faculty of Pharmacy, University of Montreal (Canada). Research interests include the rational design and evaluation of pharmaceutical nanocarriers for delivery of therapeutics and diagnostics via various routes of administrations, for which he has received several prestigious awards.

Karen L. Wooley received a B.S. in Chemistry from Oregon State University in 1988 and a Ph.D. in polymer/organic chemistry from Cornell University in 1993. She began as an Assistant Professor at Washington University in St. Louis in 1993, was promoted in 1999 to Full Professor, and was installed as a James S. McDonnell Distinguished University Professor in Arts & Sciences in 2006. In 2009, Karen relocated to Texas A&M University, undertaking a position as the W. T. Doherty-Welch Chair in Chemistry, and being awarded the title of University Distinguished Professor in 2011.
References
- 1.Nystrom AM, Wooley KL. The importance of chemistry in creating well-defined nanoscopic embedded therapeutics: devices capable of the dual functions of imaging and therapy. Acc. Chem. Res. 2011;44:969–978. doi: 10.1021/ar200097k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Maltzahn G, Park JH, Lin KY, Singh N, Schwoppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 4.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang K, Fang H, Wang Z, Li Z, Taylor JS, Wooley KL. Structure-activity relationships of cationic shell-crosslinked knedel-like nanoparticles: shell composition and transfection efficiency/cytotoxicity. Biomaterials. 2010;31:1805–1813. doi: 10.1016/j.biomaterials.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K, Fang H, Shen G, Taylor JS, Wooley KL. Well-defined cationic shell crosslinked nanoparticles for efficient delivery of DNA or peptide nucleic acids. Proc. Am. Thorac. Soc. 2009;6:450–457. doi: 10.1513/pats.200902-010AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacken PJ, Zeelenberg IS, Cruz LJ, van Hout-Kuijer MA, van de Glind G, Fokkink RG, Lambeck AJ, Figdor CG. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood. 2011;118:6836–6844. doi: 10.1182/blood-2011-07-367615. [DOI] [PubMed] [Google Scholar]
- 9.Elsabahy M, Dufresne MH, Leroux JC. In: in Materials for Nanomedicine. Torchilin V, Amiji M, editors. Hackensack: Pan Stanford Publishing; 2010. pp. 169–234. [Google Scholar]
- 10.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 11.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain S, Vanoirbeek JA, Hoet PH. Interactions of nanomaterials with the immune system. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;4:169–183. doi: 10.1002/wnan.166. [DOI] [PubMed] [Google Scholar]
- 13.Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 2009;4:411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- 14.Dwivedi PD, Tripathi A, Ansari KM, Shanker R, Das M. Impact of nanoparticles on the immune system. J Biomed Nanotechnol. 2011;7:193–194. doi: 10.1166/jbn.2011.1264. [DOI] [PubMed] [Google Scholar]
- 15.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34:J234–J246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Breslin S. Cytokine-release syndrome: overview and nursing implications. Clin J Oncol Nurs. 2007;11:37–42. doi: 10.1188/07.CJON.S1.37-42. [DOI] [PubMed] [Google Scholar]
- 17.Schneider CK, Kalinke U, Lower J. TGN1412--a regulator's perspective. Nat Biotechnol. 2006;24:493–496. doi: 10.1038/nbt0506-493. [DOI] [PubMed] [Google Scholar]
- 18.Rajananthanan P, Attard GS, Sheikh NA, Morrow WJ. Novel aggregate structure adjuvants modulate lymphocyte proliferation and Th1 and Th2 cytokine profiles in ovalbumin immunized mice. Vaccine. 1999;18:140–152. doi: 10.1016/s0264-410x(99)00213-3. [DOI] [PubMed] [Google Scholar]
- 19.Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5:7. [PubMed] [Google Scholar]
- 20.Ma CS, Tangye SG, Deenick EK. Human Th9 cells: inflammatory cytokines modulate IL-9 production through the induction of IL-21. Immunol Cell Biol. 2010;88:621–623. doi: 10.1038/icb.2010.73. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R, Shevach EM, Trinchieri G, Mellor AL, Munn DH, Gordon S, Libby P, Hansson GK, Shortman K, Dong C, Gabrilovich D, Gabrysova L, Howes A, O'Garra A. Highlights of 10 years of immunology in Nature Reviews Immunology. Nat Rev Immunol. 2011;11:693–702. doi: 10.1038/nri3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J Immunol. 2005;175:5–14. [PubMed] [Google Scholar]
- 23.Wang J, Chen B, Jin N, Xia G, Chen Y, Zhou Y, Cai X, Ding J, Li X, Wang X. The changes of T lymphocytes and cytokines in ICR mice fed with Fe3O4 magnetic nanoparticles. Int J Nanomedicine. 2011;6:605–610. doi: 10.2147/IJN.S16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Rantapaa Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen TH, Casale TB. Immune modulation for treatment of allergic disease. Immunol Rev. 2011;242:258–271. doi: 10.1111/j.1600-065X.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 26.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 27.Lan Q, Wang SS, Menashe I, Armstrong B, Zhang Y, Hartge P, Purdue MP, Holford TR, Morton LM, Kricker A, Cerhan JR, Grulich A, Cozen W, Zahm SH, Yeager M, Vajdic CM, Schenk M, Leaderer B, Yuenger J, Severson RK, Chatterjee N, Chanock SJ, Zheng T, Rothman N. Genetic variation in Th1/Th2 pathway genes and risk of 58 non-Hodgkin lymphoma: a pooled analysis of three population-based case-control studies. Br J Haematol. 2011;153:341–350. doi: 10.1111/j.1365-2141.2010.08424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002;8:567–573. doi: 10.1038/nm0602-567. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini E, Coico R, Sunshine G. In: Immunology. Margulies DH, editor. New York: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 30.Zhang LW, Yu WW, Colvin VL, Monteiro-Riviere NA. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol Appl Pharmacol. 2008;228:200–211. doi: 10.1016/j.taap.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- 32.Kim BH, Lee YS, Kang KS. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol Lett. 2003;146:65–73. doi: 10.1016/j.toxlet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Grone A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 34.Beyerle A, Irmler M, Beckers J, Kissel T, Stoeger T. Toxicity pathway focused gene expression profiling of PEI-based polymers for pulmonary applications. Mol. Pharm. 2010;7:727–737. doi: 10.1021/mp900278x. [DOI] [PubMed] [Google Scholar]
- 35.Powers MR, Davies MH, Eubanks JP. Increased expression of chemokine KC, an interleukin-8 homologue, in a model of oxygen-induced retinopathy. Curr Eye Res. 2005;30:299–307. doi: 10.1080/02713680590923276. [DOI] [PubMed] [Google Scholar]
- 36.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, Sentman CL, Kedl R, Conejo-Garcia JR. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanier L. In: Basic human immunology. Stites DP, Terr AI, editors. Norwalk, CA: Appleton & Lange; 1991. [Google Scholar]
- 38.Felber AE, Dufresne MH, Leroux JC. pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv. Drug Deliv. Rev. 2012;64:979–992. doi: 10.1016/j.addr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 40.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 41.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-Assembled Targeted Nanoparticles: Evolution of Technologies and Bench to Bedside Translation. Acc. Chem. Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 42.Jain KK. Advances in the field of nanooncology. BMC Med. 2010;8:83. doi: 10.1186/1741-7015-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG, Chan M, Li H, Yew NS, Cheng SH, Boyd AC, Davies JC, Griesenbach U, Porteous DJ, Sheppard DN, Munkonge FM, Alton EW, Gill DR. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat. Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 44.Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10:2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 45.Singer JW. Paclitaxel poliglumex (XYOTAX, CT-2103): a macromolecular taxane. J Control Release. 2005;109:120–126. doi: 10.1016/j.jconrel.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 46.Singer JW, Shaffer S, Baker B, Bernareggi A, Stromatt S, Nienstedt D, Besman M. Paclitaxel poliglumex (XYOTAX; CT-2103): an intracellularly targeted taxane. Anticancer Drugs. 2005;16:243–254. doi: 10.1097/00001813-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Stanberry LR, Simon JK, Johnson C, Robinson PL, Morry J, Flack MR, Gracon S, Myc A, Hamouda T, Baker JR., Jr Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W(80)5EC combined with approved seasonal influenza antigens. Vaccine. 2012;30:307–316. doi: 10.1016/j.vaccine.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 48.Gianella A, Jarzyna PA, Mani V, Ramachandran S, Calcagno C, Tang J, Kann B, Dijk WJ, Thijssen VL, Griffioen AW, Storm G, Fayad ZA, Mulder WJ. Multifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011;5:4422–4433. doi: 10.1021/nn103336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 50.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 51.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control. Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Elsabahy M, Wooley KL. Strategies toward Well-Defined Polymer Nanoparticles Inspired by Nature: Chemistry versus Versatility. J Polym Sci Part A: Polym Chem. 2012;50:1869–1880. doi: 10.1002/pola.25955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuhrmann K, Schulz JD, Gauthier MA, Leroux JC. PEG Nanocages as Non-sheddable Stabilizers for Drug Nanocrystals. ACS Nano. 2012;6:1667–1676. doi: 10.1021/nn2046554. [DOI] [PubMed] [Google Scholar]
- 54.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 55.Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv. Drug Deliv. Rev. 2008;60:886–898. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 57.Simard P, Leroux JC. In vivo evaluation of pH-sensitive polymer-based immunoliposomes targeting the CD33 antigen. Mol. Pharm. 2010;7:1098–1107. doi: 10.1021/mp900261m. [DOI] [PubMed] [Google Scholar]
- 58.Movassaghian S, Moghimi HR, Shirazi FH, Torchilin VP. Dendrosomedendriplex inside liposomes: as a gene delivery system. J Drug Target. 2011;19:925–932. doi: 10.3109/1061186X.2011.628396. [DOI] [PubMed] [Google Scholar]
- 59.Bedi D, Musacchio T, Fagbohun OA, Gillespie JW, Deinnocentes P, Bird RC, Bookbinder L, Torchilin VP, Petrenko VA. Delivery of siRNA into breast cancer cells via phage fusion protein-targeted liposomes. Nanomedicine. 2011;7:315–323. doi: 10.1016/j.nano.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Wolff JA, Rozema DB. Breaking the bonds: non-viral vectors become chemically dynamic. Mol. Ther. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 62.Elsabahy M, Wazen N, Puxan N, Deleavey G, Servant M, Damha MJ, Leroux JC. Delivery of nucleic acids through the controlled disassembly of multifunctional nanocomplexes. Adv. Funct. Mater. 2009;19:3862–3867. [Google Scholar]
- 63.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 64.Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dreaden EC, Mackey MA, Huang X, Kang B, El-Sayed MA. Beating cancer in multiple ways using nanogold. Chem Soc Rev. 2011;40:3391–3404. doi: 10.1039/c0cs00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee JH, Lee K, Moon SH, Lee Y, Park TG, Cheon J. All-in-one target-cellspecific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed. 2009;48:4174–4179. doi: 10.1002/anie.200805998. [DOI] [PubMed] [Google Scholar]
- 67.Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155:344–357. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008;41:60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hattori K, Nishikawa M, Watcharanurak K, Ikoma A, Kabashima K, Toyota H, Takahashi Y, Takahashi R, Watanabe Y, Takakura Y. Sustained exogenous expression of therapeutic levels of IFN-gamma ameliorates atopic dermatitis in NC/Nga mice via Th1 polarization. J Immunol. 2010;184:2729–2735. doi: 10.4049/jimmunol.0900215. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y, Jiao F, Qiu Y, Li W, Lao F, Zhou G, Sun B, Xing G, Dong J, Zhao Y, Chai Z, Chen C. The effect of Gd@C82(OH)22 nanoparticles on the release of Th1/Th2 cytokines and induction of TNF-alpha mediated cellular immunity. Biomaterials. 2009;30:3934–3945. doi: 10.1016/j.biomaterials.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Mohanan D, Slutter B, Henriksen-Lacey M, Jiskoot W, Bouwstra JA, Perrie Y, Kundig TM, Gander B, Johansen P. Administration routes affect the quality of immune responses: A cross-sectional evaluation of particulate antigen-delivery systems. J Control Release. 2010;147:342–349. doi: 10.1016/j.jconrel.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 73.Schanen BC, Karakoti AS, Seal S, Drake DR, 3rd, Warren WL, Self WT. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3:2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghoneum M, Ghoneum A, Gimzewski J. Nanodiamond and nanoplatinum liquid, DPV576, activates human monocyte-derived dendritic cells in vitro. Anticancer Res. 2010;30:4075–4079. [PubMed] [Google Scholar]
- 75.Sun B, Wang X, Ji Z, Li R, Xia T. NLRP3 Inflammasome Activation Induced by Engineered Nanomaterials. Small. 2012 doi: 10.1002/smll.201201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang EJ, Kim S, Kim JS, Choi IH. Inflammasome formation and IL-1beta release by human blood monocytes in response to silver nanoparticles. Biomaterials. 2012;33:6858–6867. doi: 10.1016/j.biomaterials.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 77.Sandberg WJ, Lag M, Holme JA, Friede B, Gualtieri M, Kruszewski M, Schwarze PE, Skuland T, Refsnes M. Comparison of non-crystalline silica nanoparticles in IL-1beta release from macrophages. Part Fibre Toxicol. 2012;9:32. doi: 10.1186/1743-8977-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meunier E, Coste A, Olagnier D, Authier H, Lefevre L, Dardenne C, Bernad J, Beraud M, Flahaut E, Pipy B. Double-walled carbon nanotubes trigger IL-1beta release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine. 2012;8:987–995. doi: 10.1016/j.nano.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Conway MA, Madrigal-Estebas L, McClean S, Brayden DJ, Mills KH. Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: effect of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine. 2001;19:1940–1950. doi: 10.1016/s0264-410x(00)00433-3. [DOI] [PubMed] [Google Scholar]
- 80.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 83.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38:1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 84.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–5057. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 85.Singh J, Pandit S, Bramwell VW, Alpar HO. Diphtheria toxoid loaded poly- (epsilon-caprolactone) nanoparticles as mucosal vaccine delivery systems. Methods. 2006;38:96–105. doi: 10.1016/j.ymeth.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Thomas C, Rawat A, Hope-Weeks L, Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8:405–415. doi: 10.1021/mp100255c. [DOI] [PubMed] [Google Scholar]
- 87.Kumar M, Kong X, Behera AK, Hellermann GR, Lockey RF, Mohapatra SS. Chitosan IFN-gamma-pDNA Nanoparticle (CIN) Therapy for Allergic Asthma. Genet Vaccines Ther. 2003;1:3. doi: 10.1186/1479-0556-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Florindo HF, Pandit S, Goncalves LM, Alpar HO, Almeida AJ. New approach on the development of a mucosal vaccine against strangles: Systemic and mucosal immune responses in a mouse model. Vaccine. 2009;27:1230–1241. doi: 10.1016/j.vaccine.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 89.Morikawa Y, Furotani M, Matsuura N, Kakudo K. The role of antigen-presenting cells in the regulation of delayed-type hypersensitivity. II. Epidermal Langerhans' cells and peritoneal exudate macrophages. Cell Immunol. 1993;152:200–210. doi: 10.1006/cimm.1993.1279. [DOI] [PubMed] [Google Scholar]
- 90.Gajewski TF, Pinnas M, Wong T, Fitch FW. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- 91.Liu T, Li L, Fu C, Liu H, Chen D, Tang F. Pathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticles. Biomaterials. 2012;33:2399–2407. doi: 10.1016/j.biomaterials.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 92.Moon C, Park HJ, Choi YH, Park EM, Castranova V, Kang JL. Pulmonary inflammation after intraperitoneal administration of ultrafine titanium dioxide (TiO2) at rest or in lungs primed with lipopolysaccharide. J Toxicol Environ Health A. 2010;73:396–409. doi: 10.1080/15287390903486543. [DOI] [PubMed] [Google Scholar]
- 93.Shin JA, Lee EJ, Seo SM, Kim HS, Kang JL, Park EM. Nanosized titanium dioxide enhanced inflammatory responses in the septic brain of mouse. Neuroscience. 2010;165:445–454. doi: 10.1016/j.neuroscience.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 94.Larsen ST, Roursgaard M, Jensen KA, Nielsen GD. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol. 2010;106:114–117. doi: 10.1111/j.1742-7843.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsao N, Kanakamma PP, Luh TY, Chou CK, Lei HY. Inhibition of Escherichia coli-induced meningitis by carboxyfullerence. Antimicrob Agents Chemother. 1999;43:2273–2277. doi: 10.1128/aac.43.9.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nazmi A, Dutta K, Basu A. Antiviral and neuroprotective role of octaguanidinium dendrimer-conjugated morpholino oligomers in Japanese encephalitis. PLoS Negl Trop Dis. 2010;4:e892. doi: 10.1371/journal.pntd.0000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doroud D, Zahedifard F, Vatanara A, Najafabadi AR, Rafati S. Cysteine proteinase type I, encapsulated in solid lipid nanoparticles induces substantial protection against Leishmania major infection in C57BL/6 mice. Parasite Immunol. 2011;33:335–348. doi: 10.1111/j.1365-3024.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 98.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 99.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 100.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- 101.Tao W, Mao X, Davide JP, Ng B, Cai M, Burke PA, Sachs AB, Sepp- Lorenzino L. Mechanistically probing lipid-siRNA nanoparticle-associated toxicities identifies Jak inhibitors effective in mitigating multifaceted toxic responses. Mol Ther. 2011;19:567–575. doi: 10.1038/mt.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bortoluci KR, Medzhitov R. Control of infection by pyroptosis and autophagy: role of TLR and NLR. Cell Mol Life Sci. 2010;67:1643–1651. doi: 10.1007/s00018-010-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 104.Kim TH, Nah JW, Cho MH, Park TG, Cho CS. Receptor-mediated gene delivery into antigen presenting cells using mannosylated chitosan/DNA nanoparticles. J Nanosci Nanotechnol. 2006;6:2796–2803. doi: 10.1166/jnn.2006.434. [DOI] [PubMed] [Google Scholar]
- 105.Chavez-Santoscoy AV, Roychoudhury R, Pohl NL, Wannemuehler MJ, Narasimhan B, Ramer-Tait AE. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using "pathogen-like" amphiphilic polyanhydride nanoparticles. Biomaterials. 2012;33:4762–4772. doi: 10.1016/j.biomaterials.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 106.Carrillo-Conde B, Song EH, Chavez-Santoscoy A, Phanse Y, Ramer-Tait AE, Pohl NL, Wannemuehler MJ, Bellaire BH, Narasimhan B. Mannose-functionalized "pathogen-like" polyanhydride nanoparticles target C-type lectin receptors on dendritic cells. Mol Pharm. 2011;8:1877–1886. doi: 10.1021/mp200213r. [DOI] [PubMed] [Google Scholar]
- 107.Hamdy S, Haddadi A, Somayaji V, Ruan D, Samuel J. Pharmaceutical analysis of synthetic lipid A-based vaccine adjuvants in poly (D,L-lactic-co-glycolic acid) nanoparticle formulations. J Pharm Biomed Anal. 2007;44:914–923. doi: 10.1016/j.jpba.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 108.Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 109.Cui Z, Han SJ, Vangasseri DP, Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2:22–28. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 110.Heng BC, Zhao X, Tan EC, Khamis N, Assodani A, Xiong S, Ruedl C, Ng KW, Loo JS. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 2011;85:1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- 111.Iversena TG, Skotlanda T, Sandvig K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today. 2011;6:176–185. [Google Scholar]
- 112.Hussain S, Boland S, Baeza-Squiban A, Hamel R, Thomassen LC, Martens JA, Billon-Galland MA, Fleury-Feith J, Moisan F, Pairon JC, Marano F. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. Toxicology. 2009;260:142–149. doi: 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 115.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, Donaldson K. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64:609–615. doi: 10.1136/oem.2005.024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh S, Shi T, Duffin R, Albrecht C, van Berlo D, Hohr D, Fubini B, Martra G, Fenoglio I, Borm PJ, Schins RP. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol Appl Pharmacol. 2007;222:141–151. doi: 10.1016/j.taap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Wittmaack K. In search of the most relevant parameter for quantifying lung inflammatory response to nanoparticle exposure: particle number, surface area, or what? Environ Health Perspect. 2007;115:187–194. doi: 10.1289/ehp.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Warheit DB, Webb TR, Sayes CM, Colvin VL, Reed KL. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci. 2006;91:227–236. doi: 10.1093/toxsci/kfj140. [DOI] [PubMed] [Google Scholar]
- 120.Rettig L, Haen SP, Bittermann AG, von Boehmer L, Curioni A, Kramer SD, Knuth A, Pascolo S. Particle size and activation threshold: a new dimension of danger signaling. Blood. 2010;115:4533–4541. doi: 10.1182/blood-2009-11-247817. [DOI] [PubMed] [Google Scholar]
- 121.Minigo G, Scholzen A, Tang CK, Hanley JC, Kalkanidis M, Pietersz GA, Apostolopoulos V, Plebanski M. Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy. Vaccine. 2007;25:1316–1327. doi: 10.1016/j.vaccine.2006.09.086. [DOI] [PubMed] [Google Scholar]
- 122.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 123.Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 124.Kreuter J, Liehl E, Berg U, Soliva M, Speiser PP. Influence of hydrophobicity on the adjuvant effect of particulate polymeric adjuvants. Vaccine. 1988;6:253–256. doi: 10.1016/0264-410x(88)90220-4. [DOI] [PubMed] [Google Scholar]
- 125.Morishige T, Yoshioka Y, Inakura H, Tanabe A, Narimatsu S, Yao X, Monobe Y, Imazawa T, Tsunoda S, Tsutsumi Y, Mukai Y, Okada N, Nakagawa S. Suppression of nanosilica particle-induced inflammation by surface modification of the particles. Arch Toxicol. 2012;86:1297–1307. doi: 10.1007/s00204-012-0823-5. [DOI] [PubMed] [Google Scholar]
- 126.Shrestha R, Elsabahy M, Florez-Malaver S, Samarajeewa S, Wooley KL. Endosomal escape and siRNA delivery with cationic shell crosslinked knedel-like nanoparticles with tunable buffering capacities. Biomaterials. 2012;33:8557–8568. doi: 10.1016/j.biomaterials.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoshida H, Nishikawa M, Yasuda S, Mizuno Y, Toyota H, Kiyota T, Takahashi R, Takakura Y. TLR9-dependent systemic interferon-beta production by intravenous injection of plasmid DNA/cationic liposome complex in mice. J. Gene Med. 2009;11:708–717. doi: 10.1002/jgm.1348. [DOI] [PubMed] [Google Scholar]
- 128.Rottembourg D, Filippi CM, Bresson D, Ehrhardt K, Estes EA, Oldham JE, von Herrath MG. Essential role for TLR9 in prime but not prime-boost plasmid DNA vaccination to activate dendritic cells and protect from lethal viral infection. J Immunol. 2010;184:7100–7107. doi: 10.4049/jimmunol.0803935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through tolllike receptor 3. J Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 130.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, Davies J, Smith SN, Browning J, Davies MG, Hodson ME, Durham SR, Li D, Jeffery PK, Scallan M, Balfour R, Eastman SJ, Cheng SH, Smith AE, Meeker D, Geddes DM. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353:947–954. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 131.Ruiz FE, Clancy JP, Perricone MA, Bebok Z, Hong JS, Cheng SH, Meeker DP, Young KR, Schoumacher RA, Weatherly MR, Wing L, Morris JE, Sindel L, Rosenberg M, van Ginkel FW, McGhee JR, Kelly D, Lyrene RK, Sorscher EJ. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum Gene Ther. 2001;12:751–761. doi: 10.1089/104303401750148667. [DOI] [PubMed] [Google Scholar]
- 132.Freimark BD, Blezinger HP, Florack VJ, Nordstrom JL, Long SD, Deshpande DS, Nochumson S, Petrak KL. Cationic lipids enhance cytokine and cell influx levels in 72 the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998;160:4580–4586. [PubMed] [Google Scholar]
- 133.Scheule RK, St George JA, Bagley RG, Marshall J, Kaplan JM, Akita GY, Wang KX, Lee ER, Harris DJ, Jiang C, Yew NS, Smith AE, Cheng SH. Basis of pulmonary toxicity associated with cationic lipid-mediated gene transfer to the mammalian lung. Hum Gene Ther. 1997;8:689–707. doi: 10.1089/hum.1997.8.6-689. [DOI] [PubMed] [Google Scholar]
- 134.Yew NS, Wang KX, Przybylska M, Bagley RG, Stedman M, Marshall J, Scheule RK, Cheng SH. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid:pDNA complexes. Hum Gene Ther. 1999;10:223–234. doi: 10.1089/10430349950019011. [DOI] [PubMed] [Google Scholar]
- 135.Goodchild A, Nopper N, King A, Doan T, Tanudji M, Arndt GM, Poidinger M, Rivory LP, Passioura T. Sequence determinants of innate immune activation by short interfering RNAs. BMC Immunol. 2009;10:40. doi: 10.1186/1471-2172-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sioud M. RNA interference and innate immunity. Adv Drug Deliv Rev. 2007;59:153–163. doi: 10.1016/j.addr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 137.Judge A, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Hum Gene Ther. 2008;19:111–124. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- 138.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 139.Kim JY, Choung S, Lee EJ, Kim YJ, Choi YC. Immune activation by siRNA/liposome complexes in mice is sequence- independent: lack of a role for Toll-like receptor 3 signaling. Mol Cells. 2007;24:247–254. [PubMed] [Google Scholar]
- 140.Judge AD, Bola G, Lee AC, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 141.Sepp-Lorenzino L, Ruddy M. Challenges and opportunities for local and systemic delivery of siRNA and antisense oligonucleotides. Clin Pharmacol Ther. 2008;84:628–632. doi: 10.1038/clpt.2008.174. [DOI] [PubMed] [Google Scholar]
- 142.Abrams MT, Koser ML, Seitzer J, Williams SC, DiPietro MA, Wang W, Shaw AW, Mao X, Jadhav V, Davide JP, Burke PA, Sachs AB, Stirdivant SM, Sepp- Lorenzino L. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol Ther. 2010;18:171–180. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 144.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 145.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 146.Yeung TK, Germond C, Chen X, Wang Z. The mode of action of taxol: apoptosis at low concentration and necrosis at high concentration. Biochem Biophys Res Commun. 1999;263:398–404. doi: 10.1006/bbrc.1999.1375. [DOI] [PubMed] [Google Scholar]
- 147.Javeed A, Ashraf M, Riaz A, Ghafoor A, Afzal S, Mukhtar MM. Paclitaxel and immune system. Eur J Pharm Sci. 2009;38:283–290. doi: 10.1016/j.ejps.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 148.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 149.Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, Mistry Y, Dilger P, Liefooghe E, Cludts I, Fox B, Tarrant G, Robinson J, Meager T, Dolman C, Thorpe SJ, Bristow A, Wadhwa M, Thorpe R, Poole S. "Cytokine storm" in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 151.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 152.Jones CF, Grainger DW. In vitro assessments of nanomaterial toxicity. Adv Drug Deliv Rev. 2009;61:438–456. doi: 10.1016/j.addr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006;6:1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- 154.Dobrovolskaia MA, Neun BW, Clogston JD, Ding H, Ljubimova J, McNeil SE. Ambiguities in applying traditional Limulus amebocyte lysate tests to quantify endotoxin in nanoparticle formulations. Nanomedicine (Lond) 2010;5:555–562. doi: 10.2217/nnm.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reisetter AC, Stebounova LV, Baltrusaitis J, Powers L, Gupta A, Grassian VH, Monick MM. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem. 2011;286:21844–21852. doi: 10.1074/jbc.M111.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Inoue K, Takano H. Aggravating impact of nanoparticles on immune-mediated pulmonary inflammation. ScientificWorldJournal. 2011;11:382–390. doi: 10.1100/tsw.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Inoue K, Takano H, Yanagisawa R, Hirano S, Kobayashi T, Fujitani Y, Shimada A, Yoshikawa T. Effects of inhaled nanoparticles on acute lung injury induced by lipopolysaccharide in mice. Toxicology. 2007;238:99–110. doi: 10.1016/j.tox.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 159.Inoue K, Takano H, Yanagisawa R, Hirano S, Sakurai M, Shimada A, Yoshikawa T. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ Health Perspect. 2006;114:1325–1330. doi: 10.1289/ehp.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi Y, Yadav S, Wang F, Wang H. Endotoxin promotes adverse effects of amorphous silica nanoparticles on lung epithelial cells in vitro. J Toxicol Environ Health A. 2010;73:748–756. doi: 10.1080/15287391003614042. [DOI] [PubMed] [Google Scholar]
- 161.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual 76 inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 162.Murray AR, Kisin E, Leonard SS, Young SH, Kommineni C, Kagan VE, Castranova V, Shvedova AA. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology. 2009;257:161–171. doi: 10.1016/j.tox.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 163.Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 164.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 166.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 167.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 168.Morand EF. Effects of glucocorticoids on inflammation and arthritis. Curr Opin Rheumatol. 2007;19:302–307. doi: 10.1097/BOR.0b013e32805e87d0. [DOI] [PubMed] [Google Scholar]
- 169.Moynagh PN. Toll-like receptor signalling pathways as key targets for mediating the anti-inflammatory and immunosuppressive effects of glucocorticoids. J Endocrinol. 2003;179:139–144. doi: 10.1677/joe.0.1790139. [DOI] [PubMed] [Google Scholar]
- 170.Val S, Hussain S, Boland S, Hamel R, Baeza-Squiban A, Marano F. Carbon black and titanium dioxide nanoparticles induce pro-inflammatory responses in bronchial 77 epithelial cells: need for multiparametric evaluation due to adsorption artifacts. Inhal Toxicol. 2009;21:115–122. doi: 10.1080/08958370902942533. [DOI] [PubMed] [Google Scholar]
- 171.Witzmann FA, Monteiro-Riviere NA. Multi-walled carbon nanotube exposure alters protein expression in human keratinocytes. Nanomedicine. 2006;2:158–168. doi: 10.1016/j.nano.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 172.Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, Macnee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- 173.Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett. 2011;203:97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 174.Khan IH, Krishnan VV, Ziman M, Janatpour K, Wun T, Luciw PA, Tuscano J. A comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom. 2009;76:159–168. doi: 10.1002/cyto.b.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shiraki T, Takayama E, Magari H, Nakata T, Maekita T, Enomoto S, Mori Y, Shingaki N, Moribata K, Deguchi H, Ueda K, Inoue I, Mizuno-Kamiya M, Yashiro K, Iguchi M, Tamai H, Kameyama Y, Kato J, Kondoh N, Ichinose M. Altered cytokine levels and increased CD4+CD57+ T cells in the peripheral blood of hepatitis C virus-related hepatocellular carcinoma patients. Oncol Rep. 2011;26:201–208. doi: 10.3892/or.2011.1258. [DOI] [PubMed] [Google Scholar]
- 176.Vistnes M, Waehre A, Nygard S, Sjaastad I, Andersson KB, Husberg C, Christensen G. Circulating cytokine levels in mice with heart failure are etiology dependent. J Appl Physiol. 2010;108:1357–1364. doi: 10.1152/japplphysiol.01084.2009. [DOI] [PubMed] [Google Scholar]
- 177.Tashiro H, Shimokawa H, Yamamoto K, Momohara M, Tada H, Takeshita A. Altered plasma levels of cytokines in patients with ischemic heart disease. Coron Artery Dis. 1997;8:143–147. doi: 10.1097/00019501-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 178.Murray AR, Kisin E, Inman A, Young SH, Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V, Fadeel B, Kagan VE, Riviere JE, Monteiro-Riviere N, Shvedova AA. Oxidative Stress and Dermal Toxicity of Iron Oxide Nanoparticles In Vitro. Cell Biochem Biophys. doi: 10.1007/s12013-012-9367-9. [DOI] [PubMed] [Google Scholar]
- 179.Cho WS, Duffin R, Poland CA, Duschl A, Oostingh GJ, Macnee W, Bradley M, Megson IL, Donaldson K. Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology. 2012;6:22–35. doi: 10.3109/17435390.2011.552810. [DOI] [PubMed] [Google Scholar]
- 180.Gui S, Zhang Z, Zheng L, Cui Y, Liu X, Li N, Sang X, Sun Q, Gao G, Cheng Z, Cheng J, Wang L, Tang M, Hong F. Molecular mechanism of kidney injury of mice caused by exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;195:365–370. doi: 10.1016/j.jhazmat.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 181.McNeil SE, editor. Characterization of nanoparticles intended for drug delivery. New York: Human Press; 2011. [Google Scholar]
- 182.Arora S, Rajwade JM, Paknikar KM. Nanotoxicology and in vitro studies: the need of the hour. Toxicol Appl Pharmacol. 2012;258:151–165. doi: 10.1016/j.taap.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 183.Stone V, Johnston H, Schins RP. Development of in vitro systems for nanotoxicology: methodological considerations. Crit Rev Toxicol. 2009;39:613–626. doi: 10.1080/10408440903120975. [DOI] [PubMed] [Google Scholar]
- 184.Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: limitations and challenges. Eur J Pharm Biopharm. 2009;72:370–377. doi: 10.1016/j.ejpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 185.Marquis BJ, Love SA, Braun KL, Haynes CL. Analytical methods to assess nanoparticle toxicity. Analyst. 2009;134:425–439. doi: 10.1039/b818082b. [DOI] [PubMed] [Google Scholar]
- 186.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat. Rev. Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mahvi DM, Henry MB, Albertini MR, Weber S, Meredith K, Schalch H, Rakhmilevich A, Hank J, Sondel P. Intratumoral injection of IL-12 plasmid DNA-results of a phase I/IB clinical trial. Cancer Gene Ther. 2007;14:717–723. doi: 10.1038/sj.cgt.7701064. [DOI] [PubMed] [Google Scholar]
- 188.Chapman P, Reyes C, Gupta V. Normal Physiological Levels of Human Cytokines Using Bio-Plex Pro™ Cytokine Assays. Bio-Rad Tech Note. 2010;6029 [Google Scholar]
- 189.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bernhard OK, Mathias RA, Barnes TW, Simpson RJ. A fluorescent microsphere-based method for assay of multiple analytes in plasma. Methods Mol Biol. 2011;728:195–206. doi: 10.1007/978-1-61779-068-3_12. [DOI] [PubMed] [Google Scholar]
- 192.Munoz C, Misset B, Fitting C, Bleriot JP, Carlet J, Cavaillon JM. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol. 1991;21:2177–2184. doi: 10.1002/eji.1830210928. [DOI] [PubMed] [Google Scholar]
- 193.Beutler BA, Milsark IW, Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985;135:3972–3977. [PubMed] [Google Scholar]
- 194.Sood A, Salih S, Roh D, Lacharme-Lora L, Parry M, Hardiman B, Keehan R, Grummer R, Winterhager E, Gokhale PJ, Andrews PW, Abbott C, Forbes K, Westwood M, Aplin JD, Ingham E, Papageorgiou I, Berry M, Liu J, Dick AD, Garland RJ, Williams N, Singh R, Simon AK, Lewis M, Ham J, Roger L, Baird DM, Crompton LA, Caldwell MA, Swalwell H, Birch-Machin M, Lopez-Castejon G, Randall A, Lin H, Suleiman MS, Evans WH, Newson R, Case CP. Signalling of DNA damage and cytokines across cell barriers exposed to nanoparticles depends on barrier thickness. Nat Nanotechnol. 2011;6:824–833. doi: 10.1038/nnano.2011.188. [DOI] [PubMed] [Google Scholar]
- 195.Kocbach A, Totlandsdal AI, Lag M, Refsnes M, Schwarze PE. Differential binding of cytokines to environmentally relevant particles: a possible source for misinterpretation of in vitro results? Toxicol Lett. 2008;176:131–137. doi: 10.1016/j.toxlet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 196.Veranth JM, Kaser EG, Veranth MM, Koch M, Yost GS. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part Fibre Toxicol. 2007;4:2. doi: 10.1186/1743-8977-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thasneem YM, Sajeesh S, Sharma CP. Effect of thiol functionalization on the hemo-compatibility of PLGA nanoparticles. J Biomed Mater Res A. 2011;99:607–617. doi: 10.1002/jbm.a.33220. [DOI] [PubMed] [Google Scholar]
- 198.Sun G, Hagooly A, Xu J, Nystrom AM, Li Z, Rossin R, Moore DA, Wooley KL, Welch MJ. Facile, efficient approach to accomplish tunable chemistries and variable biodistributions for shell cross-linked nanoparticles. Biomacromolecules. 2008;9:1997–2006. doi: 10.1021/bm800246x. [DOI] [PubMed] [Google Scholar]
- 199.Fukukawa K, Rossin R, Hagooly A, Pressly ED, Hunt JN, Messmore BW, Wooley KL, Welch MJ, Hawker CJ. Synthesis and characterization of core-shell star copolymers for in vivo PET imaging applications. Biomacromolecules. 2008;9:1329–1339. doi: 10.1021/bm7014152. [DOI] [PubMed] [Google Scholar]
- 200.Pressly ED, Rossin R, Hagooly A, Fukukawa K, Messmore BW, Welch MJ, Wooley KL, Lamm MS, Hule RA, Pochan DJ, Hawker CJ. Structural effects on the biodistribution and positron emission tomography (PET) imaging of well-defined (64)Cu-labeled 81 nanoparticles comprised of amphiphilic block graft copolymers. Biomacromolecules. 2007;8:3126–3134. doi: 10.1021/bm700541e. [DOI] [PubMed] [Google Scholar]
- 201.Chono S, Li SD, Conwell CC, Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008;131:64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Watts JK, Deleavey GF, Damha MJ. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]